Abstract
This study was carried out to investigate the efficacy of sn-2 palmitic acid-fortified vegetable oil (Sn2PA) on calcium absorption and to confirm the synergistic effects of fructooligosaccharide on calcium absorption. Male SD rats were fed 6 kinds of casein based diets containing vegetable oil (control), sn-2 palmitic acid-fortified vegetable oil (Sn2PA) and Sn2PA with fructooligosaccharide(Sn2PAFO) in two levels of calcium (normal 0.5% and high 1.0%) for 3 weeks. Total lipids, cholesterol, triglyceride and calcium in blood were measured. Feces were collected using cages for 4 days. Serum concentrations of total lipids and calcium were not significantly different among groups. However, serum triglyceride was significantly decreased by fructooligosaccharide supplementation regardless of dietary calcium level. The lipid absorption was not significantly different among experimental groups. Calcium absorption was significantly higher in Sn2PAFO group than other groups. Calcium solubility of intestine was increased by sn-2 palmitic acid supplementation. These results suggest that sn-2 palmitic acid and fructooligosaccharide supplementation could be beneficial for baby foods including infant formula, with regard to increasing absorption of calcium by more soluble calcium in the small intestinal content.
Keywords: Sn-2 palmitic acid-fortified oil, fructooligosaccharide, lipid metabolism, calcium absorption, baby food
Introduction
Lipids in human breast milk or infant formula provide 50% of total dietary energy (Breckenridge, 1978; Jensen, 1989). Although most lipids are well digested and absorbed in adulthood, lipid absorption during infancy is not good, especially in premature infant to 1 week after birth (Atkinson et al., 1981; Brooke et al., 1982; Jarvenpa, 1983). That is caused by low concentration of pancreatic lipases and bile salt (Zoppi et al., 1972). The positional distribution of fatty acids in dietary triglycerides, as well as the fatty acid composition, is an important factor of fat digestion and absorption (Murphy & Signer, 1974). Triglycerides in human breast milk contain approximately 20-25% palmitic acid (16:0), with over 70% of the 16:0 esterified to the sn-2 position of the milk triglyceride (Martin et al., 1993). The specific positioning of 16:0 at the 2-position of human breast milk triglycerides has been suggested as one of the reasons for the high efficiency of fat absorption from human milk. It has been supported by studies showing better absorption of total dietary fat or 16:0 by infants fed formula with 16:0 esterified to the 2 position rather than the 1, 3 positions of the dietary triglyceride (Filer et al., 1969). Palm olein oil has been applied to fat source for infant formula similar to human milk. The positional distribution of palmitic acid in palm olein oil is composed mainly of sn-1 and 3; which is divided into two free fatty acids and a 2-monoglyceride by enzyme (Breckenridge, 1978). It has shown strong tendency of unesterified 16:0 to form insoluble soaps with divalent cations such as calcium and magnesium. So it results in decreasing the absorption rate of lipid and calcium. When the position of palmitic acid is mainly in sn-1 and 3, fecal hardness is changed into harder state because of low lipid absorption rate and calcium soap formation (Lien et al., 1997; Lucas et al., 1997). Therefore, it has been discussed to humanize the fat source stereospecifically by fortifying with sn-2 palmitic acid in the infant formula to increase the absorption rate of lipids and calcium. It was commercially synthesized through enzymatic interesterification processing (Lien et al., 1997; Lucas et al., 1997).
Human milk is further known to be a rich source of oligosaccharides (Coppa et al., 2004). Oligosaccharide in human milk resembles receptors found on the surface of retropharyngeal cell which bind bacteria. The human milk oligosaccharide may bind potential pathogens by mimicking the receptors on retropharyngeal cells and thereby protect infants (Engfer et al., 2000). Oligosaccharides in infant formula are being mainly used as a form of galactooligosaccharide and fructooligosaccharide. Fructooligosaccharide has been well known for prebiotic effect, stimulating the growth of bifidobacteria in the colon of infant (Scholz-Ahrens et al., 2001). Moreover, two studies have shown a beneficial effect of fructooligosaccharide on intestinal calcium absorption in human (Coudray et al., 1997; Van den Heuvel et al., 1999). We used a fructooligosaccharide form in this study and hypothesized that it could facilitate the absorption of calcium in the intestine.
