Abstract
There exists a remarkable diversity of neurotransmitter compounds in the striatum, a pivotal brain region in the pathology of Parkinson’s disease, a movement disorder characterized by rigidity, tremor and bradykinesia. The striatal dopaminergic system, which is particularly vulnerable to neurodegeneration in this disorder, appears to be the major contributor to these motor problems. However, numerous other neurotransmitter systems in the striatum most likely also play a significant role, including the nicotinic cholinergic system. Indeed, there is an extensive anatomical overlap between dopaminergic and cholinergic neurons, and acetylcholine is well known to modulate striatal dopamine release both in vitro and in vivo. Nicotine, a drug that stimulates nicotinic acetylcholine receptors (nAChRs), influences several functions relevant to Parkinson’s disease. Extensive studies in parkinsonian animals show that nicotine protects against nigrostriatal damage, findings that may explain the well-established decline in Parkinson’s disease incidence with tobacco use. In addition, recent work shows that nicotine reduces L-dopa-induced abnormal involuntary movements, a debilitating complication of L-dopa therapy for Parkinson’s disease. These combined observations suggest that nAChR stimulation may represent a useful treatment strategy for Parkinson’s disease for neuroprotection and symptomatic treatment. Importantly, only selective nAChR subtypes are present in the striatum including the α4β2*, α6β2* and α7 nAChR populations. Treatment with nAChR ligands directed to these subtypes may thus yield optimal therapeutic benefit for Parkinson’s disease, with a minimum of adverse side effects.
Keywords: L-Dopa-induced dyskinesias, Neuroprotection, Nicotine, Nicotinic, Nigrostriatal, Parkinson’s disease
1. Overview
The basal ganglia are key in the pathogenesis of Parkinson’s disease, a movement disorder characterized by a predominant loss of nigrostriatal dopaminergic neurons [1-3]. A major component of the basal ganglia is the striatum which receives projections from dopaminergic cell bodies in the substantia nigra. In addition to dopamine, the striatum contains a wide diversity of neuroactive substances including serotonin, glutamate, GABA, noradrenaline, cannabinoids, opioids, adenosine, and numerous neuropeptides, any of which may contribute to the regulation of dopaminergic activity [4-18]. Furthermore, extensive evidence shows that acetylcholine influences striatal dopamine release predominantly through an action at nAChRs [19-21], and also muscarinic receptors to a lesser extent [22-24]. These interactions of acetylcholine at the cellular level most likely have important behavioral consequences. The focus of this review is on a putative involvement of the nicotinic cholinergic system in the control of striatal function and the pathophysiology of Parkinson’s disease. Extensive work shows that nicotine, which acts at nAChRs, protects against nigrostriatal damage [25-27]. In addition, our recent studies demonstrate that nicotine administration reduces a major side effect of L-dopa, the primary treatment for Parkinson’s disease [28, 29]. These observations raise the possibility that nAChR stimulation may prove useful for the long-term management of Parkinson’s disease.
2. Role for nicotine in neuroprotection against nigrostriatal damage
Cigarette smoking is a well-known health hazard, and one of the leading avoidable causes of mortality and morbidity. It is associated with a risk of serious chronic disorders, including cardiovascular disorders, lung disease, and cancers. Unexpectedly, however, cigarette use appears to confer beneficial effects against Parkinson’s disease. Since initial epidemiological findings in the early 1960’s, numerous case-report and cohort studies report a reduced incidence of Parkinson’s disease in smokers [30-39]. This apparent neuroprotection against Parkinson’s disease is correlated with increased intensity and duration of smoking, is reduced with smoking cessation, and occurs with different types of tobacco products [30-39]. Importantly, it does not appear to be due to selective survival of Parkinson’s disease cases or reporting bias [30-39]. These combined findings suggest that the decline in Parkinson’s disease with smoking is a true biological effect.
Although there can never be a rationale for smoking, these data are useful in that they may provide insight about mechanisms to reduce the occurrence of Parkinson’s disease. Such knowledge has the potential to yield novel treatment strategies for the management of this disorder. In fact, extensive studies have been/are being done in experimental animal models to investigate whether nicotine in smoke protects against nigrostriatal damage. Converging evidence from work with different parkinsonian animal models indicates that nicotine exposure improves dopaminergic markers and function in lesioned striatum, the brain region predominantly affected in Parkinson’s disease [25-27, 40] (also see Table 1). Such data provide a basis for the suggestion that the nicotine in smoke may contribute, at least in part, to the apparent neuroprotective effect of tobacco use in Parkinson’s disease.
Table 1. Nicotine pre-treatment protects against nigrostriatal damage in different parkinsonian animal models.
| Animal model | Nigrostriatal insult | Nicotine treatment regimen | Protection | References |
|---|---|---|---|---|
| Rats | 6-OHDA | Injection, minipump, drinking water | Improvement in behavioral deficits and molecular/ functional measures in striatum | [73, 74, 78, 79, 83-91] |
| Monkeys | MPTP | Drinking water | Improvement in molecular and functional measures in striatum | [42, 92, 93] |
| Mice | MPTP | Injection, minipump, drinking water | Variable effects - protection in some studies but not others | [87, 91, 94-103] |
| Paraquat | Drinking water | Improvement in molecular markers in striatum | [104] | |
| Methamphetamine | Injection | Improvement in molecular markers in striatum | [74] |
3. Nicotine is neuroprotective against nigrostriatal damage but not neurorestorative
The observation that nicotine treatment improves striatal dopaminergic integrity in animals with continuing nigrostriatal degeneration raised the question whether nicotine only protects against developing damage and/or whether it can also restore integrity of damaged dopaminergic neurons. To distinguish between these alternatives, we tested whether nicotine given before and/or after nigrostriatal damage improved striatal dopaminergic function in two well-established parkinsonian animal models, unilateral-lesioned 6-hydroxydopamine (6-OHDA) rats and MPTP-treated monkeys [41, 42].
In the rat study, animals were given nicotine both before and after a unilateral 6-OHDA lesion of the medial forebrain bundle and the data compared to that from rats exposed to nicotine given two weeks after lesioning only. As expected, combined nicotine pre- and post-treatment improved behavioral deficits and ameliorated lesion-induced losses of striatal dopaminergic markers [41] (Fig. 1). However, nicotine administered two weeks after lesioning, when 6-OHDA-induced neurodegenerative effects are essentially complete, did not improve these same measures. Similarly in the monkey study, there was improvement in striatal dopaminergic markers when nicotine was given before and during the development of nigrostriatal damage. However, nicotine treatment did not improve dopaminergic integrity in the striatum of monkeys with pre-existing nigrostriatal damage [41, 42] (Table 2).
