Summary
Vibration-induced nystagmus, as clinical sign, was recently introduced in outpatient clinical practice for the study and evaluation of otoneurological patients. This response, which can only be evoked by bone conducted vibratory stimulation in the mastoid region or at the location on the forehead in the midline at the hairline, was essentially designed for patients with persistent unilateral vestibular deficit and was interpreted as the result of excitatory functional activity of the vestibular system on the non-affected side. Vibratory stimulation is, in fact, considered to reach both systems, which in the case of functional asymmetry, respond asymmetrically with greater excitatory activation on the more responsive side. On the other hand, little information is available concerning vibration-induced nystagmus in subjects with symmetrical vestibular function. The limited experience with this recently proposed test and incomplete knowledge regarding its mechanisms suggest that it must be investigated in clinical conditions, having a known pathophysiological basis: the responses obtained could help provide insight into the potential of this test and contribute to the diagnostic definition of the superior semicircular canal dehiscence or otosclerosis. Analysis of Vibration-induced nystagmus, recently proposed to study transmission of excitatory stimuli by bone conduction, may be appropriate for altered input caused by defects of the labyrinthine capsule. This promises to be an interesting new field of research.
Keywords: Vibration induced nystagmus, Vestibular otosclerosis, Superior semicircular canal dehiscence, Vestibulo-ocular reflex
Riassunto
Il nistagmo indotto dalla vibrazione è stato recentemente introdotto nella pratica clinica per lo studio dei pazienti otoneurologici. Questa risposta che può essere evocata solo con stimolazione vibratoria prolungata nella regione mastoidea oppure alla posizione della testa che coincide con la linea mediana in corrispondenza dell’attaccatura dei capelli, è stata essenzialmente designata per pazienti con deficit unilaterale vestibolare stabilizzato ed è stata interpretata come il risultato dell’attività del sistema vestibolare del lato sano. Infatti si ritiene che lo stimolo vibratorio possa eccitare l’intero sistema, che nel caso di una asimmetria funzionale, risponde in modo appunto asimmetrico con una grande risposta eccitatoria del lato sano o in buona sostanza maggiormente responsivo. Dall’altro lato, poche informazioni sono disponibili circa la stimolazione vibratoria prolungata in soggetti con funzione vestibolare simmetrica. Le limitate conoscenze ed esperienze nell’utilizzo di tale metodica e circa il meccanismo d’azione dello stimolo in questione, suggeriscono che il test vibratorio debba comunque essere usato in ambito clinico, partendo da ben note conoscenze di fisiopatologia; le risposte così ottenute possono essere di aiuto e contribuire alla diagnosi e/o al sospetto diagnostico in due condizioni oggetto di questo studio: la deiscenza del canale semicircolare superiore e l’otosclerosi. Dunque l’analisi del nistagmo indotto dalla vibrazione, proposto di recente per lo studio della trasmissione di uno stimolo eccitatorio per via ossea, può essere considerato appropriato per la valutazione dell’alterata immissione di tale stimolo alla capsula labirintica, modificata dalla deiscenza del canale semicircolare superiore e dalla otosclerosi. Questo appare evidenziarsi come un nuovo e promettente campo di ricerca.
Introduction
Bone-conducted vibration (BCV) of the head causes, at low frequencies (60-100 Hz), linear acceleration stimulation of both inner ears and this linear acceleration is an effective way of activating probably only semicircular canals and not otolithic afferent neurons as recently demonstrated with mini vibrations 1 2. High frequency (500 Hz) BCV, at this point, is a selective means of activating otoliths whereas low frequency (50-100 Hz) BCV is an effective means of activating semicircular canals. For this reason, we have applied the term prolonged to the Bone Conducted Vibration (P-BCV) thus differentiating this modality from the cited experiences necessary in order to evoke answers from the otoliths organs.
This vibration-induced vestibular activation results in a variety of vestibulo-spinal and vestibulo-ocular responses, and one of the latter is the object of this review in two conditions: superior semicircular canal dehiscence (SSCD) and otosclerosis.
BCV delivered at the mastoids and at the midline of the forehead at the hairline (Fz) causes simultaneous and approximately equal amplitude linear acceleration stimulation at both mastoids and results in ocular movements, nystagmus.
Vibration on the bone behind the ear may elicit eye movements with horizontal and torsional components in patients with vestibular problems. Lücke 3 introduced this test for the first time in 1973, as a simple bedside method of detecting static anomalies of peripheral vestibular function. Vibration has been shown to excite the semicircular canals and otolithic afferents in animals 4 5 and humans 3 6–8. Three responses to bone trans-mastoid vibratory stimulus are considered in humans: ocular, postural and perceptual. Vibration-induced nystagmus (VIN) was introduced in outpatient clinical practice for the study of otoneurological patients by Lackner 9. This response, which can only be evoked by vibratory stimulation in the mastoid region, was essentially designed for patients with persistent unilateral vestibular deficit (UVD) 10 and was interpreted as the result of excitatory functional activity of the vestibular system on the non-affected side 11. In recent studies, however, the VIN to mastoids and bone-conducted vibration delivered to Fz (Fz BCV) stimulation has been recorded in a large number of unselected healthy subjects 12. Vibratory stimulation is, in fact, considered to reach both systems, which in the case of functional asymmetry, respond asymmetrically with greater excitatory activation on the more responsive side. On the other hand, little information is available about vibration-induced nystagmus in subjects with symmetrical vestibular function.
