Abstract
Taurine supplementation has been shown to have an effect on lowering blood lipids in ovariectomized (OVX) rats. It therefore seemed desirable to find out whether the beneficial effect of taurine on OVX rats fed calcium-deficient diet could also be reproduced. Forty female Sprague-Dawley rats were divided into two groups. One group was OVX and the other group received a sham operation (Sham). Each rat group was further divided into the control diet and the taurine supplemented (2.0 g/100 g diet) diet group. All rats were fed on calcium-deficient diet and deionized water ad libitum for 6 weeks. Plasma and liver lipids were determined by using commercial kits. LDL-cholesterol concentrations were estimated with the equation of Friedewald et al. (1972). There were no significant differences in body weight gain and food intake between the control and taurine group within Sham and OVX groups, but body weight gain, food intake, and food efficiency ratio was higher in the OVX group. Concentrations of plasma total cholesterol, triglyceride, LDL-cholesterol were significantly lower in the taurine fed group of OVX rats fed Ca deficient diet, while HDL-cholesterol concentration was increased in the taurine fed group. Therefore, in this study, we examined whether taurine also prevented hypercholesterolemia induced by ovarian hormone deficiency in ovariectomized rats when they were fed a calcium-deficient diet. These results indicate that taurine may have some beneficial effects on hypercholesterolemia and hypertriglyceridemia in OVX rats fed calcium-deficient diet.
Keywords: Taurine, lipids, Ca deficient, OVX
Introduction
Coronary heart disease is the leading cause of death in the U.S. and in most developed countries. Taurine, 2-aminoethanesulfonic acid, is one of the most abundant free amino acids in animal cells and tissues and is thought to have such functions as antioxidation, anti-inflammation, osmoregulation and nerve regulation (Huxtable & Sebring, 1983).
Since high serum cholesterol is one of the major risk factors for atherosclerosis and coronary heart diseases, taurine is thought to prevent the development of atherosclerosis. The effect of taurine on plasma lipids and coronary heart disease has been the subject of extensive research. The average US diet contains only 600 mg calcium a day and thus falls far below the recommended intakes (Nieves, 2005). Korean women on average consume only about one-third of their RI for calcium (Ministry for Health Welfare and Family Affairs, 2005). However, the effect of taurine in an ovariectomized animal model has not been studied in the calcium deficient rats. The purpose of this study was to investigate the effect of dietary taurine supplementation on plasma, liver lipid concentrations in ovariectomized rats fed calcium-deficient diet. Therefore, in this study, we examined whether taurine also prevented hypercholesterolemia induced by ovarian hormone deficiency in ovariectomized rats when they were fed a calcium-deficient diet. The diet contained 50% calcium level in AIN-93 diet.
Materials and Methods
Animals and diet
Forty female Sprague-Dawley rats (Biogenomics, Seoul, Korea) weighing about 195 g were divided into two groups. One group was ovariectomized and the other group was sham operated (Sham). Then the animals further divided into the control and taurine supplemented diet groups. The level of taurine supplementation was 2.0 g/100 g diet. All rats were fed on experimental diet and deionized water ad libitum for 6 weeks. The compositions of the diets are shown in Table 1. Rats were individually housed in stainless steel cages in a room with controlled temperature (23℃) and humidity (55%). Rats were maintained a 12 h light (07:00-19:00 h) and dark cycle. Prior to termination of the study, rats were transferred to individual metabolic cages for twelve hours of urine collection from 9 PM to 9 AM.
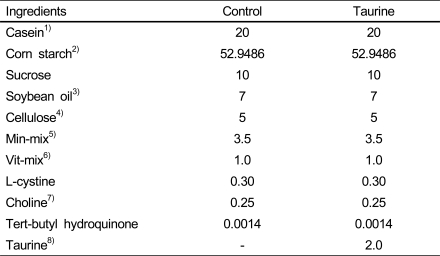
Table 1.
Composition of experimental diets (g/100g diet)
1)Lactic Casein, 30mesh, New Zealand Dairy Board, Willington, N.Z.
2)Corn Starch, Doosan Co. 234-17 Maam-Ri, Bubal-Eup, Inchon-City, Kyunggi-Do
3)Soybean oil, CJ CheilJedang Co. Seoul, Korea.
4)Cellulose, supplied by SIGMA Chemical Company.
5)Calcium deficient Mineral Mixture (AIN-93), supplied by U.S. CORNING Laboratory Services Company. TEKLAD TEST DIETS, Madison, Wisconsin.
6)Vitamin mixture (AIN-93), supplied by U.S. CORNING Laboratory Services Company. TEKLAD TEST DIETS, Madison, Wisconsin.
