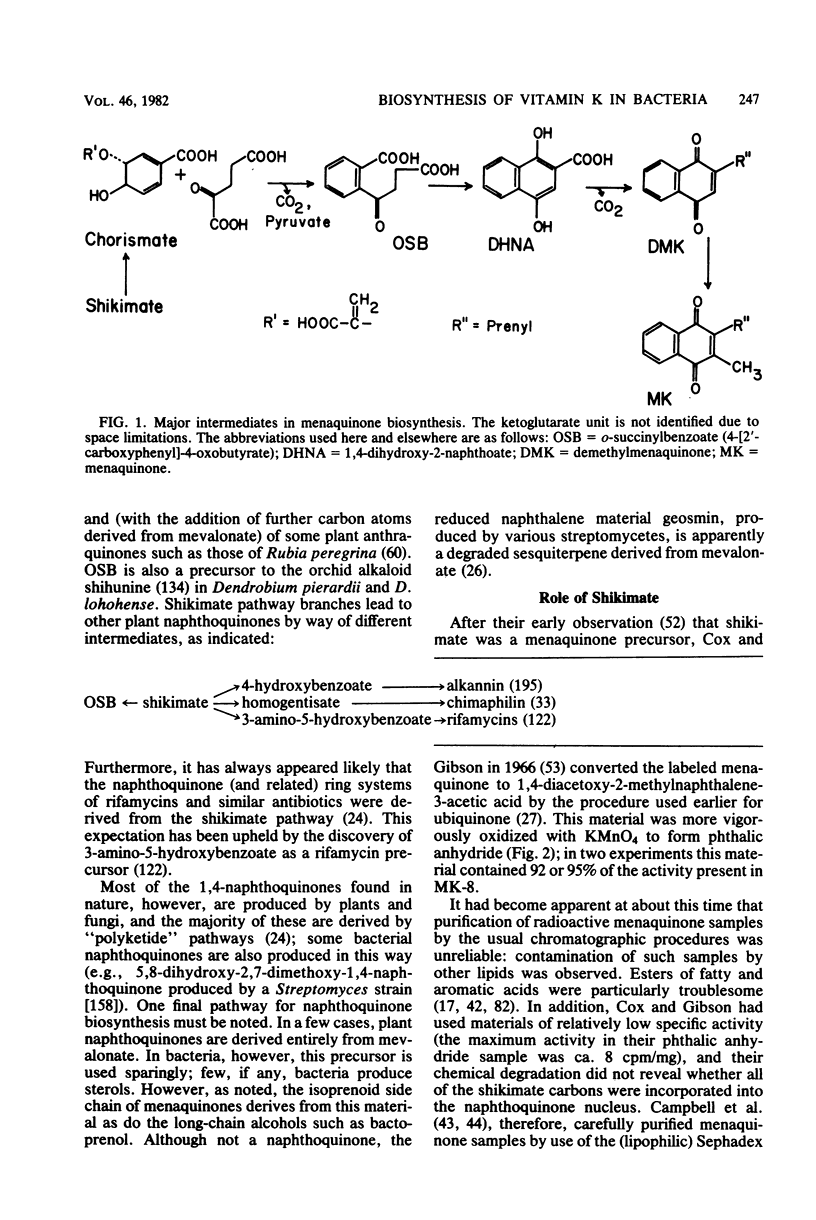
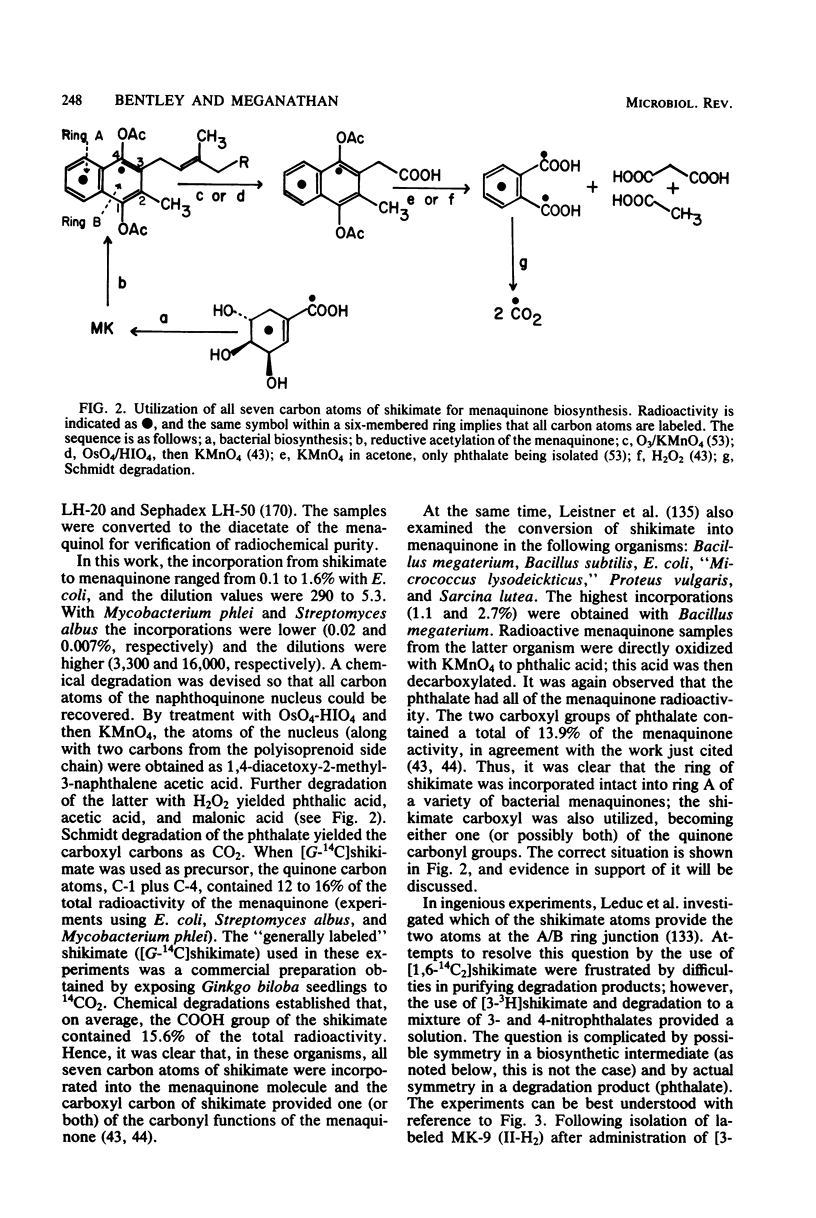
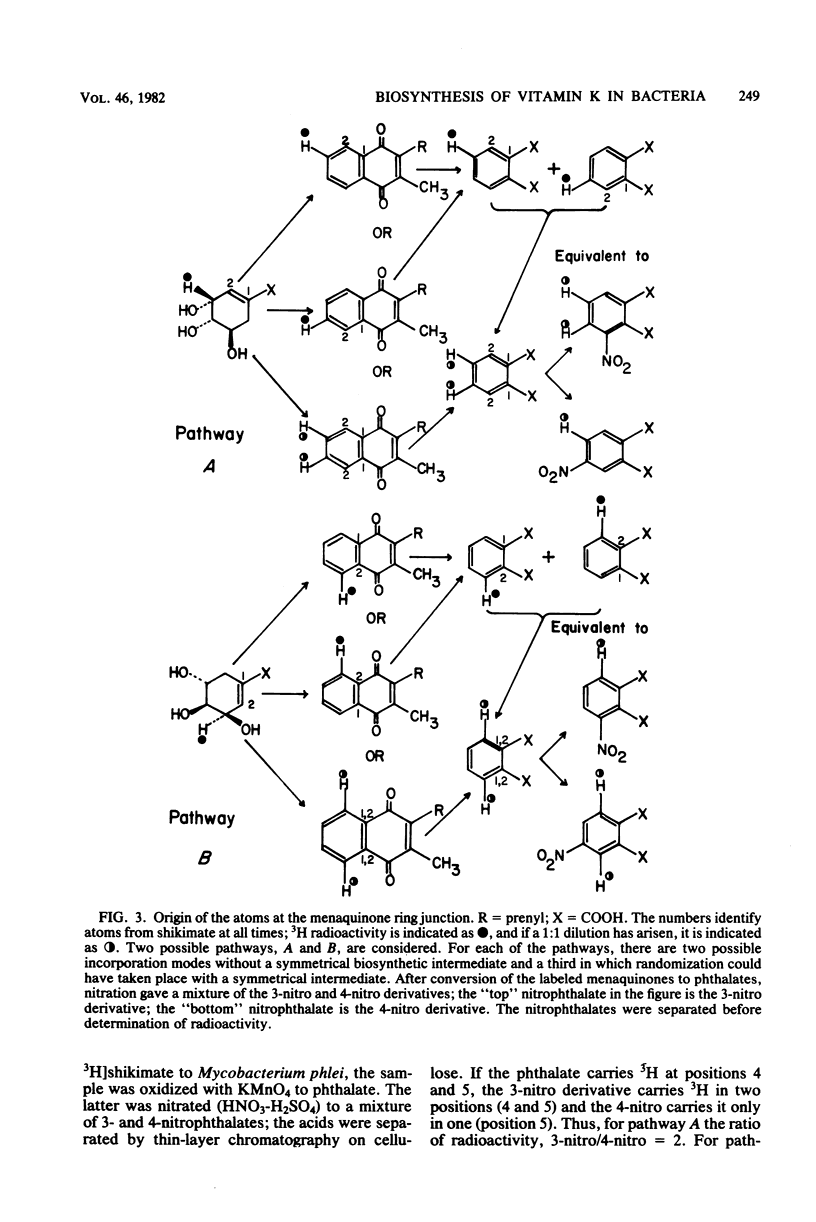
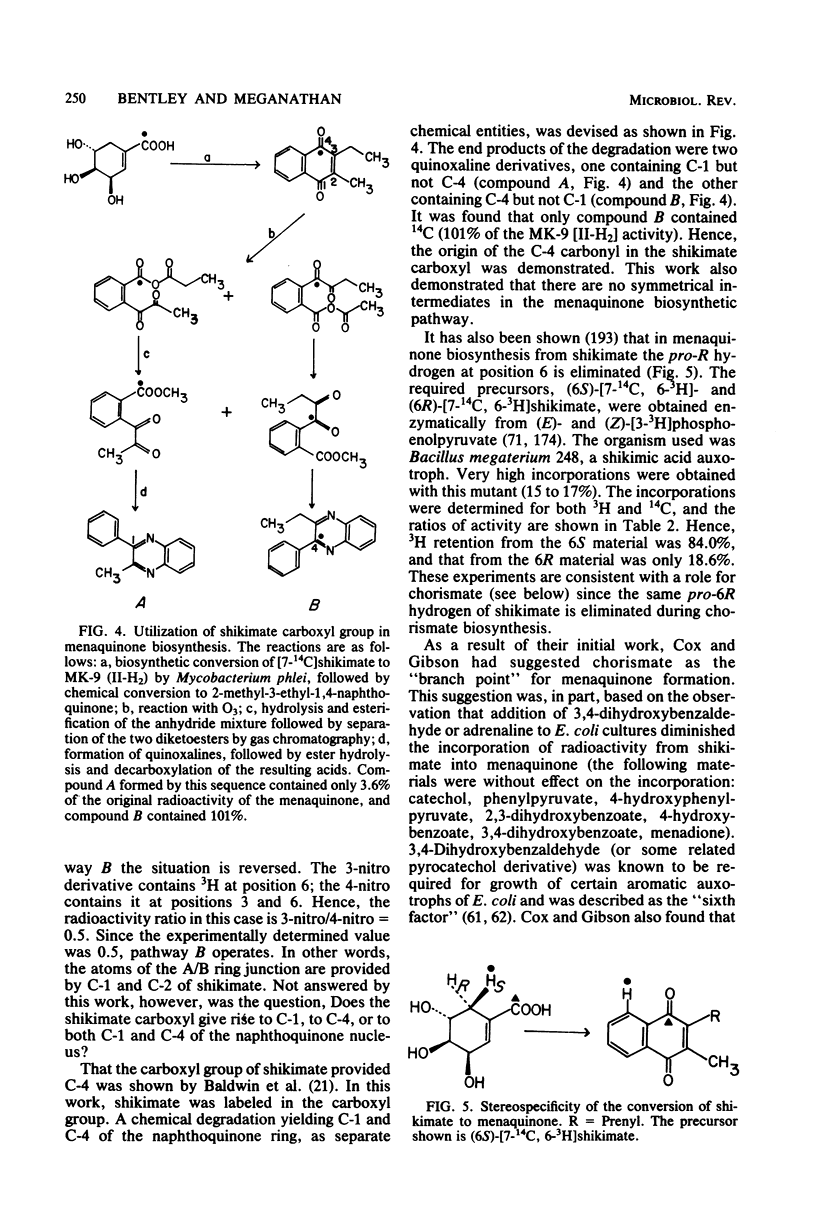
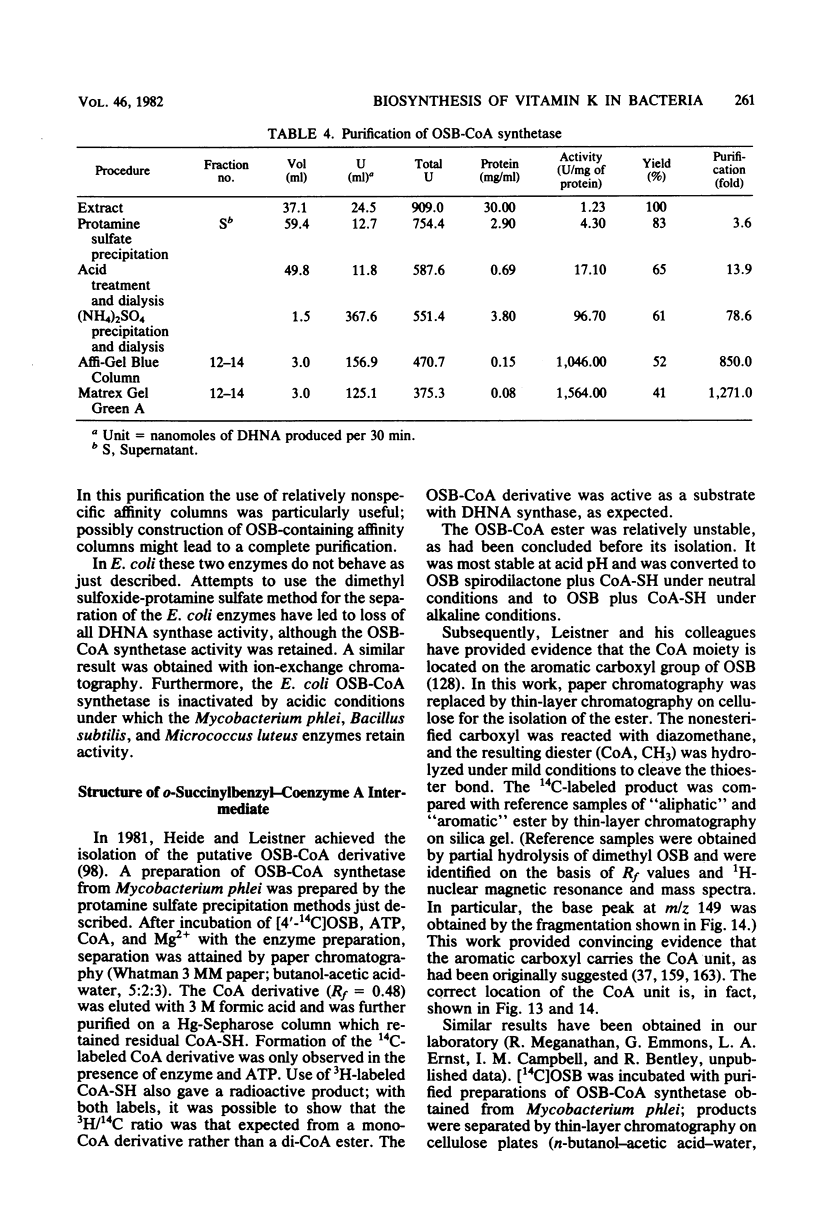
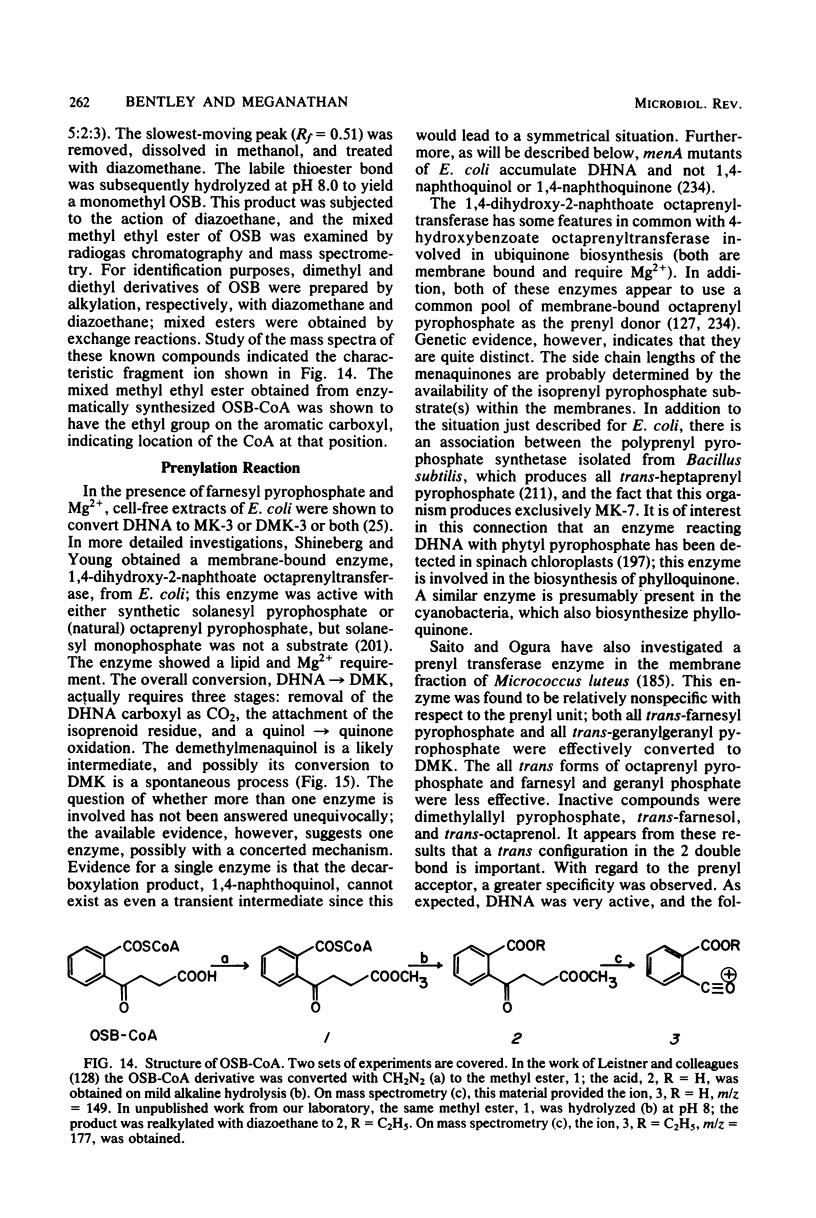
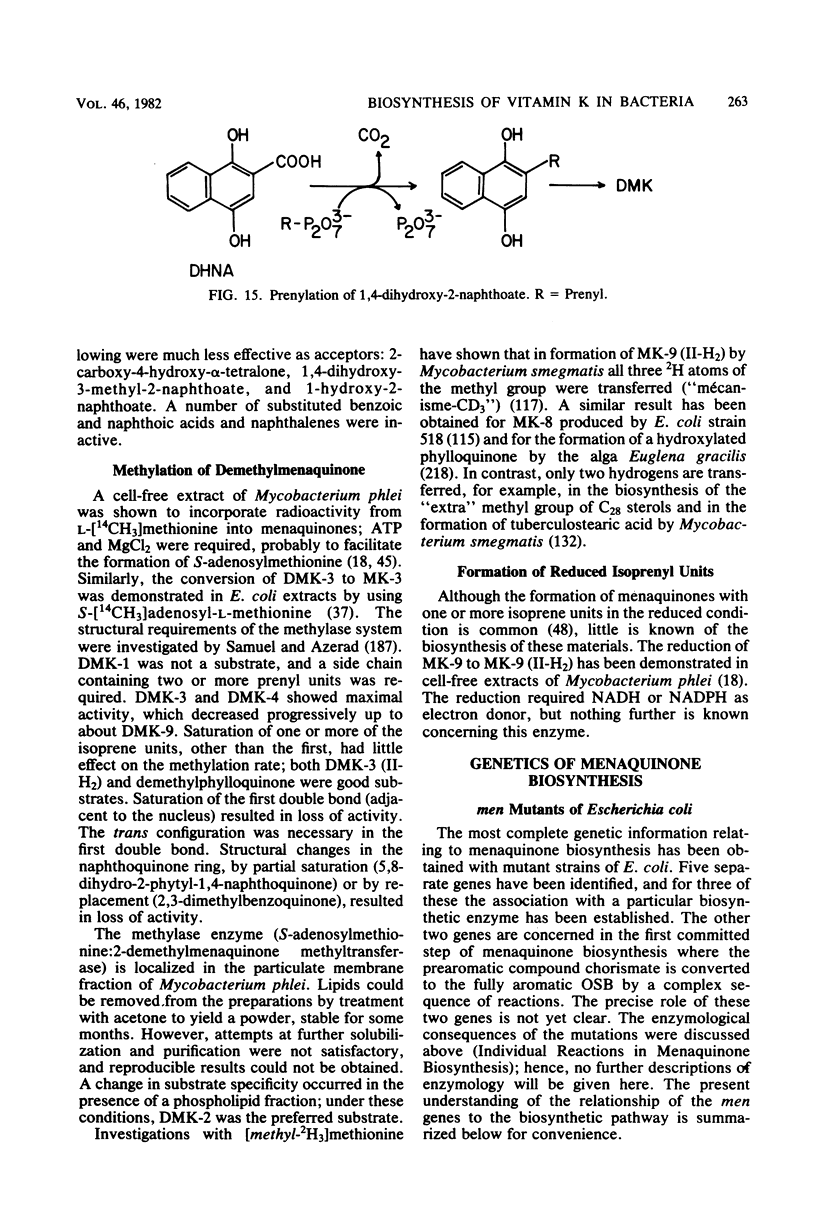
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZERAD R., BLEILER-HILL R., LEDERER E. BIOSYNTHESIS OF A VITAMIN K2 BY CELL-FREE EXTRACTS OF MYCOBACTERIUM PHLEI. Biochem Biophys Res Commun. 1965 Apr 9;19:194–197. doi: 10.1016/0006-291x(65)90503-6. [DOI] [PubMed] [Google Scholar]
- Acar J. F., Goldstein F. W., Lagrange P. Human infections caused by thiamine- or menadione-requiring Staphylococcus aureus. J Clin Microbiol. 1978 Aug;8(2):142–147. doi: 10.1128/jcm.8.2.142-147.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander K., Young I. G. Alternative hydroxylases for the aerobic and anaerobic biosynthesis of ubiquinone in Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4750–4755. doi: 10.1021/bi00615a024. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Robinson I. M. Biosynthesis of alpha-ketoglutarate by the reductive carboxylation of succinate in Bacteroides ruminicola. J Bacteriol. 1970 Oct;104(1):50–56. doi: 10.1128/jb.104.1.50-56.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almquist H. J. The early history of vitamin K. Am J Clin Nutr. 1975 Jun;28(6):656–659. doi: 10.1093/ajcn/28.6.656. [DOI] [PubMed] [Google Scholar]
- Almquist H. J. Vitamin K: discovery, identification, synthesis, functions. Fed Proc. 1979 Dec;38(13):2687–2689. [PubMed] [Google Scholar]
- Andrews S., Cox G. B., Gibson F. The anaerobic oxidation of dihydroorotate by Escherichia coli K-12. Biochim Biophys Acta. 1977 Oct 12;462(1):153–160. doi: 10.1016/0005-2728(77)90197-9. [DOI] [PubMed] [Google Scholar]
- Arvidson S., Holme T., Wadström T. Formation of bacteriolytic enzymes in batch and continuous culture of Staphylococcus aureus. J Bacteriol. 1970 Oct;104(1):227–233. doi: 10.1128/jb.104.1.227-233.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft J. R., Haddock B. A. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975 May;148(2):349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azerad R., Bleiler-Hill R., Catala F., Samuel O., Lederer E. Biosynthesis of dihydromenaquinone-9 by Mycobacterium phlei. Biochem Biophys Res Commun. 1967 Apr 20;27(2):253–257. doi: 10.1016/s0006-291x(67)80070-6. [DOI] [PubMed] [Google Scholar]
- Azerad R., Cyrot-Pelletier M. O. Structure and configuration of the polyprenoid side chain of dihydromenaquinones from Myco- and Corynebacteria. Biochimie. 1973 May;55(5):591–603. doi: 10.1016/s0300-9084(73)80421-3. [DOI] [PubMed] [Google Scholar]
- BENTLEY R., RAMSEY V. G., SPRINGER C. M., DIALAMEH G. H., OLSON R. E. APPLICATION OF A CHEMICAL DEGRADATION OF COENZYME Q TO PROBLEMS OF BIOSYNTHESIS. Biochemistry. 1965 Jan;4:166–176. doi: 10.1021/bi00877a025. [DOI] [PubMed] [Google Scholar]