Limited experience with this recently proposed test and incomplete knowledge regarding its mechanisms suggest that it be tried in clinical conditions having a known pathophysiological basis: the responses obtained could help provide insights into the potential of this test and contribute to the diagnostic definition of the condition. More specifically, analysis of VIN response in conditions involving altered bone conduction of acoustic stimuli could be an interesting study model.
Superior semicircular canal dehiscence
Superior semicircular canal dehiscence (SSCD) is a new clinical entity described by L.B. Minor et al. in 1998 14 and characterized by the onset of specific vestibular and cochlear symptoms induced by hypersensitivity of labyrinthine receptors due to a bone defect, usually located in the roof or external wall of the superior semicircular canal.
Diagnosis cannot be based on typical symptoms as these may include dizziness, Tullio phenomenon, positional vertigo, pulsatile tinnitus, conductive and/or neurosensory hearing loss. The possibility of SSCD must be evaluated on the basis of particular signs (such as Hennebert sign, nystagmus induced by Valsalva manoeuvre) and reinforced by the results of certain tests (essentially air-conducted cervical and bone-conducted ocular vestibular evoked myogenic potentials, cervical vestibular evoked myogenic potential [cVEMPs] and ocular vestibular evoked myogenic potential ([oVEMPs]), with threshold analysis 1 2–16. Briefly, simple pressure stimulation of the middle ear or an increase in intra-cranial pressure 12 can evoke a typical nystagmus, detected as a specific oculomotor pattern recorded by three-dimensional scleral search coils 4 13 while analysis of air cVEMPs 16 demonstrates a peculiar response with increased amplitude and depressed detection threshold. Final confirmation is only obtained by high resolution computed tomography (HRCT) with sagittal reconstructions following and mapping the circumference of the superior semicircular canal 1 2 13 15.
Since eye-movement recording with three-dimensional scleral search coils is rarely performed and the electrophysiological pattern of VEMPs is often difficult to interpret, especially in subjects with conductive hearing loss, selecting cases for HRCT to obtain a definitive diagnosis is not easy. A diagnostic protocol based on symptoms and HRCT could also be very expensive, considering the relative rarity of SSCD. These considerations show the need for a low-cost, such as the vibratory test, an easily reproducible test that can be used when SSCD is suspected.
Otosclerosis
Otosclerosis is a term used to describe a primary disorder of the bony capsule of the labyrinth, first identified and reported by Adam Politzer 17. This lesion is found only in the human otic capsule and may produce symptoms of the audiological sphere (conductive hearing loss of up to 50 dB or sensorineural hearing loss) or of the vestibular sphere. These conditions depend on the site, size and histological features of the pathologically involved area 18. Otosclerosis usually affects both ears. Hearing loss is the most frequent symptom and may appear gradually. Other symptoms include dizziness, loss of balance and tinnitus. In order to diagnose otosclerosis, various hearing tests, including tympanometry, audiometry and tuning fork tests, are performed to determine the extent and nature of hearing loss.
Diagnosis is usually made on the basis of family medical history, conductive hearing loss pattern and CT scan of the temporal bone. Hearing tests may initially show a sensory pattern and later a typical conductive loss pattern. Acoustic reflexes may even be absent, and early on may show an “on-off” effect. Tympanometry often shows stiffening of the ossicular chain. CT scan is specific but has low sensitivity.
Balance problems, including unsteadiness, dizziness, vertigo and other sensations of motion, may also occur. These symptoms have long since been observed in otosclerotic patients 20 21. Dizziness may occur in these patients: in one study, it was reported to occur in 15% 20, while in another, clinical evidence has shown that 3-35% of otosclerosis patients have vestibular involvement 21. One pathological sign is degeneration of the vestibular ganglion (Scarpa’s ganglion). The mechanism of dizziness is unknown, though it may be related to release of enzymes into the inner ear by metabolically active bone.
Material and methods
Overall, 10 normal subjects (controls), with no history of auditory or vestibular disease (5 female, 5 male; mean age 35 yrs, range 17-60), volunteered to take part in the study. Recruited and included in the study were 20 patients OTS (age range 22-57 yrs; 6 male; mean age 39 yrs), Figure 1A and B, and 16 patients with SSCD (6 male, 10 female, mean age 49 yrs (range 18-68), Figure 2A and B. These patients underwent the vibration test while seated. We used a hand-held vibrator (A.DE.LE. International, Bologna, Italy, 60 to 110 Hz vibration frequency, 100 Hz used stimuli). This device was applied to the mastoid region and to the vertex for at least 40 seconds; a retest was carried out within 10 minutes later.
