Abstract
Background
In men with chronic prostatitis–chronic pelvic pain syndrome, treatment with alpha-adrenergic receptor blockers early in the course of the disorder has been reported to be effective in some, but not all, relatively small randomized trials.
Methods
We conducted a multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy of alfuzosin, an alpha-adrenergic receptor blocker, in reducing symptoms in men with chronic prostatitis–chronic pelvic pain syndrome. Participation in the study required diagnosis of the condition within the preceding 2 years and no previous treatment with an alpha-adrenergic receptor blocker. Men were randomly assigned to treatment for 12 weeks with either 10 mg of alfuzosin per day or placebo. The primary outcome was a reduction of at least 4 points (from baseline to 12 weeks) in the score on the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) (range, 0 to 43; higher scores indicate more severe symptoms). A 4-point decrease is the minimal clinically significant difference in the score.
Results
A total of 272 eligible participants underwent randomization, and in both study groups, 49.3% of participants had a decrease of at least 4 points in their total NIH-CPSI score (rate difference associated with alfuzosin, 0.1%; 95% confidence interval, −11.2 to 11.0; P = 0.99). In addition, a global response assessment showed similar response rates at 12 weeks: 33.6% in the placebo group and 34.8% in the alfuzosin group (P = 0.90). The rates of adverse events in the two groups were also similar.
Conclusions
Our findings do not support the use of alfuzosin to reduce the symptoms of chronic prostatitis–chronic pelvic pain syndrome in men who have not received prior treatment with an alpha-blocker.
Prostatitis is a common and costly medical condition, with chronic prostatitis–chronic pelvic pain syndrome the most frequent subtype encountered by family physicians, internists, and urologists.1-3 Men with chronic prostatitis–chronic pelvic pain syndrome have chronic genitourinary pain, the hallmark symptom of this syndrome, but also report urinary and sexual dysfunction,4 both of which have a negative effect on the quality of life.5,6 The prevalence rate of physician-diagnosed prostatitis in one U.S. community was 9%7; population-based surveys of symptoms estimate the prevalence of prostatitis-like symptoms to be between 6 and 12%.8 In the majority of men whose symptoms persist for more than 3 months, the cause of symptoms is believed to be noninfectious.1 What was previously referred to as a diagnosis of nonbacterial prostatitis and prostatodynia is now called chronic prostatitis–chronic pelvic pain syndrome.9
Despite a lack of supporting evidence from clinical trials10-12 and the likelihood of a noninfectious cause,1,9 antimicrobial and antiinflammatory agents are often considered the mainstay of drug therapy for this syndrome.13 A recent survey showed that for men with symptoms that are characteristic of chronic prostatitis–chronic pelvic pain syndrome, more than 75% of primary care physicians prescribe antibiotics at least half the time, whereas more than 50% regularly recommend antiinflammatory agents.2
The findings of several placebo-controlled trials,14-17 but not all of them,11 suggest that treatment with alpha-adrenergic receptor antagonists may be effective for reducing symptoms in men with this syndrome, especially in those who have not previously been treated with these drugs and who have had symptoms for a relatively short time (less than a year). We performed a multicenter, randomized, double-blind, placebo-controlled trial of the alpha-adrenergic receptor blocker alfuzosin to determine whether the symptoms of chronic prostatitis–chronic pelvic pain syndrome could be reduced in men who had recently received a diagnosis of chronic prostatitis–chronic pelvic pain syndrome and who had not previously been treated with this class of drug.
Methods
Participants
Men who were at least 18 years of age and who had been seen by a physician for symptoms of chronic prostatitis–chronic pelvic pain syndrome within the previous 2 years were recruited from 10 sites in the United States and 1 site each in Canada and Malaysia. Eligibility criteria included pain or discomfort in the pelvic region for at least 6 weeks and a total score of at least 12 on the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) (on a scale of 0 to 43, with higher scores indicating more severe symptoms).18 The institutional review board at each of the participating clinical centers approved the study, and all the men provided written informed consent.
Major exclusion criteria were previous treatment with alfuzosin or any other alpha-adrenergic receptor blocker for symptoms of chronic prostatitis–chronic pelvic pain syndrome or for any other reason, a documented urinary tract infection (midstream urine culture with at least 100,000 colony-forming units per milliliter), symptomatic genital herpes in the previous 3 months, use of 5-alpha reductase inhibitors in the previous 12 months, unilateral orchialgia without pelvic symptoms, a history of genitourinary cancer, inflammatory bowel disease, active urethral stricture, prostate or bladder surgery, neurologic disease affecting the bladder, or use of exclusionary medications such as potent cytochrome P-3A4 inhibitors (e.g., ketoconazole, itraconazole, or ritonavir) or erythromycin.
Study Design and Procedures
Men at each clinical site were randomly assigned in a 1:1 ratio to receive either 10 mg of alfuzosin or an identical-looking placebo once daily for 12 weeks with the use of a centrally controlled, Web-based data-management system. A permuted-block randomization procedure with randomly assigned block sizes of 4, 6, and 8 was used. Study investigators and subjects were unaware of the treatment assignments. There were four research-clinic visits during which data for the primary and secondary outcome measures were collected: visit 1 involved screening, visit 2 involved collection of baseline data and randomization, visit 3 was the 6-week evaluation, and visit 4 was the 12-week evaluation of the primary end point. Data on adverse events were collected on visits 2, 3, and 4. The trial was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, and Sanofi-Aventis provided the study drug and placebo at no cost. Sanofi-Aventis was not involved in the design of the study, the analysis of the data, or the preparation of the manuscript.