- BILLETER M., BOLLIGER W., MARTIUS C. UNTERSUCHUNGEN UEBER DIE UMWANDLUNG VON VERFUETTERTEN K-VITAMINEN DURCH AUSTAUSCH DER SEITENKETTE UND DIE ROLLE DER DARMBAKTERIEN HIERBEI. Biochem Z. 1964 Aug 11;340:290–303. [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. M., Snyder C. D., Rapoport H. Biosynthesis of bacterial menaquinones. Dissymmetry in the naphthalenic intermediate. Biochemistry. 1974 Mar 26;13(7):1523–1530. doi: 10.1021/bi00704a031. [DOI] [PubMed] [Google Scholar]
- Bentley R., Meganathan R. Geosmin and methylisoborneol biosynthesis in streptomycetes. Evidence for an isoprenoid pathway and its absence in non-differentiating isolates. FEBS Lett. 1981 Mar 23;125(2):220–222. doi: 10.1016/0014-5793(81)80723-5. [DOI] [PubMed] [Google Scholar]
- Bollag J. M., Czaplicki E. J., Minard R. D. Bacterial metabolism of 1-naphthol. J Agric Food Chem. 1975 Jan-Feb;23(1):85–90. doi: 10.1021/jf60197a020. [DOI] [PubMed] [Google Scholar]
- Brown B. S., Whistance G. R., Threlfall D. R. Studies on alpha-naphthol as a precursor of microbial menaquinone. FEBS Lett. 1968 Oct;1(5):323–325. doi: 10.1016/0014-5793(68)80145-0. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kowand S. K., Van Den Elzen H. M. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob Agents Chemother. 1979 Jan;15(1):7–13. doi: 10.1128/aac.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. W., Jr, Bentley R. Menaquinone biosynthesis: conversion of o-succinylbenzoic acid to 1,4-dihydroxy-2-naphthoic acid and menaquinones by Escherichia coli extracts. Biochemistry. 1976 Nov 2;15(22):4792–4796. doi: 10.1021/bi00667a007. [DOI] [PubMed] [Google Scholar]
- COX G. B., GIBSON F. BIOSYNTHESIS OF VITAMIN K AND UBIQUINONE. RELATION TO THE SHIKIMIC ACID PATHWAY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 9;93:204–206. doi: 10.1016/0304-4165(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Bentley R. Inhomogeneity of vitamin K2 in Mycobacterium phlei. Biochemistry. 1968 Oct;7(10):3323–3327. doi: 10.1021/bi00850a002. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Coscia C. J., Kelsey M., Bentley R. Origin of the aromatic nucleus in bacterial menaquinones. Biochem Biophys Res Commun. 1967 Jul 10;28(1):25–29. doi: 10.1016/0006-291x(67)90400-7. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Robins D. J., Kelsey M., Bentley R. Biosynthesis of bacterial menaquinones (vitamins K 2 ). Biochemistry. 1971 Aug 3;10(16):3069–3078. doi: 10.1021/bi00792a014. [DOI] [PubMed] [Google Scholar]
- Catala F., Azerad R., Lederer E. Sur les propriétés de la desméthylménaquinone C-méthylase de Mycobacterium phlei. Int Z Vitaminforsch. 1970;40(3):363–373. [PubMed] [Google Scholar]