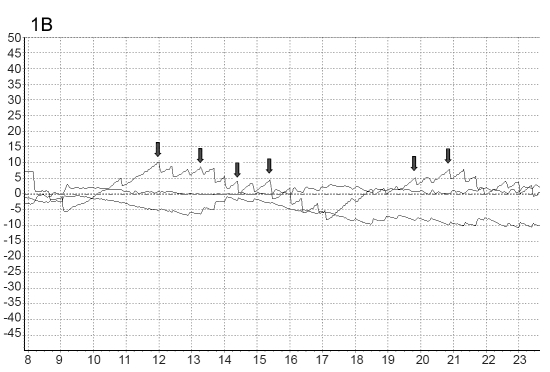
Fig. 1.
Figs. 1A, B. Typical three-dimensional eye movements in response to prolonged BVIN in patient no. 9 with bilateral conductive hearing loss and otosclerosis. The three components of nystagmus (horizontal, vertical and torsional) are plotted. The main SP horizontal component of nystagmus was directed towards the opposite side.
Fig. 2.
Figs. 2A, B. Original 3D videoculography recordings of VIN from subject with superior semicircular canal dehiscence. The three components of nystagmus (horizontal, vertical and torsional) are plotted. The main SP horizontal (H), vertical (V) and torsional (T) are plotted against time for the left eye. Positive value are assigned to rightward (R); upward (UP) and clockwise (CW) eye movements, whereas negative values are assigned to leftward (L), downward (D) and counterclockwise (CCW) eye movements.
The pattern of horizontal, torsional and vertical eye movement induced by vibratory stimulation was evaluated and recorded with three-dimensional infrared video oculography (50 Hz sampling; Torsio VNG Ulmer; Synapsys, Marseille, France). Patients were seated with Reid’s line (the line joining the inferior margin of the orbit and the middle of the external auditory meatus) approximately earth horizontal, a defined standard position that is considered comfortable and provides a very similar orientation of the otoliths and the patient is invited to maintain the chin fixed to the device as shown in Figure 3. Testing, in all cases, was conducted in complete darkness. The subjects wore a mask, with a camera fixed in front of the left eye as can be seen in Figure 3. The subjects were instructed to keep their eyes open as soon as possible and to try to keep their gaze close to the centre to control for influences on torsional eye position at eccentric gaze positions, such as those described by Listing’s Law and Alexander’s Law 4. The same examiner (LM) delivered vibration to all subjects. The horizontal, vertical, and torsional positions of the eye were processed on line using contrast image detection of the iris. The description of torsional eye movements follows the standard nomenclature.
Fig. 3.

Modality of recording nystagmus and 3D eye movements in SSCD and otosclerosis patients.
The terms used are with respect to the patient’s point of view. For example, clockwise means torsion of the superior pole of the eye to the patient’s clockwise direction. Normative data (mean ± 2 standard deviations [SD]) were obtained from 50 normal subjects (27 male, 23 female; mean age, 33 yrs; range, 18-51 yrs).
The presence and slow phase velocity (SPV), of VIN were analysed for correlations with anatomical, audiological and electrophysiological characteristics.
Comparison of the results of the vibratory-induced nystagmus between patients and control groups were analysed by means of a non-parametric Mann-Whitney U test using SPSS Version 11.0 (software SPSS, Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
Results
We have described two clinical series of subjects with bony labyrinthine capsule alterations.
VIN was recorded in 6 normal subjects (Table III) and in all cases (100%, p > 0.001) of SSCD and otosclerosis patients when stimulation was applied on both mastoids.
Table III. Results of 3D VOG evaluation after vibration over mastoids in normal subjects.
| SPV, °/s* | |||||
| Patient | Sex | Age (years) | Horizontal | Vertical | Torsional |
| 1 | M | 17 | -0.1/0.3 | 1.6/-1.2 | 0.6/-0.4 |
| 2 | M | 22 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 |
| 3 | F | 57 | 0.4/0.3 | 1.3/-1.2 | 1.2/-0.9 |
| 4 | M | 43 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 |
| 5 | F | 56 | 0.2/0.5 | 1.5/-1.7 | 0.7/-0.4 |
| 6 | M | 55 | 0.8/0.6 | 1.7/-1.3 | 0.5/-0.8 |
| 7 | F | 34 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 |
| 8 | F | 60 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 |
| 9 | F | 44 | 0.4/-0.3 | 1.6/-1.2 | 0.6/-0.4 |
| 10 | M | 42 | 0.3/0.4 | 1.2/-12.9 | 0.1/-0.5 |
*: SPV, °/s: slow phase velocity of nystagmus, degree per second.
At the onset of vibration (Figs. 1A, B and 2A, B), a change occurred in the subjects’ horizontal eye position and horizontal nystagmus appeared.
In the healthy group, VIN was recorded in 6 subjects with mainly horizontal component, little represented the others two components.