This study was carried out to investigate the efficiency of sn-2 palmitic acid-fortified oil (Sn2PA) on calcium absorption in growing SD rats fed casein-based diet and to confirm the synergistic effects of oligosaccharide supplementation.
Materials and Methods
Experimental animals and diets
This study was conducted in conformity with the policies and procedures of the Institutional Animal Care and Use Committee of the Seoul National University (SNU). Three week-old male SD (Sprague-Dawley) rats were obtained from the SNU animal laboratories (Seoul, Korea) and housed individually in stainless steel wire-mesh case in a room kept at 23 ± 1℃ with a 12-h light-dark cycle (light period: 8:00 ~ 20:00 h), and given free access to diet and distilled water.
Male SD rats were fed 6 different kinds of experimental diets for 3 weeks containing vegetable oil (Control), sn-2 palmitic acid-fortified oil (Sn2PA) and sn-2 palmitic acid-fortified oil + fructooligosaccharide (Sn2PAFO) in two calcium levels (0.5% and 1.0%). Milk protein, especially sodium caseinate (Maeil Dairy Co., Korea) was used as a protein source (Table 1). Weight gain and diet intake were recorded every day. The balance study of calcium and lipid was performed by four-day collecting of feces during the experimental period.
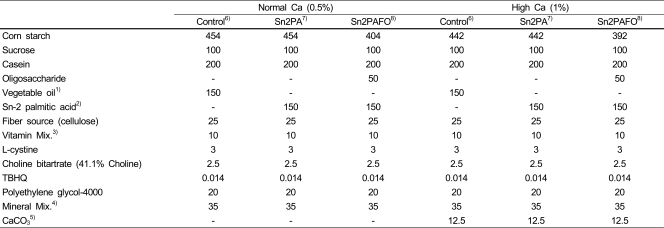
Table 1.
Composition of experimental diet (g/kg diet)
1)Vegetable oil : fat blend of soy oil, coconut oil, palmolein, high-oleic sunflower oil
2)Sn-2 palmitic acid : Sn-2 pamitic acid fortified oil : fat bled of soy oil, palm kernel oil, betapol base, high oleic sunflower oil
3)AIN-93-VX ; ICN
4)AIN-93G-MX ; ICN
5)High Ca diets supplemented with CaCo3
6)Control : Vegetable oil diet
7)Sn2PA :Sn-2 palmitic acid-fortified oil diet
8)Sn2PAFO : Sn-2 palmitic acid-fortified oil + fructooligosaccharide diet
Experimental diets (Table 1) were patterned after the AIN-93G diet (Reeves et al., 1993). The vitamin mixture (AIN-93-VX) was purchased from ICN Biochemicals (Costa Mesa, CA), and the mineral mixture was prepared using the composition of AIN-93G-MX. As a marker of diet movement in the intestinal tract, PEG (Polyethylene Glycol, 4000) was used.
Samples collection and biochemical analysis
Before sacrificing, all of the animals were fasted overnight and then fed for 1.5 hr to normalize for a post-absorptive state. One hour after feeding, blood was collected from the abdominal aorta under anesthesia. Serum was separated by a centrifugation at 3,000 rpm for 20 min and stored at a deep-freezer of -80℃ until analyzed. The samples of intestinal content were rinsed twice with cold isotonic saline, separated into soluble and insoluble fraction by a centrifugation at 10,000 rpm for 20 min and stored at a deep-freezer of -80℃ until analyzed. Feces were collected for 4 days and freeze dried.
Total lipid in serum was determined according to the method described by Fringe and Dunn (Fringe & Dumm, 1980). Concentrations of total cholesterol, triglyceride and calcium in serum were determined by blood auto analyzer (fully automated dry chemistry system: SPOCHEM: Daiichi Kagaku Co., Japan). Triglyceride in feces was determined after extracting the total lipid by Folch method (Folch et al., 1957). Calcium contents of the small intestinal content and feces were determined at 422.7 nm by atomic absorption spectrophotometer (GBC904AA, USA), using samples burned to ashes in 550-600℃ furnace and dissolved in 6N HCl solution.
