Abstract
The main objective of the present study was to test the hypothesis that exogenous insulin would enhance colon carcinogenesis. Thirty-six female F344 rats, fed ad libitum a low fat rodent chow, received a single azoxymethane injection (20 mg/kg), and were randomized a week later to two groups. Control rats were given 5 days a week a s.c. saline injection, and experimental rats were given ultralente bovine insulin, 20 U/kg. The promoting effect of insulin injections was assessed by the multiplicity (number of crypts) of aberrant crypt foci after 100 d of treatment (72 injections). The rats given insulin ate more and were heavier than controls (215 ± 11 vs. 182 ± 7 g, p<0.001). Insulin injections also increased the amount of abdominal fat, the plasma triglycerides, and the insulinemia, and decreased blood glucose (all p<0.05). The number of aberrant crypt foci was the same in both groups, but their multiplicity was significantly increased by the insulin injections (2.8 ± 0.3 vs. 2.5 ± 0.2 crypt/focus in controls, p=0.007). Besides, the proportion of sialomucin producing foci was higher in insulin injected rats than in controls (p=0.04). These data show that exogenous insulin can promote colon carcinogenesis in rats, and suggest that lifestyle and diets leading to low blood insulin might protect humans against colorectal cancer.
Keywords: insulin, colon : carcinogenesis, cancer promotion, rat, azoxymethane, ACF, aberrant crypt foci abdominal fat, plasma triglycerides, blood glucose, sialomucin, sulfomucin, colorectal cancer, prevention, diet
Introduction
The current hypothesis linking diet and colon cancer is that a high fat, low fiber and low calcium diet increases the cancer risk, by increasing the concentration of promoting bile acids in the lumen of the gut (1). However randomized intervention trials assessing the recurrence of polyps in volunteers given highfiber low-fat diets do not support this hypothesis (2, 3). Although most studies focus on luminal factors in the fecal stream, it is also possible that the risk factor reaches the colonic cells by way of the general circulation (4). The major risk factors for colorectal cancer are the followings: sedentary lifestyle, high caloric intake, and diets high in fat, sucrose and ethanol, and low in fiber, resistant starch and n-3 polyunsaturated fatty acids (1, 4). These factors are associated with elevated serum triglycerides and plasma glucose (5). High blood glucose and lipids influence circulating hormones, such as insulin, and can lead to insulin resistance, abdominal obesity, and eventually, non-insulin dependant diabetes (5). McKeown-Eyssen speculated that blood triglycerides, glucose, or insulin could promote cancer development (5). The idea was later supported by other reviews on insulin and colon cancer (6), or insulin and breast cancer (7, 8). We thus decided to study the direct effect of repeated insulin injections on rat colon carcinogenesis.
Promotion of cancer was assessed with the aberrant crypt focus (ACF) assay (9). ACF are putative precursors of colon cancer (10), specifically induced by colon carcinogens (11), promoted by promoting diets (12), inhibited by inhibitors of carcinogenesis (13). In rodents and humans, ACF display mutations and histologic changes observed in colonic tumors (14, 15, 16), and the growth of ACF correlates with the adenocarcinoma yield (17, 18, 19). The number of ACF per animal is an assay for initiators of colon cancer, although the number of crypts per focus (multiplicity) is a measure of promotion effect (17, 19, 20).
