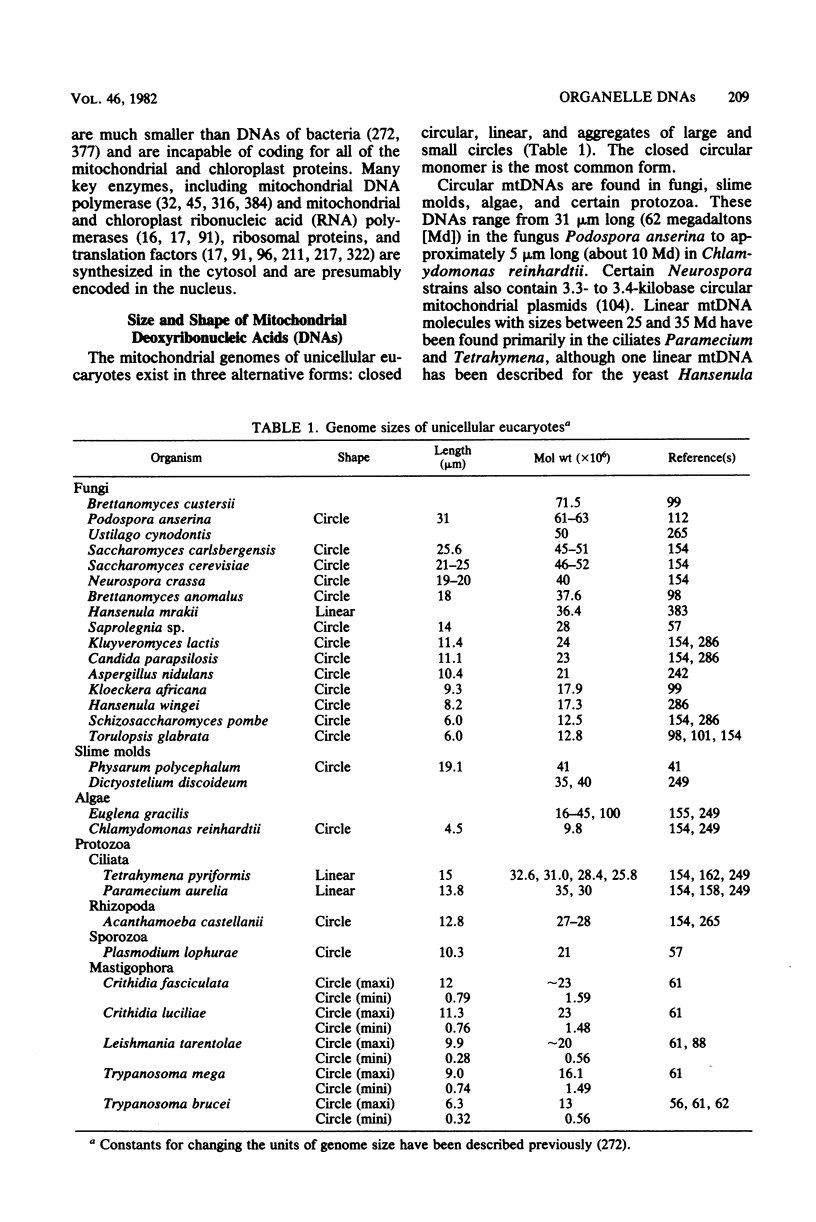
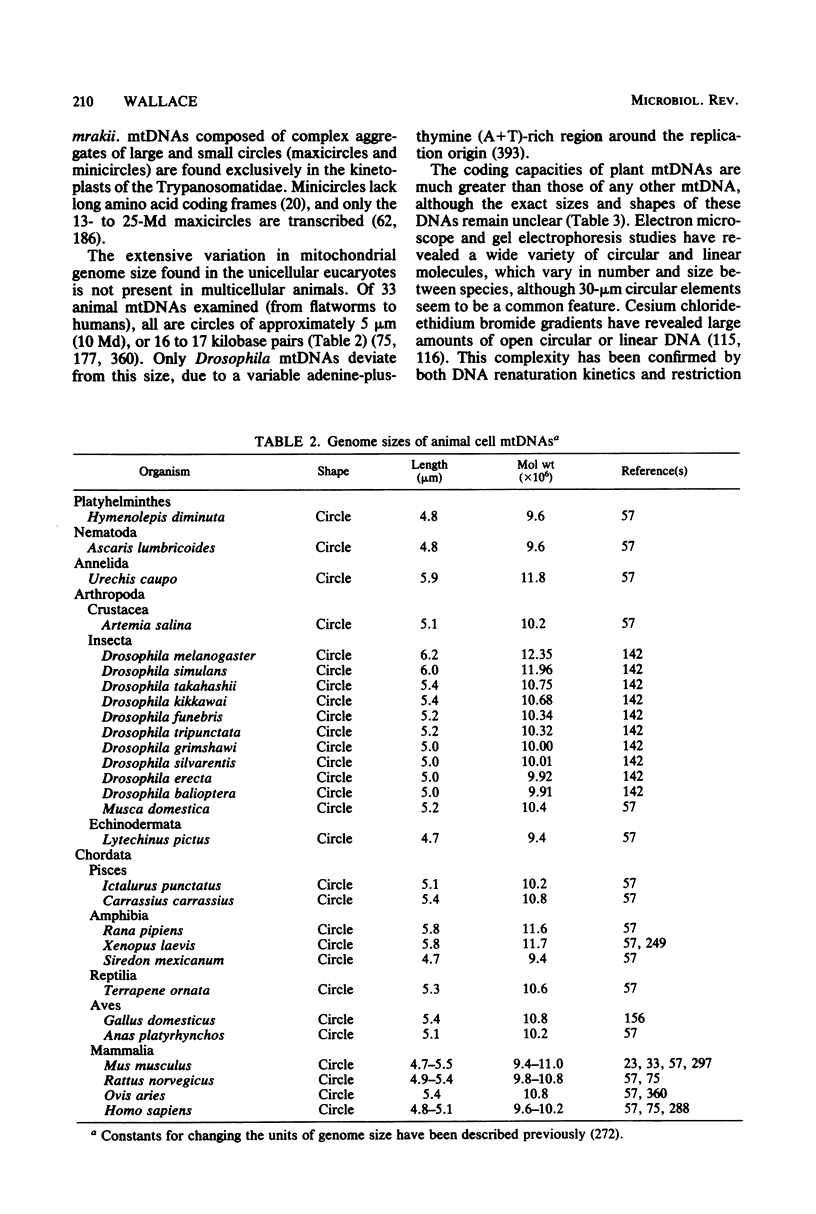
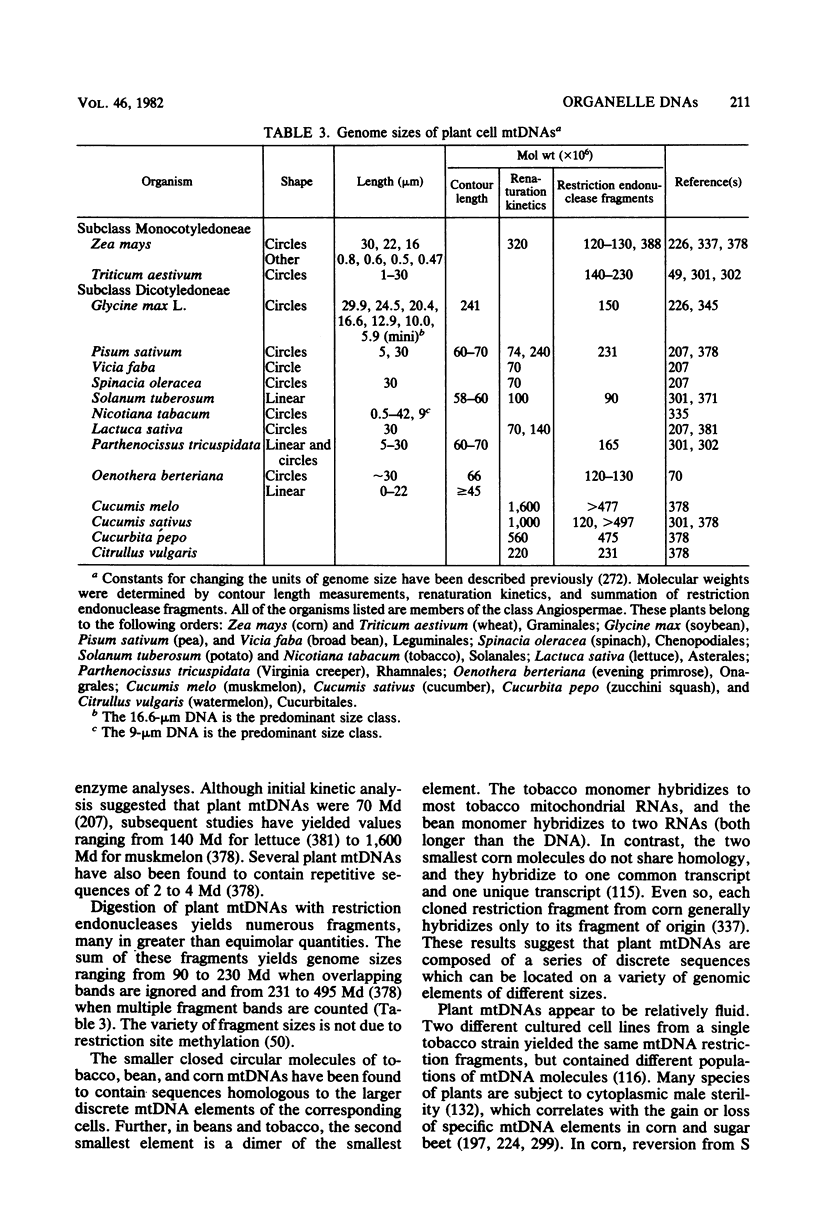
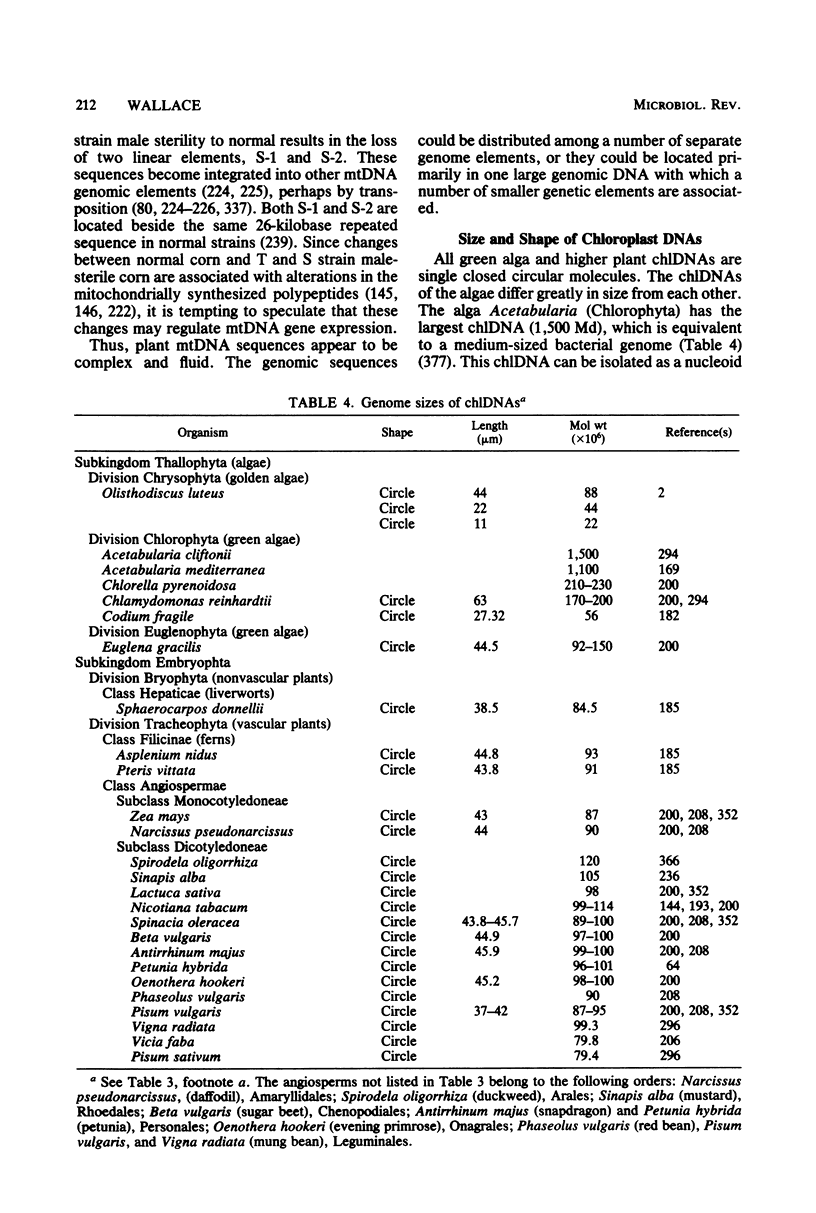
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Rochaix J. D. Structure analysis at the ends of the intervening DNA sequences in the chloroplast 23S ribosomal genes of C. reinhardii. Cell. 1979 Sep;18(1):55–60. doi: 10.1016/0092-8674(79)90353-2. [DOI] [PubMed] [Google Scholar]
- Amalric F., Merkel C., Gelfand R., Attardi G. Fractionation of mitochondrial RNA from HeLa cells by high-resolution electrophoresis under strongly denaturing conditions. J Mol Biol. 1978 Jan 5;118(1):1–25. doi: 10.1016/0022-2836(78)90241-3. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Baer R. J., Dubin D. T. Methylated regions of hamster mitochondrial ribosomal RNA: structural and functional correlates. Nucleic Acids Res. 1981 Jan 24;9(2):323–337. doi: 10.1093/nar/9.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle F. E., Brownlee G. G. AUG is the only recognisable signal sequence in the 5' non-coding regions of eukaryotic mRNA. Nature. 1978 Jul 6;274(5666):84–87. doi: 10.1038/274084a0. [DOI] [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Cooperation of mitochondrial and nuclear genes specifying the mitochondrial genetic apparatus in Neurospora crassa. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1371–1374. doi: 10.1073/pnas.69.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Induction of mitochondrial RNA polymerase in Neurospora crassa. Nat New Biol. 1972 Dec 13;240(102):195–197. doi: 10.1038/newbio240195a0. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of human mitochondrial DNA implicates transfer RNA excision as a major processing event. J Biol Chem. 1980 Dec 10;255(23):11599–11606. [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Bechmann H., Haid A., Schweyen R. J., Mathews S., Kaudewitz F. Expression of the "split gene" COB in yeast mtDNA. Translation of intervening sequences in mutant strains. J Biol Chem. 1981 Apr 10;256(7):3525–3531. [PubMed] [Google Scholar]
- Bedbrook J. R., Coen D. M., Beaton A. R., Bogorad L., Rich A. Location of the single gene for the large subunit of ribulosebisphosphate carboxylase on the maize chloroplast chromosome. J Biol Chem. 1979 Feb 10;254(3):905–910. [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: topology of circular daughter molecules and dynamics of catenated oligomer formation. J Mol Biol. 1976 Jan 5;100(1):85–92. doi: 10.1016/s0022-2836(76)80036-8. [DOI] [PubMed] [Google Scholar]
- Berlani R. E., Bonitz S. G., Coruzzi G., Nobrega M., Tzagoloff A. Transfer RNA genes in the cap-oxil region of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5017–5030. doi: 10.1093/nar/8.21.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Blanc H., Adams C. W., Wallace D. C. Different nucleotide changes in the large rRNA gene of the mitochondrial DNA confer chloramphenicol resistance on two human cell lines. Nucleic Acids Res. 1981 Nov 11;9(21):5785–5795. doi: 10.1093/nar/9.21.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L. Evolution of organelles and eukaryotic genomes. Science. 1975 May 30;188(4191):891–898. doi: 10.1126/science.1138359. [DOI] [PubMed] [Google Scholar]
- Bohnert H. J., Driesel A. J., Crouse E. J., Gordon K., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Presence of a transfer RNA gene in the spacer sequence between the 16 S and 23 S rRNA genes of spinach chloroplast DNA. FEBS Lett. 1979 Jul 1;103(1):52–56. doi: 10.1016/0014-5793(79)81248-x. [DOI] [PubMed] [Google Scholar]
- Bohnert H. J. Size and structure of mitochondrial DNA from Physarum polycephalum. Exp Cell Res. 1977 May;106(2):426–430. doi: 10.1016/0014-4827(77)90195-1. [DOI] [PubMed] [Google Scholar]
- Bolden A., Noy G. P., Weissbach A. DNA polymerase of mitochondria is a gamma-polymerase. J Biol Chem. 1977 May 25;252(10):3351–3356. [PubMed] [Google Scholar]
- Bonen L., Cunningham R. S., Gray M. W., Doolittle W. F. Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 1977 Mar;4(3):663–671. doi: 10.1093/nar/4.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. On the prokaryotic nature of red algal chloroplasts. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2310–2314. doi: 10.1073/pnas.72.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Partial sequences of 16S rRNA and the phylogeny of blue-green algae and chloroplasts. Nature. 1976 Jun 24;261(5562):669–673. doi: 10.1038/261669a0. [DOI] [PubMed] [Google Scholar]
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Huh T. Y., Gray M. W. Can partial methylation explain the complex fragment patterns observed when plant mitochondrial DNA is cleaved with restriction endonucleases? FEBS Lett. 1980 Mar 10;111(2):340–346. doi: 10.1016/0014-5793(80)80823-4. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Physical map of the Oxi3 locus of yeast mitochondrial DNA. J Biol Chem. 1980 Dec 25;255(24):11922–11926. [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Borbely G., Simoncsits A. 3'-Terminal conserved loops of 16S rRNAs from the cyanobacterium Synechococcus AN PCC 6301 and maize chloroplast differ only in two bases. Biochem Biophys Res Commun. 1981 Aug 14;101(3):846–852. doi: 10.1016/0006-291x(81)91827-1. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F. The maxi-circle of Trypanosoma brucei kinetoplast DNA. Biochim Biophys Acta. 1979 Nov 22;565(1):1–12. doi: 10.1016/0005-2787(79)90078-9. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. One gene's intron is another gene's exon. Nature. 1981 Feb 5;289(5797):439–440. doi: 10.1038/289439a0. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. Small is beautiful--portrait of a mitochondrial genome. Nature. 1981 Apr 9;290(5806):443–444. doi: 10.1038/290443a0. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Hecht N. B., Tabak H. F., Van Ommen G. J., Borst P. Splice point sequence and transcripts of the intervening sequence in the mitochondrial 21S ribosomal RNA gene of yeast. Cell. 1980 May;20(1):207–214. doi: 10.1016/0092-8674(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Bovenberg W. A., Kool A. J., Nijkamp H. J. Isolation, characterization and restriction endonuclease mapping of the Petunia hybrida chloroplast DNA. Nucleic Acids Res. 1981 Feb 11;9(3):503–517. doi: 10.1093/nar/9.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega A., Baglioni C. A study of mitochondrial protein synthesis in intact HeLa cells. Eur J Biochem. 1971 Oct 14;22(3):415–422. doi: 10.1111/j.1432-1033.1971.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Breitenberger C. A., Graves M. C., Spremulli L. L. Evidence for the nuclear location of the gene for chloroplast elongation factor G. Arch Biochem Biophys. 1979 Apr 15;194(1):265–270. doi: 10.1016/0003-9861(79)90617-9. [DOI] [PubMed] [Google Scholar]
