Abstract
Inspired by nature’s ability to fabricate supramolecular nanostructures from the bottom-up, materials scientist have become increasingly interested in the use of biomolecules like DNA, peptides, or proteins as templates for the creation of novel nanostructures and nanomaterials. Although the advantages of self-assembling biomolecular structures clearly lie in their chemical diversity, spatial control, and numerous geometric architectures, it is challenging to elaborate them into functional hybrid inorganic–bionanomaterials without rendering the biomolecular scaffold damaged or dysfunctional. In this study, attachment of gold nanoparticles to collagen-related self-assembling peptides at L-lysine residues incorportated within the peptides sequence and the N-terminus led to metal nanoparticle–decorated fibers. After electroless silver plating, these fibers were completely metalized, creating electrically conductive nanowires under mild conditions while leaving the peptide fiber core intact. This study demonstrates the bottom-up assembly of synthetic peptidic fibers under mild conditions and their potential as templates for other complex inorganic–organic hybrid nanostructures.
Introduction
Research in the field of nanosensors, opto- or nanoelectronics, and microfluidic devices for applications in biomedical imaging and diagnosis, environmental monitoring, and the like calls for the development of novel, innovative nanomaterials and their organization into complex nanostructures. Top-down lithographic methods have been traditionally employed, but as dimensions shrink below one hundred nanometers these techniques become increasingly expensive and difficult.1, 2 To meet this challenge, alternative strategies have recently been developed to generate complex nanostructures from self-assembling organic macromolecules in a bottom-up manner. Nature’s ability to create sophisticated three-dimensional nanostructures has provided a rich source of inspiration for such bottom-up assembly strategies.3 Key concepts that can be adopted from self-assembly found in nature include molecular recognition of the single building blocks and the formation of predictable nanostructures.2, 4–7
Pioneered by Braun, Belcher, and their coworkers, there have been numerous examples of DNA or viruses as scaffolds for complex nanostructured inorganic materials.8–11 Since the nucleobase or amino-acid sequence encodes how these scaffolds self-assemble, a variety of programed nanostructures can be produced.12, 13 Despite this potential, viruses are not accessible by chemical synthesis. Although DNA can be readily synthesized, it is composed of a small number of similar monomers and virtually all functional groups are engaged in duplex formation, such that there is limited opportunity for external interactions or further functionalisation. As a result, some approaches have combined DNA and proteins to create functional nanomaterials.14 In contrast, peptides and proteins are built from 20 proteinogenic plus a wide variety of non-natural amino-acids. This degeneracy leads to chemical diversity, evident by the display of aliphatic, acidic, basic, or aromatic side chains from a peptide backbone, and structural complexity, manifested by the multitude of possible molecular architectures like helices, β-sheets, and tubules.15–17 Diverse nanostructures and, more importantly, controllable display of desired functionalities on their surface make peptides/proteins excellent scaffolds.18–20 A bottle neck common to all approaches utilizing biomolecular scaffolds to assemble nanomaterials is the delicate combination of organic and inorganic components to produce functional hybrid nanomaterials, which, based on their chemical/structural diversity, peptides are better suited to address.