- Coates M. E. Gnotobiotic animals in nutrition research. Proc Nutr Soc. 1973 Sep;32(2):53–58. doi: 10.1079/pns19730015. [DOI] [PubMed] [Google Scholar]
- Coletsos P. La vitamine K hydrosoluble (méthyl-2-naphthohydroquinone di-benzoyl-disulfonate de sodium), facteur de croissance permettant d'assurer l'isolement et la pérennité en culture de Mycobacterium johneï (M.j.) in vitro. C R Acad Sci Hebd Seances Acad Sci D. 1970 Nov 9;271(19):1717–1719. [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Pirouz T., Goodfellow M., Minnikin D. E. Distribution of menaquinones in actinomycetes and corynebacteria. J Gen Microbiol. 1977 Jun;100(2):221–230. doi: 10.1099/00221287-100-2-221. [DOI] [PubMed] [Google Scholar]
- Cook S. F., Scott K. G. APPARENT INTOXICATION IN POULTRY DUE TO NITROGENOUS BASES. Science. 1935 Nov 15;82(2133):465–467. doi: 10.1126/science.82.2133.465. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Pittard J. Mutant strains of Escherichia coli K-12 unable to form ubiquinone. J Bacteriol. 1968 May;95(5):1591–1598. doi: 10.1128/jb.95.5.1591-1598.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. The role of shikimic acid in the biosynthesis of vitamin K2. Biochem J. 1966 Jul;100(1):1–6. doi: 10.1042/bj1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Succinate dehydrogenase-dependent nutritional requirement for succinate in mutants of Escherichia coli K12. J Gen Microbiol. 1978 Jul;107(1):1–13. doi: 10.1099/00221287-107-1-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Aromatic biosynthesis. IV. Preferential conversion, in incompletely blocked mutants, of a common precursor of several metabolites. J Bacteriol. 1952 Nov;64(5):729–748. doi: 10.1128/jb.64.5.729-748.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam H. International symposium on recent advances in research on vitamins K and related quinones (vitamins K, ubiquinones or coenzymes Q, plastoquinones) in honor of Professor Henrik Dam. Historical survey and introduction. Vitam Horm. 1966;24:295–306. [PubMed] [Google Scholar]
- Dam H. The antihaemorrhagic vitamin of the chick. Biochem J. 1935 Jun;29(6):1273–1285. doi: 10.1042/bj0291273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansette P., Azerad R. A new intermediate in naphthoquinone and menaquinone biosynthesis. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1090–1095. doi: 10.1016/0006-291x(70)90906-x. [DOI] [PubMed] [Google Scholar]
- Ellestad G. A., Kunstmann M. P., Whaley H. A., Patterson E. L. The structure of frenolicin. J Am Chem Soc. 1968 Feb 28;90(5):1325–1332. doi: 10.1021/ja01007a039. [DOI] [PubMed] [Google Scholar]