Materials and Methods
Animals and Treatments
Thirty-six five-week-old female F344 rats were obtained from Iffa-Credo (Lyon, France). They were acclimatized to the colony for one week, housed two rats per stainless steel wire drop-bottom cage, at 22°C with light-dark cycle 12h-12h, “day” starting at 7h30 a.m., and fed a laboratory chow (6% fat, UAR, Villemoisson, France) and water ad libitum. The rats were initiated between 9 and 10 a.m. with a single i.p. injection of azoxymethane (Sigma, St. Quentin, France) at a dose of 20 mg/kg in NaCl 9 g/l. They were randomly allocated to the treatments 7 d later. Rats in the control group were given subcutaneous injection of sterile saline (NaCl 9 g/l, 2.5 ml/kg, id est 0.21 to 0.52 ml/rat). In the experimental group they were given s.c. injection of insulin (20 U/kg/d, UltralenteMC Novo Nordish Pharm., Boulogne, France, containing per ml 40 U bovine insulin and 0.08 mg zinc) diluted with saline (8 U/ml in NaCl 9 g/l). Injections were given five times per week, between 8h30 and 9 a.m.. A preliminary experiment showed that, provided they have free access to food, the rats may be given insulin up to 48 U/kg/d without major problems. Food intake, water intake and body weight were measured weekly. The animals were sacrificed 106 d after the carcinogen injection, 99 d after the first insulin injection, the day of the 72nd insulin injection, by cervical dislocation between 2 and 3h15 p.m.. Blood was taken from the heart, the abdominal fat was excised and weighed, and the colon was measured, and kept in formalin for ACF scoring.
Assay of Blood Insulin, Glucose and Triglycerides
Blood samples (1.5 ml) were collected by cardiac puncture in a syringe containing 5 μl heparin within 1 min after death, within 5h30 and 6h15 after the last insulin injection. After centrifugation for 10 min at 1300 g the plasma was divided in coded microtubes kept at −80°C for further analyses. Insulin was measured by radioimmunoassay, using a kit designed for human plasma, giving a 100% cross-reaction with bovine and rat insulin (Insik-5, Sorin Biomedica, Antony France). Glucose and triglycerides were measured by enzymatic-spectrophotometric methods (kits PAP-500 and -150, BioMérieux, Marcy France).
Assay of Aberrant Crypt Foci
ACF were scored 106 d after the carcinogen injection using the procedure described by Bird (10). Immediately after sacrifice, colons were removed and flushed with Kreb’s Ringer solution (Sigma), then opened longitudinally and fixed flat between coded filter paper in 10% buffered formalin (Sigma). The colons were stained with methylene blue (0.1 %) for 10–15 min, then the mucosal side was observed at 32 × magnification. ACF were distinguished by their slit-like opening, increased staining, size and pericryptal zone. The multiplicity (number of crypts per ACF) was recorded for each ACF in each colon. Those were scored blindly by a single observer (C.J.).
After ACF methylene-blue scoring, the colons were rinsed then stained with high-iron diamine Alcian blue procedure to determine the type of mucin secretion (21). As already described (22, 23), the normal crypts in the proximal part of rat colon were stained blue (sialomucin). The normal crypts were stained dark brown (sulphomucin) in the distal colon, like in the normal human colorectal mucosa (22). ACF mucus staining was examined in this distal part (length 6.8 ± 0.3 cm in controls vs 7.1 ± 0.9 cm insulin rats, p=0.1). The sialomucin secreting blue ACF were easily identified against the background of normal brown crypts. It has been suggested that a shift from sulphomucin to sialomucin production may represent an early feature of malignancy (22, 23). Colons were scored again blindly by a single observer (G.P.), to determine the type of mucin secreted by ACF.
Statistics
Data are given as mean ± standard deviations. The statistical analysis of ACF assay for colon cancer promotion was based on ACF multiplicity as defined by the mean number of aberrant crypts per focus (19). Data were examined by two-sided Student’s t-test, or Welsh’s test when variances were unequal.
Results
Weights
Rats given insulin grew faster than controls as shown on fig. 1. The difference reached significance on day 28 (p=0.015) and after. The food intake also was higher in insulin injected rats, even before they become heavier than control, e.g., between days 20 and 23, they ate (mean ± SD) 16.6 ± 0.9 vs. 13.7 ± 0.4 g/d respectively (p<0.001). Differences in food intake, water intake, fecal output, and body weight remained significant at all time points (data no shown), except during weekends: the injections were not done Saturday and Sunday, and rats in the insulin group tended to eat less than controls those days, e.g., 13.0 ± 0.7 vs. 14.1 ± 0.7 (p=0.05) between days 17 and 20. As shown on fig. 1 the body weight difference between groups increased particularly during the last week, but weights were taken after 2 p.m. instead of 8h30 a.m. usually. We guess that control rats do not eat during daytime (and can loose weight via fecal excretion), while insulin injected rats eat mostly between 9 a.m. and 2 p.m., i.e., just after the insulin injection.
