Abstract
Beneficial effects of dehydroepiandrosterone (DHEA) supplement on age-associated chronic diseases such as cancer, cardiovascular disease, insulin resistance and diabetes, have been reported. However, its mechanism of action in hepatocellular carcinoma in vivo has not been investigated in detail. We have previously shown that during hepatocellular carcinogenesis, DHEA treatment decreases formation of preneoplastic glutathione S-transferase placental form-positive foci in the liver and has antioxidant effects. Here we aimed to determine the mechanism of actions of DHEA, in comparison to vitamin E, in a chemically-induced hepatocellular carcinoma model in rats. Sprague-Dawley rats were administered with control diet without a carcinogen, diets with 1.5% vitamin E, 0.5% DHEA and both of the compounds with a carcinogen for 6 weeks. The doses were previously reported to have anti-cancer effects in animals without known toxicities. With DHEA treatment, cytosolic malate dehydrogenase activities were significantly increased by ~5 fold and glucose 6-phosphate dehydrogenase activities were decreased by ~25% compared to carcinogen treated group. Activities of Se-glutathione peroxidase in the cytotol was decreased significantly with DHEA treatment, confirming its antioxidative effect. However, liver microsomal cytochrome P-450 content and NADPH-dependent cytochrome P-450 reductase activities were not altered with DHEA treatment. Vitamin E treatment decreased cytosolic Se-glutathione peroxidase activities in accordance with our previous reports. However, vitamin E did not alter glucose 6-phosphate dehydrogenase or malate dehydrogenase activities. Our results suggest that DHEA may have decreased tumor nodule formation and reduced lipid peroxidation as previously reported, possibly by increasing the production of NADPH, a reducing equivalent for NADPH-dependent antioxidant enzymes. DHEA treatment tended to reduce glucose 6-phosphate dehydrogenase activities, which may have resulted in limited supply for de novo synthesis of DNA via inhibiting the hexose monophophaste pathway. Although both DHEA and vitamin E effectively reduced preneoplastic foci in this model, they seemed to function in different mechanisms. In conclusion, DHEA may be used to reduce hepatocellular carcinoma growth by targeting NADPH synthesis, cell proliferation and anti-oxidant enzyme activities during tumor growth.
Keywords: Hepatocellular carcinoma, DHEA, malate dehydrogenase, NADPH and glucose 6-phosphate dehydrogenase
Introduction
DHEA and DHEA-sulfate are the precursors of androgens and estrogens produced from adrenal glands (Roberge et al., 2007). Their levels start to decrease after middle age and animal and human studies have reported that age-associated chronic diseases involving dysregulation of metabolism may be due to the decline in the levels of these compounds (Labrie, 2007). Although there have been in vivo studies about the protective effects of DHEA against cancer, obesity, diabetes, postmenopausal osteoporosis, sexual dysfunction and cardiovascular disease as modulators of adrenocortical steroid synthesis (Allolio et al., 2007; Kim and Choi, 2005), its mechanism of action needs to be studied more in detail before safely used as supplements in humans for different diseases.
Reactive oxygen species are continuously formed as by-products of aerobic metabolism and from reactions of drugs and environmental toxins such as carcinogens (Cerutti, 1985). These play important roles in tumor progression by damaging DNA, proteins and unsaturated lipids (Champe & Harvey, 1987). The cells have protective mechanisms that can minimize the toxic effects from these compounds. These mechanisms include antioxidant chemicals (e.g. vitamin E), enzymes that catalyze antioxidant reactions using NADPH as a source of reducing electrons and a liver microsomal cytochrome P-450 monooxygenase system, which also utilizes NADPH to convert steroids and drugs to soluble forms (Wu et al., 1989).
We have previously shown that DHEA decreases glutathione S-transferase placental from (GST-p) foci in the liver and lipid peroxdation using a hepatocellular carcinoma model in rats (Kim & Choi, 2005). Here, we aimed to determine the mechanism of protective effects of DHEA supplement during chemical hepatocellular carcinogenesis in vivo in comparsion to those with vitamin E supplement, an effective intracellular reducing agent.