- Ellis J. R., Glover J. Some aspects of menaquinone biosynthesis in Escherichia col E 106. Biochem J. 1968 Nov;110(2):22P–22P. doi: 10.1042/bj1100022pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS J., MACTURK H. M., MADINAVEITIA J., SNOW G. A. Mycobactin, a growth factor for Mycobacterium johnei. I. Isolation from Mycobacterium phlei. Biochem J. 1953 Nov;55(4):596–607. doi: 10.1042/bj0550596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. A., Jr, Cummins C. S. Nutritional requirements of anaerobic coryneforms. J Bacteriol. 1978 Sep;135(3):858–867. doi: 10.1128/jb.135.3.858-867.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss H. G., Onderka D. K., Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736–744. [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., ENGLE L. P. VITAMIN K COMPOUNDS IN BACTERIA THAT ARE OBLIGATE ANAEROBES. Science. 1964 Dec 4;146(3649):1307–1309. doi: 10.1126/science.146.3649.1307. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLICK M. C., ZILLIKEN F., GYORGY P. Supplementary growth promoting effect of 2-methyl-1,4-naphthoquinone of Lactobacillus bifidus var. pennsylvanicus. J Bacteriol. 1959 Feb;77(2):230–236. doi: 10.1128/jb.77.2.230-236.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., DAFT F. S., McDANIEL E. G., SMITH J. C., FITZGERALD R. J. Effects of vitamin K-active compounds and intestinal microorganisms in vitamin K-deficient germfree rats. J Nutr. 1962 Dec;78:461–468. doi: 10.1093/jn/78.4.461. [DOI] [PubMed] [Google Scholar]
- Gelbart S. M., Juhasz S. E. Mycobacterium sp. strain 80: Mycobacterium phlei or Mycobacterium smegmatis? J Gen Microbiol. 1969 Nov;59(1):141–143. doi: 10.1099/00221287-59-1-141. [DOI] [PubMed] [Google Scholar]
- Goldenbaum P. E., Keyser P. D., White D. C. Role of vitamin K2 in the organization and function of Staphylococcus aureua membranes. J Bacteriol. 1975 Feb;121(2):442–449. doi: 10.1128/jb.121.2.442-449.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Menaquinone biosynthesis: mutants of Escherichia coli K-12 requiring 2-succinylbenzoate. J Bacteriol. 1977 Jun;130(3):1038–1046. doi: 10.1128/jb.130.3.1038-1046.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Shaw D. J. Molecular cloning of menaquinone biosynthetic genes of Escherichia coli K12. Mol Gen Genet. 1981;181(3):379–383. doi: 10.1007/BF00425615. [DOI] [PubMed] [Google Scholar]
- Guérin M., Azerad R., Lederer E. Métabolisme des acides benzoique, phénylacétique et phénylbutyrique par des cellules entières et des extraits acellulaires de Mycobacterium phlei. Bull Soc Chim Biol (Paris) 1968 Mar 2;50(1):187–193. [PubMed] [Google Scholar]
- Guérin M., Azerad R., Lederer E. Sur l'origine biogénétique du méthyle en position 2 de la vitamine K2 de Mycobacterium phlei. Bull Soc Chim Biol (Paris) 1965;47(11):2105–2114. [PubMed] [Google Scholar]
- Guérin M., Leduc M. M., Azerad R. G. Biosynthèse du noyau naphtoquinonique des ménaquinones bactériennes. Eur J Biochem. 1970 Sep;15(3):421–427. doi: 10.1111/j.1432-1033.1970.tb01024.x. [DOI] [PubMed] [Google Scholar]