Fig. 1.
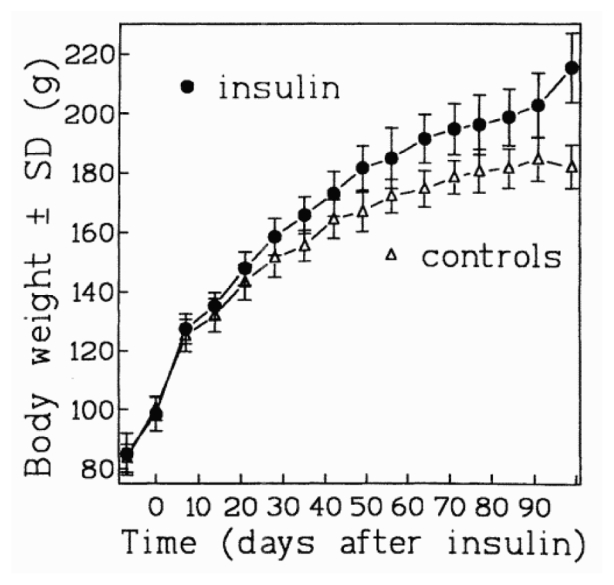
Mean body weights of F344 female rats during daily injections of saline or insulin, starting 0 day.
On the day of sacrifice, the rats given insulin had more abdominal fat (10.4 ± 1.8 vs. 7.7 ± 1.3 g), and a longer colon (13.8 ± 1.1 vs. 12.5 ± 0.7 cm) than controls. These differences were significant (both p<0.005), but only the difference between abdominal fat remained so after adjustment for body weight (p<0.05).
Blood Values
On the day of sacrifice, about 6h after the last insulin injections, the blood insulin was higher in rats given insulin than in controls (440 ± 230 vs. 80 ± 25 μU/ml, p<0.0001). The blood glucose was lower in rats given insulin than in controls (4.2 ± 1.2 vs. 10.8 ± 1.6 mmol/l, p<0.0001). The blood triglycerides were higher in rats given insulin than in controls (1.19 ± 0.25 vs. 0.97 ± 0.21 mmol/L, p=0.03).
Promotion of ACF
A total of 9355 ACF was detected in the 36 colons. The number of ACF per colon, which is not associated with tumor promotion, was the same in both groups (see Table 1). By contrast, the crypt multiplicity (or number of crypts/focus), a predictor of the tumor incidence, was higher in insulin treated rats than in controls (an 11 % increase, p=0.007). The number of large ACF, containing 7 crypts or more, which is another measure for ACF promotion, was higher in rats given insulin than in controls, with borderline significance (p=0.056). Thus data show that ACF grew faster in rats given insulin.
Table 1.
Effect of repeated insulin injections on aberrant crypt foci (ACF) in F344 rats after a single azoxymethane injectiona
| Group n | ACF/Rat | Multiplicity crypts/ACF | Large ACF b |
|---|---|---|---|
| Saline 19c | 259±78 | 2.49±0.21 | 7.5±5.4 |
| Insulin 17 | 260±66 | 2.76±0.33 | 10.8±4.5 |
| p value | 0.98 | 0.007 | 0.056 |
72 insulin injections (20 U/kg) were given during 106 days after a single azoxymethane injection (20 mg/kg)
Large ACF: > 6 crypts
Values are means ± SD; n, no. of rats;
Nature of Mucus
The proportion of ACF excreting sialomucin was higher in insulin injected rats than in controls (43 ± 11 vs. 34 ± 8%, p=0.038), which suggests enhanced carcinogenesis. The proportion of large ACF excreting sialomucin was not changed by insulin. As shown on fig. 2, the proportion of sialomucin excreting ACF in the distal colon (mean 7 cm from anus) increased with ACF multiplicity. The correlation between the proportion of sialomucin marked ACF and the number of crypt per ACF was very high (r=0.99, p<0.0001). Thus, the sialomucin might just be another marker for large ACF.