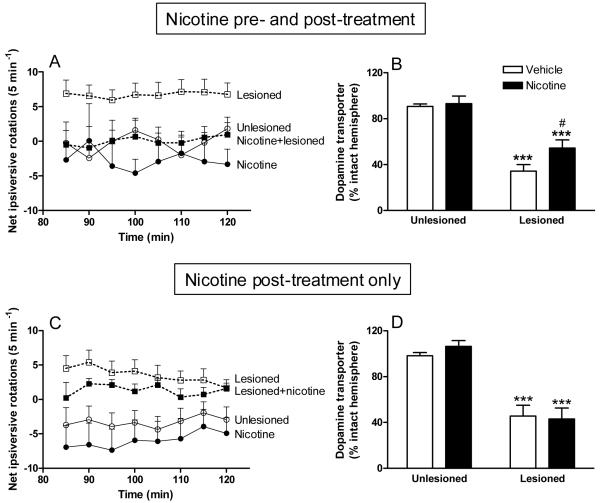
Fig. 1. Nicotine exposure is neuroprotective against ongoing nigrostriatal damage, but not neurorestorative in rats.
In the top panels (A, B), nicotine (50 μg/ml) was administered to unlesioned rats in saccharin-containing drinking water, as previously described [41]. Two weeks later, the rats received intracranial injections of 6-OHDA or vehicle into the right medial forebrain bundle. To assess the effect of nicotine treatment on motor deficits that arise with nigrostriatal damage, amphetamine-induced ipsiversive rotation was assessed 2-3 weeks after lesioning, with the nicotine [41]. The rats were placed in the chamber for 30 min for acclimatization, after which amphetamine (4.0 mg/kg ip) was administered. Circling behavior was assessed between 85 to 120 min after amphetamine administration when effects of nicotine on circling had returned to control levels. The rats were then killed and dopamine transporter levels measured using autoradiography. This treatment regimen resulted in an improvement in aberrant motor behavior and in striatal dopamine transporter levels. In the nicotine post-treatment only study (C, D), rats were first lesioned with 6-OHDA, as described above. Two to 3 weeks later, nicotine (50 μg/ml) treatment was initiated and maintained throughout. Ipsiversive rotation was evaluated after 3-4 weeks on nicotine. The rats were then killed and dopamine transporter levels evaluated. By contrast, there was no improvement in aberrant turning behavior or striatal dopamine transporter levels when nicotine was administered after nigrostriatal damage was complete. Results are the mean ± SEM of 6-9 rats per group. With nicotine pre-treatment (A), there was a significant (p < 0.001) main effect of 6-OHDA lesioning on rotations assessed using three-way ANOVA and a significant (p < 0.05) interaction between nicotine treatment and 6-OHDA lesioning. By contrast, nicotine post-treatment (C) yielded a significant main effect of 6-OHDA lesioning (p < 0.001) but no interaction, by three-way ANOVA, suggesting no neuroprotection. Significance of difference from unlesioned group (B,D) using two-way ANOVA followed by a Bonferroni post hoc test; ***p < 0.001, from lesioned #p < 0.05. Reproduced in modified form with permission from reference [41].
Table 2. Nicotine exposure is neuroprotective against ongoing nigrostriatal damage, but not neurorestorative in monkeys.
| Group | Striatal dopamine levels | |||
|---|---|---|---|---|
| Nicotine pre- and post-treatment | Nicotine post-treatment only | |||
| (ng/mg protein) | % Control | (ng/mg protein) | % Control | |
| Control | 89.6 ± 2.8 | 100 ± 3.1 | 110 ± 5.2 | 100 ± 4.7 |
| Nicotine | 103 ± 7.6 | 115 ± 8.5 | 96.5 ± 3.1 | 87 ± 2.8 |
| MPTP | 29.1 ± 5.3 | 32 ± 5.9 | 50.8 ± 9.9 | 46 ± 9.0 |
| MPTP + nicotine | 54.2 ± 8.1* | 60 ± 9.0* | 44.2 ± 5.5 | 40 ± 4.6 |
In the pre- plus post-treatment group (left columns), monkeys were given nicotine in the drinking water for several months. They were subsequently lesioned by injection of three doses of 1.5 mg/kg MPTP given at 2-month intervals with the nicotine continued throughout. This relatively low dose chronic regimen was used to optimize the neuroprotective potential of nicotine. The monkeys were then euthanized ~2 months after the last MPTP injection [42, 92]. Administration of nicotine prior to, during and after lesioning improved dopamine levels in the striatum of lesioned animals compared to monkeys not receiving nicotine [42, 92], indicating this treatment strategy leads to neuroprotection. In the post-treatment only study (right columns), monkeys were first lesioned with one dose of 2.0 mg/kg MPTP, a larger dose than that described above to generate a more rapid lesion [41]. The different MPTP treatment regimen was used, since the objective was to determine whether nicotine could restore damaged dopaminergic neurons. One month after MPTP, when the toxin-induced nigrostriatal damage is essentially complete, nicotine was added to the drinking solution, with the monkeys maintained on nicotine for ~2 more months until they were euthanized. No improvement was observed in dopamine levels when nicotine was administered after nigrostriatal damage is essentially complete, suggesting that nicotine does not repair damaged neurons. Values are the mean ± SEM of 3 to 7 monkeys. Significance of difference from MPTP using two-way ANOVA followed by a Bonferroni post hoc test.
p < 0.05.
Altogether these data show that nicotine does not restore integrity/function once dopaminergic neurons have been damaged, but rather, that it attenuates ongoing neurodegenerative processes. Since Parkinson’s disease is a progressive neurodegenerative disorder, the results from experimental animal models would suggest that nicotine might reduce Parkinson’s disease progression.
4. Inconsistent effects of nicotine on Parkinson’s disease symptoms
The observed inverse relationship between tobacco use and Parkinson’s disease incidence also raised the question whether nicotine may directly improve motor symptoms. This is not an unreasonable assumption since nicotine is well known to modulate dopamine release from striatum [19-21]. Enhanced synaptic dopamine levels could conceivably ameliorate motor deficits that arise because of a nigrostriatal dopaminergic deficiency.
In fact, a number of studies have been done to determine whether nicotine may improve Parkinson’s disease symptoms. There is no definitive conclusion at present, with positive results in only about half of the reported studies [43-51] (Table 3). The reason for this inconsistency may relate to variations in the mode of nicotine administration (patch, intravenous, gum), nicotine dose and duration/timing of nicotine treatment (days to weeks) among studies [43-51] (Table 3). Interestingly, there was a dramatic improvement in Parkinson’s disease symptoms in a recent study using high dose nicotine, possibly suggesting this is a critical factor [52]. A final consideration that may help explain the observed variability in outcome is study design, that is, open-label versus double-blinded [43-51]. Notably, improvement was observed in 5/6 open-label studies but only 1/4 double-blinded placebo-control studies, possibly indicating that the observed improvement is due to a placebo effect.