Materials and Methods
Animals
Six-week-old male Sprague Dawley rats were purchased from Seould National University and housed at the animal care facility at Seoul National University (Seoul, Korea). All rats were kept under standard temperature, humidity, and timed lighting conditions and were provided with rat chow and water ad libitum. Animals were induced with hepatocellular carcinoma by i.p. injection of diethylnitrosamine (200 mg/kg body weight) followed by administration of diets containing 0.01% of 2-acetylaminofluorene for 6 weeks. Partial hepatectomy surgeries were performed 1 week after the start of 2-acetylaminofluorene diet to effectively induce progression of tumor as previously published (Ito et al., 1988).
Diets and materials
Rat chow was purchased from Purina (purified rodent diet 5053, St. Louis, Missouri) and was supplemented with 1.5% vitamin E (Sigma T-3376, DL-α tocopheryl acetate, St. Louis, MO) and 0.5% DHEA (Sigma D-400, DHEA 3-acetate, St. Louis, MO) as described previously (Kim & Choi, 2005).
Biochemical assays
Frech or frozen livers were weighed and five volumes of ice-cold homogenization buffer [154 mM KCl, 50 mM Tris-HCl, 1mM EDTA, pH 7.5] were added. The tissue was homogenized and was fractionated by spinning at 1,000 g for 13 min at 4℃. The middle layer was centrifugated at 10,000 g for 13 min at 4℃. Then the supernatant was centrifugated at 100,000 g for 65 min at 4℃ to obtain cytosolic and microsomal fractions as described in detail previously (Kim & Choi, 2005).
Malate dehydrogenase activities
Malate dehydrogenase activities were measured in the cytosolic fraction using modified methods of Ochoa (1969). Briefly, 500 µl of triethanolamine buffer [0.4 M, pH 7.4], 50µl of L-malate [30 mM], 100 µl of MnCl2, 4H2O [0.12 M] and 200µl of NADP [3.4 mM] were mixed with 1,400 µl of cytosolic fraction using a vortex. Absorption at 270 nm during 1 min at 26℃ was measured every 6 seconds. One unit is increased in absoption by 0.01 during 1 min.
Glucose 6-phophate dehydrogenase activities
Glucose 6-phophate dehydrogenase activities were measured in the cytosolic fraction using modified methods of Lohr and Waller (1974). Briefly, 2.4 ml of triethanolamine buffer [50 mM, pH 7.5] and 0.5 ml of cytosolic fraction (~0.8 mg/ml protein) were mixed using a vortex. Then, 50 µl of NADP solution was added and was kept at 25℃ for 5 minutes. Next, 50 µl of glucose 6-phosphate was added and increases in absorption was immediately measured at 340 nm for every 2 minutes up to 6 minutes. Specific activity was calculated as µmole of substrate converted per minute per mg protein.
Se-glutathione peroxidase
Cytosolic Se-glutathione peroxidase activites were measured using a modified method of Tappel (1978) as previously described.
Microsomal cytochrome P-450
Hepatic microsomal fraciton was used to measure cytochrome P-450 content (Omura & Sato, 1964). Fresh microsomal fraction was diluted with phosphate buffer (pH 7.4) to conatin ~ 1 mg/ml protein and sodium dithionate was added and was saturated with CO gas for 1 minute. Then, absorptions of reduced carbon monoxide at 450 and 490 nm were determined spectrophotometrically. Molar extinction coefficient used was 91/mM/cm.
Protein concentration
Protein concentrations were determined by the Bradford assay using the Biorad Protein assay reagent (Biorad, Hercules, CA).
Statistical analysis
Data are expressed as mean ± SE. SAS software was used for Duncan's multiple range test and one-way ANOVA to determine statistical differences (p<0.05).