Fig. 2.
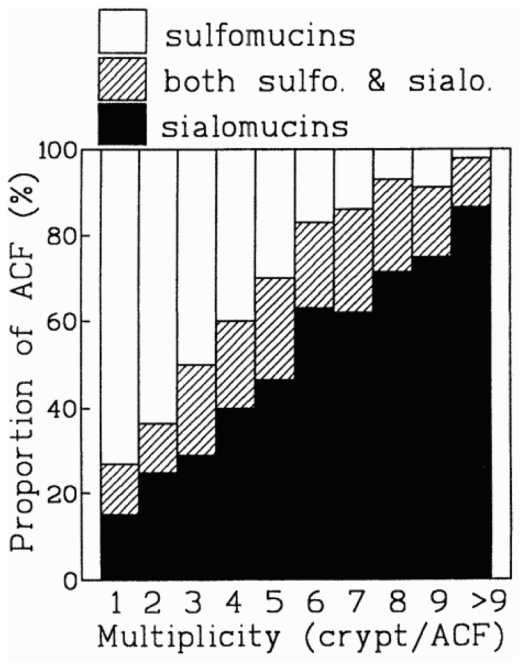
Type of mucus produced by the aberrant crypt foci in the distal colon of rats according to their multiplicity. Percentage of ACF/total ACF, secreting sialomucins, sulphomucins, or both types of mucins together. Data are based on 2444 ACF.
Discussion
The results of this study show that exogenous insulin leads to increased food intake, higher body weight and more abdominal fat, increased levels of plasma insulin and triglycerides, and reduced levels of plasma glucose. Insulin also increased the growth of aberrant crypt foci, with more crypts per ACF, and more large ACF per colon in insulin treated rats than in controls. Thus, insulin injections promoted colon carcinogenesis in this model. We also observed more sialomucin producing ACF in insulin treated rats than in controls. This might either suggest a more advanced phenotype (22, 23), or simply reflect the increased size of ACF.
A recent report by Tran et al. also shows that insulin is a colon tumor promoter in rats (24). After azoxymethane initiation and daily injections of insulin, the fraction of rats with colon adenomatous polyps was greater in rats receiving insulin than in rats receiving saline (79% vs. 50% respectively, p<0.05). The number of tumors per rat was also greater in the insulin group (2.0 vs. 0.7, p<0.05). Insulin did not increase body weight gain (24). Design differences between Tran’s study and this one may explain the discrepancies in body weight gain and tumor outcome. Tran et al. used male rats (vs. female here), starting at 240 g body weight (vs. 85 g), fed an AIN93M diet (vs. rodent chow), given medium-acting porcine insulin (vs. long-action bovine) at 15 U/kg for 132 days (vs. 20 U/kg for 100 d), after two 15 mg/kg azoxymethane injections (vs. a single dose 20 mg/kg), with a light cycle shifted 6 h earlier than daylight (24). It is surprising that in Tran et al. study the insulin did not increase body weight, but it may be explained by differences in starting weight, gender, diet and insulin quickness. Although both studies show a significant promotion of carcinogenesis by insulin, at first glance the magnitude of effect appears smaller in the present study. However the crypt multiplicity increased here by a 1.11 factor, which may be compared to the 1.20 increase published by Zhang et al. (17) and Magnuson et al. (19). According to their data, the diets that increase the crypt multiplicity by 1.2 at 100 d also increase the adenocarcinoma incidence by 3 later (17, 19). This increase is higher than the 1.6 increased tumor incidence observed by Tran (24). Thus, the promotion potency of insulin in both studies may be similar.
Previous reports show that insulin is a growth factor for rats and for tumors. Daily insulin injections (25 U/kg for 28 d) increase body weight, fat tissue weight, and fat cell size in female rats (25). Daily insulin injections to hypophysectomized rats enable the growth of liver tumors, initiated by dietary methyldimethyl-aminobenzene (26). Conversely, diabetes induced by alloxan in female rats previously initiated with dimethylbenzenthra- dimethylbenzenthracene produces the rapid regression of 90% of established mammary tumors. These tumors were shown to be insulin dependent in vitro (27).