7)Choline, supplied by SIGMA Chemical Company.
8)Taurine, Dong-A Pharm. Co. Ltd. 434-4 Moknae-dong, Ansan-City, Kyunggi-Do
During this 12-hour urine collection period, rats had access to deionized water but no food was provided. At the termination of the study, animals were weighted and then anesthetized with an intramuscular injection of a combination of lumpon (1.0 mg/100 g body weight) and entobal (5 mg/100 g body weight) before blood was drawn from their abdominal aortas. On the last day of the experimental period, a blood sample was collected from the abdominal aorta. The plasma was separated from blood by centrifugation (1600 × g, 15 min 4℃) and was stored at -70℃ until analysis. After blood collection, the liver was immediately removed, washed with cold saline (9 g NaCl/L), blotted dry on filter paper, weighted, and stored at -70℃ until analysis. Plasma was deproteinized by using sulfosalicilic acid, and taurine concentrations in plasma were measured with an automatic amino acid analyzer based on ion exchange chromatography (Biochrom 20, Pharmacia Biotech, Cambridge, England).
Plasma and hepatic lipids
Plasma lipids (total cholesterol, HDL-cholesterol, and triglycerides) were determined by using commercial kits (Wako Pure Chemical, Osaka, Japan). LDL-cholesterol concentrations were estimated with the equation of Friedewald (Friedewald et al., 1972). About 2 g of liver were homogenized, and lipids were extracted with a chloroform: methanol mixture (2:1, v/v) as described by Folch (1957). The concentrations of cholesterol and triglyceride in liver were determined enzymatically with a commercial kit (Waco, Japan).
Statistical analysis
The statistical significance of differences among the groups was evaluated by two-way ANOVA, using a computer software package (version 9.13, SAS Institute Inc, Cary, NC). Individual comparisons were made by Duncan's multiple range test using ANOVA. Differences were considered to be significant at p<0.05. Data were expressed as means ± SD.
Results
Weight gain and food efficiency ratio(FER)
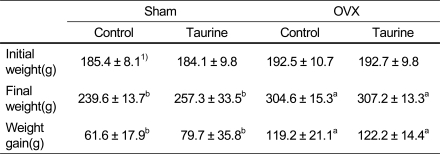
All rats were weighted initially and at the termination of the experiment. In the present study, initial body weights were not significantly different between two groups (Table 2). At the end of experiment, OVX rats gained more weight than the sham treated group. Supplementation of taurine to the diet did not affect body weight in comparison with control group. The results of this study indicate that body weight gain was higher in OVX groups than in Sham groups regardless of diets.
Table 2.
The effect of diets on body weight and weight gain in rats fed Ca deficient diet
1)Mean ± SD
2)Values with different superscript within a row are significantly different at p<0.05 by Duncan's multiple range test.
Blood and hepatic lipids
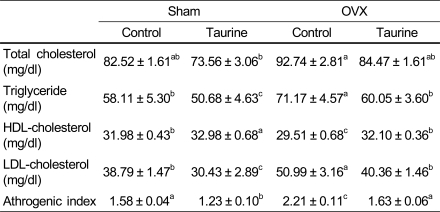
Rats fed taurine diet had significantly lower total cholesterol, triglyceride, LDL-cholesterol and athrogenic index than those fed control diet in OVX rats fed calcium-deficient diet. Rats fed taurine diet had significantly higher HDL-cholesterol than those fed control diet in OVX rats fed calcium-deficient diet.
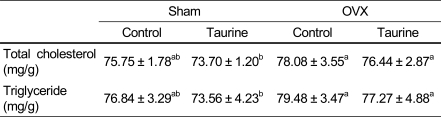
Liver total cholesterol and triglyceride levels were not significantly reduced in rats fed taurine diet compared to those fed control diet in OVX rats fed calcium-deficient diet.
Plasma taurine concentrations were not significantly different between two groups, however, concentration of plasma taurine tended to be high in the taurine supplemented group than in the control group (Table 6). This finding is in agreement with Choi and co-workers who reported that taurine supplementation had no influence on the plasma taurine concentration (Choi et al., 2006).
Table 6.
Plasma taurine concentrations of rats fed experimental diet (µmol/L)
1)Mean ± SD
2)Values with different superscript within a row are significantly different at p<0.05 by Duncan's multiple range test.