Table 3. Clinical trials shows variable effectiveness of nicotine in improving Parkinson’s disease symptoms.
|
| Conclusion: Beneficial effects on Parkinson’s disease symptoms may be associated with high dose nicotine. Alternatively, nicotine-mediated improvement in Parkinson’s disease symptoms may be due to a placebo effect. |
These combined observations may suggest that a relatively high nicotine dose is required to obtain symptomatic improvement or that beneficial effects are related to a placebo effect. Well-controlled double-blinded trials are necessary for a clear understanding of the role of nicotine in the treatment of Parkinson’s disease motor symptoms.
5. Nicotine does not improve motor deficits in parkinsonian rats or monkeys
As an alternate approach to investigate whether nicotine may reduce motor deficits that arise with nigrostriatal damage, we initiated studies in parkinsonian animal models. Such work offers the advantage that parkinsonism can be evaluated by raters blinded to the treatment status of the animals.
For the rat studies, the effect of nicotine was tested on two motor tests frequently used to evaluate parkinsonism in lesioned animals (Fig. 2A). One of these was dopaminergic drug-induced rotational behavior which is monitored via computer and thus provides an objective measure of nicotine-induced changes [53]. Nicotine treatment did not change either amphetamine or L-dopa-induced turning behavior in unilateral 6-OHDA-lesioned rats compared to control [28]. We also assessed the effect of nicotine on limb use asymmetry, a non-drug induced exploratory activity that evaluates limb dysfunction that arises with nigrostriatal damage [53]. In agreement with the results of the previous tests, nicotine did not affect limb use in lesioned rats treated with or without L-dopa as compared to controls [28] (Fig. 2A).
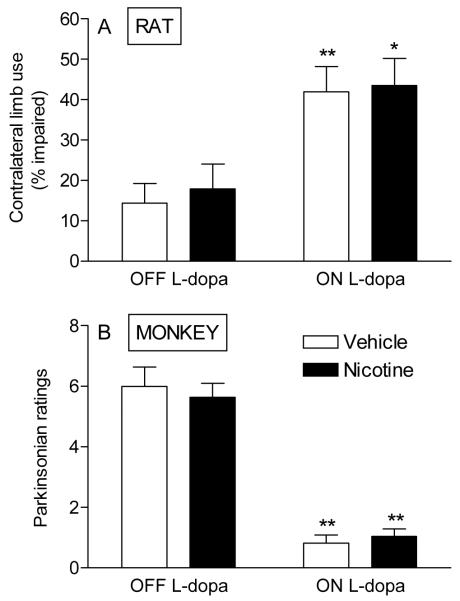
Fig. 2. Nicotine treatment does not improve motor function in parkinsonian rats or monkeys.
In A, rats were lesioned by injection of 6-OHDA into the right medial forebrain bundle. When the lesion was complete, they were given nicotine in the drinking water for several weeks. Forelimb use (cylinder test) was then evaluated before and 1 h after administration of 8 mg/kg L-dopa methyl ester plus 15 mg/kg benserazide. There were no significant differences in motor performance with nicotine treatment as compared to vehicle either OFF or ON L-dopa. Each value represents the mean ± SEM of 6-8 rats. In B, parkinsonism was assessed in MPTP-treated monkeys immediately prior 5 mg/kg L-dopa plus carbidopa dose, as well as ~2 hours after L-dopa treatment when a maximal antiparkinsonian effect is observed. L-dopa treatment alone resulted in significant declines in parkinsonism, as expected. However, nicotine resulted in no appreciable effect on motor behavior either ON or OFF L-dopa. Each value is the mean ± SEM of 5 monkeys. Significance of difference from the same group with no L-dopa treatment using two-way ANOVA followed by a Bonferroni post hoc test; **p < 0.01, *p < 0.05. Reproduced in modified form with permission from reference [28, 29].
A study was also done in which the effect of nicotine treatment was determined on parkinsonism in MPTP-lesioned monkeys (Fig. 2B). This species offers the advantage that motor deficits after nigrostriatal damage are very reminiscent of those in Parkinson’s disease, including bradykinesia, rigidity, tremor, posture and manual dexterity [29]. As expected, there was a significant reduction in parkinsonism in the monkeys with L-dopa administration [29] (Fig. 2B). As above, nicotine treatment did not influence parkinsonian behavior in monkeys not receiving L-dopa, nor did it modify the improvement in motor function observed with L-dopa treatment [29] (Fig. 2B).
Altogether, these results show that nicotine does not improve nor worsen motor function associated with nigrostriatal damage in either parkinsonian rats or monkeys, at least under the conditions tested. Such an outcome is in agreement with the results of the clinical trials reviewed in the previous section, which found no consistent improvement in motor symptoms in Parkinson’s disease patients with nicotine treatment.
6. Nicotine reduces L-dopa-induced dyskinetic-like movements in parkinsonian rats and monkeys
During the course of our studies to evaluate the effect of nicotine on parkinsonism in L-dopa-treated animals, we also tested whether it influenced the occurrence of the dyskinetic-like movements that accompanied L-dopa treatment. Although L-dopa remains the most effective drug for the symptomatic treatment of Parkinson’s disease, chronic therapy leads to abnormal involuntary movements (AIMs) or dyskinesias. These movements, which develop in the majority of Parkinson’s disease patients with continued L-dopa use, can be very debilitating and impair a patient’s quality of life. Indeed, they represent one of the major drawbacks to L-dopa treatment [17, 54-58]. Amantadine is one of the few pharmacological treatments available for the treatment of L-dopa-induced AIMs, but its effects are limited and short-lived. Another approach is deep brain stimulation; however, this is a very serious intervention with all the potential adverse effects associated with brain surgery. Novel pharmacological approaches are therefore urgently required. In fact, studies are in progress in different laboratories to test the effect of drugs that influence function of the serotonergic, glutamatergic, GABAergic, adrenergic, cannabinoid, opioid, adenosine and other neurotransmitter systems in the striatum [4-16], any one of which has the potential to improve dyskinesias.