Collagen is an exemplary type of robust biological nanostructure built from simple building blocks. It is the most abundant protein in modern vertebrates, comprising approximately 30% of the total protein content and 70% of the dry weight of human skin, and provides the three-dimensional matrix for connective tissue types such as bone and cartilage.21–24 Its unique tertiary structure is a right-handed triple helix composed of three helical peptide strands (left-handed polyproline II-type). The general amino-acid sequence of the single strands is Xaa-Yaa-Gly, with 2S-proline (L-proline, one letter code: P) being the most abundant aminoacid in the Xaa-position and (2S,4R)-4-hydroxyproline (L-hydroxyproline, one letter code: O) in the Yaa-position. Other amino-acids can occupy these positions without significantly perturbing the collagen structure, providing diverse functional groups along the helical backbone.25, 26 This robustness in conjunction with the modular design and straightforward solid-phase synthesis make collagen-related peptides a promising platform for generating new and interesting nanostructured materials.27, 28
In this report, micrometer-long fibers were assembled from collagen-related peptides through π–π stacking interactions between C- and N-terminal aromatic residues, as reported by Maryanoff and coworkers.29 Furthermore, these peptides feature a lysine residue within their amino-acid sequence which allowed the attachment of gold nanoparticles to both the side-chain lysine residue and the N-terminal amino functionality. After subsequent electroless silver plating under mild conditions, the peptidic fibers were grown into metallic nanowires (Scheme 1). The dimensions and morphology of these nanowires were assessed by transmission electron microscopy (TEM) and scanning electron microscopy (SEM), and their composition confirmed by energy dispersive X-ray spectroscopy (EDS). Finally, the conductivity of such metallic nanowires were measured using electron-beam (e-beam) lithography to define two terminal devices of single nanowires.
Scheme 1.
Self-assembly of collagen related synthetic peptides and their use as templates for the assembly of metallic nanowires: 1) modification with colloidal gold nanoparticles; 2) self-assembly to collagen-like fibers; 3) electroless silver plating to yield metallic nanowires.
Experimental
Materials and instrumentation
Amino-acids and resins were purchased from Novabiochem and Fmoc-pentafluoro-L-phenylalanine from Synthetech. Fmoc-ProHypGly-OH tripeptide was synthesized as described previously.30 Mono-Sulfo-N-Hydroxy-Succinimido-Nano-gold® Labeling Reagent was purchased from Nanoprobes and Aurion R-Gent SE-EM Silver Enhancement Reagent from EMScience.
Automated peptide synthesis was performed on an Applied Biosystems Synergy 432A. HPLC-purification was carried out on a ZORBAX Rx-C8 column (9.4 mm × 250 mm), and Vivaspin 500 (5000 MWCO) columns were used for ultrafiltration. MALDI-TOF mass spectrometry was performed on a PerSeptive Biosystems Voyager-DE™PRO. TEM images were recorded on a Tecnai T12 fitted with a Gatan CCD digital camera and an Erlangshen ES500W digital camera (operating at an accelerating voltage of 80 kV). SEM and EDS studies were carried out on a Zeiss 1500XB CrossBeam workstation equiped with a Thermo Electron Nanotrace X-ray detector. E-beam lithography was performed using a LEO 55VP SEM equiped with a Raith beam blanker. Metal electrode deposition was accomplished using an Angstrom Engineering e-beam evaporator. Device measurements were made using a costum-built I–V transport setup on a Cascade Microtech probe station.
Peptide synthesis and gold labeling
The peptide was synthesized by standard Fmoc-chemistry on Fmoc-L-phenylalanine loaded Wang resin utilizing Fmoc-ProHypGly-OH tripeptides. After acidic cleavage from the solid support the crude product was precipitated from tert-butylmethyl ether. A solution of the crude product was heated to 70 °C for 10 min and purified by preparative HPLC at 65 °C and analysed by MALDI-TOF mass spectrometry ([M+H]+ calculated for C139H190F5N33O42, 3088.37; found 3088.26).
For the gold labeling, the peptide (0.5 mg, 120 nmol) was dissolved in 0.1 mL PBS buffer (pH 8.2) and added to a solution of Mono-Sulfo-NHS-Nanogold (6 nmol) in 0.2 mL of water (prepared according to the manufacturer’s protocol) and agitated for 18 h at 4 °C. Unreacted peptide was removed by ultrafiltration. The retained peptide solution was lyophilized and stored at −18 °C.