Exogenous insulin may promote tumor growth by at least three mechanisms.
- First, exogenous insulin increases food intake and weight gain. Overfeeding and overweight increase, and caloric restriction decreases, carcinogenesis in many studies (28). Overfeeding modifies the hormonal balance and increases the energy available through the circulation for neoplastic cells.
- Second, exogenous insulin leads to hyperinsulinemia, which may directly promote tumor growth (27), for instance by easing the uptake of nutrients by tumor cells.
- Last, insulin may modify other blood-borne factor(s), for instance the insulin-like growth factor IGF1. Most colon cell lines have receptors for IGF1, and high fat diets induce both insulin and IGF1 resistance in rats (29).
A study in pair-fed rats receiving exogenous insulin, but as many calories as controls, might tell if hyperinsulinemia by itself can promote tumors. In human beings, however, hyperinsulinemia is always associated with hyperphagia.
Diets high in calories, fat, sucrose, fructose, amylopectin starch, and low in fibers, resistant starch and n-3 unsaturated lipids, favor the insulin resistance syndrome in rodents. These diets increase blood insulin, and lead to abdominal fat store, obesity, and non-insulinodependant diabetes in many animal models (30, 31, 32, 33), and probably in humans too (34, 35). These same diets are also known to promote experimental carcinogenesis in rodents, and are considered risk factors for colorectal cancer in human populations (5, 6). It is usually considered that bile acids in the fecal stream can explain the promoting effect of these diets on colon cancer, but blood insulin may also explain the promotion by these diets (4). The present results support the idea that in humans, hyperinsulinemia and/or insulin resistance may explain the high risk of colorectal cancer associated with sedentary lifestyle, overfeeding, and nutriments cited above (4, 5, 6).
This study is the second to show a promotion of colon carcinogenesis by exogenous insulin. If nutritional studies, in progress in our laboratory, also show the promoting effect of endogenous insulin, it will be necessary to study the protection afforded by interventions that reduce insulinemia in humans.
Acknowledgments
We thank Gail McKeown-Eyssen, Giovanna Caderni, and John Cummings for their comments on the study protocol, Raymond Gazel for taking care of the rats, Jacques Alary for insulin supply, and W. Robert Bruce for stimulating discussions.
The study was supported in part by the Direction Générale de l’Enseignement et de la Recherche du Ministère de l’Agriculture (France), and by a grant of the Association de la Recherche contre le Cancer.
References
- 1.Potter JD. Risk Factors for Colon Neoplasia - Epidemiology and Biology. Europ J Cancer. 1995;31A:1033–1038. doi: 10.1016/0959-8049(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 2.McKeown-Eyssen GE, Bright-See E, Bruce WR, Jazmaji V Toronto-Polyp-Prevention-Group. A Randomized Trial of a Low Fat High Fibre Diet in the Recurrence of Colorectal Polyps. J Clin Epidemiol. 1994;47:525–536. doi: 10.1016/0895-4356(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan R, Macrae F, Bain C, Battistutta D, Chapuis P, et al. Randomized Trial of Intake of Fat, Fiber, and Beta Carotene to Prevent Colorectal Adenomas. J Natl Cancer Inst. 1995;87:1760–1766. doi: 10.1093/jnci/87.23.1760. [DOI] [PubMed] [Google Scholar]