We recently did experiments in parkinsonian rats and monkeys to evaluate whether nAChR stimulation may improve dyskinesias [28, 29] (Fig. 3). In the rat studies, animals were first given a unilateral 6-OHDA lesion, which resulted in hemiparkinsonism [28] (Fig. 3A). They were then administered vehicle or nicotine either intermittently in the drinking water or in a constant fashion via minipump, at doses that yielded plasma levels similar to those in smokers. The rats were next injected with 8 mg/kg L-dopa methyl ester plus 15 mg/kg benserazide to induce AIMs. Both modes of nicotine administration led to a ≥50% decline in L-dopa-induced AIMs in these L-dopa naïve rats [28] (Fig. 3A). Furthermore, nicotine reduced AIMs in L-dopa-primed rats [28].
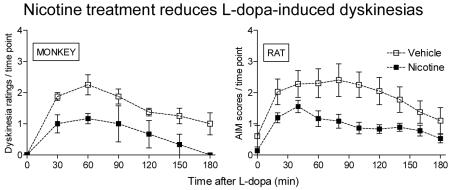
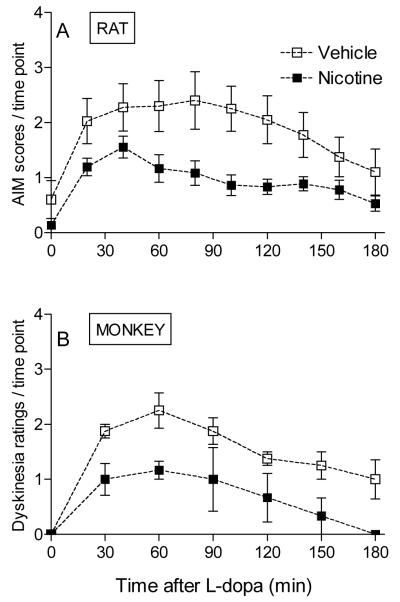
Fig. 3. Nicotine administration reduces L-dopa-induced dyskinetic-like movements in rats and monkeys.
In A, unilateral 6-OHDA-lesioned rats were given saccharin containing drinking water without or with nicotine (25 μg/ml) for several weeks as described [28]. They were then given 8 mg/kg L-dopa methyl ester plus 15 mg/kg benserazide, and the AIMs scores evaluated over a 3-hour period. Nicotine treatment significantly reduced AIMs compared to the vehicle-treated group (p < 0.05 using repeated measures ANOVA). Each symbol is the mean ± SEM of 9-10 rats. In B, MPTP-lesioned monkeys were given either nicotine or vehicle in drinking water, as described [29]. They were then gavaged with L-dopa 5 mg/kg plus carbidopa and dyskinesias rated over a 3-hour interval. Each symbol is the mean ± SEM of 3 to 4 monkeys. Dyskinesias were significantly reduced in nicotine-treated animals compared to monkeys not receiving nicotine (p < 0.05 using repeated measures ANOVA). Reproduced in modified form with permission from reference [28, 29].
We also tested the effect of nicotine treatment in parkinsonian monkeys (Fig. 3B). For these studies MPTP-lesioned monkeys were administered L-dopa (5 mg/kg) plus carbidopa twice daily by oral gavage, a regimen which resulted in an effective reduction in parkinsonism (see Fig. 2) [29]. As expected, it also led to L-dopa-induced dyskinesias, that is, abnormal involuntary movements of the limbs and trunk that range in severity from mild to severe and are very similar to those observed in Parkinson’s disease [29]. Nicotine treatment decreased L-dopa-induced dyskinesias, with ~50% decline in their occurrence [29] (Fig. 3B). They also reduced dyskinesias in L-dopa-primed monkeys [29].
These combined data suggest that nicotine may represent a useful treatment strategy to reduce L-dopa-induced dyskinesias in patients with Parkinson’s disease.
7. Striatal nAChR subtypes that may mediate the effects of nicotine in the nigrostriatal system
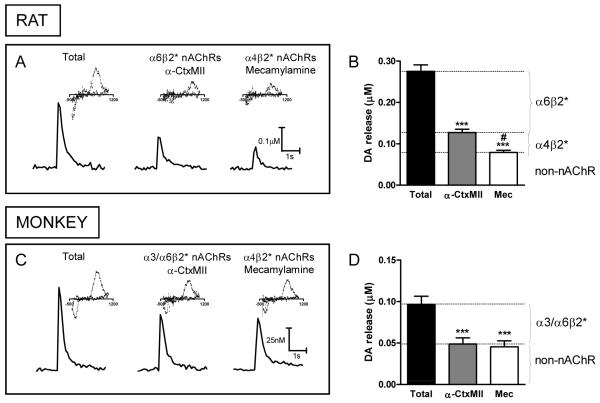
Knowledge of the mechanism(s) whereby nicotine results in its beneficial effects in Parkinson’s disease is very important because this would allow for the development of selective therapies, with a minimum of adverse side effects. Nicotine generally exerts its effects in the peripheral and central nervous system by stimulating nAChRs. These are pentameric ligand-gated ion channels composed of α and β subunits or only of α subunits [20, 59-63]. Importantly, the nAChRs in the peripheral nervous system and the brain are distinct from each other, providing an opportunity of selective targeting of the desired receptors. The nAChRs of most relevance to the pathophysiology of Parkinson’s disease are most likely the ones in the nigrostriatal tract, although receptors in other brain regions may also play critical roles. Identification of these nAChRs has proved quite challenging because multiple subtypes are expressed with six different α (α2, α3, α4, α5, α6, α7) and three different β (β2, β3, β4) subunits in the nigrostriatal pathway. Accumulating studies indicate that the primary subtypes in the striatum are the α4β2* and α6β2* receptors, together with a smaller population of α7 nAChRs [19, 20, 59, 60, 63] (the asterisk indicates the possible presence of other subunits in the receptor complex). Evidence for their presence stems from a wide variety of approaches, including work with nAChR null mutant mice, nAChR subtype selective antibodies and lesion studies [20, 59, 60, 64]. Acetylcholine, released from tonically active striatal cholinergic interneurons, interacts with the α4β2* and α6β2* nAChRs to modulate dopamine release and consequently dopaminergic function [65]. Recent studies in striatal slices using fast scan cyclic voltammetry, show that the α6β2* nAChR population may play a particularly prominent role in modulating evoked dopamine release, with a considerable proportion of electrically stimulated dopamine release regulated by this subtype in both rat and monkey striatum [19, 66-69] (Fig. 4).
Fig. 4. Stimulated dopamine release from rat and monkey striatal slices is primarily influenced by α6β2* nAChRs.