Monitoring of all adverse events was conducted with the use of standardized queries by the research coordinator at each study site. All adverse signs and symptoms, as well as worsening of preexisting conditions, whether or not they were considered to be related to the study drug, were reported and categorized in accordance with the codes in the Medical Dictionary for Regulatory Activities (MedDRA), version 6.0.19
Outcomes
The primary outcome was a decrease (improvement) in the NIH-CPSI of at least 4 points from baseline to 12 weeks.18,20 The NIH-CPSI measures aspects of the three most important symptom domains of the chronic prostatitis–chronic pelvic pain syndrome: pain (location, frequency, and severity; score range, 0 to 21), voiding problems (irritative and obstructive symptoms; score range, 0 to 10), and negative effects on the quality of life (score range, 0 to 12), with a total score ranging from 0 to 43.18 A 4-point decrease in the NIH-CPSI score has been shown to be the minimal clinically significant difference perceived by patients as beneficial.20 Early withdrawal from the study was classified as treatment failure, and men who withdrew early were included in the denominator for determining the primary-outcome response rate in an intention-to-treat analysis.
A number of secondary outcomes were assessed using a 7-point global response assessment.21 Men who reported moderate or marked improvement in the global response assessment at the end of the study were identified as treatment responders. Comparison of the global response assessment scores between study groups included men who withdrew early (11 in the placebo group and 19 in the alfuzosin group), as prespecified in the data analysis and monitoring plan.
Other measures included assessment of general pain and urinary urgency on a Likert scale (range, 0 [none] to 10 [most severe]), the McGill Pain Questionnaire (ranges, 0 to 45, 0 to 33, and 0 to 12 for total, sensory, and affective scores, respectively, with higher scores indicating greater pain)22; the Medical Outcomes Study Short Form Health Survey 12 (range, 0 to 100 for the physical component summary and mental component summary, with the mean set at 50 and higher scores indicating better quality of life)23; the Hospital Anxiety and Depression Scale (range, 0 to 42, with higher scores indicating greater anxiety and depression)24; the International Index of Erectile Function (range, 0 to 75, with higher scores indicating better sexual function)25; and the Male Sexual Health Questionnaire (range, 0 to 40, with higher scores indicating better function with respect to erection and ejaculation and greater satisfaction with sexual life).26
Safety Assessment
Adverse events were summarized on the basis of the body system, according to the Common Toxicity Criteria. Toxicity was assessed for each subject both overall and within each body system. Each participant was counted only once in the assessment for each body system. In the case of multiple events occurring in the same body system for a given participant, the highest grade of severity reported by that participant was recorded. Toxicity rates, both overall and within each study group, were estimated.
Statistical Analysis
Descriptive statistics were used to compare baseline demographic characteristics (age, race or ethnic group, and clinical center) and all primary and secondary measures. For participants who did not complete the study, the time to withdrawal was compared between the two groups with the use of the log-rank test. For each group, the rate of adherence to treatment, based on pill counts at the 6-week visit and the 12-week visit, was calculated as the average of the percentage of pills taken.
The primary analysis compared rates for the primary outcome between study groups, using the exact conditional test version of the Mantel–Haenszel test to control for clustering by clinical center.27 The pooled rate difference (i.e., the between-group difference in response rates across clinical centers)28 and the 95% confidence interval for this difference were calculated with the use of the “metan” module in SAS software, version 9.0, to implement a Mantel–Haenszel estimator for the difference. For secondary efficacy outcomes, both cross-sectional descriptive statistics and changes from baseline were calculated.
For the safety analysis, the frequency of each grade of toxicity in each body system was calculated. Between-group comparisons of overall adverse-event rates, with each patient classified according to the worst grade reported across all body systems, were performed with the use of an exact Kruskal–Wallis test. Since there were 26 individual categories of adverse events, the Hochberg procedure was applied to correct for inflation of event rates as a result of multiple comparisons.29
Sample-size calculations were based on 80% power to detect a difference (effect size) of 20 percentage points between response rates in the two groups (40% in the placebo group and 60% in the alfuzosin group) for the primary outcome, defined as a decline of 4 or more points in the NIH-CPSI total score. The estimated response rate of 40% for the placebo group was based on previous studies of chronic prostatitis–chronic pelvic pain syndrome,11,14-17 although we recognized that limited information on response rates was available for men who received the diagnosis recently (i.e., those with symptoms of short duration) and who had not previously been treated with an alpha-blocker. On the basis of a two-sided alpha level of 0.05 for Fisher's exact test, we calculated that a total sample of 270 participants would be required (135 per study group). This proposed sample size included a 20% increase to adjust for clustering within clinical sites and a 5% increase for interim monitoring.