- 4.Bruce WR, Corpet DE. The Colonic Protein Fermentation and Insulin Resistance Hypotheses for Colon Cancer Etiology: Experimental Tests Using Precursor Lesions. Europ J Cancer Prev. 1996 doi: 10.1097/00008469-199612002-00007. in press. [DOI] [PubMed] [Google Scholar]
- 5.McKeown-Eyssen G. Epidemiology of Colorectal Cancer Revisited: are Serum Triglycerides and/or Plasma Glucose Associated with Risk ? Cancer Epidemiol Biom Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 6.Giovannucci E. Insulin and Colon Cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BA. Nutrition and Breast Cancer Risk: Can an Effect via Insulin Resistance be Demonstrated? Breast Cancer Res Treat. 1996;38:239–246. doi: 10.1007/BF01806141. [DOI] [PubMed] [Google Scholar]
- 8.Kaaks R. Nutrition, Hormones, and Breast Cancer: Is Insulin the Missing Link? Cancer Causes Control. 1996 Nov; doi: 10.1007/BF00051703. in press. [DOI] [PubMed] [Google Scholar]
- 9.Bruce WR, Archer MC, Corpet DE, Medline A, Minkin S, et al. Diet, Aberrant Crypt Foci and Colorectal Cancer. Mutation Res. 1993;290:111–118. doi: 10.1016/0027-5107(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 10.Bird RP. Observation and Quantification of Aberrant Crypts in Murine Colon Treated with a Colon Carcinogen: Preliminary Findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 11.McLellan EA, Medline A, Bird RP. Dose Response and Proliferative Characteristics of Aberrant Crypt Foci - Putative Preneoplastic Lesions in Rat Colon. Carcinogenesis. 1991;12:2093–2098. doi: 10.1093/carcin/12.11.2093. [DOI] [PubMed] [Google Scholar]
- 12.Tang ZC, Shivapurkar N, Frost A, Alabaster O. The Effect of Dietary Fat on the Promotion of Mammary and Colon Cancer in a Dual-organ Rat Carcinogenesis Model. Nutr Cancer. 1996;25:151–159. doi: 10.1080/01635589609514437. [DOI] [PubMed] [Google Scholar]
- 13.Wargovich MJ, Harris C, Chen CD, Palmer C, Steele VE, et al. Growth Kinetics and Chemoprevention of Aberrant Crypts in the Rat Colon. J Cell Biochem. 1992;S 16G:51–54. doi: 10.1002/jcb.240501110. [DOI] [PubMed] [Google Scholar]
- 14.Stopera SA, Murphy LC, Bird RP. Evidence for a Ras Gene Mutation in Azoxymethane-Induced Colonic Aberrant Crypts in Sprague-Dawley Rats - Earliest Recognizable Precursor Lesions of Experimental Colon Cancer. Carcinogenesis. 1992;13:2081–2085. doi: 10.1093/carcin/13.11.2081. [DOI] [PubMed] [Google Scholar]
- 15.Pretlow TP, Brasitus TA, Fulton NC, Cheyer C, Kaplan EL. K-ras Mutations in Putative Preneoplastic Lesions in Human Colon. J Natl Cancer Inst. 1993;85:2004–2007. doi: 10.1093/jnci/85.24.2004. [DOI] [PubMed] [Google Scholar]
- 16.Losi L, Roncucci L, Digregorio C, Deleon MP, Benhattar J. K-ras and p53 Mutations in Human Colorectal Aberrant Crypt Foci. J Pathol. 1996;178:259–263. doi: 10.1002/(SICI)1096-9896(199603)178:3<259::AID-PATH473>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XM, Stamp D, Minkin S, Medline A, Corpet DE, et al. Promotion of Aberrant Crypt Foci and Cancer in Rat Colon by Thermolyzed Protein. J Natl Cancer Inst. 1992;84:1026–1030. doi: 10.1093/jnci/84.13.1026. [DOI] [PubMed] [Google Scholar]
- 18.Pretlow TP, Oriordan MA, Somich GA, Amini SB, Pretlow TG. Aberrant Crypts Correlate with Tumor Incidence in F344 Rats Treated with Azoxymethane and Phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509. [DOI] [PubMed] [Google Scholar]
- 19.Magnuson BA, Carr I, Bird RP. Ability of Aberrant Crypt Foci Characteristics to Predict Colonic Tumor Incidence in Rats Fed Cholic Acid. Cancer Res. 1993;53:4499–4504. [PubMed] [Google Scholar]