In all patients, the 3D analysis of nystagmus revealed that the direction of nystagmus was mainly vertical and torsional, consistent with excitatory or inhibitory stimulation of the affected or more affected superior semicircular canal, in the case of superior semicircular canal dehiscence (Table I, Figs. 1A, B and 2A, B). At the onset of vibration, there was a change in subjects’ ocular torsional position and torsional nystagmus.
Table I. Results of 3d VOG evaluation after vibration over mastoids in patients with superior canal dehiscence syndrome.
| SPV, °/s* | |||||
| Patient | Age (years) | Dehiscence | Horizontal | Vertical | Torsional |
| 1 | 27 | LB > RB | -3.1/4.3 | -10.6/15.2 | -23.6/26.4 |
| 2 | 18 | RA | 0.8/0.6 | 9.7/6.3 | 15.8/9.8 |
| 3 | 57 | LC > RB | -4/1.3 | 12.3/-15.2 | 16.2/-26.9 |
| 4 | 43 | LB | -2.8/2.5 | 2.7/-9.4 | -3.2/18.8 |
| 5 | 66 | LA | -1.2/1.5 | 2.5/-8.7 | 2.7/-15.4 |
| 6 | 65 | LA | 1.8/1.6 | 1.7/-6.3 | 5/-13.8 |
| 7 | 34 | LC > RC | 1.6/1.5 | -4/-4.9 | 5.6/-4 |
| 8 | 36 | LC | -1.5/1.2 | 2.8/-8.9 | -8.2/-20.3 |
| 9 | 54 | RB > LC | -4/1.3 | -6/-5.2 | 26/-16.4 |
| 10 | 52 | RB | 0.9/0.4 | 6.2/5.9 | 21.1/4.5 |
| 11 | 66 | LB | -1.7/1.8 | 2.8/-12.9 | 4.2/-21.5 |
| 12 | 35 | LB | -1.1/1.9 | 4.8/-11.1 | 10.2/-22.2 |
| 13 | 29 | LB > RA | 1.1/0.9 | 3.1/-3.9 | 6.6/-7.1 |
| 14 | 68 | LC | -0.5/1.1 | 9.8/-10.9 | 18.2/-27.3 |
| 15 | 67 | LC > RB | 1.7/1.9 | 6.1/-9.9 | 16.6/-14.1 |
| 16 | 65 | RB > LA | -1.2/1.9 | -9.3/-8.2 | 16.5/-12.4 |
L: left; R: right; A,B,C: width of dehiscence into one of three ranges: A)< 2 mm; B) from 2 to 4 mm; c)> 4 mm; *: SPV, °/s: slow phase velocity of nystagmus, degree per second.
In the event of the SSCD, the subjects were divided into four further groups as shown in Table I and mean nystagmus responses evoked by mastoid vibration were analysed, considering patients with right, left and bilateral SSCD. The latter group was divided into two further groups: those with left SSCD greater than right SSCD and vice versa.
Although there was considerable between-subject variability in the magnitude of the various components of the oculomotor response to VIN, three patterns of vertical and torsional nystagmus component were detected (Table I):
vertical and torsional, consistent with excitatory response of the affected side; 17 out of 23 ears (73.9%);
vertical, horizontal and rotatory, consistent with inhibitory response of the affected side; two out of 23 ears (8.6%);
vertical and rotatory component with SPV directed in the opposite direction in the affected ear. Four out of 23 ears were affected (17.4%).
In one patient, VIN finished a few seconds after the end of the vibratory stimulus (post-stimulus response). In all cases, VIN was accompanied by a mild/intense sensation of spinning and all patients were able to tolerate the vibratory stimulation for 40 seconds (test-retest).
Analysis of quantitative characteristics of VIN SPV (degrees/sec) peak velocity, mean amplitude and frequency (extent and direction) and the other factors (width of dehiscence, mono- or bilaterality) did not reveal any correlations (Table I).
In the same way, therefore, in the otosclerosis group, VIN was detected in all cases (100%, p > 0.001) when stimulation was applied to mastoids. But 3D (three-dimensional) analysis of nystagmus revealed that the direction of nystagmus was mainly horizontal, consistent with inhibitory stimulation and ampullifugal flow in the normal ear, while stimulation on the affected side revealed horizontal eye movement, consistent with excitatory stimulation and ampullipetal flow in the lateral semicircular canal. These data indicate an asymmetry of the function of the vestibular system (Table II).
Table II. Results of 3D VOG evaluation after vibration over mastoids in patients with otosclerosis and conductive hearing loss.