- Breitenberger C. A., Spremulli L. L. Purification of Euglena gracilis chloroplast elongation factor G and comparison with other prokaryotic and eukaryotic translocases. J Biol Chem. 1980 Oct 25;255(20):9814–9820. [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calagan J. L., Pirtle R. M., Pirtle I. L., Kashdan M. A., Vreman H. J., Dudock B. S. Homology between chloroplast and prokaryotic initiator tRNA. Nucleotide sequence of spinach chloroplast methionine initiator tRNA. J Biol Chem. 1980 Oct 25;255(20):9981–9984. [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Canaday J., Dirheimer G., Martin R. P. Yeast mitochondrial methionine initiator tRNA: characterization and nucleotide sequence. Nucleic Acids Res. 1980 Apr 11;8(7):1445–1457. doi: 10.1093/nar/8.7.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Gloeckler R., Weil J. H. The nucleotide sequence of spinach chloroplast tryptophan transfer RNA. Nucleic Acids Res. 1981 Jan 10;9(1):47–53. doi: 10.1093/nar/9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Weil J. H. The nucleotide sequences of the initiator transfer RNAs from bean cytoplasm and chloroplasts. Nucleic Acids Res. 1980 Mar 11;8(5):999–1008. doi: 10.1093/nar/8.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-tsmith T. The origin of nuclei and of eukaryotic cells. Nature. 1975 Aug 7;256(5517):463–468. doi: 10.1038/256463a0. [DOI] [PubMed] [Google Scholar]
- Cedergren R. J., LaRue B., Sankoff D., Lapalme G., Grosjean H. Convergence and minimal mutation criteria for evaluating early events in tRNA evolution. Proc Natl Acad Sci U S A. 1980 May;77(5):2791–2795. doi: 10.1073/pnas.77.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg S. S., Englund P. T. Heterogeneity of minicircles in kinetoplast DNA of Leishmania tarentolae. J Mol Biol. 1980 Apr 15;138(3):447–472. doi: 10.1016/s0022-2836(80)80012-x. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Hecker L. I., Brum C. K., Schnabel J. J., Heckman J. E., Silberklang M., RajBhandary U. L., Barnett W. E. The nucleotide sequence of Euglena cytoplasmic phenylalanine transfer RNA. Evidence for possible classifications of Euglena among the animal rather than the plant kingdom. Nucleic Acids Res. 1981 Jul 10;9(13):3199–3204. doi: 10.1093/nar/9.13.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Hunkapiller M. W., Attardi G. Alignment of the amino terminal amino acid sequence of human cytochrome c oxidase subunits I and II with the sequence of their putative mRNAs. Nucleic Acids Res. 1981 Feb 25;9(4):867–877. doi: 10.1093/nar/9.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri O., Di Pasquale G., Tiboni O. Chloroplast elongation factors are synthesized in the chloroplast. Eur J Biochem. 1979 Dec 17;102(2):331–335. doi: 10.1111/j.1432-1033.1979.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Ciferri O., Tiboni O. Elongation factors for chloroplast and mitochondrial protein synthesis in Chlorella vulgaris. Nat New Biol. 1973 Oct 17;245(146):209–211. doi: 10.1038/newbio245209a0. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Partial duplication of the large ribosomal RNA sequence in an inverted repeat in circular mitochondrial DNA from Kloeckera africana. Implications for mechanisms of the petite mutation. J Mol Biol. 1981 Apr 15;147(3):399–415. doi: 10.1016/0022-2836(81)90492-7. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Sriprakash K. S. Sequence rearrangements between mitochondrial DNAs of Torulopsis glabrata and Kloeckera africana identified by hybridization with six polypeptide encoding regions from Saccharomyces cerevisiae mitochondrial DNA. J Mol Biol. 1981 Sep 25;151(3):367–387. doi: 10.1016/0022-2836(81)90002-4. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. Are/were mitochondria and chloroplasts microorganisms? Am Sci. 1970 May-Jun;58(3):281–289. [PubMed] [Google Scholar]
- Coleman A. W., Heywood P. Structure of the chloroplast and its DNA in chloromonadophycean algae. J Cell Sci. 1981 Jun;49:401–409. doi: 10.1242/jcs.49.1.401. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Stohl L. L., Cole M. D., Lambowitz A. M. Characterization of a novel plasmid DNA found in mitochondria of N. crassa. Cell. 1981 May;24(2):443–452. doi: 10.1016/0092-8674(81)90335-4. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Costantino P., Attardi G. Identification of discrete electrophoretic components among the products of mitochondrial protein synthesis in HeLa cells. J Mol Biol. 1975 Aug 5;96(2):291–306. doi: 10.1016/0022-2836(75)90349-6. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Kelly J. M. Mature 23 SrRNA of prokaryotes appears homologous with the precursor of 25--28 rRNA of eukaryotes: comments on the evolution of 23--28 rRNA. FEBS Lett. 1981 Jul 20;130(1):1–6. doi: 10.1016/0014-5793(81)80652-7. [DOI] [PubMed] [Google Scholar]
- Crews S., Attardi G. The sequences of the small ribosomal RNA gene and the phenylalanine tRNA gene are joined end to end in human mitochondrial DNA. Cell. 1980 Mar;19(3):775–784. doi: 10.1016/s0092-8674(80)80053-5. [DOI] [PubMed] [Google Scholar]