- HAGER L. P., KORNBERG H. L. On the mechanism of alpha-oxoglutarate oxidation in Escherichia coli. Biochem J. 1961 Jan;78:194–198. doi: 10.1042/bj0780194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Formation of vitamin K2 isoprenologues by Staphylococcus aureus. J Bacteriol. 1969 Nov;100(2):573–578. doi: 10.1128/jb.100.2.573-578.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Inhibition of vitamin K2 and carotenoid synthesis in Staphylococcus aureus by diphenylamine. J Bacteriol. 1970 Sep;103(3):611–615. doi: 10.1128/jb.103.3.611-615.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Separation of vitamin K2 isoprenologues by reversed-phase thin-layer chromatography. J Chromatogr. 1969 Dec 23;45(3):446–452. doi: 10.1016/s0021-9673(01)86242-7. [DOI] [PubMed] [Google Scholar]
- Hanks J. H. Host-dependent microbes. Bacteriol Rev. 1966 Mar;30(1):114–135. doi: 10.1128/br.30.1.114-135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide L., Leistner E. Enzymatic synthesis of the coenzyme A ester of o-succinylbenzoic acid, an intermediate in menaquinone (vitamin K2) biosynthesis. FEBS Lett. 1981 Jun 15;128(2):201–204. doi: 10.1016/0014-5793(81)80080-4. [DOI] [PubMed] [Google Scholar]
- Hollander D., Muralidhara K. S., Rim E. Colonic absorption of bacterially synthesized vitamin K2 in the rat. Am J Physiol. 1976 Feb;230(2):251–255. doi: 10.1152/ajplegacy.1976.230.2.251. [DOI] [PubMed] [Google Scholar]
- Holländer R. Correlation of the function of demethylmenaquinone in bacterial electron transport with its redox potential. FEBS Lett. 1976 Dec 15;72(1):98–100. doi: 10.1016/0014-5793(76)80821-6. [DOI] [PubMed] [Google Scholar]
- Holst W. F., Halbrook E. R. A "SCURVY-LIKE" DISEASE IN CHICKS. Science. 1933 Apr 7;77(1997):354–354. doi: 10.1126/science.77.1997.354. [DOI] [PubMed] [Google Scholar]
- Hooper C. A., Haney B. B., Stone H. H. Gastrointestinal bleeding due to vitamin K deficiency in patients on parenteral cefamandole. Lancet. 1980 Jan 5;1(8158):39–40. doi: 10.1016/s0140-6736(80)90571-1. [DOI] [PubMed] [Google Scholar]
- Hopkins F. G. Feeding experiments illustrating the importance of accessory factors in normal dietaries. J Physiol. 1912 Jul 15;44(5-6):425–460. doi: 10.1113/jphysiol.1912.sp001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. The enzymic degradation of alkyl-substituted gentisates, maleates and malates. Biochem J. 1971 Mar;122(1):29–40. doi: 10.1042/bj1220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. T., Bentley R. Utilization of shikimic acid for the formation of mycobactin S and salicylic acid by Mycobacterium smegmatis. Biochemistry. 1970 Sep 29;9(20):3984–3987. doi: 10.1021/bi00822a017. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Threlfall D. R. Formation of spirodilactone of 4-(2'-carboxyphenyl)-4,4-dihydroxybutyrate from 2-succinylbenzoate in cell-free extracts of Micrococcus luteus. Biochim Biophys Acta. 1978 Jul 25;530(1):1–8. doi: 10.1016/0005-2760(78)90120-0. [DOI] [PubMed] [Google Scholar]