An independent data and safety monitoring board reviewed safety and efficacy data in April 2006, when we had obtained data on the primary outcome for a total of 129 patients (47%), and at the end of the study. At the time of the interim review, the criterion for early termination of the study (P<0.003 for the difference in efficacy between the study groups) was not met, and the board recommended continuation of the trial.
Results
Study Participants
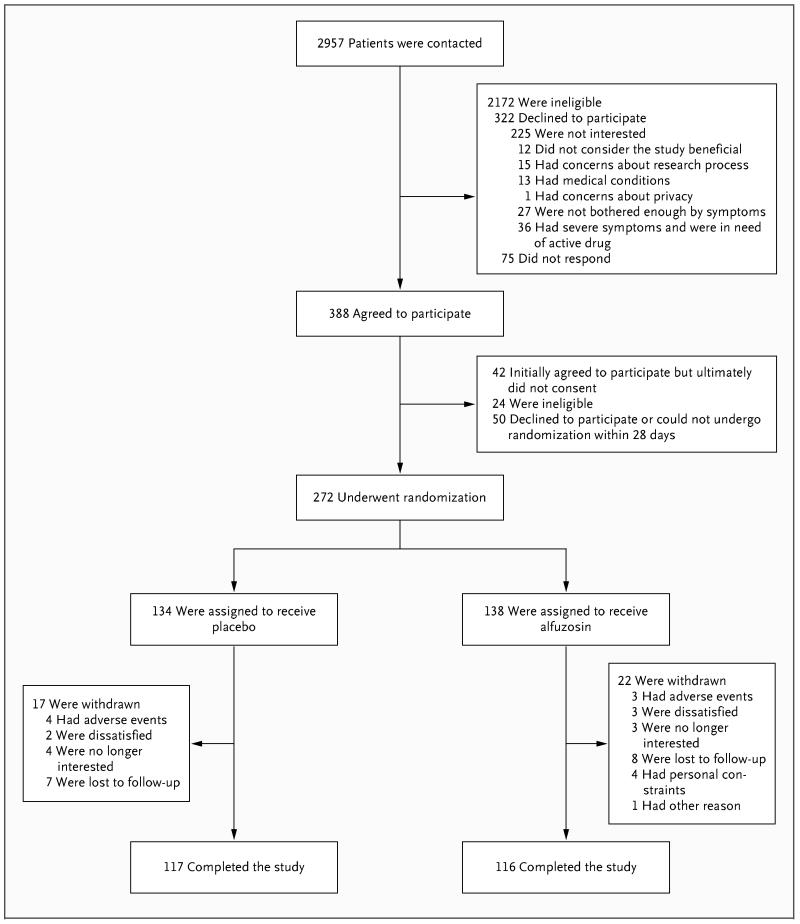
Of 388 men who agreed to participate at the beginning of the screening phase, 272 underwent randomization, and 233 completed 12 weeks of follow-up and had primary and secondary outcomes ascertained (Fig. 1). On the basis of the inclusion criteria specified by the protocol, the enrollment of four patients was deferred, with plans to enroll them in the study when they qualified for participation, but none were enrolled; these four patients provided informed consent but did not undergo randomization (Fig. 1). Withdrawal rates did not differ significantly between study groups (15.9% of men assigned to alfuzosin and 12.6% of those assigned to placebo, P = 0.52). Among the 233 subjects who completed the study and whose adherence to treatment could be determined, 158 (68%) had pill counts indicating an adherence rate of 95% or higher, and another 61 (26%) had pill counts indicating adherence rates of 75% to up to (but not including) 95%.
Figure 1. Flow of Subjects through Study Phases.
The most common reasons for ineligibility were preexisting symptoms of chronic prostatitis–chronic pelvic pain syndrome of more than 2 years at the start of the study and previous treatment with alpha-adrenergic receptor blockers for symptoms. Among the subjects who declined to participate, seven gave two reasons.
The baseline characteristics of each study group are presented in Table 1. The median duration of symptoms of chronic prostatitis–chronic pelvic pain syndrome was 1.2 years. There were no significant differences in the distributions of demographic characteristics between the two groups. The mean NIH-CPSI total score was slightly higher in the placebo group than in the alfuzosin group, but the difference was not significant (25.1 vs. 23.8, P = 0.06), and there were no significant differences between the groups in any of the NIH-CPSI subscale scores.
Table 1. Baseline Characteristics of the Study Subjects*.