| SPV, °/s* | ||||||
| Patient | Sex | Age (years) | Ear affected | Horizontal | Vertical | Torsional |
| 1 | M | 27 | L | -0.1/0.3 | 6.6/15.2 | -0.6/0.4 |
| 2 | M | 22 | R | 0.3/0.1 | -9.7/-6.3 | 0.8/0.8 |
| 3 | F | 57 | R | 0.4/1.3 | -12.3/-15.2 | 1.2/-0.9 |
| 4 | F | 43 | L | -0.8/0.2 | 4.7/9.4 | -0.2/-1.8 |
| 5 | F | 56 | L | -0.2/0.2 | 6.5/8.7 | 0.7/-0.4 |
| 6 | F | 55 | L | 0.8/0.3 | 11.7/16.3 | 0.5/-0.8 |
| 7 | F | 34 | L | 0.6/0.5 | 14/14.9 | 0.6/-0.4 |
| 8 | F | 36 | R | -0.3/0.2 | -12.8/-18.9 | -0.2/-0.3 |
| 9 | F | 44 | Bil | -0.4/-0.3 | -6/5.2 | 0.6/-0.4 |
| 10 | F | 42 | R | 0.2/0.4 | -11.2/-12.9 | 0.1/0.5 |
| 11 | M | 36 | L | -0.7/0.0 | 10.8/12.9 | 0.2/-0.5 |
| 12 | M | 35 | L | -0.1/0.2 | 12.8/11.1 | 0.2/-0.2 |
| 13 | M | 29 | Bil | 0.1/0.0 | -3.1/3.9 | 0.6/-0.6 |
| 14 | F | 38 | L | -0.0/0.1 | 9.8/10.9 | 0.2/-0.3 |
| 15 | F | 47 | L | 0.2/0.2 | 6.1/9.9 | 0.6/-0.8 |
| 16 | F | 35 | R | -0.2/0.1 | -9.3/-8.2 | 0.5/-0.4 |
| 17 | F | 36 | R | 0.2/0.1 | -7.1/-8.3 | 0.6/-0.6 |
| 18 | F | 31 | L | 0.3/0.1 | 9.4/6.9 | 0.2/-0.3 |
| 19 | M | 44 | R | 1.5/1.2 | -8.3/-5.8 | 0.5/-0.4 |
| 20 | F | 50 | R | 1.7/1.3 | -8.6/-9.6 | -0.1/-0.5 |
Bil: bilateral; L: left; R: right; *: SPV, °/s: slow phase velocity of nystagmus, degree per second.
Comparing separately SSCD slow phase velocity nystagmus and normals (Table IV) and consequently otosclerosis slow phase velocity nystagmus and normals, it clearly appears that, in the first case, the great differences in the average of the SPV were obtained in all the components. In the second group, it is obvious instead as the single one horizontal component is in a marked manner different from the pathological ones regarding the normals (Table V).
Table IV. Comparison of vibration-induced slow phase nystagmus in SSCD patients and normal subjects.
| SPV, °/S | |||
| SSCD | Horizontal | Vertical | Torsional |
| Right (affected) = 2 | RS: -0,85 (-0,8 to 0,9) | RS: 7,5 (6,2 to 9,7) | RS: 18,45 (15,8 to 21,1) |
| LS: 0,5 (0,4 to 0,6) | LS: 6,1 (5,9 to 6,3) | LS: 7,5 (4,5 to 9,8) | |
| Left (affected) = 7 | LS: 1,657 (1,1 to 2,5) | LS: -9,743 (-6,3 to -12,9) | LS: -19,8 (-13,8 to 27,3) |
| RS: -1,0 (-2,8 to 1,8) | RS: 3,871 (1,7 to 9,8) | RS: 4,8 (-8,2 to 18,2) | |
| Bilateral L > R = 5 | LS: 1,98 (0,9 to 4,3) | LS: -9,82 (-15,2 to -3,9) | LS: -15,7 (-26,41 to -4,0) |
| RS: -0,54 (-4 to 1,7) | RS: -1,38 (-10,6 to 12,3) | RS: 4,28 (-23,6 to 16,6) | |
| Bilateral R > L = 2 | LS: 1,6 (1,3 to 1,9) | LS: -6,7 (-8,2 to -5,2) | LS: -14,4 (-16,4 to -12,4) |
| RS: -2,6 (-4 to 1,2) | RS: -7,65 (-9,3 to 6,0) | RS: 21,25 (16,5 to 26,0) | |
| Normal = 10 | LS: -0,6 (-0,9 to 0) | LS: 0,18 (-0,3 to 0,6) | LS: -0,26 (-0,9 to 0) |
| RS: 0,89 (0 to 1,7) | RS: 0,2 (-0,2 to 0,8) | RS: 0,37 (0 to 1,2) | |
| Total: 23 ears affected | |||
RS: Right stimulation; LS: Left stimulation; L: Left; R: Right; SSCD: Superior semicircular canal dehiscence; SPV: Slow phase velocità; °/S: degree/second.