- Jackman L. M., O'Brien I. G., Cox G. B., Gibson F. Methionine as the source of methyl groups for ubiquinone and vitamin K: a study using nuclear magnetic resonance and mass spectrometry. Biochim Biophys Acta. 1967 Jun 13;141(1):1–7. doi: 10.1016/0304-4165(67)90239-5. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J. The late steps of anaerobic heme biosynthesis in E. coli: role for quinones in protoporphyrinogen oxidation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):429–433. doi: 10.1016/0006-291x(77)91272-4. [DOI] [PubMed] [Google Scholar]
- Joyce G. H., Hammond R. K., White D. C. Changes in membrane lipid composition in exponentially growing Staphylococcus aureus during the shift from 37 to 25 C. J Bacteriol. 1970 Oct;104(1):323–330. doi: 10.1128/jb.104.1.323-330.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klubes P., Cerna I. Actions of 2-methyl-1,4-naphthoquinone in Bacillus cereus. Biochem Pharmacol. 1972 Jan 15;21(2):249–261. doi: 10.1016/0006-2952(72)90275-4. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V. On the role of quinones in bacterial electron transport. The respiratory system of Bacillus megaterium. Eur J Biochem. 1969 Dec;11(2):328–340. doi: 10.1111/j.1432-1033.1969.tb00776.x. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LEV M. Apparent requirement for vitamin K of rumen strains of Fusiformis nigrescens. Nature. 1958 Jan 18;181(4603):203–204. doi: 10.1038/181203a0. [DOI] [PubMed] [Google Scholar]
- LEV M., REITER B. Growth response of a strain of Haemophilus parainfluenzae to menadione (vitamin K3). Nature. 1962 Mar 3;193:905–906. doi: 10.1038/193905a0. [DOI] [PubMed] [Google Scholar]
- LEV M. The growth-promoting activity of compounds of the vitamin K group and analogues for a rumen strain of Fusiformis nigrescens. J Gen Microbiol. 1959 Jun;20(3):697–703. doi: 10.1099/00221287-20-3-697. [DOI] [PubMed] [Google Scholar]
- Leahy G. J., Currie D. J., Holmes H. L., Maltman J. R. Growth-inhibitory activities of some 1,4-naphthoquinones and related compounds on Staphylococcus aureus and Escherichia coli. Can J Microbiol. 1968 Jun;14(6):661–666. doi: 10.1139/m68-110. [DOI] [PubMed] [Google Scholar]
- Lederer E. The origin and function of some methyl groups in branched-chain fatty acids, plant sterols and quinones. Biochem J. 1964 Dec;93(3):449–468. doi: 10.1042/bj0930449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M. M., Dansette P. M., Azerad R. G. Incorporation de l'acide shikimique dans le noyau des naphtoquinones d'origine bactérienne et végétale. Eur J Biochem. 1970 Sep;15(3):428–435. doi: 10.1111/j.1432-1033.1970.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Leistner E., Schmitt J. H., Zenk M. H. Alpha-naphthol: a precursor of vitamin K2. Biochem Biophys Res Commun. 1967 Sep 27;28(6):845–850. doi: 10.1016/0006-291x(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Leistner E., Zenk M. H. Zur Biogenese von 5-Hydroxy-1.4-naphthochinon (Juglon) in Juglans regia L. Z Naturforsch B. 1968 Feb;23(2):259–268. [PubMed] [Google Scholar]