| Characteristic | Placebo Group (N = 134) |
Alfuzosin Group (N = 138) |
P Value |
|---|---|---|---|
| Age — yr | 0.99 | ||
| Mean | 40.1±12.3 | 40.1±11.4 | |
| Range | 19–66 | 19–68 | |
| Race — no. (%)† | 0.19 | ||
| White | 97 (72.4) | 88 (63.8) | |
| Black | 16 (11.9) | 20 (14.5) | |
| Asian | 14 (10.4) | 13 (9.4) | |
| Other | 7 (5.2) | 17 (12.3) | |
| NIH-CPSI | |||
| Total score | 25.1±5.9 | 23.8 ±6.3 | 0.06 |
| Pain score | 11.5±3.4 | 11.1±3.3 | 0.15 |
| Urinary score | 4.9±2.9 | 4.5±2.8 | 0.30 |
| Quality-of-life score | 4.7±0.9 | 4.5±1.1 | 0.15 |
| Likert Pain and Urinary Urgency Scale | |||
| Average pain score | 5.0±1.9 | 4.9±2.0 | 0.52 |
| Urgency score | 4.7±2.6 | 4.4±2.6 | 0.40 |
| McGill Pain Questionnaire | |||
| No. evaluated | 133 | 134 | |
| Total score | 11.6±8.7 | 11.2±8.6 | 0.60 |
| Sensory score | 8.9±6.2 | 8.6±5.9 | 0.55 |
| Affective score | 2.6±3.2 | 2.6±3.3 | 0.83 |
| SF-12 | |||
| No. evaluated | 130 | 136 | 0.84 |
| Physical-component summary | 45.6±8.4 | 45.5±9.6 | 0.50 |
| Mental-component summary | 44.9±10.2 | 44.0±10.9 | |
| Hospital Anxiety and Depression Scale | |||
| No. evaluated | 133 | 137 | |
| Total score | 12.8±7.1 | 12.8±7.6 | 0.81 |
| International Index of Erectile Function | |||
| No. evaluated | 126 | 133 | |
| Total score | 52.8±17.4 | 53.5±17.9 | 0.57 |
| Male Sexual Health Questionnaire | |||
| No. evaluated | 126 | 130 | |
| Total score | 30.4±6.6 | 30.0±7.4 | 0.81 |
Plus–minus values are means ±SD. For the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI), higher scores indicate more severe symptoms (for the quality-of-life score, higher scores indicate a more negative effect). Score ranges are as follows: total score, 0 to 43; pain score, 0 to 21; urinary score, 0 to 10, quality-of-life score, 0 to 12; and average pain and urgency scores, 0 to 10. For the Likert Pain and Urinary Urgency Scale, a score of 0 indicates no pain or urgency and a score of 10 indicates the most severe pain or urgency. For the McGill Pain Questionnaire, higher scores indicate greater pain. Score ranges are as follows: total score, 0 to 45; sensory score, 0 to 33; affective score, 0 to 12. For the Medical Outcomes Study Short Form Health Survey 12 (SF-12), higher scores indicate better quality of life. Score range for both the physical and mental component summaries is 0 to 100. For the Hospital Anxiety and Depression Scale, higher scores indicate greater anxiety and depression; range, 0 to 42. For the International Index of Erectile Function, higher scores indicate better sexual function; range, 0 to 75. For the Male Sexual Health Questionnaire, higher scores indicate better function with respect to erection and ejaculation and greater satisfaction with sexual life; range, 0 to 40.
Race was self-reported.
Study End Points
The proportion of men with a decrease of at least 4 points in their total NIH-CPSI score from baseline to 12 weeks was 49.3% in both study groups (difference between groups, 0.1%; 95% confidence interval, −11.2 to 11.0) (Table 2). The results of the global response assessment were similar in the placebo and alfuzosin groups, with response rates at 12 weeks of 33.6% and 34.8%, respectively (P = 0.90).
Table 2. Response Rates for NIH-CPSI and Global Response Assessment According to Study Group*.
| Measure | Placebo Group (N = 134) |
Alfuzosin Group (N = 138) |
Absolute Difference in Rates |
|---|---|---|---|
| no. (%) | % (95% CI) | ||
| NIH-CPSI score, decline of ≥4 points (primary efficacy end point) | 66 (49.3) | 68 (49.3) | 0.1 (−11.2 to 11.0) |
| Global response assessment | |||
| Marked or moderate improvement | 45 (33.6) | 48 (34.8) | 1.8 (−9.0 to 2.5) |
| Marked improvement | 16 (11.9) | 23 (16.7) | |
| Moderate improvement | 29 (21.6) | 25 (18.1) | |
| Slight improvement | 32 (23.9) | 26 (18.8) | |
| No change | 36 (26.9) | 36 (26.1) | |
| Slight worsening | 5 (3.7) | 6 (4.3) | |
| Moderate worsening | 4 (3.0) | 2 (1.4) | |
| Marked worsening | 1 (0.7) | 1 (0.7) | |
The response rate for the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) was based on a decline in the total score of ≥4 at 12 weeks (P = 0.99); higher scores indicate more severe symptoms. Men without responses included 17 subjects in the placebo group and 22 subjects in the alfuzosin group who were withdrawn from the study. When adjusting for site (different sites had different weights owing to sample size and within-site variation), the adjusted rate difference becomes 0.1%. The confidence interval was also calculated with site adjustment. The response rate for the global response assessment was based on marked or moderate improvement at 12 weeks (P = 0.90 by Fisher's exact test).
Table 3 shows the change from baseline to week 12 for all secondary end points. Of the 233 men who completed the trial, those assigned to the alfuzosin group had a 7.1-point mean decrease in the total score for the NIH-CPSI, as compared with a 6.5-point mean decrease in the placebo group (treatment effect, 0.6 point; P = 0.70). There also were no significant differences between the two groups in the changes over time in the other measures. The only measure for which there was a significant difference between the groups in the change from baseline to week 12 was the score for ejaculation on the Male Sexual Health Questionnaire (which showed significant improvement in the alfuzosin group [1.5±4.1, range −14.0 to 14.0] as compared with the placebo group [0.3±6.5, range −29.0 to 20.0], P = 0.04). There was no significant difference between the alfuzosin and placebo groups with respect to changes in any of the five subdomains of the International Index of Erectile Function.