Statistical analysis
Statistical analysis of data was carried out using an SAS 8.1 version (Cary, NC) program. All data were expressed as mean ± SE values. Statistical significance among the groups was tested by analysis of variance (two-way ANOVA), followed by Duncan's multiple range test. Differences were considered significant at p<0.05 and p<0.01.
Results
Body weight and food intake
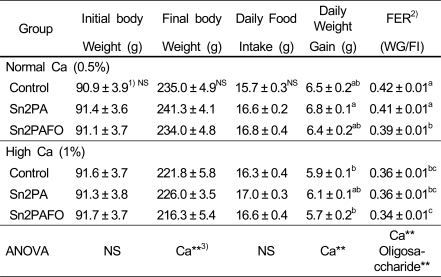
Table 2 shows body weight, food intake and food efficiency ratio (FER). There was no significant difference in final body weight and daily food intake among experimental groups. The daily weight gain and FER in high calcium diet groups were lower than those in normal calcium groups. However, the weight gains did not vary by dietary lipid sources.
Table 2.
Body weight, food intake and food efficiency ratio (FER) of the rats fed experimental diets
1)Mean ± SE of 9 rats per group
2)FER (Food Efficiency Ratio) = (Weight gain/food intake) ×100
3)*p<0.05, **p<0.01
4)Values with different superscripts in columns are significantly different at p<0.05.
5)NS is not significant.
Lipid profile and calcium in serum
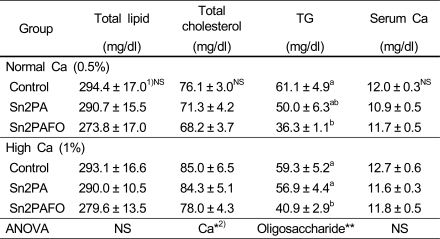
The concentrations of lipid profile and calcium in serum of the rats were shown in Table 3. There were no significant differences in serum concentrations of total lipid, total cholesterol and calcium. But, the concentration of triglyceride in serum was significantly low in the Sn2PAFO in both normal and high calcium level, suggesting a serum triglyceride-lowering effect of dietary fructooligosaccharide.
Table 3.
The concentration of total lipid, total cholesterol, triglyceride and calcium in serum
1)Mean ± SE of 9 rats per group
2)*p<0.05, **p<0.01
3)Values with different superscripts in columns are significantly different at p<0.05.
4)NS is not significant.
Fecal excretion and intestinal absorption of lipid
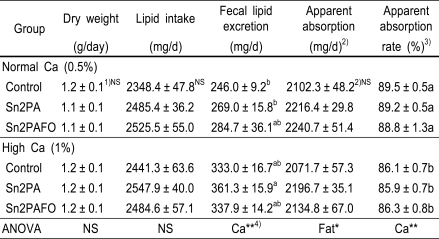
Fecal lipid excretion and apparent lipid absorption were shown in Table 4. There were no differences in fecal weight and lipid intake in each group, but fecal lipid excretion was increased significantly in high calcium diet groups. The lipid absorption in Sn2PA supplement diet groups tended to be increased as compared with control diet groups. It might be explained by increasing the intake of lipid in Sn2PA diet groups, and not by the difference of fecal lipid excretion. Moreover, lipid absorption was more increased in normal calcium diet groups than high calcium diet group. In this study, we found out that fecal lipid excretion was increased in high calcium diet groups.
Table 4.
Fecal lipid excretion and apparent lipid absorption
1)Mean ± SE of 9rats per group
2)Apparent absorption (mg) = lipid intake (mg) - Fecal lipid excretion (mg)
3)Apparent absorption rate (%) = [Apparent absorption (mg)/Intake (mg)]×100
4)*p<0.05, **p<0.01
5)Values with different superscripts in columns are significantly different at p<0.05.
6)NS is not significant.