Traces of evoked dopamine release in slices of rat dorsal striatum (A) in the absence (Total) and presence of the α3/α6β2* antagonist α-CtxMII (100 nM), or the general nAChR blocker mecamylamine (mec, 100 μM). Quantitative analyses (B) of peak striatal dopamine release show that ~75% nAChR-modulated dopamine release occurs through α6β2* nAChRs and ~25% by α4β2* nAChRs in the rat [68]. The values represent the mean ± SEM of 4-6 rats. Traces of evoked dopamine release in slices of monkey dorsal putamen (C) in the absence (Total) and presence of α-CtxMII (100 nM), or mecamylamine (100 μM). Quantitative analyses of peak dopamine release show that almost all of the nAChR-modulated dopamine release in monkey dorsal striatum is modulated by α3/α6β2* nAChRs. Insets are typical voltammograms for dopamine with an oxidation peak at 500-600 mV and a reduction peak around -200 mV. The values represent the mean ± SEM of 3-7 monkeys. Significance of difference from control using one-way ANOVA followed by a Bonferroni post hoc test, after a 1 pulse stimulus, ***p < 0.001; from α-CtxMII, #p < 0.05. Reproduced in modified form with permission from reference [68].
Work now indicates that the α6β2* nAChRs are composed of several subtypes, including the α6α4β2* and α6(nonα4)β2* populations [70, 71]. Interestingly, these two α6β2* subtypes appear to be differentially regulated with chronic nicotine dosing [69]. Evidence for this stems from recent results using cyclic voltammetry, which show a differential regulation of α6β2* nAChR mediated dopamine release from striatal slices under non-burst (1 pulse) and burst (4-pulse) stimulus conditions in control rats. This is no longer observed after long-term nicotine treatment, suggesting that the normal regulation of burst-stimulated dopamine release is disrupted [69]. Further work with nAChR null mutant mice indicate that the α6α4β2* nAChR population may be important for this differential effect of higher frequency stimulation on dopamine release [69].
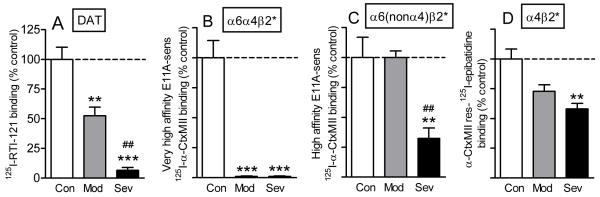
Not only are the α6α4β2* and α6(nonα4)β2* nAChRs subtypes variably affected by nicotine exposure, but they also appear to be differentially affected by nigrostriatal damage. For instance, the α6α4β2* nAChR subtype is more susceptible to lesioning than the α6(nonα4)β2* or α4β2* nAChR populations in parkinsonian rodents and monkeys [72]. Similar findings were also observed in the brains of Parkinson’s disease patients [72] (Fig. 5). These data may indicate that the α6α4β2* nAChR subtype is expressed on dopaminergic terminals that are more susceptible to nigrostriatal degeneration. Such a possibility is consistent with studies showing that some dopaminergic neuron populations are more sensitive to neurodegenerative insults than others, which may relate to the presence of specific molecular markers on certain dopaminergic neurons but not others. Examples include calbindin, whose presence is associated with nigrostriatal dopamine neuron survival (Gerfen et al., 1987a; German et al., 1992; Liang et al., 1996). By contrast, neuromelanin has been negatively linked to dopaminergic neuron survival in some studies (Herrero et al., 1993; Hirsch et al., 1988; McCormack et al., 2004; Zecca et al., 2003). The preferential loss of the α6α4β2* subtype with initial nigrostriatal damage, subsequently followed by the α6(nonα4)β2* and α4β2* nAChR subtypes, may suggest that these different nAChR subtypes are expressed on dopaminergic neurons with varying repertoires of molecular markers.
Fig. 5. Differential sensitivity of varying striatal nAChRs in Parkinson’s disease striatum.
The results depict changes in nAChR subtypes in human putamen with ~50% (mod - moderate) and ~90% (sev - severe) declines in the striatal dopamine transporter measured using 125I-RTI-121, as shown in A. 125I-α-CtxMII binding studies in the absence and presence of the α-CtxMII analog E11A distinguished between at least two E11A sensitive α6β2* nAChR populations, the α6α4β2* (B) and the α6(nonα4)β2* (C) nAChR subtype [72]. With 50% declines in the dopamine transporter, there is almost complete loss of the α6α4β2* subtype, with no significant reduction in the α6(nonα4)β2* nAChR subtype. By contrast, there are major deficits in α6(nonα4)β2* receptors with a ~90% decline in the dopamine transporter. α4β2* nAChRs, determined using 125I-epibatidine binding in the presence of 100 nM α-CtxMII (D), are much less affected than the α6β2* nAChR subtypes with a small nonsignificant decline with 50% decrease and an ~40% loss with a near complete loss in dopamine transporter. Thus, the α6α4β2* subtype appears to be the most vulnerable to nigrostriatal damage, the α6(nonα4)β2* receptor is next affected, and the α4β2* nAChR is the most resistant to nigrostriatal damage. Each value is the mean ± SEM of 4-6 control (Con) or 4-6 Parkinson’s disease cases (Mod or Sev). Significance of difference from control using one-way ANOVA followed by a Bonferroni post hoc test; ***p < 0.001, **p < 0.01; from a moderate lesion, ##p < 0.01, #p < 0.05. Reproduced in modified form with permission from reference [72, 105].
Nicotine-mediated neuroprotection against nigrostriatal damage also appears to involve various nAChR subtypes. That protection is receptor-mediated is apparent from work showing a decline with nAChR blockade [73]. Evidence for an involvement of the α4β2* nAChR subtype is based on studies showing a loss of nicotine-mediated protection in α4β2* nAChR knockout mice with nigrostriatal damage [74]. A role for select α6β2* nAChR subtypes in neuroprotection stems from work in lesioned rats [41], using the neurotoxin α-CtxMII E11A to differentiate between α6α4β2* and α6(nonα4)β2* subtypes [72]. Receptor competition studies in control and lesioned rats treated with and without nicotine showed that the α6α4β2* subtype is present only when nicotine-mediated protection is observed. This suggests that this latter subtype may be necessary for neuroprotection and a target for the development of protective strategies against Parkinson’s disease. The mechanisms whereby an interaction at α6α4β2* nAChR results in protection against nigrostriatal damage are not yet known as the intracellular signaling pathways linked to this specific nAChR subtype remain to be elucidated. However, based on knowledge of other nAChR subtypes, one might anticipate that changes in multiple downstream pathways are involved, such as alterations in various kinases, including phosphatidylinositol 3-kinase (PI3K), Akt, mitogen-activated protein kinase (MAPK) and jnk kinase, caspases 3, 8 and 9, nitric oxide synthase, the cell survival protein Bcl-2, and other intracellular events [25, 60, 75]. Activation of these intracellular messengers may modulate immune responsiveness to enhance neuronal function/integrity [76, 77]. Alternatively, or as well, they may activate different trophic factors, such as brain-derived neurotrophic factor (BDNF) and/or basic fibroblast growth factor-2 (FGF-2) levels, which are implicated in neuroprotection against nigrostriatal damage [78-81].