- Crews S., Ojala D., Posakony J., Nishiguchi J., Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979 Jan 18;277(5693):192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. II. Properties of mutant DNA and multimeric circular DNA from senescent cultures. Mol Gen Genet. 1979 Mar 27;171(3):239–250. doi: 10.1007/BF00267578. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Maki R. A., Conlon P. J., Laping J. Anatomy of mitochondrial DNA from Paramecium aurelia. Mol Gen Genet. 1980;178(3):499–510. doi: 10.1007/BF00337854. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Duesing J. H., Keene D. Supercoiled mitochondrial DNAs from plant tissue culture cells. Nucleic Acids Res. 1981 Sep 25;9(18):4583–4593. doi: 10.1093/nar/9.18.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M. Sequence homology among different size classes of plant mtDNAs. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4453–4457. doi: 10.1073/pnas.78.7.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Schwartz R. M. Evidence on the origin of eukaryotic mitochondria from protein and nucleic acid sequences. Ann N Y Acad Sci. 1981;361:92–104. doi: 10.1111/j.1749-6632.1981.tb46513.x. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. Cytochrome c and the evolution of energy metabolism. Sci Am. 1980 Mar;242(3):137–153. [PubMed] [Google Scholar]
- Doolittle W. F., Bonen L. Molecular sequence data indicating an endosymbiotic origin for plastids. Ann N Y Acad Sci. 1981;361:248–259. doi: 10.1111/j.1749-6632.1981.tb46522.x. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Edwards K., Kössel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981 Jun 25;9(12):2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Eigen M., Winkler-Oswatitsch R. Transfer-RNA: the early adaptor. Naturwissenschaften. 1981 May;68(5):217–228. doi: 10.1007/BF01047323. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Erion J. L., Tarnowski J., Weissbach H., Brot N. Cloning, mapping, and in vitro transcription-translation of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from spinach chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3459–3463. doi: 10.1073/pnas.78.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. Mitochondria and chloroplasts as descendants of prokaryotes. Biochem Genet. 1972 Jun;6(4):275–291. doi: 10.1007/BF00486121. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Leaver C. J. Nuclear and cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci U S A. 1980 Jan;77(1):418–422. doi: 10.1073/pnas.77.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Magrum L. J., Balch W. E., Wolfe R. S., Woese C. R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fox L., Erion J., Tarnowski J., Spremulli L., Brot N., Weissbach H. Euglena gracilis chloroplast EF-Ts. Evidence that it is a nuclear-coded gene product. J Biol Chem. 1980 Jul 10;255(13):6018–6019. [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Gatenby A. A., Castleton J. A., Saul M. W. Expression in E. coli of maize and wheat chloroplast genes for large subunit of ribulose bisphosphate carboxylase. Nature. 1981 May 14;291(5811):117–121. doi: 10.1038/291117a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E. Evolution of organelle genomes and protein-synthesizing systems. Ann N Y Acad Sci. 1981;361:20–43. doi: 10.1111/j.1749-6632.1981.tb46509.x. [DOI] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Mitochondrial DNA replication in Paramecium aurelia. Cross-linking of the initiation end. J Mol Biol. 1977 Jan 15;109(2):327–344. doi: 10.1016/s0022-2836(77)80037-5. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from the genus Drosophila. Nucleic Acids Res. 1980 Feb 25;8(4):741–757. [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Bollen-de Boer J. E., van Bruggen E. F., Borst P. Conservation of the sequence and position of the ribosomal RNA genes in Tetrahymena pyriformis mitochondrial DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):187–197. doi: 10.1016/0005-2787(78)90261-7. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Bollen-de Boer J. E., van Bruggen E. F., Borst P. Replication of the linear mitochondrial DNA of Tetrahymena pyriformis. Biochim Biophys Acta. 1979 May 24;562(3):400–417. doi: 10.1016/0005-2787(79)90104-7. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Graf L., Kössel H., Stutz E. Sequencing of 16S--23S spacer in a ribosomal RNA operon of Euglena gracilis chloroplast DNA reveals two tRNA genes. Nature. 1980 Aug 28;286(5776):908–910. doi: 10.1038/286908a0. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Spencer D. F. Is wheat mitochondrial 5S ribosomal RNA prokaryotic in nature? Nucleic Acids Res. 1981 Jul 24;9(14):3523–3529. doi: 10.1093/nar/9.14.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Hallick R. B. Physical mapping of the Euglena gracilis chloroplast DNA and ribosomal RNA gene region. Biochemistry. 1978 Jan 24;17(2):284–289. doi: 10.1021/bi00595a015. [DOI] [PubMed] [Google Scholar]
- Green B. R. Covalently closed minicircular DNA associated with Acetabularia chloroplasts. Biochim Biophys Acta. 1976 Oct 4;447(2):156–166. doi: 10.1016/0005-2787(76)90339-7. [DOI] [PubMed] [Google Scholar]
- Green B. R. Protein synthesis by isolated Acetabularia chloroplasts. In vitro synthesis of the apoprotein of the P-700-chlorophyll alpha-protein complex (CP i). Biochim Biophys Acta. 1980 Aug 26;609(1):107–120. doi: 10.1016/0005-2787(80)90205-1. [DOI] [PubMed] [Google Scholar]
- Green M. R., Grimm M. F., Goewert R. R., Collins R. A., Cole M. D., Lambowitz A. M., Heckman J. E., Yin S., RajBhandary U. L. Transcripts and processing patterns for the ribosomal RNA and transfer RNA region of Neurospora crassa mitochondrial DNA. J Biol Chem. 1981 Feb 25;256(4):2027–2034. [PubMed] [Google Scholar]
- Grohmann K., Amairic F., Crews S., Attardi G. Failure to detect "cap" structures in mitochondrial DNA-coded poly(A)-containing RNA from HeLa cells. Nucleic Acids Res. 1978 Mar;5(3):637–651. doi: 10.1093/nar/5.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. J., Haselkorn R. Counting the genes for stabel RNA in the nucleus and chloroplasts of Euglena. Biochim Biophys Acta. 1976 Sep 20;447(1):82–95. doi: 10.1016/0005-2787(76)90098-8. [DOI] [PubMed] [Google Scholar]