Self-assembly of micrometer length peptide fibers
A peptide solution in water (0.05 mg/mL) was heated to 70 °C for 10 min, filtered (0.45 μm), and then, following a variation of the protocol by Maryanoff and coworkers,29 stored at 4 °C for 16 to 24 h, incubated at room temperature for 2 to 4 h, and heated to 37 °C for 16 to 24 h. Afterwards the solution was divided into aliquots, lyophilized, and stored at −18 °C until further use.
Analysis of the peptide fibers by electron microscopy and energy dispersive X-ray spectroscopy
For TEM, 5 μL of a 0.5 mg/mL pepide solution in water were deposited on pioloform–coated Ni TEM grids (300 mesh) and dried at 40 °C. Electroless silver plating of the gold nanoparticle decorated fibers was carried out by placing the grids upside down on a droplet of silver enhancement reagent for 30–60 min per enhancement step, and subsequently washed by placing on a water droplet (3 × 5 min). After drying at 40 °C, the samples were positive-stained with uranyl acetate or lead citrate, washed with water and dried at 40 °C.
Samples for SEM were prepared by depositing drops (2–5 μL) of a 0.5 mg/mL pepide solution in water onto Si/SiO2 substrate. After an incubation time of 20 min, the remaining solution was rinsed off, the substrates were briefly sonicated in water (30–60 s), and the surface was dried with a stream of air. Electroless silver plating was achieved in a similar fashion as described for the TEM grids by floating the substrates upside down on a drop of the enhancement solution. After rinsing, brief sonication and drying under a stream of air, SEM imaging and EDS elemental analysis was performed.
Collagen nanowire device fabrication and conductivity measurements
Following deposition of gold-labeled collagen fibers onto a Si substrate coated with a 600–nm thermal oxide layer with predefined markers, electroless silver plating was carried out as described in the previous section. Standard e-beam lithography procedures, which were modified where necessary to avoid exposing fibers to temperatures above their melting temperature, were performed to define two electrode patterns before metal electrodes (80 nm titanium covered by 10 nm gold) were deposited using e-beam evaporation. Further silver plating can be carried out post evaporation if necessary. All devices were then characterized using cyclic voltage sweeps while monitoring current.
Results and Discussion
Design and self-assembly of synthetic collagen-like fibers
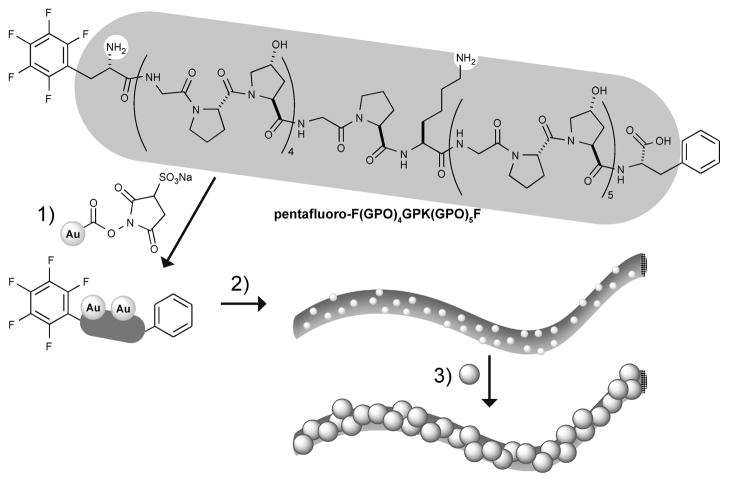
Our peptide design is based on the collagen-related peptides reported by Maryanoff and coworkers,29 but we deviated from the original amino-acid sequence, which was built only from glycine, L-proline, and L-hydroxyproline resembling a collagen type I sequence. We substituted one Lhydroxyproline residue with L-lysine, which served as an attachment point for further modification with amine-reactive gold nanoparticles. This lysine-substituted peptide still formed micrometer-long fibers after self-assembly. The modification was expected to only have a moderate destabilizing effect on the formation of collagen-like fibers as compared to the traditional POG-repeats. Melting experiments for the substituted and unsubstituted synthetic peptides showed a melting temperature drop of 4 °C for the triple helix, confirming this expectation (see CD spectra in supplementary information). These fibers seemed to be flexible, as they frequently curled into knots and loops (Fig. 1a) and their dimensions were comparable to the fibers reported by Maryanoff and coworkers. The thickness of the fiber-like assemblies ranged from 50 to 100 nm, which is much thicker than one would expect for a single triple helix (~5 nm) suggesting lateral stacking or bundling of numerous triple helices forming the observed peptide fibers. We attribute this to additional π–π stacking interactions perpendicular to the fiber axis, therefore leading to the formation of triple-helical-bundles of appreciably larger diameter. This explanation is in agreement with findings that hydrophobic interactions between tyrosine and phenylalanine residues in the C-telopeptide region of native collagen I are crucial for fibril assembly in in vitro experiments.31
Fig. 1.