Results
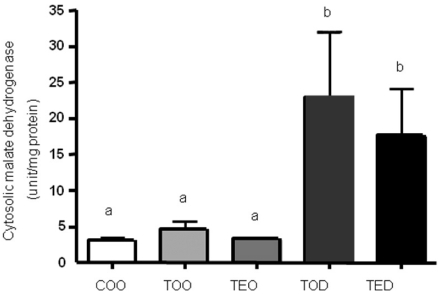
DHEA treament increased cytosolic malate dehydrogenase activities
We previously showed (Kim & Choi, 2005) that DHEA treatment decreased NADPH-utilizing antioxidant enzymes in this model so we first determined if activities of enzymes that regulate NADPH synthesis were altered. Interestingly, DHEA treatment significantly increased malate dehydrogenase activities by almost 5 fold vs. carcinogen-treated controls (Fig. 1). This effect was not seen in vitamin E-treated animals. Malate dehydrogenase activities were not different between non-carcninogen treated and carcinogen-treatd groups (Fig. 1, COO vs. TOO). Based on these results, we focused our study on determining the activities of another NADPH producing enzyme, glucose 6-phosphate dehydrogenase. a and b; one-way ANOVA, p<0.05
Fig. 1.
DHEA treament increases cytosolic malate dehydrogenase activities. The level of cytosolic malate dehydrogenase activity was determined by biochmical enzymatic assay. Hepatocellular carcinoma was induced with diethylnitrosamine, 2-acetylaminofluorene and ~70% partial hepatectomy using male Sprague-Dawley rats. The animals were treated with control diet without a carcinogen, carcinogen diets with 1.5% vitamin E, 0.5% dehydroepiandrosterone (DHEA) and both 1.5% vitamin E and 0.5% DHEA. After ultracentrifugation of freshly-obtained liver, cytosolic fraction was obtained and used to measure malate dehydrogenase activities. Activities were normlaized against protein concentration. One-way ANOVA test was used to determine significance (n=6~9 each). Means with different subscripts are significantly different at p<0.05 by Duncan's multiple range test.
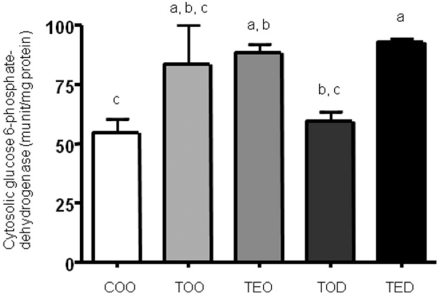
Activities of cytosolic glucose 6-phosphate dehydrogenase had a tendency to decrease with DHEA treatment
Although DHEA treatment increased malate dehydrogenase activities, it had a strong tendency to decrease glucose 6-phosphate dehydrogenase activities (Fig. 2). Glucose 6-phosphate dehydrogenase activity was increased by almost 1.5 fold with the carcinogen treatment and DHEA supplement decreased it to the level of those of non-carcinogen treated group (COO vs. TOD). However, in the combination group (TED), the enzyme activity was not suppressed as seen in DHEA-only treated animals. a, b, c; one-way ANOVA, p<0.05.
Fig. 2.
Activities of cytosolic glucose 6-phosphate dehydrogenase tended to decrease with DHEA treatment. The level of glucose 6-phosphate dehydrogenase activity was determined by biochmical enzymatic assay. Similar to the malate dehydrogenase, after ultracentrifugation of freshly-obtained liver, cytosolic fraction was obtained and used to measure glucose 6-phosphate dehydrogenase activities. Activities were normalized against protein concentration. One-way ANOVA test was used to determine significance (n=6~9 each). Means with different subscripts are significantly different at p<0.05 by Duncan's multiple range test.