- Hahn U., Lazarus C. M., Lünsdorf H., Küntzel H. Split gene for mitochondrial 24S ribosomal RNA of Neurospora crassa. Cell. 1979 May;17(1):191–200. doi: 10.1016/0092-8674(79)90307-6. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Ching E., Attardi G. Isolation, subunit composition, and site of synthesis of human cytochrome c oxidase. Biochemistry. 1980 May 13;19(10):2023–2030. doi: 10.1021/bi00551a003. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Laipis P. J., Gilman M. E., O'Brien T. W., Michaels G. S., Rayfield M. A. Genetic mapping of bovine mitochondrial DNA from a single animal. Gene. 1980 Jan;8(2):193–209. doi: 10.1016/0378-1119(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Hecker L. I., Schwartzbach S. D., Barnett W. E., Baumstark B., RajBhandary U. L. Structure and function of initiator methionine tRNA from the mitochondria of Neurospora crassa. Cell. 1978 Jan;13(1):83–95. doi: 10.1016/0092-8674(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Yin S., Alzner-DeWeerd B., RajBhandary U. L. Mapping and cloning of Neurospora crassa mitochondrial transfer RNA genes. J Biol Chem. 1979 Dec 25;254(24):12694–12700. [PubMed] [Google Scholar]
- Hedberg M. F., Huang Y. S., Hommersand M. H. Size of the Chloroplast Genome in Codium fragile. Science. 1981 Jul 24;213(4506):445–447. doi: 10.1126/science.213.4506.445. [DOI] [PubMed] [Google Scholar]
- Heidemann S. R., Sander G., Kirschner M. W. Evidence for a functional role of RNA in centrioles. Cell. 1977 Mar;10(3):337–350. doi: 10.1016/0092-8674(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Grivell L. A., Borst P., Bos J. L. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. RNA from the insect trypanosome Crithidia luciliae contains transcripts of the maxi-circle and not of the mini-circle component of kinetoplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):407–411. doi: 10.1016/0005-2787(78)90282-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Jackl G., Sebald W. Identification of two products of mitochondrial protein synthesis associated with mitochondrial adenosine triphosphatase from Neurospora crassa. Eur J Biochem. 1975 May;54(1):97–106. doi: 10.1111/j.1432-1033.1975.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Jacq B. Sequence homologies between eukaryotic 5.8S rRNA and the 5' end of prokaryotic 23S rRNa: evidences for a common evolutionary origin. Nucleic Acids Res. 1981 Jun 25;9(12):2913–2932. doi: 10.1093/nar/9.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni B., Stutz E. Physical mapping of the ribosomal DNA region of Euglena gracilis chloroplast DNA. Eur J Biochem. 1978 Jul 17;88(1):127–134. doi: 10.1111/j.1432-1033.1978.tb12429.x. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975 Apr 10;254(5500):495–498. doi: 10.1038/254495a0. [DOI] [PubMed] [Google Scholar]
- Jurgenson J. E., Bourque D. P. Mapping of rRNA genes in an inverted repeat in Nicotiana tabacum chloroplast DNA. Nucleic Acids Res. 1980 Aug 25;8(16):3505–3516. doi: 10.1093/nar/8.16.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Kearsey S. E., Craig I. W. Altered ribosomal RNA genes in mitochondria from mammalian cells with chloramphenicol resistance. Nature. 1981 Apr 16;290(5807):607–608. doi: 10.1038/290607a0. [DOI] [PubMed] [Google Scholar]
- Keller M., Burkard G., Bohnert H. J., Mubumbila M., Gordon K., Steinmetz A., Heiser D., Crouse E. J., Weil J. H. Transfer RNA genes associated with the 16S and 23S rRNA genes of Euglena chloroplast DNA. Biochem Biophys Res Commun. 1980 Jul 16;95(1):47–54. doi: 10.1016/0006-291x(80)90702-0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Klink F. Archaebacterial elongation factor is ADP-ribosylated by diphtheria toxin. Nature. 1980 Sep 18;287(5779):250–251. doi: 10.1038/287250a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of the tRNAMMet from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Sep 11;9(17):4387–4390. doi: 10.1093/nar/9.17.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Knapp G., Ogden R. C., Peebles C. L., Abelson J. Splicing of yeast tRNA precursors: structure of the reaction intermediates. Cell. 1979 Sep;18(1):37–45. doi: 10.1016/0092-8674(79)90351-9. [DOI] [PubMed] [Google Scholar]
- Koch W., Edwards K., Kössel H. Sequencing of the 16S-23S spacer in a ribosomal RNA operon of Zea mays chloroplast DNA reveals two split tRNA genes. Cell. 1981 Jul;25(1):203–213. doi: 10.1016/0092-8674(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Inverted repeats in chloroplast DNA from higher plants. Proc Natl Acad Sci U S A. 1979 Jan;76(1):41–45. doi: 10.1073/pnas.76.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Pepe G., Bakker H., Holtrop M., Bollen J. E., Van Bruggen E. F., Cantatore P., Terpstra P., Saccone C. The restriction fragment map of rat-liver mitochondrial DNA: a reconsideration. Biochim Biophys Acta. 1977 Sep 20;478(2):128–145. doi: 10.1016/0005-2787(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Küntzel H., Heidrich M., Piechulla B. Phylogenetic tree derived from bacterial, cytosol and organelle 5S rRNA sequences. Nucleic Acids Res. 1981 Mar 25;9(6):1451–1461. doi: 10.1093/nar/9.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H. Proteins of mitochondrial and cytoplasmic ribosomes from Neurospora crassa. Nature. 1969 Apr 12;222(5189):142–146. doi: 10.1038/222142a0. [DOI] [PubMed] [Google Scholar]
- Lagerkvist U. Unorthodox codon reading and the evolution of the genetic code. Cell. 1981 Feb;23(2):305–306. doi: 10.1016/0092-8674(81)90124-0. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Chua N. H., Luck D. J. Mitochondrial ribosome assembly in Neurospora. Preparation of mitochondrial ribosomal precursor particles, site of synthesis of mitochondrial ribosomal proteins and studies on the poky mutant. J Mol Biol. 1976 Nov 5;107(3):223–253. doi: 10.1016/s0022-2836(76)80003-4. [DOI] [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Mitochondrial protein synthesis in mouse L-cells: effect of selective nicking of mitochondrial DNA. J Mol Biol. 1975 Dec 25;99(4):777–793. doi: 10.1016/s0022-2836(75)80184-7. [DOI] [PubMed] [Google Scholar]