TEM images of representative gold-labeled collagen-like peptide fibers before (a) and after one hour of electroless silver plating (b). All scale bars are 1 μm.
Electroless silver plating of nanoparticle-modified peptide fibers
Electroless silver plating, also termed “silver enhancement”, has been used in the fabrication of nanowires from DNA8 and recombinant amyloid proteins.32 For recombinant amyloid proteins, gold nanoparticles attached to the protein surface serve as nucleation sites to promote the electroless reduction of silver and gold enabling nanowire growth. Only one metal nanoparticle per 250 amino-acids was needed to create conductive nanowires.32 Therefore, we expected treatment of our lysine-substituted peptides with amine-reactive gold nanoparticles, which will attach to amine functionalities at the N-terminus and lysine sidechain groups, followed by self-assembly under analogous conditions to yield nanoparticle decorated fibers suitable for nanowire growth from electroless silver plating. We found that self-assembly was not affected by this gold nanoparticle treatment as micrometer-long fibers could still be observed by TEM (Fig. 1a). Direct observation of gold nanoparticles was difficult due to their small diameter (1.4 nm). After electroless silver plating, however, the nanoparticles were visualised easily. Specifically, a single–silver plating step (1 × 1 h) led to a substantial increase in metal nanoparticle diameter, which then show up as black spots on TEM images and are heavily enriched along the fibers (Fig. 1b overview, Fig. 2a high magnification).
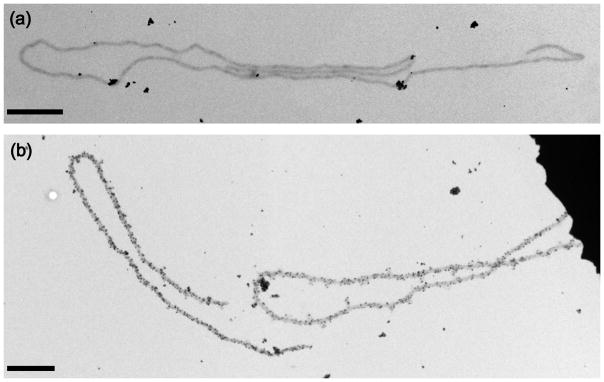
Fig. 2.
TEM images showing the evolution of collagen-like nanofibers (grey) coated by silver nanoparticles (darker) through multiple stages of electroless silver plating: (a) single 1-h deposition; (b) two 1-h depositions; and (c) three 1-h depositions. All scale bars are 100 nm.
Metal particles not attached to the fibers are a result of homogeneous metallic silver precipitation from the enhancement mixture. It was found that unspecific homogeneous precipitation could be minimized only by using freshly prepared solutions of the collagen fibers and the enhancement mixture. Additional washing steps did not improve the result further. One step of silver enhancement treatment does not lead to complete coverage of the fiber surface with metallic nanoparticles (Fig. 2a). Multiple cycles of alternating enhancement and washing steps were necessary, leading to coarsening of the metallic nanoparticles and, eventually, to continous metal coverage of the collagen-like fibers. Two or three 1–h silver enhancement steps yielded templated nanowires (Fig. 2b,c).