Discussion
Dairy consumption has been postulated to reduce the risk of obesity and metabolic disturbances. Several observational studies have found inverse associations between calcium intake or intake of dairy products and body weight, composition, or both (Loos et al., 2004). Low daily calcium intake is associated with greater adiposity, particularly in women. In both sexes, high calcium intake is associated with plasma lipoprotein-lipid profile predictive of lower risk of coronary heart disease risk compared with low calcium intake (Snijder et al., 2007). Some previous studies (Azadbakht et al., 2005; Choi et al., 2005; Liu et al., 2006; Marques-Vidal et al., 2006; Mirmiran et al., 2005; Moore et al., 2006; Steffen et al., 2005) showed an inverse relation between total dairy intake and body weight or metabolic risk, but others (Berkey et al., 2005; Rajpathak et al., 2006) did not (Shapses et al., 2004).
A large proportion of the worlds' population consumes low-calcium diets. Western diets are also high in protein, especially animal protein. The international epidemiologic data show an association between protein consumption and osteoporotic fractures (Abelow et al., 1992; Feskanich et al., 1996; Frassetto et al., 2000).
Although the sulfur amino acids in animal proteins such as meat are thought to cause hypercalciuria, the high phosphorus content of these proteins has been found to negate this effect (Spencer et al., 1988).
Taurine is a sulfur-containing amino acid that is best known for its conjugation with bile acids, but it is also involved in the coordination of nerve function, stabilization of the cell membrane, detoxification, antioxidant reactions and modulation of osmotic pressure (Huxtable, 1992). In the pancreas, taurine is highly concentrated in the islets and stimulates insulin release by fetal β-cells in vitro (Bustamante et al., 1998; Cherif et al., 1996). Body weight gain and food intake of rats fed the taurine diet (20 g taurine/kg diet) did not differ from those of rats fed the control diet. Taurine is thought to be quite safe and there is little concern about the side effects of excessive intake of taurine (Furukawa et al., 1991).
Rats fed taurine diet had significantly lower total cholesterol, triglyceride, LDL-cholesterol and athrogenic index than those fed control diet in OVX rats fed calcium-deficient diet. Rats fed taurine diet had significantly higher HDL-cholesterol than those fed control diet in OVX rats fed calcium-deficient diet.
Numerous studies suggest that taurine, 2-aminoethanesulfonic acid, decreases hypercholesterolemia and its effects (Murakami et al., 1996).
Taurine up-regulated low density lipoprotein receptor (LDLR) activity in a human hepatoma cell line (Metz et al., 1993). Therefore, up-regulation of hepatic low density lipoprotein receptor (LDLR) activity may be involved in the hypocholesterolemic effect of taurine.
These results indicate that taurine may have some beneficial effects on hypercholesterolemia and hypertriglyceridemia in OVX rats fed calcium-deficient diet.
Table 3.
The effect of diets on food intake and food efficiency ratio(FER) in rats fed Ca deficient diet
1)Mean ± SD
2)Values with different superscript within a row are significantly different at p<0.05 by Duncan's multiple range test.
Table 4.
The effect of diets on lipid concentrations in rats fed Ca deficient diet
1)Mean ± SD
2)Values with different superscript within a row are significantly different at p<0.05 by Duncan's multiple range test.
Table 5.
The effect of diets on liver lipid concentrations in rats fed Ca deficient diet
1)Mean ± SD
2)Values with different superscript within a row are significantly different at p<0.05 by Duncan's multiple range test.
Acknowledgment
We thank the Dong-A Pharmaceutical Co. Korea, which donated taurine.