- Lazarus C. M., Lünsdorf H., Hahn U., Stepień P. P., Küntzel H. Physical map of Aspergillus nidulans mitochondrial genes coding for ribosomal RNA: an intervening sequence in the large rRNA cistron. Mol Gen Genet. 1980 Feb;177(3):389–397. doi: 10.1007/BF00271477. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Kim B. D., Pring D. R., Conde M. F., Mans R. J., Laughnan J. R., Gabay-Laughnan S. J. Cytoplasmic Reversion of cms-S in Maize: Association with a Transpositional Event. Science. 1980 Aug 29;209(4460):1021–1023. doi: 10.1126/science.209.4460.1021. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochloron and the theory of symbiogenesis. Ann N Y Acad Sci. 1981;361:325–329. doi: 10.1111/j.1749-6632.1981.tb46528.x. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochlorophyta as a proposed new division of algae. Nature. 1976 Jun 24;261(5562):697–698. doi: 10.1038/261697b0. [DOI] [PubMed] [Google Scholar]
- Li M., Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979 Sep;18(1):47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- Link G., Bogorad L. Sizes, locations, and directions of transcription of two genes on a cloned maize chloroplast DNA sequence. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1832–1836. doi: 10.1073/pnas.77.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G. Cloning and mapping of the chloroplast DNA sequences for two messenger RNAs from mustard (Sinapis alba L.). Nucleic Acids Res. 1981 Aug 11;9(15):3681–3694. doi: 10.1093/nar/9.15.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Nagley P. Mitochondrial genetics in perspective: the derivation of a genetic and physical map of the yeast mitochondrial genome. Plasmid. 1978 Jun;1(3):324–345. doi: 10.1016/0147-619x(78)90049-5. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M., Luck D. J. The intracellular site of synthesis of mitochndrial ribosomal proteins in Neurospora crassa. J Cell Biol. 1972 Jul;54(1):56–74. doi: 10.1083/jcb.54.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Fox G. E., Kilpatrick M. W., Walker R. T., Domdey H., Krupp G., Gross H. J. The nucleotide sequence of the 5S rRNA from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Feb 25;9(4):965–970. doi: 10.1093/nar/9.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machatt M. A., Ebel J. P., Branlant C. The 3'-terminal region of bacterial 23S ribosomal RNA: structure and homology with the 3'-terminal region of eukaryotic 28S rRNA and with chloroplast 4.5s rRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1533–1549. doi: 10.1093/nar/9.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macino G., Scazzocchio C., Waring R. B., Berks M. M., Davies R. W. Conservation and rearrangement of mitochondrial structural gene sequences. Nature. 1980 Nov 27;288(5789):404–406. doi: 10.1038/288404a0. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system. The DNA sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. J Biol Chem. 1979 Jun 10;254(11):4617–4623. [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin W. B., Meyer D. J., Woodward D. O., Erickson S. K. Chloramphenicol-sensitive labelling of protein in microsomes of Neurospora crassa. Nature. 1977 Sep 29;269(5627):447–450. doi: 10.1038/269447a0. [DOI] [PubMed] [Google Scholar]
- Mahler H. R. Mitochondrial evolution: organization and regulation of mitochondrial genes. Ann N Y Acad Sci. 1981;361:53–75. [PubMed] [Google Scholar]
- Malnoë P., Rochaix J. D., Chua N. H., Spahr P. F. Characterization of the gene and messenger RNA of the large subunit of ribulose 1,5-diphosphate carboxylase in Chlamydomonas reinhardii. J Mol Biol. 1979 Sep 25;133(3):417–434. doi: 10.1016/0022-2836(79)90401-7. [DOI] [PubMed] [Google Scholar]
- Malnoë P., Rochaix J. D. Localization of 4S RNA genes on the chloroplast genome of Chlamydomonas reinhardii. Mol Gen Genet. 1978 Nov 9;166(3):269–275. doi: 10.1007/BF00267618. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Collins R. A., Green M. R., Lambowitz A. M. Defective splicing of mitochondrial rRNA in cytochrome-deficient nuclear mutants of Neurospora crassa. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2635–2639. doi: 10.1073/pnas.76.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis L. Evolutionary criteria in thallophytes: a radical alternative. Science. 1968 Sep 6;161(3845):1020–1022. doi: 10.1126/science.161.3845.1020. [DOI] [PubMed] [Google Scholar]
- Martens P. A., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: localization and sequence of the light-strand origin of replication. J Mol Biol. 1979 Dec 5;135(2):327–351. doi: 10.1016/0022-2836(79)90440-6. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Miller D., Hartley J., Moynihan P., Donelson J. E. The tRNAAGYSer and tRNACGYArg genes from a gene cluster in yeast mitochondrial DNA. Cell. 1980 Feb;19(2):339–343. doi: 10.1016/0092-8674(80)90508-5. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Pham H. D., Underbrink-Lyon K., Miller D. l., Donelson J. E. Yeast mitochondrial tRNATrp can recognize the nonsense codon UGA. Nature. 1980 Jun 19;285(5766):579–581. doi: 10.1038/285579a0. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Underbrink-Lyon K. A mitochondrial locus is necessary for the synthesis of mitochondrial tRNA in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4743–4747. doi: 10.1073/pnas.78.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Dirheimer G., de Henau S., Grosjean H. Yeast mitochondrial tRNATrp injected with E. coli activating enzyme into Xenopus oocytes suppresses UGA termination. Nature. 1981 Sep 17;293(5829):235–237. doi: 10.1038/293235a0. [DOI] [PubMed] [Google Scholar]