Structural/elemental analysis using SEM and EDS
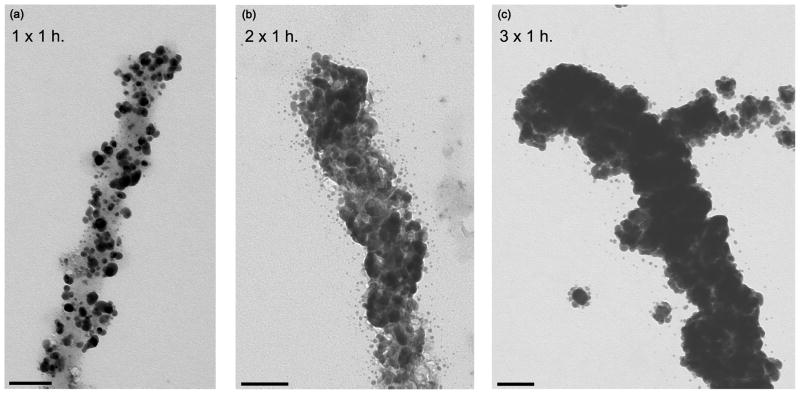
SEM imaging of gold-labeled collagen-like fibers and EDS analysis of silver-enhanced collagen nanowires (Fig. 3) were performed to gain more information on structural and elemental composition. As with TEM studies, multiple micrometer-long gold-labeled collagen-like fibers are easily found under SEM (Fig. 3a). The better depth of field provided by SEM reveals surface topography information of individual fibers/nanowires that complement high magnification TEM studies. Specifically, SEM (Fig. 3a inset) clearly resolves individual silver-enhanced gold nanoparticles as well as the peptide fiber backbone they coat, making it possible to gauge important structural details such as grain size and continuity that are critical to creating conductive nanowires. The typical grain size was ~20–30 nm after 30 min of enhancement and ~100–150 nm after 3 h of enhancement. EDS elemental analysis (Fig. 3c) show that metallic silver and gold are the primary elemental components of the nanowire samples, as expected.
Fig. 3.
Representative SEM images of micrometer-long gold-labeled collagen-like fibers after 30 min of electroless silver plating (a) and after 2 h of electroless silver plating (b). Additionally, a representative EDS spectrum (c) of the nanowire from (b) confirms the presence of both silver and gold in the sample.
Electronic properties of metallic collagen nanowires
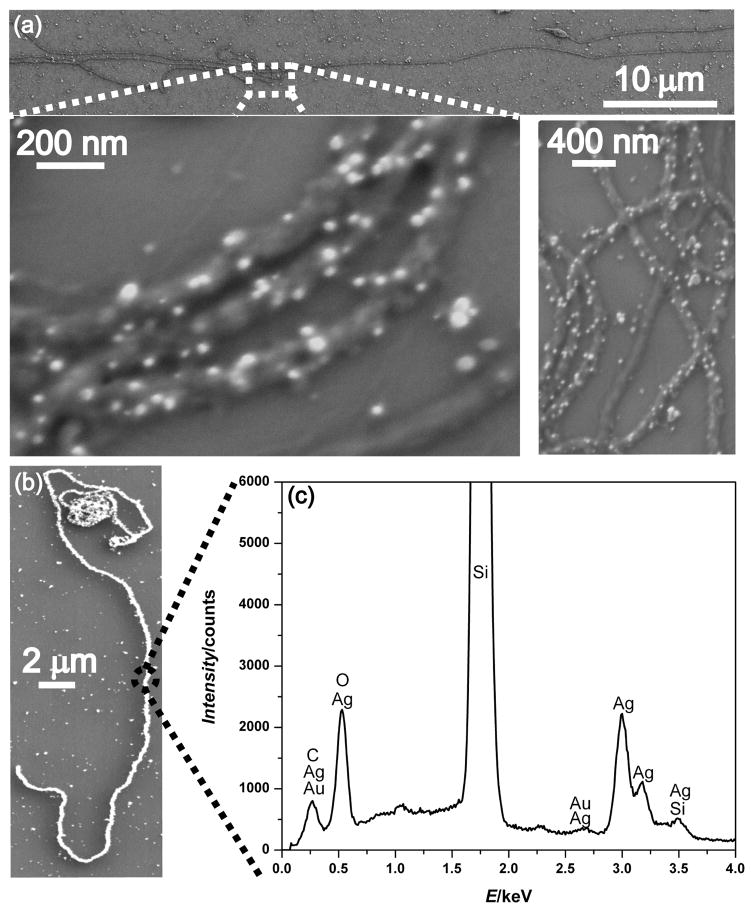
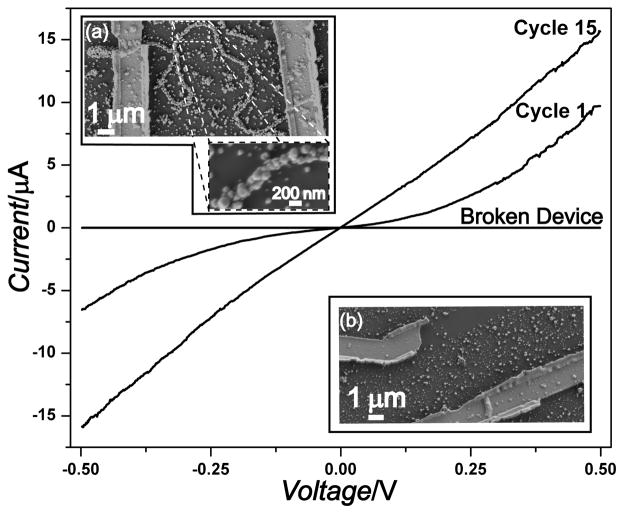
To demonstrate potential applications in nanoelectronics or nanosensing, we investigated the electrical properties of these assembled nanowires by fabricating two terminal devices around single nanowires. The continous coverage of the collagen-like fibers with metallic particles shown by electron microscopy suggested electrical conductivity. The current-voltage (I-V) curve of one representative device along with the SEM images of this device are presented in Fig. 4. As can be seen, the initial I–V sweep (cycle 1) is not ohmic; rather, there