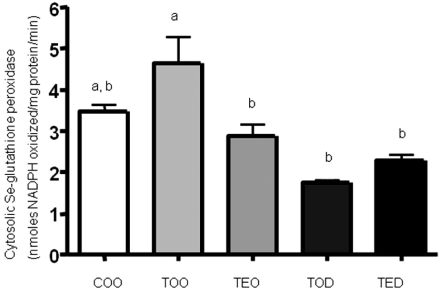
DHEA treatment decreased hepatic cytosolic Se-glutathione peroxidase activities.
Activities of cytosolic Se-glutathione peroxidase activities tended to increase with carcinogen treatment (COO vs. TOO). The enzyme activities were inhibited almost 50-60% with DHEA treamtment comapred to controls (COO and TOO vs. TOD). Vitamin E treatment also decreased the activities significantly and a synergistic inhibitory effects were seen in combination treatment group (TOO vs. TED).
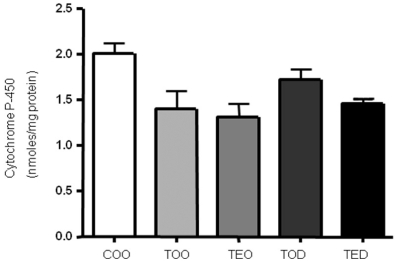
Hepatic microsomal cytochrome P-450 content was not altered with DHEA treatment.
To examine the mechanism of action of DHEA on hepatic microsomal cytochrome P-450 mono-oxygenase system, cytochrome P-450 content was measured. DHEA treatment did not affect cytochrome P-450 content (Fig. 4). We also measured microsomal NADPH cytochrome P-450 reductase activites but they were also not affected with DHEA treatment (data not shown).
Fig. 4.
Hepatic microsomal cytochrome P-450 content was not different with DHEA treatment. Content of cytochrome P-450 was measured in the hepatic microsomal fractions using reduced carbon monoxide. Microsomal fraction was obtained as described in the methods. One-way ANOVA test was used to determine significance (n=6~9 each).
Discussion
Here we studied the mechanism of action of DHEA during hepatocellular carcinogenesis in vivo. We found that DHEA significantly increased activities of malate dehydrogenase, an enzyme important for supplying biochemical reductant NADPH in the cytosol. On the other hand, glucose 6-phosphate dehydrogenase activites were reduced by ~25% with DHEA treatment possibly due to the high level of NADPH/NADP+ ratio in the cytosol, provided by increased malate dehydrogenase activity. Reports showed that DHEA treatment decreased mRNA levels of hepatic malate dehydrogenase in broiler chickens (Zhao et al., 2007) and increased the activity in non-insulin dependent diabetic rats (Ladriere et al., 1997). However, mRNA levels do not always correlate with levels of enzyme activity and the dose, duration and the disease model used in these studies were different from the current study. Marrero, et al. showed that DHEA treatment in female mice increased hepatic malate dehydrogenase activites by 2-3 fold (Marrero et al., 1990) but did not alter glucose 6-phosphate dehydrogenase activity, similar to the data from this study. Further studies using different organ systems in various disases models are needed to clarify effect of DHEA on these NADP-dependent enzymes.
Under most metabolic conditions, the ratio of NADPH/NADP+ is high enough to inhibit glucose 6-phosphate dehydrogenase activity. However, with increased demand for NADPH in the cells (e.g. toxic and oxidative stress), the ratio of NADPH/NADP+ decreases and flux through the hexose monophosphate pathway is enhanced by increasing the activity of glucose 6-phosphate dehydrogenase (Champe & Harvey, 1987). Glucose 6-phosphate dehydrogenase is the key enzyme regulating the first and irreversible step of the pentose phsophate pathway. This pathway can then provide ribose-5-phosphates, a precursor for DNA synthesis in cell proliferation. During promotional phase of carcinogenesis, the demand for ribose-5-phosphate for de novo synthesis of DNA may be increased and it is thus particularly important that DHEA treatment suppressed this rate limiting enzyme of the pentose phosphate pathway, glucose 6-phosphate dehydrogenase. This may be one of the protective mechanisms of DHEA during carcinogenesis as reported previously (Kim & Choi, 2005). Although malate dehydrogenase activites increased with DHEA treamtment in this study, it has been reported that its activity is ~15% of that of glucose 6-phosphate dehydrogenase in rat adrenal cortex (Frederiks et al., 1990). If this is the case in rat liver, the decrease in glucose 6-phosphate dehydrogenase would surpass the relative change in the malate dehydrogenase activity in the inhibition of pentose phosphate pathway.