The ability of nicotine to reduce L-dopa-induced dyskinetic-like movements most likely also occurs via an interaction at nAChRs since improvement is not observed in the presence of the nAChR blocker mecamylamine [82]. It is currently not known which striatal nAChRs populations are involved in the nicotine-mediated decline in L-dopa-induced dyskinesias. However, since the primary receptors in the brain, and specifically the nigrostriatal pathway, are the α4β2*, α6β2* and α7 subtypes, we anticipate these may be important [19, 20, 59, 60, 63]. With respect to brain region, we expect that the receptors present in the nigrostriatal pathway play a role; however, it is also possible that nAChRs in other brain regions are key. This idea is based on extensive evidence that multiple neurotransmitter systems are implicated in the development of L-dopa-induced dyskinetic-like movements [17, 56]. Such a possibility is not unexpected since the striatum functions in an integrated fashion with the globus pallidus, thalamus, various cortical areas, subthalamic nucleus, cerebellum, and other areas [17, 56].
Altogether these findings suggest a role for α4β2*, α6β2* and/or α7 nAChR-directed ligands for Parkinson’s disease therapeutics. Continued work is critical to identify the specific nAChR subtypes involved in neuroprotection and for improvement in L-dopa-induced dyskinesias. Such knowledge will allow for the development of nAChR drugs with optimal beneficial and a minimum of adverse effects for Parkinson’s disease management.
Acknowledgements
This work was supported by NIH grants NS42091, NS47162 and the California TRDRP 17RT-0119. We thank Dr. Sharon Grady, University of Colorado Boulder, for helpful comments on the manuscript.
Abbreviations
- ANOVA
analysis of variance
- α-CtxMII
α-conotoxinMII
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- nAChR
nicotinic acetylcholine receptor
- 6-OHDA
6-hydroxydopamine
- RTI-121
2β-carboxylic acid isopropyl ester-3β-(4-iodophenyl) tropane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Toulouse A, Sullivan AM. Progress in Parkinson’s disease-where do we stand? Prog Neurobiol. 2008;85:376–92. doi: 10.1016/j.pneurobio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [2].Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- [3].Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–27. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- [4].Brotchie JM, Lee J, Venderova K. Levodopa-induced dyskinesia in Parkinson’s disease. J Neural Transm. 2005;112:359–91. doi: 10.1007/s00702-004-0251-7. [DOI] [PubMed] [Google Scholar]
- [5].Cenci MA, Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem. 2006;99:381–92. doi: 10.1111/j.1471-4159.2006.04124.x. [DOI] [PubMed] [Google Scholar]
- [6].Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0925s41. Chapter 9:Unit 9 25. [DOI] [PubMed] [Google Scholar]
- [7].Fox SH, Lang AE, Brotchie JM. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord. 2006;21:1578–94. doi: 10.1002/mds.20936. [DOI] [PubMed] [Google Scholar]
- [8].Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, et al. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat Disord. 2005;11(Suppl 1):S25–9. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- [9].Guigoni C, Li Q, Aubert I, Dovero S, Bioulac BH, Bloch B, et al. Involvement of sensorimotor, limbic, and associative basal ganglia domains in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci. 2005;25:2102–7. doi: 10.1523/JNEUROSCI.5059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Linazasoro G. New ideas on the origin of L-dopa-induced dyskinesias: age, genes and neural plasticity. Trends Pharmacol Sci. 2005;26:391–7. doi: 10.1016/j.tips.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [11].Mercuri NB, Bernardi G. The ‘magic’ of L-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci. 2005;26:341–4. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [12].Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5:677–87. doi: 10.1016/S1474-4422(06)70521-X. [DOI] [PubMed] [Google Scholar]
- [13].Samadi P, Bedard PJ, Rouillard C. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol Sci. 2006;27:512–7. doi: 10.1016/j.tips.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [14].Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007 doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- [15].Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23:1641–52. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Linazasoro G, Van Blercom N, Ugedo L, Ruiz Ortega JA. Pharmacological treatment of Parkinson’s disease: life beyond dopamine D2/D3 receptors? J Neural Transm. 2008;115:431–41. doi: 10.1007/s00702-007-0852-z. [DOI] [PubMed] [Google Scholar]
- [17].Carta M, Carlsson T, Munoz A, Kirik D, Bjorklund A. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog Brain Res. 2008;172:465–78. doi: 10.1016/S0079-6123(08)00922-9. [DOI] [PubMed] [Google Scholar]
- [18].Munoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T, et al. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain. 2008 doi: 10.1093/brain/awn235. [DOI] [PubMed] [Google Scholar]
- [19].Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–46. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [22].Grilli M, Patti L, Robino F, Zappettini S, Raiteri M, Marchi M. Release-enhancing pre-synaptic muscarinic and nicotinic receptors co-exist and interact on dopaminergic nerve endings of rat nucleus accumbens. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05307.x. [DOI] [PubMed] [Google Scholar]
- [23].Miller AD, Forster GL, Yeomans JS, Blaha CD. Midbrain muscarinic receptors modulate morphine-induced accumbal and striatal dopamine efflux in the rat. Neuroscience. 2005;136:531–8. doi: 10.1016/j.neuroscience.2005.08.035. [DOI] [PubMed] [Google Scholar]
- [24].Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–46. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- [25].Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- [26].Quik M, O’Neill M, Perez XA. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007 doi: 10.1016/j.tips.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [27].O’Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Target CNS Neurol Disord. 2002;1:399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- [28].Bordia T, Campos C, Huang LZ, Quik M. Continuous and intermittent nicotine treatment reduces L-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–47. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- [29].Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of Neurology. 2007;62:588–96. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- [30].Thacker EL, O’Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–8. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–7. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- [32].O’Reilly EJ, McCullough ML, Chao A, Henley SJ, Calle EE, Thun MJ, et al. Smokeless tobacco use and the risk of Parkinson’s disease mortality. Mov Disord. 2005;20:1383–4. doi: 10.1002/mds.20587. [DOI] [PubMed] [Google Scholar]