Table V. Comparison of Vibration-Induced slow phase nystagmus in otosclerosis patients and normal subjects.
| SPV, °/S | |||
| Otosclerosis | Horizontal | Vertical | Torsional |
| Right (affected) = 8 | RS: -9,9 (-12,3 to -7,1) | RS: 0,5 (-0,3 to 1,7) | RS: 0,425 (-0,2 to 1,2) |
| LS: -10,65 (-18,9 to -5,8) | LS: 0,6 (0,1 to 1,1) | LS: -0,225 (-0,8 to 0,9) | |
| Left (affected) = 10 | LS: 11,62 (6,9 to 16,3) | LS: 0,21 (0 to 0,5) | LS: -0,5 (-1,8 to 0,5) |
| RS: 9,24 (4,7 to 12,8) | RS: 1,4 (-0,8 to 0,8) | RS: -0,23 (-0,6 to 0,6) | |
| Bilateral = 2 | LS: 1,98 (0,9 to 4,3) | LS: 9,1 (3,9 to 5,2) | LS: -0,1 (-0,4 to 0,6) |
| RS: -0,15 (0 to 0,3) | RS: -5,68 (-6 to -3,1) | RS: 0,6 | |
| Normal = 10 | LS: -0,6 (-0,9 to 0) | LS: 0,18 (-0,3 to 0,6) | LS: -0,26 (-0,9 to 0) |
| RS: 0,89 (0 to 1,7) | RS: 0,2 (-0,2 to 0,8) | RS: 0,37 (0 to 1,2) | |
| Normal = 10 | LS: -0,6 (-0,9 to 0) | LS: 0,18 (-0,3 to 0,6) | LS: -0,26 (-0,9 to 0) |
| RS: 0,89 (0 to 1,7) | RS: 0,2 (-0,2 to 0,8) | RS: 0,37 (0 to 1,2) | |
| Total: 22 ears affected | |||
RS: Right stimulation; LS: Left stimulation; L: Left; R: Right; SPV: Slow phase velocità; °/S: degree/second.
Discussion
Skull vibration of different frequencies has been shown to excite saccular afferents in frogs 19, lateral semicircular canal afferents in pigeons 22 and both semicircular canal and otolith afferents in squirrel monkeys 21.
The most compelling evidence of vibratory stimulation of vestibular receptors is that stimulation of receptors of vertical semicircular canals induces torsional eye movements 5–23.
Stimulation of neck muscle spindles has not, to our knowledge, been reported to cause torsional eye movements. Presumably, this skull vibratory stimulation causes waves in the cerebrospinal fluid which exert forces on the vestibule via the vestibular and cochlear aqueduct, causing deflection of the cupula of the semicircular canals.
First of all, however, it must be emphasized that we used a very low frequency stimulation (60-100 Hz) and it is remarkable that VIN was detected in all cases (100%, p > 0.001) of SSCD and otosclerosis with conductive hearing loss (CHL) when stimulation was applied to either mastoid both conditions indicate an asymmetry of the function of the vestibular system with different modality of answer of ocular movements. It is important to emphasize the fact of the level of the frequency, since recent studies on the mini vibration of the skull to 500 Hz 1 2 have demonstrated that the canalar vestibular system does not appear to respond to less vigorous stimuli. At this point, it is, therefore, reasonable to consider VIN as a response of both labyrinths (canals) to low prolonged vibratory stimulation. This means that in a hypothetical normal model, the normal response (healthy subject) is “absence of nystagmus” in response to low frequency vibrations applied to, mastoids. In fact, stimulation of both labyrinths activates symmetrical responses of the vestibular receptors (mainly, semicircular canals, and, less, otolith organs).
Obviously, these predictions are a huge simplification. In fact, in an article 11, it was proposed that the eye movements induced by vibration can be explained by vestibular and proprioceptive stimulation and that vibration induces eye movements (horizontal nystagmus) in normal subjects, although they tend to be of small amplitude, with the slow phase of the horizontal component directed toward the stimulated ear.
Mastoid vibration appears to be a double stimulation (kinetic and sonic) and to activate both labyrinths when applied to the skull, producing CSF waves like an earthquake. It induces postural, proprioceptive and oculomotor reactions in humans. In this report, only the latter has been considered.
An example of the comparison of the components in the 2 groups (Fig. 4) induces a series of considerations that render the P-BCV a complementary test to other procedures and of fundamental importance in the study of the canalar system.
Fig. 4.

SPV values of horizontal, vertical and torsional components of VIN in 2 subjects with right SSCD, 8 subjects with right otosclerosis and 1 normal.
The predominantly horizontal component of nystagmus in otosclerosis patients draws our attention to the ampulla of the affected lateral semicircular canal and the adjacent utricle that becomes more sensitive. The cupula in the semicircular canal ampulla functions as a diaphragm separating the semicircular canals from the open space of the vestibule or utricle. It is unclear how this separation is achieved, as the cupula is not firmly connected to the ampulla wall but adheres to it by a mechanism that remains to be fully elucidated 22.
In response to a vibratory stimulus, applied to the affected right side of a patient with otosclerosis and CHL, we observed horizontal, leftward, slow-phase nystagmus (and rightward, slow-phase nystagmus in the case of an affected left ear) consistent with excitatory stimulation and ampullipetal flow in the lateral semicircular canal of the affected vestibular end organ (Fig. 1A, B).
This observation suggests a pattern of response arising from impaired vestibular receptors, as shown in Figure 1. Vibration produces a valid response: nystagmus in the plane of the lateral semicircular canal axis triggered by excitatory stimulation (ampullipetal deflection - “on” direction). Despite experimental results demonstrating that this nystagmus arises from the lateral semicircular canal, recent reports 1 2 suggest that the utricle can also generate nystagmus and vertigo.