are symmetric current plateaus centered around zero voltage, indicative of electron–tunneling barriers probably at the grain boundaries and the specimen/electrode contact.8, 33 Because these nanowires were generated by overcoating gold nanoparticle seeds through multiple electroless silver plating steps, there are many grain boundaries (visible using both SEM and TEM) and this behavior is not surprising based on hopping conduction through many metallic domains. Cycling the device reduced these effects until almost purely ohmic behaviour was observed after 15 cycles of I–V scans. Presumably, this cycling process anneals the metal grains and reduces contamination at both the grain boundaries and the electrode/specimen interface, therefore improving the current transport through the device. The resistance value of the device after stability is achieved after multiple cycles, along with the length and diameter of the nanowire, can be used to estimate the nanowire resistivity. For the collagen nanowire device presented in Fig. 4a the resistivity was estimated to be 4 × 10−5Ωm which is considerably higher than that expected for polycrystalline silver33 but on par with previous reports of biomolecule templated Ag nanowires.8, 33, 34 To ensure that conductivity is from the nanowires and not random silver precipitates that accidentally bridged the electrodes, control measurements were taken on both a broken device (Fig. 4b) and between unconnected electrodes in the immediate vicinity of the device. In all cases, no current was detectable unless a nanowire bridged the electrodes. Further SEM examination of the device and the surrounding area did not reveal any conductive pathways other than the nanowire itself. Although device performance could be improved by further optimizing e-beam lithography conditions or the extent/quality of the metallic coatings, the large currents (over 15 μA at 0.5 V) and reasonable resistivities observed here demonstrate the feasibility of integrating these peptidic fiber templated nanowires into more complex devices, such as nanosensors.