- 20.Corpet DE, Stamp D, Medline A, Minkin S, Archer MC, et al. Promotion of Colonic Microadenoma Growth in Mice and Rats Fed Cooked Sugar or Cooked Casein and Fat. Cancer Res. 1990;50:6955–6958. [PubMed] [Google Scholar]
- 21.Luceri C, Caderni G, Lancioni L, Aiolli S, Dolara P, et al. Effects of Repeated Boluses of Sucrose on Proliferation and on AOM-induced Aberrant Crypt Foci in Rat Colon. Nutr Cancer. 1996;25:187–196. doi: 10.1080/01635589609514441. [DOI] [PubMed] [Google Scholar]
- 22.Filipe MI. Mucous Secretion in Rat Colonic Mucosa During Carcinogenesis Induced by DMH. A Morphological and Histochemical Study. Br J Cancer. 1975;32:60–77. doi: 10.1038/bjc.1975.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caderni G, Giannini A, Lancioni L, Luceri C, Biggeri A, et al. Characterisation of Aberrant Crypt Foci in Carcinogen-treated Rats: Association with Intestinal Carcinogenesis. Br J Cancer. 1995;71:763–769. doi: 10.1038/bjc.1995.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran TT, Medline A, Bruce WR. Insulin Promotion of Colon Tumors in Rats. Cancer Epidemiol Biomarkers & Prevention. 1996 in press. [PubMed] [Google Scholar]
- 25.Krotkiewski M, Bjorntorp P. The Effect of Progesterone and of Insulin Administration on Regional Adipose Tissue Cellularity in the Rat. Acta Physiol Scand. 1976;96:122–127. doi: 10.1111/j.1748-1716.1976.tb10177.x. [DOI] [PubMed] [Google Scholar]
- 26.Dodge BG, O’Neal MA, Chang JP. Effects of Adrenocorticotropin and Insulin on Carcinogenesis in Hypophysectomized Rats. J Natl Cancer Inst. 1961;27:817–825. [PubMed] [Google Scholar]
- 27.Heuson JC, Legros N. Influence of Insulin Deprivation on Growth of the 7,12-dimethylbenz(a)anthracene-induced Mammary Carcinoma in Rats Subjected to Alloxan Diabetes and Food Restriction. Cancer Res. 1972;32:226–232. [PubMed] [Google Scholar]
- 28.Kritchevsky D. Colorectal Cancer - The Role of Dietary Fat and Caloric Restriction. Mutation Res. 1993;290:63–70. doi: 10.1016/0027-5107(93)90033-c. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Baracos VE, Quinney HA, Lebricon T, Clandinin MT. Parallel Insulin-like Growth Factor I and Insulin Resistance in Muscles of Rats Fed a High Fat Diet. Endocrinology. 1995;136:3318–3324. doi: 10.1210/endo.136.8.7628366. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JA, Miller JCB, Denyer GS. Development of Insulin Resistance in the Rat is Dependent on the Rate of Glucose Absorption from the Diet. J Nutr. 1996;126:596–602. doi: 10.1093/jn/126.3.596. [DOI] [PubMed] [Google Scholar]
- 31.Ikemoto S, Thompson KS, Takahashi M, Itakura H, Lane MD, et al. High Fat Diet Induced Hyperglycemia: Prevention by Low Level Expression of Glucose Transporter (GLU4) Minigene in Transgenic Mice. Proc Natl Acad Sci USA. 1995;92:3096–3099. doi: 10.1073/pnas.92.8.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafrir E. Development and Consequences of Insulin Resistance: Lessons from Animals with Hyperinsulinaemia. Diabetes Metab. 1996;22:122–131. [PubMed] [Google Scholar]
- 33.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, et al. Differential Effects of Fat and Sucrose on the Development of Obesity and Diabetes in c57BL/6j and a/j Mice. Metab Clin Exper. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 34.Frayn KN, Klingman SM. Dietary Sugars and Lipid Metabolism in Humans. Am J Clin Nutr. 1995;62:S250–S263. doi: 10.1093/ajcn/62.1.250S. [DOI] [PubMed] [Google Scholar]
- 35.Zemel MB. Insulin Resistance, Obesity and Hypertension: an Overview. J Nutr. 1995;125:S1715–S1717. doi: 10.1093/jn/125.suppl_6.1715S. [DOI] [PubMed] [Google Scholar]