In fact, data in the literature, on the Tullio phenomenon, suggest that these signs may arise from the utricle. The anatomical explanation is the close proximity of the stapes footplate to the utricle. However, eye movements originating from the utricular macula are torsional, not horizontal. In these cases, hypomobility of the stapes is the probable reason for hypersensitivity of the lateral semicircular canal. Endolymph circulation can induce an intense nystagmic reaction with the fast component beating towards the affected ear. In otosclerosis patients, VIN can, in our opinion, lead to a combined, high intensity response resulting from ampullipetal flow and excitation of the lateral semicircular canal.
Vibratory stimulation of the mastoid bone induced nystagmus in patients with SSCD, due inter alia to the abnormally low inner ear impedance, typical nystagmus of SSCD, with vertical and torsional components, even in the absence of a vestibular deficit.
In other words, SSCD patients could represent a different mechanical model with a response pattern depending on the underlying anatomical alterations.
This could also explain the recent report of horizontal VIN towards the affected side in patients with Ménière’s disease 25, a condition that could be related to an increase in inner ear impedance due to endolymphatic hydrops. In our study, however, some patients with SSCD showed VIN not aligned with excitatory stimulation (ampullofugal deflection - “on” direction) of the affected canal but with inhibitory stimulation (ampullopetal deflection - “off” direction) of dehiscent canal.
Both on and off direction responses were found by Ewald, in 1892 26, when he surgically fenestrated canals in animals and then applied positive and negative stimuli at the site of the opening.
There is another possible explanation for these conclusions regarding ampullopetal deflection. In accordance with Cremer et al. 2000 27, we agree with the postulate that a large region of bony roof dehiscence allows the overlying dura of the temporal lobe to compress the membranous superior canal impeding the flow of endolymph towards the utricle with ampullofugal deflection.
At this point, the lack of correspondence between the plane of the affected canal and that of the eye movements evoked does not exclude the possibility that, besides the ampulla, other vestibular receptors, i.e., utricular otolith macula, are excited by these stimuli 5–22. The main reason for implicating the utricle is the presence of a sustained torsional eye movement, or rotation about the line of sight, for the duration of the vibration. The utricle, in fact, produces sustained ocular torsional position and eye movement as part of the ocular tilt reaction whereas the semicircular canals induce nystagmus. In our opinion, the novelty of this proposed test, modifying previous procedures 7 8–11, is that VIN can result in a high intensity response of the affected canal and/or of the otolith organs (utricular macula) if the canal is impaired. In patients with third mobile window, the vibratory stimulus is probably easier to relate to the otolith organs (utricle) at higher frequency 1 2. Indeed, Suzuky et al., in 1969 29, reported that the response to isolated stimulation of the utricular nerve was predominantly rotatory in both eyes, with the upper portion of the eyeball rotating away from the side of the stimulation. In conjunction with this observation, there were two other induced movements: i) small vertical movements, upbeating in the ipsilateral eye and downbeating in the contralateral eye; ii) very small horizontal movements of the contralateral eye. In such cases, disruption of the bony labyrinth with concomitant development of a third mobile window in the vestibule has been postulated as a mechanism 30 31.
Conclusions
Although the pathogenesis of VIN has not been exhaustively studied, the constant recording of nystagmus in patients with Superior Semicircular Canal Dehiscence & Otosclerosis with CHL seems to be of some significance. The Bone (Mastoid) VIN test is easy to perform, economical, not costly and well tolerated by patients, furthermore, our preliminary results suggest that it could be a promising test when superior semicircular canal dehiscence and otosclerosis with CHL are suspected 30 31.
Comparing the results and the constant recording of high vertical and torsional (CW or CCW) component responses in the series of patients with SSCD and horizontal component responses in the series of patients with otosclerosis appears to be helpful for the clinician. These findings combined with a medical history can suggest a diagnosis of alteration of the bony capsule.
The bone VIN test, as already pointed out, is easy to perform, inexpensive and well tolerated by patients. Although further studies are needed, our preliminary results suggest that when SSCD or otosclerosis with CHL is suspected, especially when it is impossible to perform air cervical VEMPs (as in patients with a middle ear disorder or substantial conductive hearing loss), VIN could also be a useful test, therefore, in order to reveal a different modality of behaviour of the vestibular system in these two conditions.