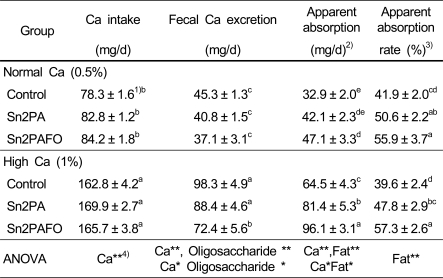
Fecal excretion and intestinal absorption of calcium
Daily calcium intake, fecal calcium excretion and apparent absorption were shown in Table 5. In this experiment, fecal excretion and apparent absorption of calcium were significantly increased in high calcium diet groups, but apparent calcium absorption rate (%) was not significantly affected by dietary calcium level. Calcium absorption was higher in Sn2PA or/and fructooligosaccharide diet groups than control group.
Table 5.
Daily calcium intake, fecal calcium excretion and apparent calcium absorption
1)Mean ± SE of 9rats per group
2)Apparent absorption (mg) = Ca intake (mg) - fecal Ca excretion (mg)
3)Apparent absorption rate (%) = [Apparent absorption (mg)/Intake (mg)]×100
4)*p<0.05, **p<0.01
5)Values with different superscripts in columns are significantly different at p<0.05.
6)NS is not significant.
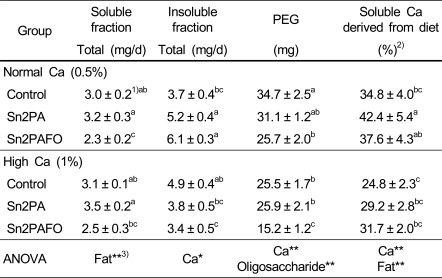
Solubility of calcium in small intestinal contents
Table 6 showed soluble and insoluble calcium in the small intestinal content. Soluble calcium derived from diet in the small intestinal content was increased by the intake of Sn2 PA supplement. We found that insoluble calcium from the small intestinal content was significantly increased in Sn-2PA group and Sn-2PAFO group whereas insoluble calcium from the small intestinal content was significantly decreased in high calcium groups. The PEG content was significantly decreased in high calcium group and in inverse proportional to fructooligosaccharide. In this experiment, the ratio of soluble calcium (%) derived from diet in the small intestinal content was more increased by intake of sn-2PA and sn-2PA with fructooligosaccharide than control.
Table 6.
Soluble and insoluble calcium in small intestinal contents
1)Mean ± SE of 9rats per group
2)Soluble Ca derived from diet (%) = (Soluble Ca/PEG in the small intestine) / (Ca/PEG in diet)×100
3)*p<0.05, **p<0.01
4)Values with different superscripts in columns are significantly different at p<0.05.
5)NS is not significant.
Discussion
There are numerous differences between the composition of human milk and that of infant formulas. The absorption of calcium and lipid in infant formula is lower than in human breast milk. Especially, in case of infant fed infant formula, the absorption of calcium depends on the composition of the formula. We examined that of sn-2 palmitic acid-fortified oil (Sn2PA) on calcium absorption in growing SD rats fed casein-based diet to confirm the synergistic effects of oligosaccharide supplementation.
With regard to the calcium absorption of dietary Sn2PA intake, Carnielli (Carnielli et al., 1995) reported that calcium excretion was reduced in Sn2PA group with the increasing tendency of possession rate of calcium. Moreover, they reported that urinary excretion of calcium was significantly higher in Sn2PA group than in other groups. Nelson (Nelson et al., 1998) reported that when infant fed palm olein abundant of sn-1, 2 and 3 palmitic acid, calcium possession rate was significantly higher in sn-2 palmitic acid group than in palm olein group. The fact that fecal excretion of calcium was correlated with fecal excretion of palmitic acid and total fat reinforces the interpretation that the formation of insoluble soaps occurs when saturated fatty acids are liberated in the presence of calcium (Quinlan et al., 1995). In this study, dietary Sn2PA and fructooligosaccharide increased remarkably the intestinal absorption of calcium at both normal and high calcium levels, whereas fecal calcium excretion was decreased greatly.