Table 3. Changes at 12 Weeks in Scores for Measures of Secondary Outcomes According to Study Group*.
| Measure | Placebo Group (N = 134) |
Alfuzosin Group (N = 138) |
Absolute Difference between Groups (95% CI) |
P Value |
|---|---|---|---|---|
| NIH-CPSI | ||||
| No. evaluated | 117 | 116 | ||
| Total score (0–43) | −6.5±8.5 | −7.1±9.0 | −0.6 (−2.7 to 1.5) | 0.70 |
| Pain score (0–21) | −3.0±4.4 | −3.3±4.5 | −0.3 (−1.4 to 0.8) | 0.64 |
| Urinary score (0–10) | −1.0±2.6 | −1.2±2.6 | −0.2 (−0.8 to 0.4) | 0.62 |
| Quality-of-life score (0–12) | −1.2±1.5 | −1.2±1.5 | 0 (−0.4 to 0.4) | 0.99 |
| McGill Pain Questionnaire | ||||
| No. evaluated | 116 | 112 | ||
| Total score (0–45) | −3.1±6.5 | −3.4±6.4 | −0.3 (−1.8 to 1.2) | 0.45 |
| Sensory score (0–33) | −2.3±4.9 | −2.5±5.0 | −0.2 (−1.4 to 1.0) | 0.47 |
| Affective score (0–12) | −0.9±2.3 | −1.0±2.1 | −0.1 (−0.6 to 0.4) | 0.89 |
| SF-12 | ||||
| No. evaluated | 113 | 115 | ||
| Physical component summary (0–100) | 3.5±8.1 | 3.0±7.4 | −0.5 (−2.3 to 1.3) | 0.60 |
| Mental-component summary (0–100) | 1.9±10.6 | 4.0±10.5 | 2.1 (−0.4 to 4.6) | 0.16 |
| Hospital Anxiety and Depression Scale | ||||
| No. evaluated | 117 | 115 | ||
| Total score | −1.5±5.5 | −2.6±5.7 | −1.1 (−2.4 to 0.2) | 0.08 |
| International Index of Erectile Function | ||||
| No. evaluated | 109 | 110 | ||
| Total score | −0.2±14.7 | 0.5±12.7 | 0.7 (−2.6 to 4.0) | 0.94 |
| Male Sexual Health Questionnaire | ||||
| No. evaluated | 111 | 107 | ||
| Total score (0–40) | 0.6±6.8 | 1.7±4.5 | 1.1 (−0.3 to 2.5) | 0.06 |
Plus–minus values are means ±SD. For the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI), higher scores indicate more severe symptoms (for the quality-of-life score, higher scores indicate a more negative effect). Score ranges are as follows: total score, 0 to 43; pain score, 0 to 21; urinary score, 0 to 10, quality-of-life score, 0 to 12; and average pain and urgency scores, 0 to 10. For the McGill Pain Questionnaire, higher scores indicate greater pain. Score ranges are as follows: total score, 0 to 45; sensory score, 0 to 33; affective score, 0 to 12. For the Medical Outcomes Study Short Form Health Survey 12 (SF-12), higher scores indicate better quality of life. Score range for both the physical and mental component summaries is 0 to 100. For the Hospital Anxiety and Depression Scale, higher scores indicate greater anxiety and depression; range, 0 to 42. For the International Index of Erectile Function, higher scores indicate better sexual function; range, 0 to 75. For the Male Sexual Health Questionnaire, higher scores indicate better function with respect to erection and ejaculation and greater satisfaction with sexual life; range, 0 to 40.
Adverse Events
Overall, 77 (28%) of the 272 participants who underwent randomization reported at least one adverse event. Most events were classified as mild or moderate (Table 4). Seven men (5%) in the placebo group reported one severe adverse event each, whereas three men (2%) in the alfuzosin group reported a total of five severe adverse events (arrhythmia, heartburn, nausea, dizziness, and increased pain). There were no significant differences between the groups in the overall rates of adverse events (P = 0.79) or the rates for any of the 26 individual adverse-event categories.
Table 4. Adverse Events According to Study Group.
| Adverse Events | Placebo Group (N = 134) |
Alfuzosin Group (N = 138) |
P Value |
|---|---|---|---|
| no. (%) | |||
| Overall events | 39 (29.1) | 38 (27.5) | 0.79 |
| Mild (grade 1) | 13 (9.7) | 16 (11.6) | |
| Moderate (grade 2) | 19 (14.2) | 19 (13.8) | |
| Severe (grade 3) | 7 (5.2) | 3 (2.2) | |
| Serious* | 2 (1.5) | 1 (0.7) | |
| Events according to body system† | |||
| Constitutional events | 7 (5.2) | 4 (2.9) | 0.37 |
| Gastrointestinal events | 13 (9.7) | 10 (7.2) | 0.52 |
| Neurologic events‡ | 6 (4.5) | 11 (8.0) | 0.32 |
| Pain in any body system§ | 20 (14.9) | 15 (10.9) | 0.37 |
Four serious events were reported; one occurred before randomization. Serious adverse events were defined as any untoward (unwanted) medical occurrence that was life-threatening or resulted in death, persistent significant disability or incapacity, in-patient hospitalization or prolongation of existing hospitalization, or a congenital anomaly or birth defect. The three serious adverse events occurring after randomization included myocardial infarction and traumatic head laceration in the placebo group and spontaneous pneumothorax in the alfuzosin group.