Fig. 4.
Representative current vs. voltage (I–V) curves for the nanowire device shown in the inset (a). The first I-V scan cycle reveals barrier behavior, whereas stable ohmic behavior is observed after repeated I-V scans (Cycle #15). The I–V curve for a broken device shown in the inset (b) shows no detectable current.
Conclusions
In this study, we show that synthetic peptides related to collagen retain their ability for self-assembly into micrometer-long fibers when L-lysine was introduced into the amino-acid sequence, despite a slight decrease in triple-helix stability. Modification with colloidal gold nanoparticles does not diminish their ability to self-assemble, leading to gold-decorated collagen-like fibers which can be further made into metal nanowires by metallization through electroless silver plating under mild conditions compatible with the peptidic fibers. Structural characterization confirms the formation of nearly continuous metallic nanowires made primarily of silver with trace amount of gold as expected. Furthermore, the assembled metallic nanowires are electrically conductive, with estimated resistivities of 4 × 10−5Ωm, and stable, surviving repeated voltage cycling without break down. This study demonstrates the bottom-up assembly of synthetic peptidic fibers under mild conditions and their potential as templates for other complex inorganic-organic hybrid nanostructures. When these collagen-like fibers are modified further with functional groups or whole molecules by rational design and optimization of the peptide building blocks, we can enhance the diversity of inorganic materials and the complexity of nanostructures that can be assembled. Ultimately, elaborate molecular design of synthetic collagen-like peptidic fiber templates could be used to create new functional organic-inorganic hybrid nanomaterials with potential applications as novel nanobiosensors or other nanoelectronic devices.
Supplementary Material
Acknowledgments
R.T. Raines thanks grant AR044276 (NIH) for support. S. Jin thanks UW-Madison NSEC (NSF Grant DMR 0425880), a Dupont Young Professor Grant, and a 3M Non-tenured Faculty Award for support. D. Gottlieb thanks F.W. Kotch and M.D. Shoulders (helpful discussions), B.K. August (training in TEM microscopy), and D.R. McCaslin (training in CD spectroscopy). S.A. Morin thanks a 3M Graduate Fellowship for support.
Footnotes
Electronic Supplementary Information (ESI) available: CD spectra for POG and lysine substituted peptides. See DOI: 10.1039/b000000x/
Contributor Information
Song Jin, Email: jin@chem.wisc.edu.
Ronald T. Raines, Email: rtraines@wisc.edu.
References
- 1.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 2.Whitesides GM. Nat Biotechnol. 2003;21:1161. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 3.Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Nat Mater. 2002;1:169. doi: 10.1038/nmat758. [DOI] [PubMed] [Google Scholar]
- 4.Ma N, Sargent EH, Kelley SO. J Mater Chem. 2008;18:954. [Google Scholar]
- 5.Zhang S. Nat Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 6.Appella DH, Christianson LA, Klein DA, Powell DR, Huang XL, Barchi JJ, Gellman SH. Nature. 1997;387:381. doi: 10.1038/387381a0. [DOI] [PubMed] [Google Scholar]
- 7.Pomerantz WC, Yuwono VM, Pizzey CL, Hartgerink JD, Abbott NL, Gellman SH. Angew Chem-Int Edit. 2008;47:1241. doi: 10.1002/anie.200704372. [DOI] [PubMed] [Google Scholar]