Our results agree with those reported by Kruger (Kruger et al., 2003). They reported that fecal calcium excretion was reduced significantly in the fructooligosaccharide diet group than in inulin diet group; moreover, calcium absorption was increased significantly in the fructooligosaccharide diet group. Carnielli (Carnielli et al., 1995) reported that fructooligosaccharide containing diet increased the content of short chain fatty acids in the cecum. Short chain fatty acids promote calcium solubilization by lowering pH in the colon. Therefore, calcium absorption was increased in the fructooligosaccharide containing diet. Oligosaccharides in human milk play an important role, as prebiotic soluble fibers, in the postnatal development of the intestinal flora (Coppa et al., 2004). This study suggested that fructooligosaccharide was fermented by probiotics such as bifidobacteria in the colon and formed some organic acid such as acetic acid, propionic acid and butric acid. They will lower pH in the colon and convert insoluble calcium soap to soluble calcium or ionization so as to increase the absorption of calcium (Ohta et al., 1994). Moreover, the development of colon wall and promotion of growth of bifidobacteria by intake of oligosaccharide will promote the absorption of some divalent minerals (Takahara et al., 2000). Scholz-Ahrens (Scholz-Ahrens et al., 2001) reported that oligosaccharides in the diet increased not only the absorption of calcium in colon but also the content of calcium in bone in the rat. Moreover, we could find that moisture content in feces was relatively increased in oligosaccharide added groups. Therefore, if infant formula is formulated with oligosaccharides, the hardness of feces could be mild and soft.
Taken together, these results suggest that the combination of sn-2PAFO and fructooligosaccharide could be expected to be a major component to absorb calcium in the aspect of infant nutrition due to the increase of calcium absorption by increasing soluble calcium in the small intestine, resulting that this might contribute to the bone calcium content. Hence, sn-2PA and fructooligosaccharide in infant formula could have synergistic effects on calcium bioavailability for infant and young baby.
Further research is needed to investigate the mechanism of calcium absorption associated with different tendency of calcium possession rate in serum and feces.
References
- 1.Atkinson SA, Bryan MH, Anderson GH. Human milk feeding in premature infants: protein, fat, and carbohydrate balances in the first two weeks of life. J Pediatr. 1981;99:617–624. doi: 10.1016/s0022-3476(81)80275-2. [DOI] [PubMed] [Google Scholar]
- 2.Breckenridge WC. Stereospecific analysis of triacylglycerols. In: Kuksis A, editor. Handbook of lipid research 1-fatty acids and glycerides. NewYork. USA: Plenum Press; 1978. pp. 197–232. [Google Scholar]
- 3.Brooke OG, Wood C, Barley J. Energy balance, nitrogen balance, and growth in preterm infants fed expressed breast milk, a premature infant formula, and two low-solute adapted formulae. Arch Dis Child. 1982;57:898–904. doi: 10.1136/adc.57.12.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnielli VP, Luijendijk IH, van Beek RH, Boerma GJ, Degenhart HJ, Sauer PJ. Effect of dietary triacylglycerol fatty acid positional distribution on plasma lipid classes and their fatty acid composition in preterm infants. Am J Clin Nutr. 1995;62:776–781. doi: 10.1093/ajcn/62.4.776. [DOI] [PubMed] [Google Scholar]
- 5.Carnielli VP, Luijendijk IH, van Goudoever JB, Sulkers EJ, Boerlage AA, Degenhart HJ, Sauer PJ. Feeding premature newborn infants palmitic acid in amounts and stereoisomeric position similar to that of human milk: effects on fat and mineral balance. Am J Clin Nutr. 1995;61:1037–1042. doi: 10.1093/ajcn/61.4.1037. [DOI] [PubMed] [Google Scholar]