Only body systems for which adverse events were reported at a rate of more than 5% in either study group were included. Each participant was counted only once in each body system. In the case of multiple events occurring in the same body system for a given participant, the highest grade of severity reported by that participant was included.
The most frequent neurologic adverse event was dizziness (in five men in the placebo group and eight in the alfuzosin group).
No one specific site of pain was particularly common, and reported sites of pain did not differ significantly between the groups.
Discussion
Among men who had received a diagnosis of chronic prostatitis–chronic pelvic pain syndrome within 2 years before enrollment in the study and who had not previously been treated with an alpha-blocker, a 12-week course of alfuzosin as compared with placebo did not result in a clinically meaningful reduction in symptoms, as measured by the NIH-CPSI. On the basis of the 95% confidence interval for the difference between the groups in the proportion of men who had at least a 4-point improvement in the NIH-CPSI score, the results were compatible with an absolute difference of 11.2% at most. Similarly, there was no significant difference between the alfuzosin and placebo groups in multiple secondary outcomes, including the results of the global response assessment and measures of quality of life, depression, sexual function, and pain.
Despite a lack of good evidence to support their use, alpha-blockers have often been prescribed for men with chronic prostatitis–chronic pelvic pain syndrome. There are several reasons for this practice: alpha-blockers are considered first-line treatment for lower urinary tract symptoms (similar to those experienced by men with prostatitis–chronic pelvic pain syndrome) in older men with a diagnosis of clinical benign prostatic hyperplasia,30 alpha-receptors located in the central nervous system have been implicated in long-term pain syndromes,31 and recent preclinical data have suggested that alpha-blockers such as alfuzosin may reduce neurogenic inflammation in the lower urinary tract.32
The identical response rates in clinically meaningful symptom reduction in the present study contrast with the findings of four smaller randomized, placebo-controlled clinical trials14-17 but are consistent with those of another large, randomized, placebo-controlled trial.11 The four “positive” trials involved 37 patients17 to 90 patients,15,16 and ranged in duration from 6 weeks14 to 6 months.16,17 One of the studies enrolled patients with no previous exposure to alpha-blockers,15 one enrolled patients regardless of whether they had previous exposure,16 and two did not report whether patients had previous exposure.14,17 In addition, each study used a different primary end point. The differences in results between the current trial and these four trials may reflect their inclusion of different populations of men, different durations of therapy, or their selection of different primary outcomes. The larger trial, which was adequately powered (with 196 subjects) and used a two-by-two factorial design to assess the effects of 6 weeks of therapy with the alpha-blocker tamsulosin, ciprofloxacin, or both on the total NIH-CPSI score, showed no significant benefit of alpha-blocker therapy.11 However, men enrolled in that trial reported long-standing symptoms and had previously been treated with other drugs, including alpha-blockers. It was argued that patients whose symptoms had developed more recently and who had not previously been treated with alpha-blockers might be more likely to benefit, particularly with a longer duration of therapy.33-35 This was the hypothesis tested in the present study.
Our study enrolled men who had not previously been treated with an alpha-blocker and who reported having had symptoms for 2 years or less — features characteristic of men enrolled in several previous clinical trials that have shown a beneficial effect of this class of drug, as well as of patients seen by primary care physicians. In addition, our primary outcome was based on a validated instrument, the NIH-CPSI.18 This index has been shown to be responsive to symptom changes over time20 and has been adopted internationally as a primary end point in clinical trials involving men with chronic prostatitis–chronic pelvic pain syndrome.1,10-17 The degree of change required to meet the definition of a positive response to treatment (a decrease of 4 points or more on the NIH-CPSI) also appears to be clinically meaningful.20 Finally, we examined a wide range of patient-reported, secondary outcomes previously shown to be important in this syndrome.5,36
The limitations of our study should also be noted. We looked at only a single alpha-blocker, and the duration of our study was 12 weeks. Consequently, we cannot exclude the possibility that the drug would have had a beneficial effect if the treatment period had been longer or if the patients had had more acute symptoms (a duration of less than 1 year) or clinically significant voiding symptoms.
The results of our study will inform not only future clinical trials of alpha-blockers but also other potential therapies. Although the evidence for using alpha-blockers to treat men with newly diagnosed chronic prostatitis–chronic pelvic pain syndrome is relatively weak, authors of several systematic reviews and meta-analyses have advocated the use of this class of drug in such men.33-35 Our trial does not support these recommendations and should prompt reconsideration of the choice of initial therapy for these patients.