Increases in malate dehydrogenase activities by DHEA treatment may have also provided NADPH to reduce glutathione efficiently. This antioxidant effects of DHEA was seen in decreased Se-dependent glutathione peroxidase activities (Fig. 3), confirming less oxidative damage with DHEA treatment as previously reported (Kim & Choi, 2005). Reduced glutathione (a tripeptide-thiol) can detoxify hydrogen peroxide in most cells in a NADPH-dependent manner (Frederiks et al., 1990) catalyzed by glutathione reductase. Although the cells were producing higher amounts of NADPH through malate dehydrogenase, it may be that the cells were not triggered to increase NADPH-dependent antioxidant enzymes due to already decreasd oxidative stress level in the cell by direct or indirect antioxidant effect of DHEA. The possible mechanism of the non-enzymatic antioxidative activity of DHEA needs further studies in the future. Furthermore, the effects of DHEA may depend on the phase in which these enzymatic activities were measured during the progression of hepatocellular carcinoma.
Fig. 3.
DHEA treatment decreased hepatic cytosolic Se-glutathione peroxidase activities. Activities of Se-glutathione peroxidase activities were measured in the hepatic cytosolic fractions as mentioned above, using hydrogen peroxide as substrate. Means with different subscripts are significantly different at p<0.05 by Duncan's multiple range test.
Vitamin E has been known to be a strong nonenzymic antioxidant, preventing oxidative damage in the cell components by reducing free radicals as reported (Muller et al., 2003). Here, vitamin E decreased glutathione peroxidase activities (Fig. 3) probably through different mechanism than that from DHEA; vitamin E did not alter malate dehydrogenase or glucose 6-phosphate dehydrogenase activities in this study.
Lastly, the content of cytochrome P-450 (Fig. 4) and NADPH-dependent cytochrome P-450 reductase activities (data not shown) were not affected by DHEA treatment. These data suggest that the mechanism of actions of DHEA is independent from these liver microsomal cytochrome P-450 mono-oxygenase system, a major pathway for the hydroxylation of aromatic and aliphatic compounds sush as stroids and alcohols and carcinogens (Wu et al., 1989) which also uses NADPH to to convert them into soluble forms. This is different from previous reports that showed that DHEA decreased cytochrome P-450 enzymes, especially P4501A and 3A (Fitzpatrick et al., 2001). It is possible that because we measured total levels of cytochrome P-450 in this study, we may not have found any difference in the overall levels and may have missed isozyme-specific effects of DHEA.
Our data suggest that DHEA has protective effects during hepatocellular carcinoma by upregulating NADPH-producing malate dehydrogenase and may thus spare cells from reactive oxygen species-induced damages during carcinogenesis. In the future, more studies with different concentration of DHEA in various stages of cancer progression will be required to find out further details of its mechanism of action in vivo.