References
- 1.Abelow BJ, Holford TR, Isogna KL. Cross-cultural association between dietary animal protein and hip fractures: a hypothesis. Calcif Tissue Int. 1992;50:14–18. doi: 10.1007/BF00297291. [DOI] [PubMed] [Google Scholar]
- 2.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82:523–530. doi: 10.1093/ajcn.82.3.523. [DOI] [PubMed] [Google Scholar]
- 3.Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159:543–550. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante J, Alonso FJ, Lobo M, Gine E, Tamarit-Rodriguez J, Solis JM, Martin del Rio R. Taurine levels and localization in pancreatic islets. Adv Exp Med Biol. 1998;442:65–69. doi: 10.1007/978-1-4899-0117-0_8. [DOI] [PubMed] [Google Scholar]
- 5.Cherif H, Reusens B, Dahri S, Remacle C, Hoet JJ. Stimulatory effects of taurine on insulin secretion by fetal rat islets cultured in vitro. J Endocrinol. 1996;151:501–506. doi: 10.1677/joe.0.1510501. [DOI] [PubMed] [Google Scholar]
- 6.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165:997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 7.Choi MJ, Kim JH, Chang KJ. The effect of dietary taurine supplementation on plasma and liver lipid concentrations and free amino acid concentrations in rats fed a high cholesterol diet. AEMB. 2003;526:75–82. doi: 10.1007/978-0-387-33504-9_25. [DOI] [PubMed] [Google Scholar]
- 8.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143:472–479. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- 9.Folch F, Lees M, Slane-Stanly GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biochem. 1957;1226:497–509. [PubMed] [Google Scholar]
- 10.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Worldwide incidence of hip fractures in elderly women: relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci. 2000;55:M585–M592. doi: 10.1093/gerona/55.10.m585. [DOI] [PubMed] [Google Scholar]
- 11.Fridewald WT, Lavy RI, Fredricson DS. Estimation of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Furukawa S, Katto M, Kouyama H, Nishida I, Kikumori M, Taniguchi Y, Toda T, Araki H. Repeated dose toxicity study of intravenous treatment with taurine for 13 weeks and recovery test for 5 weeks in rats. J Jpn Phamacol Ther. 1991;19:275–306. [Google Scholar]
- 13.Hegsted DM. Calcium and osteoporosis. J Nutr. 1986;116:2316–2319. doi: 10.1093/jn/116.11.2316. [DOI] [PubMed] [Google Scholar]
- 14.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Huxtable RJ, Sebring LA. Cardiovascular actions of taurine. In: Kuriyama K, Iwata RJ, Liss AR, editors. Sulfur Amino Acids: Biochemical and Clinical aspects. New York. USA: Clin Aspec; 1983. pp. 5–37. [Google Scholar]
- 16.Liu S, Choi HK, Ford E. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–1584. doi: 10.2337/dc06-0256. [DOI] [PubMed] [Google Scholar]
- 17.Loos RJ, Rankinen T, Leon AS. Calcium intake is associated with adiposity in black and white men and white women of the HERITAGE Family Study. J Nutr. 2004;134:1772–1778. doi: 10.1093/jn/134.7.1772. [DOI] [PubMed] [Google Scholar]
- 18.Marques-Vidal P, Goncalves A, Dias CM. Milk intake is inversely related to obesity in men and in young women: data from the Portuguese Health Interview Survey 1998-1999. Int J Obes (Lond) 2006;30:88–93. doi: 10.1038/sj.ijo.0803045. [DOI] [PubMed] [Google Scholar]
- 19.Metz JA, Anderson JJ, Gallagher PN., Jr Intakes of calcium, phosphorus, and protein, and physical-activity level are related to radial bone mass in young adult women. Am J Clin Nutr. 1993;58:537–542. doi: 10.1093/ajcn/58.4.537. [DOI] [PubMed] [Google Scholar]
- 20.Ministry for Health Welfare and Family Affairs. Korea National Health and Nutrition Examination Survey. Seoul. Republic of Korea: 2005. [Google Scholar]
- 21.Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption and body mass index: an inverse relationship. Int J Obes (Lond) 2005;29:115–121. doi: 10.1038/sj.ijo.0802838. [DOI] [PubMed] [Google Scholar]
- 22.Moore LL, Bradlee ML, Gao D, Singer MR. Low dairy intake in early childhood predicts excess body fat gain. Obesity (Silver Spring) 2006;14:1010–1018. doi: 10.1038/oby.2006.116. [DOI] [PubMed] [Google Scholar]
- 23.Murakami S, Yamagishi I, Asami Y, Ohta Y, Toda Y, Nara Y, Yamori Y. Hypolipidemic effect of taurine in stroke-prone spontaneously hypertensive rats. Pharmacology. 1996;52:303–313. doi: 10.1159/000139395. [DOI] [PubMed] [Google Scholar]
- 24.Nieves JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005;81:1232S–1239S. doi: 10.1093/ajcn/81.5.1232. [DOI] [PubMed] [Google Scholar]
- 25.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83:559–566. doi: 10.1093/ajcn.83.3.559. [DOI] [PubMed] [Google Scholar]
- 26.Shapses SA, Heshka S, Heymsfield SB. Effect of Calcium Supplementation on weight and fat loss in women. J Clin Endocrinol metab. 2004;89:632–637. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G, Heine RJ, Bouter LM, Dekker JM. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn Study. Am J Clin Nutr. 2007;85:989–995. doi: 10.1093/ajcn/85.4.989. [DOI] [PubMed] [Google Scholar]
- 28.Spencer H, Kramer L, Osis D. Do protein and phosphorus cause calcium loss? J Nutr. 1988;118:657–660. doi: 10.1093/jn/118.6.657. [DOI] [PubMed] [Google Scholar]
- 29.Steffen LM, Kroenke CH, Yu X. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2005;82:1169–1177. doi: 10.1093/ajcn/82.6.1169. [DOI] [PubMed] [Google Scholar]