- Mazza A., Casale A., Sassone-Corsi P., Bonotto S. A minicircular component of Acetabularia acetabulum chloroplast DNA replicating by the rolling circle. Biochem Biophys Res Commun. 1980 Apr 14;93(3):668–674. doi: 10.1016/0006-291x(80)91130-4. [DOI] [PubMed] [Google Scholar]
- McCoy J. M., Jones D. S. The nucleotide sequence of Scenedesmus obliquus chloroplast tRNAfMet. Nucleic Acids Res. 1980 Nov 11;8(21):5089–5093. doi: 10.1093/nar/8.21.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea J. M., Hershberger C. L. Chloroplast DNA codes for transfer RNA. Nucleic Acids Res. 1976 Aug;3(8):2005–2018. doi: 10.1093/nar/3.8.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery-Drugeon E., Crouse E. J., Schmitt J. M., Bohnert H. J., Bernardi G. The mitochondrial genomes of Ustilago cynodontis and Acanthamoeba castellanii. Eur J Biochem. 1981 Mar;114(3):577–583. doi: 10.1111/j.1432-1033.1981.tb05183.x. [DOI] [PubMed] [Google Scholar]
- Meyer R. R. On the evolutionary origin of mitochondrial DNA. J Theor Biol. 1973 Mar;38(3):647–653. doi: 10.1016/0022-5193(73)90264-6. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C., Pham H. D., Donelson J. E. Sequence analysis of two yeast mitochondrial DNA fragments containing the genes for tRNA Ser UCR and tRNA Phe UUY. J Biol Chem. 1979 Nov 25;254(22):11735–11740. [PubMed] [Google Scholar]
- Miller D. L., Sigurdson C., Martin N. C., Donelson J. E. Nucleotide sequence of the mitochondrial genes coding for tRNAglyGGR and tRNAvalGUR. Nucleic Acids Res. 1980 Mar 25;8(6):1435–1442. doi: 10.1093/nar/8.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. M., Loeblich L. A., Klotz L. C., Loeblich A. R., 3rd DNA organization of Methanobacterium thermoautotrophicum. Science. 1979 Jun 8;204(4397):1082–1084. doi: 10.1126/science.377486. [DOI] [PubMed] [Google Scholar]
- Montoya J., Ojala D., Attardi G. Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs. Nature. 1981 Apr 9;290(5806):465–470. doi: 10.1038/290465a0. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Biochemistry of protozoan microbodies: peroxisomes, alpha-glycerophosphate oxidase bodies, hydrogenosomes. Annu Rev Microbiol. 1975;29:467–483. doi: 10.1146/annurev.mi.29.100175.002343. [DOI] [PubMed] [Google Scholar]
- Nazar R. N. A 5.8 S rRNA-like sequence in prokaryotic 23 S rRNA. FEBS Lett. 1980 Oct 6;119(2):212–214. doi: 10.1016/0014-5793(80)80254-7. [DOI] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Schatz G. Biosynthesis and assembly of the proton-translocating adenosine triphosphatase complex from chloroplasts. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1361–1364. doi: 10.1073/pnas.77.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D., Pham H. D., Underbrink-Lyon K., Martin N. C. Characterization of tRNA genes in tRNA region II of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5007–5016. doi: 10.1093/nar/8.21.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Complete restriction map of the cytochrome b region of mitochondrial DNA in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9821–9827. [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Nomiyama H., Sakaki Y., Takagi Y. Nucleotide sequence of a ribosomal RNA gene intron from slime mold Physarum polycephalum. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1376–1380. doi: 10.1073/pnas.78.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Ohi S., Ramirez J. L., Upholt W. B., Dawid I. B. Mapping of mitochondrial 4 S RNA genes in Xenopus laevis by electron microscopy. J Mol Biol. 1978 May 25;121(3):299–310. doi: 10.1016/0022-2836(78)90365-0. [DOI] [PubMed] [Google Scholar]
- Ojala D. K., Montoya J., Attardi G. The putative mRNA for subunit II of human cytochrome c oxidase starts directly at the translation initiator codon. Nature. 1980 Sep 4;287(5777):79–82. doi: 10.1038/287079a0. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. A detailed physical map of HeLa cell mitochondria DNA and its alignment with the positions of known genetic markers. Plasmid. 1977 Nov;1(1):78–105. doi: 10.1016/0147-619x(77)90010-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Gray P. W., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. I. The location of transfer RNA, 5 S, 16 S, and 23 S ribosomal RNA genes. J Biol Chem. 1980 Nov 25;255(22):10991–10996. [PubMed] [Google Scholar]
- Orozco E. M., Jr, Rushlow K. E., Dodd J. R., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. II. Nucleotide sequence homology between the 16 S--23 S ribosomal RNA spacer and the 16 S ribosomal RNA leader regions. J Biol Chem. 1980 Nov 25;255(22):10997–11003. [PubMed] [Google Scholar]
- Padmanabhan U., Green B. R. The kinetic complexity of Acetabularia chloroplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):67–73. doi: 10.1016/0005-2787(78)90249-6. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Rearrangements in the chloroplast genomes of mung bean and pea. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5533–5537. doi: 10.1073/pnas.78.9.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M. Restriction endonuclease cleavage maps of rat and mouse mitochondrial DNAs. Nucleic Acids Res. 1977;4(5):1291–1299. doi: 10.1093/nar/4.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Penman S. Mitochondrial protein synthesis: resistance to emetine and response to RNA synthesis inhibitors. Biochem Biophys Res Commun. 1970 Aug 24;40(4):941–948. doi: 10.1016/0006-291x(70)90994-0. [DOI] [PubMed] [Google Scholar]
- RIS H., PLAUT W. Ultrastructure of DNA-containing areas in the chloroplast of Chlamydomonas. J Cell Biol. 1962 Jun;13:383–391. doi: 10.1083/jcb.13.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Mahler H. R. The non symbiotic origin of mitochondria. Science. 1972 Aug 18;177(4049):575–582. doi: 10.1126/science.177.4049.575. [DOI] [PubMed] [Google Scholar]
- Ramirez J. L., Dawid I. B. Mapping of mitochondrial DNA in Xenopus laevis and X. borealis: the positions of ribosomal genes and D-loops. J Mol Biol. 1978 Feb 15;119(1):133–146. doi: 10.1016/0022-2836(78)90273-5. [DOI] [PubMed] [Google Scholar]