- [33].Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson’s disease: systematic review of prospective studies. Mov Disord. 2004;19:614–21. doi: 10.1002/mds.20029. [DOI] [PubMed] [Google Scholar]
- [34].Tanner CM, Goldman SM, Aston DA, Ottman R, Ellenberg J, Mayeux R, et al. Smoking and Parkinson’s disease in twins. Neurology. 2002;58:581–8. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- [35].Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson’s disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- [36].Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Ann Neurol. 2001;50:780–6. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- [37].Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52:115–9. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- [38].Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull. 1996;52:58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- [39].Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology. 1995;45:1041–51. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- [40].Miksys S, Tyndale RF. Nicotine induces brain CYP enzymes: relevance to Parkinson’s disease. J Neural Transm Suppl. 2006:177–80. doi: 10.1007/978-3-211-45295-0_28. [DOI] [PubMed] [Google Scholar]
- [41].Huang L, Parameswaran N, Bordia T, McIntosh JM, Quik M. Nicotine is neuroprotective when administered before but not after nigrostriatal damage in rats and monkeys. Journal of Neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06011.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, et al. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98:1866–75. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- [43].Clemens P, Baron JA, Coffey D, Reeves A. The short-term effect of nicotine chewing gum in patients with Parkinson’s disease. Psychopharmacology (Berl) 1995;117:253–6. doi: 10.1007/BF02245195. [DOI] [PubMed] [Google Scholar]
- [44].Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord. 1999;14:1011–3. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [45].Mitsuoka T, Kaseda Y, Yamashita H, Kohriyama T, Kawakami H, Nakamura S, et al. Effects of nicotine chewing gum on UPDRS score and P300 in early-onset parkinsonism. Hiroshima J Med Sci. 2002;51:33–9. [PubMed] [Google Scholar]
- [46].Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43:274–82. [PubMed] [Google Scholar]
- [47].Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57:1032–5. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- [48].Lemay S, Chouinard S, Blanchet P, Masson H, Soland V, Beuter A, et al. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:31–9. doi: 10.1016/S0278-5846(03)00172-6. [DOI] [PubMed] [Google Scholar]
- [49].Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993;117:28–32. doi: 10.1016/0022-510x(93)90150-w. [DOI] [PubMed] [Google Scholar]
- [50].Fagerstrom KO, Pomerleau O, Giordani B, Stelson F. Nicotine may relieve symptoms of Parkinson’s disease. Psychopharmacology (Berl) 1994;116:117–9. doi: 10.1007/BF02244882. [DOI] [PubMed] [Google Scholar]
- [51].Hanagasi HA, Lees A, Johnson JO, Singleton A, Emre M. Smoking-responsive juvenile-onset Parkinsonism. Mov Disord. 2007;22:115–9. doi: 10.1002/mds.21177. [DOI] [PubMed] [Google Scholar]
- [52].Villafane G, Cesaro P, Rialland A, Baloul S, Azimi S, Bourdet C, et al. Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol. 2007;14:1313–6. doi: 10.1111/j.1468-1331.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- [53].Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- [54].Calabresi P, Di Filippo M, Ghiglieri V, Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord. 2008;23(Suppl 3):S570–9. doi: 10.1002/mds.22019. [DOI] [PubMed] [Google Scholar]
- [55].Fahn S. How do you treat motor complications in Parkinson’s disease: Medicine, surgery, or both? Ann Neurol. 2009;64:S56–S64. doi: 10.1002/ana.21453. [DOI] [PubMed] [Google Scholar]
- [56].Fox SH, Chuang R, Brotchie JM. Parkinson’s disease--opportunities for novel therapeutics to reduce the problems of levodopa therapy. Prog Brain Res. 2008;172:479–94. doi: 10.1016/S0079-6123(08)00923-0. [DOI] [PubMed] [Google Scholar]
- [57].Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–77. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- [58].Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson’s disease: A feature inherent to the treatment or the disease? Prog Neurobiol. 2008 doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–11. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- [60].Quik M, Bordia T, O’Leary K. Nicotinic receptors as CNS targets for Parkinson’s disease. Biochem Pharmacol. 2007;74:1224–34. doi: 10.1016/j.bcp.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dani JA, Bertrand D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- [62].Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- [64].Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther. 2006;316:481–9. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- [65].Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- [66].Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. alpha6-Containing Nicotinic Acetylcholine Receptors Dominate the Nicotine Control of Dopamine Neurotransmission in Nucleus Accumbens. Neuropsychopharmacology. 2008;33:2158–66. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- [67].Meyer EL, Yoshikami D, McIntosh JM. The neuronal nicotinic acetylcholine receptors alpha 4* and alpha 6* differentially modulate dopamine release in mouse striatal slices. J Neurochem. 2008;105:1761–9. doi: 10.1111/j.1471-4159.2008.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Perez X, O’Leary K, Parameswaran N, McIntosh JM, Quik M. Prominent role of {alpha}3/{alpha}6{beta}2* nAChRs in regulating evoked dopamine release in primate putamen; effect of long-term nicotine treatment. Mol Pharmacol. 2009 doi: 10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Mol Pharmacol. 2008;74:844–53. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- [71].Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic Nicotine Differentially Regulates {alpha}6- and {beta}3-containing Nicotinic Cholinergic Receptors in Rat Brain. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- [72].Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of {alpha}6{beta}2* nAChRs in mouse, monkey and Parkinson’s disease striatum. Mol Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- [73].Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Res. 2001;888:336–42. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- [74].Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–6. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- [76].Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. Aaps J. 2005;7:E885–94. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Belluardo N, Blum M, Mudo G, Andbjer B, Fuxe K. Acute intermittent nicotine treatment produces regional increases of basic fibroblast growth factor messenger RNA and protein in the tel- and diencephalon of the rat. Neuroscience. 1998;83:723–40. doi: 10.1016/s0306-4522(97)00323-0. [DOI] [PubMed] [Google Scholar]