Appendix
The authors' affiliations are as follows: Department of Urology, Queen's University, Kingston, ON, Canada (J.C.N.); Department of Urology, University of Washington, Seattle (J.N.K., R.B.); Department of Medicine, Massachusetts General Hospital, Boston (M.M.-C.); Urology Department, Stanford University Medical Center, Stanford, CA (R.U.A.); Department of Urology, Temple University, Philadelphia (M.P.); Glickman Urologic Institute, Cleveland Clinic, Cleveland (D.A.S.); Departments of Urology and Health Services, David Geffen School of Medicine and School of Public Health, University of California, Los Angeles (M.S.L., S.C.); Department of Urology, University of Maryland, Baltimore (R.B.A.); Department of Surgery, University of Mississippi, Jackson (P.C.W.); Department of Urology, Northwestern University, Chicago (R.N., A.J.S.); Department of Surgery, Brigham and Women's Hospital, Boston (M.O.); University of Sciences, Penang, Malaysia (M.L.L.); Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia (S.Z., J.R.L.); and the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD (J.W.K., L.M.N.).
In addition to the authors, the Chronic Prostatitis Collaborative Research Network (CPCRN-2) Study Group includes the following institutions and individuals: Northwestern University — E.A. Calhoun, J.Q. Clemens, D. Marko, C.M. Fitzgerald; Harvard University — C. Williams, D. Rhodes, P. Desai; Queen's University — D.A. Tripp, D. Ardern, J. Clark-Pereira, J. Downey, R. Siemens, A. Morales; Temple University — N. Lamarr, B. Simpkiss, M. Santiago, A. Braverman, C. Dobi; University of California, Los Angeles — Y. Xie, G. Byrd, S. Freeman; University of Maryland — S. Keay, T. Chai, L. Radebaugh, Y. Underwood, J. Murray, G. Markowitz-Chrystal; University of Mississippi — J.E. Fowler, Jr., W. Duncan, D. Lumpkin, R. Tapley; Cleveland Clinic — J. Potts, D. Murphy; Charles R. Drew University — N.S. Datta, K. Mervin; Stanford University — C.K. Payne, C. Chan, R. Shinghal, E. Orenberg, V. Flores, A. Morey; University of Washington — C. Muller, J. Turner, I. Rothman, M. Frest; University of Washington–University of Sciences Malaysia —– S. Ross, L. Butler, R. Bale, Jr., R. Sweet, J. Giesler, D. Riley, K.H Yuen, S.W.H. Lee, P.Y. Cheah, L.T. Chin, J.R. Yang, W.S. Leong, C.W. Loong, L.W. Seng, H.W. Yap, N. Kahn, T. Kohr, M. Mohan, D.C. Lang, L.C. Sin, H.K. Heng (19); University of Pennsylvania School of Medicine — K.J. Propert, R. Madigan, K. Mickelberg, M. Durborow, L. Cen, E. Barrell, Y. Wang, A. Chew; National Institute of Diabetes and Digestive and Kidney Diseases — C. Mullins; Prostatitis Foundation — M. Hennenfent.
Acknowledgments
Supported by cooperative agreements (U01 DK65209, U01 DK65268, U01 DK65297, U01 DK65187, U01 DK65277, U01 DK65189, U01 DK65174, U01 DK65266, U01 DK65257, U01 DK65186, and U01 DK65287) from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Minority Health and Health Disparities. The study drugs were provided by Sanofi-Aventis, Paris.
Dr. Nickel reports receiving a lecture fee from Sanofi-Aventis, consulting fees from Pfizer and Farr Labs, and research support from Allergan and American Medical Systems; Drs. O'Leary and Landis, receiving consulting and advising fees from Sanofi-Aventis; Dr. Krieger, receiving consulting and advising fees from Pfizer; Dr. Alexander, receiving lecture fees from Boehringer Ingelheim; Dr. Shoskes, receiving consulting fees from Farr Labs and holding stock in Triurol; Dr. Kusek, holding stock in Eli Lilly, Pfizer, and deCODE Genetics; and Dr. Schaeffer, receiving consulting fees from Alita Pharmaceuticals, NovaBay Pharmaceuticals, IMS Health, and Regeneron and lecture fees from the Wright Resource and cme2. No other potential conflict of interest relevant to this article was reported.
We thank the men who participated in this clinical trial.