- Rastl E., Dawid I. B. Expression of the mitochondrial genome in Xenopus laevis: a map of transcripts. Cell. 1979 Oct;18(2):501–510. doi: 10.1016/0092-8674(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Raven P. H. A multiple origin for plastids and mitochondria. Science. 1970 Aug 14;169(3946):641–646. doi: 10.1126/science.169.3946.641. [DOI] [PubMed] [Google Scholar]
- Reijnders L. The origin of mitochondria. J Mol Evol. 1975 Aug 5;5(3):167–176. doi: 10.1007/BF01741239. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Saccone C., Cantatore P., Gadaleta G., Gallerani R., Lanave C., Pepe G., Kroon A. M. The nucleotide sequence of the large ribosomal RNA gene and the adjacent tRNA genes from rat mitochondria. Nucleic Acids Res. 1981 Aug 25;9(16):4139–4148. doi: 10.1093/nar/9.16.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Galli M. G., Levi M., Burroni D., Parisi B., Pedrali-Noy G., Spadari S. Functional roles of the plant alpha-like and gamma-like DNA polymerases. FEBS Lett. 1981 Feb 9;124(1):112–118. doi: 10.1016/0014-5793(81)80064-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sawada M., Osawa S., Kobayashi H., Hori H., Muto A. The number of ribosomal RNA genes in Mycoplasma capricolum. Mol Gen Genet. 1981;182(3):502–504. doi: 10.1007/BF00293942. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Schmitt H. Analysis and site of synthesis of ribosomal proteins from yeast mitochondria. FEBS Lett. 1972 Oct 1;26(1):215–220. doi: 10.1016/0014-5793(72)80576-3. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Chloroplast origins: inferences from protein and nucleic acid sequences. Ann N Y Acad Sci. 1981;361:260–272. doi: 10.1111/j.1749-6632.1981.tb46523.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Schwartzbach S. D., Hecker L. I., Barnett W. E. Transcriptional origin of Euglena chloroplast tRNAs. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1984–1988. doi: 10.1073/pnas.73.6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Z., Jolly S. O., Steinmetz A. A., Bogorad L. Overlapping divergent genes in the maize chloroplast chromosome and in vitro transcription of the gene for tRNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3423–3427. doi: 10.1073/pnas.78.6.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Z., Kössel H., Schwarz E., Bogorad L. A gene coding for tRNA is located near 5' terminus of 16S rRNA gene in Zea mays chloroplast genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4748–4752. doi: 10.1073/pnas.78.8.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy D. G., Stein D. B., Searcy K. B. A mycoplasma-like archaebacterium possibly related to the nucleus and cytoplasms of eukaryotic cells. Ann N Y Acad Sci. 1981;361:312–324. doi: 10.1111/j.1749-6632.1981.tb46527.x. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Kobayashi M., Seki T., Koike K. Nucleotide sequence of a cloned fragment of rat mitochondrial DNA containing the replication origin. Gene. 1980 Oct;11(1-2):53–62. doi: 10.1016/0378-1119(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. M., Kidane G., Livingston L., Spithill T. W. The kinetoplast DNA of the hemoflagellate protozoa. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1053–1063. doi: 10.4269/ajtmh.1980.29.1053. [DOI] [PubMed] [Google Scholar]
- Spremulli L. L., Breitenberger C. A., Graves M. C., Beck C. M., Steege D. A. Comparison of the Euglena chloroplast protein synthesizing system with those of prokaryotes and eukaryotes. Ann N Y Acad Sci. 1981;361:461–471. doi: 10.1111/j.1749-6632.1981.tb46538.x. [DOI] [PubMed] [Google Scholar]
- Sriprakash K. S., Clark-Walker G. D. The size of yeast mitochondrial ribosomal RNAs. Biochem Biophys Res Commun. 1980 Mar 13;93(1):186–193. doi: 10.1016/s0006-291x(80)80264-6. [DOI] [PubMed] [Google Scholar]
- Stephenson G., Marzuki S., Linnane A. W. Biogenesis of mitochondria. Two-dimensional electrophoretic analysis of mitochondrial translation products in yeast. Biochim Biophys Acta. 1980 Sep 19;609(2):329–341. doi: 10.1016/0005-2787(80)90245-2. [DOI] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., van der Laan J., Osinga K. A., Schouten J. P., van Boom J. H., Veeneman G. H. Use of a synthetic DNA oligonucleotide to probe the precision of RNA splicing in a yeast mitochondrial petite mutant. Nucleic Acids Res. 1981 Sep 25;9(18):4475–4483. doi: 10.1093/nar/9.18.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. Cloning and characterization of 4.5S and 5S RNA genes in tobacco chloroplasts. Gene. 1980 Jul;10(2):95–103. doi: 10.1016/0378-1119(80)90127-4. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., Clayton D. A. Mechanism of replication of human mitochondrial DNA. Localization of the 5' ends of nascent daughter strands. J Biol Chem. 1981 May 25;256(10):5109–5115. [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]
- Thorne S. W., Newcomb E. H., Osmond C. B. Identification of chlorophyll b in extracts of prokaryotic algae by fluorescence spectroscopy. Proc Natl Acad Sci U S A. 1977 Feb;74(2):575–578. doi: 10.1073/pnas.74.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Shinozaki K., Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5399–5406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G., Imam G., Küntzel H. Mitochondrial ATPase complex of Aspergillus nidulans and the dicyclohexylcarbodiimide-binding protein. Eur J Biochem. 1979 Jul;97(2):565–571. doi: 10.1111/j.1432-1033.1979.tb13145.x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Macino G., Sebald W. Mitochondrial genes and translation products. Annu Rev Biochem. 1979;48:419–441. doi: 10.1146/annurev.bi.48.070179.002223. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Uzzell T., Spolsky C. Mitochondria and plastids as endosymbionts: a revival of special creation? Am Sci. 1974 May-Jun;62(3):334–343. [PubMed] [Google Scholar]