- Lev M. Glutamine-stimulated amino acid and peptide incorporation in Bacteroides melaninogenicus. J Bacteriol. 1980 Aug;143(2):753–760. doi: 10.1128/jb.143.2.753-760.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Keudell K. C., Milford A. F. Succinate as a growth factor for Bacteroides melaninogenicus. J Bacteriol. 1971 Oct;108(1):175–178. doi: 10.1128/jb.108.1.175-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Milford A. W. Water-soluble factors with vitamin K activity from pig liver and from Fusiformis nigrescens. Nature. 1966 Jun 11;210(5041):1120–1122. doi: 10.1038/2101120a0. [DOI] [PubMed] [Google Scholar]
- MACDONALD J. B., GIBBONS R. J., SOCRANSKY S. S. Bacterial mechanisms in periodontal disease. Ann N Y Acad Sci. 1960 Mar 29;85:467–478. doi: 10.1111/j.1749-6632.1960.tb49975.x. [DOI] [PubMed] [Google Scholar]
- MACDONALD J. B., SUTTON R. M., KNOLL M. L., MADLENER E. M., GRAINGER R. M. The pathogenic components of an experimental fusospirochetal infection. J Infect Dis. 1956 Jan-Feb;98(1):15–20. doi: 10.1093/infdis/98.1.15. [DOI] [PubMed] [Google Scholar]
- MACDONALD J. B., SUTTON R. M., KNOLL M. L. The production of fusospirochetal infections in guinea pigs with recombined pure cultures. J Infect Dis. 1954 Nov-Dec;95(3):275–284. doi: 10.1093/infdis/95.3.275. [DOI] [PubMed] [Google Scholar]
- MARTIUS C., LEUZINGER W. UBER DIE UMWANDLUNG VON K-VITAMINEN IN EINEM K-HETEROTROPHEN ANAEROBIER(FUSIFORMIS NIGRESCENS) Biochem Z. 1964 Aug 11;340:304–315. [PubMed] [Google Scholar]
- MORRISON N. E. CIRCUMVENTION OF THE MYCOBACTIN REQUIREMENT OF MYCOBACTERIUM PARATUBERCULOSIS. J Bacteriol. 1965 Mar;89:762–767. doi: 10.1128/jb.89.3.762-767.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. The fat-soluble vitamins in modern medicine. Vitam Horm. 1974;32:131–154. doi: 10.1016/s0083-6729(08)60009-6. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Ratledge C. Conversion of chorismic acid and isochorismic acid to salicylic acid by cell-free extracts of Mycobacterium smegmatis. Biochim Biophys Acta. 1971;230(3):643–645. doi: 10.1016/0304-4165(71)90201-7. [DOI] [PubMed] [Google Scholar]
- Matschiner J. T. Characterization of vitamin K from the contents of bovine rumen. J Nutr. 1970 Feb;100(2):190–192. doi: 10.1093/jn/100.2.190. [DOI] [PubMed] [Google Scholar]