References
- 1.Schaeffer AJ. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355:1690–8. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JQ, Calhoun EA, Litwin MS, McNaughton Collins M. Primary care physician practice patterns in the management of chronic prostatitis/chronic pelvic pain syndrome. J Urol; Presented at the AUA 2007 Annual Meeting; May 19-24, 2007; Anaheim, CA. 2007. pp. 30–1. abstract. [Google Scholar]
- 3.Calhoun EA, McNaughton Collins M, Pontari MA, et al. The economic impact of chronic prostatitis. Arch Intern Med. 2004;164:1231–6. doi: 10.1001/archinte.164.11.1231. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer AJ, Landis JR, Knauss JS, et al. Demographic and clinical characteristics of men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort (CPC) study. J Urol. 2002;168:593–8. [PubMed] [Google Scholar]
- 5.Wenninger K, Heiman JR, Rothman I, Berghuis JP, Berger RE. Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol. 1996;155:965–8. [PubMed] [Google Scholar]
- 6.McNaughton Collins M, Pontari MA, O'Leary MP, et al. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16:656–62. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology. 1998;51:578–84. doi: 10.1016/s0090-4295(98)00034-x. [DOI] [PubMed] [Google Scholar]
- 8.McNaughton Collins M, Joyce GF, Wise M, Pontari MA. Prostatitis. In: Litwin MS, Saigal CS, editors. Urologic diseases in America. Vol. 2004. Washington, DC: Government Printing Office; pp. 9–41. [Google Scholar]
- 9.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 10.Nickel JC, Downey J, Clark J, et al. Levofloxacin for chronic prostatitis/ chronic pelvic pain syndrome in men: a randomized placebo-controlled multicenter trial. Urology. 2003;62:614–7. doi: 10.1016/s0090-4295(03)00583-1. [DOI] [PubMed] [Google Scholar]
- 11.Alexander RB, Propert KJ, Schaeffer AJ, et al. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004;141:581–9. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 12.Nickel JC, Pontari M, Moon T, et al. A randomized, placebo-controlled, multicenter study to evaluate the safety and efficacy of rofecoxib in the treatment of chronic nonbacterial prostatitis. J Urol. 2003;169:1401–5. doi: 10.1097/01.ju.0000054983.45096.16. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC. The three As of chronic prostatitis therapy: antibiotics, alpha-blockers, and anti-inflammatories: what is the evidence? BJU Int. 2004;94:1230–3. doi: 10.1111/j.1464-410X.2004.05148.x. [DOI] [PubMed] [Google Scholar]
- 14.Nickel JC, Narayan P, McKay J, Doyle C. Treatment of chronic prostatitis/chronic pelvic pain syndrome with tamsulosin: a randomized double-blind trial. J Urol. 2004;171:1594–7. doi: 10.1097/01.ju.0000117811.40279.19. [DOI] [PubMed] [Google Scholar]
- 15.Cheah PY, Liong ML, Yuen KH, et al. Terazosin therapy for chronic prostatitis/ chronic pelvic pain syndrome: a randomized, placebo controlled trial. J Urol. 2003;169:592–6. doi: 10.1097/01.ju.0000042927.45683.6c. [DOI] [PubMed] [Google Scholar]
- 16.Tuğcu V, Taşçi AI, Fazlioğlu A, et al. A placebo-controlled comparison of the efficiency of triple- and monotherapy in category III B chronic pelvic pain syndrome (CPPS) Eur Urol. 2007;51:1113–8. doi: 10.1016/j.eururo.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Mehik A, Alas P, Nickel JC, Sarpola A, Helström PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62:425–9. doi: 10.1016/s0090-4295(03)00466-7. [DOI] [PubMed] [Google Scholar]
- 18.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, et al. The National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI): development and validation of a new outcomes measure. J Urol. 1999;162:369–75. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 19.Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Bethesda, MD: National Cancer Institute; 2006. [November 24, 2008]. at http://ctep.cancer.gov/forms/CTCAEv3.pdf. [Google Scholar]
- 20.Propert KJ, Litwin MS, Wang Y, et al. Responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) Qual Life Res. 2006;15:299–305. doi: 10.1007/s11136-005-1317-1. [DOI] [PubMed] [Google Scholar]
- 21.Propert KJ, Alexander RB, Nickel JC, et al. Design of a multicenter randomized clinical trial for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:870–6. doi: 10.1016/s0090-4295(02)01601-1. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Kosinski M, Dewey J. How to score version 2 of the SF-36 Health Survey. 3rd. Lincoln, RI: QualityMetric; 2000. [Google Scholar]
- 24.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 26.Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777–82. doi: 10.1016/j.urology.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR, Oakes DO. Analysis of survival data. London: Chapman & Hall/CRC Press; 1984. [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 30.AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 31.Andersson KE, Gratzke C. Pharmacology of alpha1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol. 2007;4:368–78. doi: 10.1038/ncpuro0836. [DOI] [PubMed] [Google Scholar]
- 32.Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008;101(Suppl):2–6. doi: 10.1111/j.1464-410X.2008.07493.x. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer AJ, Anderson RU, Krieger JN, et al. In: McConnell J, Abrams P, Denis L, Khoury S, Roehrborn C, editors. The assessment and management of male pelvic pain syndrome, including prostatitis; Male lower urinary tract dysfunction, evaluation and management: 6th International Consultation on New Developments in Prostate Cancer and Prostate Disease; Paris. Health Publications; pp. 341–85. [Google Scholar]
- 34.Yang G, Wei Q, Li H, Yang Y, Zhang S, Dong Q. The effect of alpha-adrenergic antagonists in chronic prostatitis/chronic pelvic pain syndrome: a meta-analysis of randomized controlled trials. J Androl. 2006;27:847–52. doi: 10.2164/jandrol.106.000661. [DOI] [PubMed] [Google Scholar]
- 35.Mishra VC, Browne J, Emberton M. Role of alpha-blockers in type III prostatitis: a systemic review of the literature. J Urol. 2007;177:25–30. doi: 10.1016/j.juro.2006.08.090. [DOI] [PubMed] [Google Scholar]
- 36.Nickel JC, Tripp DA, Chuai S, et al. Psychosocial variables affect the quality of life of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2008;101:59–64. doi: 10.1111/j.1464-410X.2007.07196.x. [DOI] [PubMed] [Google Scholar]