- [79].Belluardo N, Mud G, Blum M, Cheng Q, Caniglia G, Dell’Albani P, et al. The nicotinic acetylcholine receptor agonist (+/-)-epibatidine increases FGF-2 mRNA and protein levels in the rat brain. Brain Res Mol Brain Res. 1999;74:98–110. doi: 10.1016/s0169-328x(99)00266-1. [DOI] [PubMed] [Google Scholar]
- [80].Massey KA, Zago WM, Berg DK. BDNF up-regulates alpha7 nicotinic acetylcholine receptor levels on subpopulations of hippocampal interneurons. Mol Cell Neurosci. 2006;33:381–8. doi: 10.1016/j.mcn.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhou X, Nai Q, Chen M, Dittus JD, Howard MJ, Margiotta JF. Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function. J Neurosci. 2004;24:4340–50. doi: 10.1523/JNEUROSCI.0055-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bordia T, Campos C, Quik M. Chronic but not acute nAChR stimulation reduces L-dopa-induced dyskinesias in parkinsonian rats. Society for Neuroscience Abstracts. 2009 [Google Scholar]
- [83].Janson AM, Fuxe K, Agnati LF, Kitayama I, Harfstrand A, Andersson K, et al. Chronic nicotine treatment counteracts the disappearance of tyrosine-hydroxylase-immunoreactive nerve cell bodies, dendrites and terminals in the mesostriatal dopamine system of the male rat after partial hemitransection. Brain Res. 1988;455:332–45. doi: 10.1016/0006-8993(88)90092-3. [DOI] [PubMed] [Google Scholar]
- [84].Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64:125–35. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- [85].Abin-Carriquiry JA, McGregor-Armas R, Costa G, Urbanavicius J, Dajas F. Presynaptic involvement in the nicotine prevention of the dopamine loss provoked by 6-OHDA administration in the substantia nigra. Neurotox Res. 2002;4:133–9. doi: 10.1080/10298420290015863. [DOI] [PubMed] [Google Scholar]
- [86].Visanji NP, O’Neill MJ, Duty S. Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology. 2006;51:506–16. doi: 10.1016/j.neuropharm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- [87].Janson AM, Fuxe K, Goldstein M. Differential effects of acute and chronic nicotine treatment on MPTP-(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced degeneration of nigrostriatal dopamine neurons in the black mouse. Clin Investig. 1992;70:232–8. doi: 10.1007/BF00184656. [DOI] [PubMed] [Google Scholar]
- [88].Belluardo N, Mudo G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113:21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- [89].Maggio R, Riva M, Vaglini F, Fornai F, Racagni G, Corsini GU. Striatal increase of neurotrophic factors as a mechanism of nicotine protection in experimental parkinsonism. J Neural Transm. 1997;104:1113–23. doi: 10.1007/BF01273324. [DOI] [PubMed] [Google Scholar]
- [90].Dajas F, Costa G, Abin-Carriquiry JA, McGregor R, Urbanavicius J. Involvement of nicotinic acetylcholine receptors in the protection of dopamine terminals in experimental parkinsonism. Funct Neurol. 2001;16:113–23. [PubMed] [Google Scholar]
- [91].Parain K, Marchand V, Dumery B, Hirsch E. Nicotine, but not cotinine, partially protects dopaminergic neurons against MPTP-induced degeneration in mice. Brain Res. 2001;890:347–50. doi: 10.1016/s0006-8993(00)03198-x. [DOI] [PubMed] [Google Scholar]
- [92].Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26:4681–9. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bordia T, Parameswaran N, Fan H, Langston JW, McIntosh JM, Quik M. Partial recovery of striatal nicotinic receptors in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned monkeys with chronic oral nicotine. J Pharmacol Exp Ther. 2006;319:285–92. doi: 10.1124/jpet.106.106997. [DOI] [PubMed] [Google Scholar]
- [94].Janson AM, Fuxe K, Agnati L, Sundstrom E, Goldstein M. The effect of chronic nicotine treatment on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degneration of nigrostriatal dopamine neurons in the black mouse. Advances in Pharmacological Sciences. 1991;1:323–9. [Google Scholar]
- [95].Carr LA, Rowell PP. Attenuation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity by tobacco smoke. Neuropharmacology. 1990;29:311–4. doi: 10.1016/0028-3908(90)90019-n. [DOI] [PubMed] [Google Scholar]
- [96].Shahi GS, Das NP, Moochhala SM. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: partial protection against striato-nigral dopamine depletion in C57BL/6J mice by cigarette smoke exposure and by beta-naphthoflavone-pretreatment. Neurosci Lett. 1991;127:247–50. doi: 10.1016/0304-3940(91)90804-3. [DOI] [PubMed] [Google Scholar]
- [97].Gao ZG, Cui WY, Zhang HT, Liu CG. Effects of nicotine on 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced depression of striatal dopamine content and spontaneous locomotor activity in C57 black mice. Pharmacol Res. 1998;38:101–6. doi: 10.1006/phrs.1998.0337. [DOI] [PubMed] [Google Scholar]
- [98].Parain K, Hapdey C, Rousselet E, Marchand V, Dumery B, Hirsch EC. Cigarette smoke and nicotine protect dopaminergic neurons against the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinsonian toxin. Brain Res. 2003;984:224–32. doi: 10.1016/s0006-8993(03)03195-0. [DOI] [PubMed] [Google Scholar]
- [99].Perry TL, Hansen S, Jones K. Exposure to cigarette smoke does not decrease the neurotoxicity of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neurosci Lett. 1987;74:217–20. doi: 10.1016/0304-3940(87)90152-2. [DOI] [PubMed] [Google Scholar]
- [100].Sershen H, Hashim A, Wiener HL, Lajtha A. Effect of chronic oral nicotine on dopaminergic function in the MPTP-treated mouse. Neurosci Lett. 1988;93:270–4. doi: 10.1016/0304-3940(88)90094-8. [DOI] [PubMed] [Google Scholar]
- [101].Behmand RA, Harik SI. Nicotine enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. J Neurochem. 1992;58:776–9. doi: 10.1111/j.1471-4159.1992.tb09786.x. [DOI] [PubMed] [Google Scholar]
- [102].Hadjiconstantinou M, Hubble JP, Wemlinger TA, Neff NH. Enhanced MPTP neurotoxicity after treatment with isoflurophate or cholinergic agonists. J Pharmacol Exp Ther. 1994;270:639–44. [PubMed] [Google Scholar]
- [103].Ferger B, Spratt C, Earl CD, Teismann P, Oertel WH, Kuschinsky K. Effects of nicotine on hydroxyl free radical formation in vitro and on MPTP-induced neurotoxicity in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:351–9. doi: 10.1007/pl00005264. [DOI] [PubMed] [Google Scholar]
- [104].Khwaja M, McCormack A, McIntosh JM, Di Monte DA, Quik M. Nicotine partially protects against paraquat-induced nigrostriatal damage in mice; link to alpha6beta2* nAChRs. J Neurochem. 2007;100:180–90. doi: 10.1111/j.1471-4159.2006.04177.x. [DOI] [PubMed] [Google Scholar]
- [105].Quik M, Bordia T, Forno L, McIntosh JM. Loss of alpha-conotoxinMII- and A85380-sensitive nicotinic receptors in Parkinson’s disease striatum. J Neurochem. 2004;88:668–79. doi: 10.1111/j.1471-4159.2004.02177.x. [DOI] [PubMed] [Google Scholar]