- 6.Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotics in human: human milk oligosaccharide. J Clin Gastroenterol. 2004;38:80–83. doi: 10.1097/01.mcg.0000128926.14285.25. [DOI] [PubMed] [Google Scholar]
- 7.Coudray C, Bellanger J, Castiglia-Delavaud C, Rémésy C, Vermorel M, Rayssignuier Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr. 1997;51:375–380. doi: 10.1038/sj.ejcn.1600417. [DOI] [PubMed] [Google Scholar]
- 8.Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–1596. doi: 10.1093/ajcn/71.6.1589. [DOI] [PubMed] [Google Scholar]
- 9.Filer IJ, Mattson FH, Fomon SJ. Triglyceride configuration and fat absorption by the human infant. J Nutr. 1969;99:293–298. doi: 10.1093/jn/99.3.293. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Less M, Sloane stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Frings CS, Dunn RT. The colorimetric method for determination of serum total lipids based on the sulfophospovanilin reaction. Am J Clin Pathol. 1970;53:89–91. doi: 10.1093/ajcp/53.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Jarvenpaa AL. Feeding the low-birth-weight infant IV. fat absorption as a function of diet and duodenal bile acids. Pediatrics. 1983;72:684–689. [PubMed] [Google Scholar]
- 13.Jensen RG. Lipids in human milk-composition and fat-soluble vitamins. In: Lebenthal E, editor. Textbook of gastroenterology and nutrition in infancy. 2nd ed. NewYork. USA: Raven Press; 1989. pp. 57–208. [Google Scholar]
- 14.Kruger MC, Brown KE, Collett G, Layton L, Schollum LM. The effect of fructooligosaccharides with various degrees of polymerization on calcium bioavailability in the growing rat. Exp Biol Med (Maywood) 2003;228:683–688. doi: 10.1177/153537020322800606. [DOI] [PubMed] [Google Scholar]
- 15.Lien EL, Boyle FG, Yuhas R, Tomarelli RM, Quinlan P. The effect of triglyceride positional distribution on fatty acid absorption in rats. J Pediatr Gastroenterol Nutr. 1997;25:167–174. doi: 10.1097/00005176-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lucas A, Quinlan phosphorus, Abrams S, Ryan S, Meah S, Lucas PJ. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77:F178–F184. doi: 10.1136/fn.77.3.f178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin JC, Bougnoux P, Antoine JM, Lanson M, Couet C. Triacylglycerol structure of human colostrum and mature milk. Lipids. 1993;28:637–643. doi: 10.1007/BF02536059. [DOI] [PubMed] [Google Scholar]
- 18.Murphy GM, Signer E. Bile acid metabolism in infants and children. Gut. 1974;15:151–163. doi: 10.1136/gut.15.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson SE, Frantz JA, Ziegler EE. Absorption of fat and calcium by infants fed a milk-based formula containing palm olein. J Am Coll Nutr. 1998;17:327–332. doi: 10.1080/07315724.1998.10718770. [DOI] [PubMed] [Google Scholar]
- 20.Ohta A, Ohtuki M, Takizawa T, Inaba H, Adachi T, Kimura S. Effects of fructooligosaccharides on the absorption of magnesium and calcium by cecectomized rats. Int J Vitam Nutr Res. 1994;64:316–323. [PubMed] [Google Scholar]
- 21.Quinlan PT, Lockton S, Irwin J, Lucas AL. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr. 1995;20:81–90. doi: 10.1097/00005176-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents:final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 23.Scholz-Ahrens KE, Schaafsma G, van den Heuvel EG, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73:459S–464S. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 24.Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. J Nutr. 2000;130:1792–1795. doi: 10.1093/jn/130.7.1792. [DOI] [PubMed] [Google Scholar]
- 25.Van den Heuvel EG, Muys T, van Dokkum W, Schaafsma G. Oligofructose stimulates calcium absorption in adolescents. Am J Clin Nutr. 1999;69:544–548. doi: 10.1093/ajcn/69.3.544. [DOI] [PubMed] [Google Scholar]
- 26.Zoppi G, Andreotti G, Pajno-Ferrara F, Njai DM, Gaburro D. Exocrine pancreas function in premature and full term neonates. Pediatr Res. 1972;6:880–886. doi: 10.1203/00006450-197212000-00005. [DOI] [PubMed] [Google Scholar]