- 8.Braun E, Eichen Y, Sivan U, Ben-Yoseph G. Nature. 1998;391:775. doi: 10.1038/35826. [DOI] [PubMed] [Google Scholar]
- 9.Hinds S, Taft BJ, Levina L, Sukhovatkin V, Dooley CJ, Roy MD, MacNeil DD, Sargent EH, Kelley SO. J Am Chem Soc. 2006;128:64. doi: 10.1021/ja057002+. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Mao CB, Flynn CE, Belcher AM. Science. 2002;296:892. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]
- 11.Mao C, Flynn CE, Hayhurst A, Sweeney R, Qi J, Georgiou G, Iverson B, Belcher AM. Proc Natl Acad Sci USA. 2003;100:6946. doi: 10.1073/pnas.0832310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao C. PLoS Biol. 2004;2:2036. doi: 10.1371/journal.pbio.0020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Lee SH, Mao C. Angew Chem-Int Edit. 2004;43:5335. doi: 10.1002/anie.200460789. [DOI] [PubMed] [Google Scholar]
- 14.Keren K, Krueger M, Gilad R, Ben-Yoseph G, Sivan U, Braun E. Science. 2002;297:72. doi: 10.1126/science.1071247. [DOI] [PubMed] [Google Scholar]
- 15.Sarikaya M, Tamerler C, Schwartz DT, Baneyx FO. Ann Rev Mater Res. 2004;34:373. [Google Scholar]
- 16.Reches M, Gazit E. Nat Nanotech. 2006;1:195. doi: 10.1038/nnano.2006.139. [DOI] [PubMed] [Google Scholar]
- 17.Woolfson DN, Ryadnov MG. Curr Opin Chem Biol. 2006;10:559. doi: 10.1016/j.cbpa.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Papapostolou D, Smith AM, Atkins EDT, Oliver SJ, Ryadnov MG, Serpell LC, Woolfson DN. Proc Natl Acad Sci USA. 2007;104:10853. doi: 10.1073/pnas.0700801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dublin SN, Conticello VP. J Am Chem Soc. 2008;130:49. doi: 10.1021/ja0775016. [DOI] [PubMed] [Google Scholar]
- 20.Yuwono VM, Hartgerink JD. Langmuir. 2007;23:5033. doi: 10.1021/la0629835. [DOI] [PubMed] [Google Scholar]
- 21.Birk DE, Bruckner P. Top Curr Chem. 2005;247:185. [Google Scholar]
- 22.Eyre DR, Wu JJ. Curr Chem. 2005;247:207. [Google Scholar]
- 23.Myllyharju J, Kivirikko KI. Ann Med. 2001;33:7. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 24.Ricard-Blum S, Ruggiero F, van der Rest M. Top Curr Chem. 2005;247:35. [Google Scholar]
- 25.Jenkins CL, Raines RT. Nat Prod Rep. 2002;19:49. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- 26.Ramshaw JAM, Sha NK, Brodsky BJ. J Struct Biol. 1998;122:86. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 27.Rele S, Song YH, Apkarian RP, Qu Z, Conticello VP, Chaikof EL. J Am Chem Soc. 2007;129:14780. doi: 10.1021/ja0758990. [DOI] [PubMed] [Google Scholar]
- 28.Kotch FW, Raines RT. Proceedings of the National Academy of Science. 2006;103:3028. doi: 10.1073/pnas.0508783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cejas MA, Kinney WA, Chen C, Leo GC, Tounge BA, Vinter JG, Joshi PP, Maryanoff BE. J Am Chem Soc. 2007;129:2202. doi: 10.1021/ja066986f. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Org Lett. 2005;7:2619. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]
- 31.Prockop DJ, Fertala A. J Biol Chem. 1998;273:15598. doi: 10.1074/jbc.273.25.15598. [DOI] [PubMed] [Google Scholar]
- 32.Scheibel T, Parthasarathy R, Sawicki G, Lin XM, Jaeger H, Lindquist SL. Proc Natl Acad Sci USA. 2003;100:4527. doi: 10.1073/pnas.0431081100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Park SH, Reif JH, LaBean TH. Proc Natl Acad Sci U S A. 2004;101:717. doi: 10.1073/pnas.0305860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. Science. 2003;301:1882. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.