- Uzzell T., Spolsky C. Two data sets: alternative explanations and interpretations. Ann N Y Acad Sci. 1981;361:481–504. doi: 10.1111/j.1749-6632.1981.tb46540.x. [DOI] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. EVIDENCE FOR A TRICARBOXYLIC ACID CYCLE IN MYCOPLASMA HOMINIS. J Bacteriol. 1964 Dec;88:1602–1607. doi: 10.1128/jb.88.6.1602-1607.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. RESPIRATORY PATHWAYS IN THE MYCOPLASMA. II. PATHWAY OF ELECTRON TRANSPORT DURING OXIDATION OF REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE BY MYCOPLASMA HOMINIS. J Bacteriol. 1964 Jul;88:122–129. doi: 10.1128/jb.88.1.122-129.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Van Valen L. M., Maiorana V. C. The archaebacteria and eukaryotic origins. Nature. 1980 Sep 18;287(5779):248–250. doi: 10.1038/287248a0. [DOI] [PubMed] [Google Scholar]
- Vedel F., Quetier F. Physico-chemical characterization of mitochondrial DNA from potato tubers. Biochim Biophys Acta. 1974 Apr 10;340(4):374–387. doi: 10.1016/0005-2787(74)90059-8. [DOI] [PubMed] [Google Scholar]
- Walker W. F. Proposed sequence homology between the 5'-end regions of prokaryotic 23 S rRNA and eukaryotic 28 S rRNA. Relevance to the hypothesis that 5.8 S rRNA is homologous to the 5'-end region of 23 S rRNA. FEBS Lett. 1981 Apr 20;126(2):150–151. doi: 10.1016/0014-5793(81)80228-1. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Assignment of the chloramphenicol resistance gene to mitochondrial deoxyribonucleic acid and analysis of its expression in cultured human cells. Mol Cell Biol. 1981 Aug;1(8):697–710. doi: 10.1128/mcb.1.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Morowitz H. J. Genome size and evolution. Chromosoma. 1973;40(2):121–126. doi: 10.1007/BF00321457. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Webster K. A., Oliver N. A., Wallace D. C. Assignment of an oligomycin-resistance locus to human chromosome 10. Somatic Cell Genet. 1982 Mar;8(2):223–244. doi: 10.1007/BF01538679. [DOI] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. Linear mitochondrial deoxyribonucleic acid from the yeast Hansenula mrakii. Mol Cell Biol. 1981 May;1(5):387–393. doi: 10.1128/mcb.1.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. The genetic map of transfer RNA genes of yeast mitochondria: correction and extension. Mol Gen Genet. 1979 Mar 5;170(3):261–275. doi: 10.1007/BF00267059. [DOI] [PubMed] [Google Scholar]
- Westergaard O., Marcker K. A., Keiding J. Induction of a mitochondrial DNA polymerase in Tetrahymena. Nature. 1970 Aug 15;227(5259):708–710. doi: 10.1038/227708a0. [DOI] [PubMed] [Google Scholar]
- Whatley F. R. The establishment of mitochondria: Paracoccus and Rhodopseudomonas. Ann N Y Acad Sci. 1981;361:330–340. doi: 10.1111/j.1749-6632.1981.tb46529.x. [DOI] [PubMed] [Google Scholar]
- Whatley J. M. Chloroplast evolution--ancient and modern. Ann N Y Acad Sci. 1981;361:154–165. doi: 10.1111/j.1749-6632.1981.tb46517.x. [DOI] [PubMed] [Google Scholar]
- Whatley J. M., John P., Whatley F. R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):165–187. doi: 10.1098/rspb.1979.0020. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Nazar R. N. Nucleotide sequence of wheat chloroplastid 4.5 S ribonucleic acid. Sequence homologies in 4.5 S RNA species. J Biol Chem. 1980 Dec 25;255(24):11896–11900. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Yatscoff R. W., Goldstein S., Freeman K. B. Conservation of genes coding for proteins synthesized in human mitochondria. Somatic Cell Genet. 1978 Nov;4(6):633–645. doi: 10.1007/BF01543155. [DOI] [PubMed] [Google Scholar]
- Young I. G., Anderson S. The genetic code in bovine mitochondria: sequence of genes for the cytochrome oxidase subunit II and two tRNAs. Gene. 1980 Dec;12(3-4):257–265. doi: 10.1016/0378-1119(80)90108-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Zablen L. B., Kissil M. S., Woese C. R., Buetow D. E. Phylogenetic origin of the chloroplast and prokaryotic nature of its ribosomal RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2418–2422. doi: 10.1073/pnas.72.6.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski R. E., Price C. A. Synthesis of thylakoid membrane proteins by chloroplasts isolated from spinach. Cytochrome b559 and P700-chlorophyll a-protein. J Cell Biol. 1980 May;85(2):435–445. doi: 10.1083/jcb.85.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Tu J., Holz I. Thermoproteales--a third order of thermoacidophilic archaebacteria. Nature. 1981 Sep 3;293(5827):85–86. doi: 10.1038/293085a0. [DOI] [PubMed] [Google Scholar]
- Zipkas D., Riley M. Proposal concerning mechanism of evolution of the genome of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1354–1358. doi: 10.1073/pnas.72.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Glotz C., Brimacombe R. Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species. Nucleic Acids Res. 1981 Aug 11;9(15):3621–3640. doi: 10.1093/nar/9.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong L., Holtrop M., Kroon A. M. The biogenesis of rat mitochondrial ATPase. Two subunits are synthesized inside the mitochondria. Biochim Biophys Acta. 1980 Jun 27;608(1):32–38. doi: 10.1016/0005-2787(80)90130-6. [DOI] [PubMed] [Google Scholar]
- de Jong L., Holtrop M., Kroon A. M. The biogenesis of rat-liver mitochondrial ATPase. Evidence that the N, N'-dicyclohexyl carbodiimide-binding protein is synthesized outside the mitochondria. Biochim Biophys Acta. 1980 Feb 29;606(2):331–337. doi: 10.1016/0005-2787(80)90042-8. [DOI] [PubMed] [Google Scholar]
- de Vries H., de Jonge J. C., Bakker H., Meurs H., Kroon A. Laboratory of Physiological Chemistry, State University Groningen, Netherlands. Nucleic Acids Res. 1979;6(5):1791–1803. doi: 10.1093/nar/6.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]
- van Ee J. H., Vos Y. J., Planta R. J. Physical map of chloroplast DNA of Spirodela oligorrhiza; analysis by the restriction endonucleases PstI, xhoI, SacI. Gene. 1980 Dec;12(3-4):191–200. doi: 10.1016/0378-1119(80)90101-8. [DOI] [PubMed] [Google Scholar]