References
- 1.Allolio B, Arlt W, Hahner S. DHEA: Why, when, and how much-DHEA replacement in adrenal insufficiency. Ann Endocrinol (Paris) 2007;68:268–273. doi: 10.1016/j.ando.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 3.Champe PC, Harvey RA. Hexose monophosphate pathway. In: Winters R, Schott J, editors. Biochemistry. USA: Lippincott-Raven, Philadelphia, Pennsylvania; 1987. pp. 111–113. [Google Scholar]
- 4.Fitzpatrick JL, Ripp SL, Smith NB, Pierce WM, Prough RA., Jr Metabolism of DHEA by cytochromes P450 in rat and human liver microsomal fractions. Arch Biochem Biophys. 2001;389:278–287. doi: 10.1006/abbi.2001.2341. [DOI] [PubMed] [Google Scholar]
- 5.Frederiks WM, Van Noordan CJ, Aronson DC, Marx F, Bosch KS, Jonges GN, Vogels IM, James J. Quantitative changes in acid phosphatase, alkaline phosphatase and 5'-nucleotidase activity in rat liver after experimentally induced cholestasis. Liver. 1990;10:158–166. doi: 10.1111/j.1600-0676.1990.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 6.Ito N, Tsuda H, Tatematsu M, Inoue T, Tagawa Y, Aoki T, Uwagawa S, Kagawa M, Ogiso T, Masui T. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats-an approach for a new medium-term bioassay system. Carcinogenesis. 1988;9:387–394. doi: 10.1093/carcin/9.3.387. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Choi H. Effects of vitamin E and dehydroepiandrosterone on the formation of preneoplastic lesions in rat hepatocellular carcinogenesis. The Korean Journal of Nutrition. 2005;38:364–372. [Google Scholar]
- 8.Labrie F. Drug insight: breast cancer prevention and tissue-targeted hormone replacement therapy. Nat Clin Pract Endocrinol Metab. 2007;3:584–593. doi: 10.1038/ncpendmet0559. [DOI] [PubMed] [Google Scholar]
- 9.Ladriere L, Laghmich A, Malaisse-Lagae F, Alaisse WJ. Effect of dehydroepiandrosterone in hereditarily diabetic rat. Cell Biochem Funct. 1997;15:287–292. doi: 10.1002/(SICI)1099-0844(199712)15:4<287::AID-CBF753>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Lohr GW, Waller HD. Glucose 6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods in enzymatic analysis, (2) New York. USA: AP.; 1974. pp. 636–643. [Google Scholar]
- 11.Marrero M, Prough RA, Frenkel RA, Milewich L. Dehydroepiandrosterone feeding and protein phosphorylation, phosphatases, and lipogenic enzymes in mouse liver. Proc Soc Exp Biol Med. 1990;193:110–117. doi: 10.3181/00379727-193-43010. [DOI] [PubMed] [Google Scholar]
- 12.Muller A, Pallauf J. Effect of increasing selenite concentrations, vitamin E supplementation and different fetal calf serum content on GPx1 activity in primary cultured rabbit hepatocytes. J Trace Elem Med Biol. 2003;17:183–192. doi: 10.1016/S0946-672X(03)80024-X. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa S. Malic enzyme. In: Lowenstein JM, editor. Methods in enzymology. New York, USA: AP.; 1969. pp. 230–237. [Google Scholar]
- 14.Omura T, Sato R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 15.Roberge C, Carpentier C, Langlois M, Baillargeon J, Ardilouze J, Maheux P, Gallo-Payet N. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Am J Physiol Endocrinol Metab. 2007;293:E1465–E1478. doi: 10.1152/ajpendo.00516.2007. [DOI] [PubMed] [Google Scholar]
- 16.Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 17.Wu HQ, Masset-Brown J, Tweedie DJ, Milewich L, Frenkel RA, Martin-Wixtrom C, Estabrook RW, Prough RA. Induction of microsomal NADPH-cytochrome P-450 reductase and cytochrome P-450IVA1 (P-450LA omega) by dehydroepiandrosterone in rats: a possible peroxisomal proliferator. Cancer Res. 1989;49:2337–2343. [PubMed] [Google Scholar]
- 18.Zhao S, Ma H, Zou S, Chen W. Effects of in ovo administration of DHEA on lipid metabolism and hepatic lipogenetic genes expression in broiler chickens during embryonic development. Lipids. 2007;42:749–757. doi: 10.1007/s11745-007-3068-y. [DOI] [PubMed] [Google Scholar]