References
- 1.Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colebatch JG, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology 2007;68:1227-9. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, MacDougall HG, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 2008;119:2135-47. [DOI] [PubMed] [Google Scholar]
- 3.Lücke K. Eine Method zur Provokation eines pathologischen Nystagmus durch Vibration-reize von 100 Hz. Z Laryngol Rhinol 1973;52:716-20. [PubMed] [Google Scholar]
- 4.Robinson DA, Zee D, Hain TC, Holmes A, Rosenberg LF. Alexander’s law: its behaviour and origin in the human vestibule-ocular reflex. Ann Neurol 1984;16:714-22. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Jl, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation Acta Otolaryngol 1969;68:350-62. [DOI] [PubMed] [Google Scholar]
- 6.Guild SR. Histologic otosclerosis.Ann Otol Rhinol Laryngol 1944;53:246-7. [Google Scholar]
- 7.Dumas G, Lavieille JP, Schmerber S. Vibratory test and head shaking test and caloric test: a series of 87 patients. Ann Otolaryngol Chir Cervicofac 2004;121:22-32. [DOI] [PubMed] [Google Scholar]
- 8.Hamann KF, Schuster EM. Vibration induced nystagmus - a sign of unilateral vestibular deficit. J Otorhinolaryngol Relat Spec 1999;61:74-9. [DOI] [PubMed] [Google Scholar]
- 9.Lackner JR. Elicitation of vestibular side effects by regional vibration of head. Aerospace Med 1974;45:1267-72. [DOI] [PubMed] [Google Scholar]
- 10.Karlberg M, Aw ST, Black RA, Todd MJ, MacDougall HG, Halmagyi GM. Vibration-induced ocular torsion and nystagmus after unilateral vestibular deafferentation. Brain 2003;126:956-64. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Shin J, Shim D. Mechanisms of vibration-induced nystagmus in normal subjects and patients with vestibular neuritis. Audiol Neurootol 2007;12:189-97. [DOI] [PubMed] [Google Scholar]
- 12.Minor LB, Haslwanter T, Straumann D, Zee DS. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology 1999;53:2158-68. [DOI] [PubMed] [Google Scholar]
- 13.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope 2005;115:1717-27. [DOI] [PubMed] [Google Scholar]
- 14.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg 1998;124:249-58. [DOI] [PubMed] [Google Scholar]
- 15.Halmagyi GM, Curthoys IS, Colebatch JG, Aw ST. Vestibular responses to sound. Ann NY Acad Sci 2005;1039:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Brantberg K, Bergenius J, Tribukait A. Vestibular-evoked myogenic potentials in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol 1999;119:633-40. [DOI] [PubMed] [Google Scholar]
- 17.Politzer A. Diseases of the Ear. Philadelphia, PA: Lea Brothers & Co. 1903. [Google Scholar]
- 18.Declau F, Van Spaendonck M, Timmermans JP, Michaels L, Liang J, Qiu JP, et al. Prevalence of histologic otosclerosis: an unbiased temporal bone study in Caucasian. Adv Otorhinolaryngol 2007;65:6-16. [DOI] [PubMed] [Google Scholar]
- 19.Christensen-Dalsgaard J, Narins PM. Sound and vibration sensitivity of VIIIth nerve fibers in the frogs Leptodactylus albilabris and Rana pipiens pipiens. J Comp Physiol 1993;172:653-62. [DOI] [PubMed] [Google Scholar]
- 20.Hulk J, Jongkees LBW. Vestibular examination in cases of otosclerosis. J Laryngol Otol Rhinol 1950;64:1-26. [Google Scholar]
- 21.Rasmussen H. Vestibular functions prior to and following operation for otosclerosis. Arch Otolaryngol 1949;49:402-13. [DOI] [PubMed] [Google Scholar]
- 22.Wit HP, Bleeker JD, Mulder HH. Responses of pigeons vestibular nerve fibers to sound and vibration with audiofrequencies. J Acoust Soc Am 1984;75:202-8. [DOI] [PubMed] [Google Scholar]
- 23.Young ED, Fernandez C. Responses of squirrel monkey vestibular neurons to audio frequency sound and head vibration. Acta Otolaryngol 1977;84:352-60. [DOI] [PubMed] [Google Scholar]
- 24.Hillmann DE, McLaren JW. Displacement configuration of semicircular canal cupulae. Neuroscience 1979;4:1989-2000. [DOI] [PubMed] [Google Scholar]
- 25.Ohki M, Murofushi T, Nakahara H, Sugasawa K. Vibration-induced nystagmus in patients with vestibular disorders. Otolaryngol Head Neck Surg 2003;129:255-8. [DOI] [PubMed] [Google Scholar]
- 26.Ewald JR. Physiologische Untersuchungen über das Endorgan des Nervus octavus. Wiesbaden, Germany: Bergmann 1892. [Google Scholar]
- 27.Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology 2000;55:1833-41. [DOI] [PubMed] [Google Scholar]
- 28.Belden CJ, Weg N, Minor LB, Zinreich SJ. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology 2003;226:337-43. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol 1969;68:350-62. [DOI] [PubMed] [Google Scholar]
- 30.Manzari L, Modugno GC, Brandolini C, Pirodda A. Bone vibration-induced nystagmus is useful in diagnosing superior semicircular canal dehiscence. Audiol Neurootol 2008;13:379-87. [DOI] [PubMed] [Google Scholar]
- 31.Manzari L, Modugno GC. Nystagmus induced by bone (mastoid) vibration in otosclerosis: a new perspective in the study of vestibular function in otosclerosis. Med Sci Monit 2008;14:CR505-10. [PubMed] [Google Scholar]