- Matthews P. R., McDiarmid A., Collins P., Brown A. The dependence of some strains of Mycobacterium avium on mycobactin for initial and subsequent growth. J Med Microbiol. 1978 Feb;11(1):53–57. doi: 10.1099/00222615-11-1-53. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C., Edwards T., Jensen S. Characterization of Bacteroides asaccharolyticus and B. melaninogenicus oral isolates. Can J Microbiol. 1980 Oct;26(10):1178–1183. doi: 10.1139/m80-197. [DOI] [PubMed] [Google Scholar]
- McFarlane W. D., Graham W. R., Richardson F. The fat-soluble vitamin requirements of the chick: The vitamin A and vitamin D content of fish meal and meat meal. Biochem J. 1931;25(1):358–366. doi: 10.1042/bj0250358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern E. P., Bentley R. Biosynthesis of flaviolin and 5,8-dihydroxy-2,7-dimethoxy-1,4-naphthoquinone. Biochemistry. 1975 Jul 15;14(14):3138–3143. doi: 10.1021/bi00685a016. [DOI] [PubMed] [Google Scholar]
- McGovern E. P., Bentley R. Isolation and properties of naphthoate synthetase from Mycobacterium phlei. Arch Biochem Biophys. 1978 May;188(1):56–63. doi: 10.1016/0003-9861(78)90355-7. [DOI] [PubMed] [Google Scholar]
- Meganathan R., Bentley R. Biosynthesis of o-succinylbenzoic acid in a men- Escherichia coli mutant requires decarboxylation of L-glutamate at the C-1 position. Biochemistry. 1981 Sep 1;20(18):5336–5340. doi: 10.1021/bi00521a038. [DOI] [PubMed] [Google Scholar]
- Meganathan R., Bentley R. Menaquinone (vitamin K2) biosynthesis: conversion of o-succinylbenzoic acid to 1,4-dihydroxy-2-naphthoic acid by Mycobacterium phlei enzymes. J Bacteriol. 1979 Oct;140(1):92–98. doi: 10.1128/jb.140.1.92-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan R., Bentley R., Taber H. Identification of Bacillus subtilis men mutants which lack O-succinylbenzoyl-coenzyme A synthetase and dihydroxynaphthoate synthase. J Bacteriol. 1981 Jan;145(1):328–332. doi: 10.1128/jb.145.1.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan R. Enzymes from Escherichia coli synthesize o-succinylbenzoic acid, an intermediate in menaquinone (vitamin K2) biosynthesis. J Biol Chem. 1981 Sep 25;256(18):9386–9388. [PubMed] [Google Scholar]
- Meganathan R., Folger T., Bentley R. Conversion of o-succinylbenzoate to dihydroxynaphthoate by extracts of Micrococcus luteus. Biochemistry. 1980 Feb 19;19(4):785–789. doi: 10.1021/bi00545a026. [DOI] [PubMed] [Google Scholar]
- Nyström E., Sjövall J. Separation of vitamins K2 on capillary columns of methylated Sephadex. J Chromatogr. 1966 Sep;24(1):212–214. doi: 10.1016/s0021-9673(01)98132-4. [DOI] [PubMed] [Google Scholar]
- Onderka D. K., Floss H. G. Steric course of the chorismate synthetase reaction and the 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) synthetase reaction. J Am Chem Soc. 1969 Oct 8;91(21):5894–5896. doi: 10.1021/ja01049a046. [DOI] [PubMed] [Google Scholar]
- Peterson W. H., Peterson M. S. RELATION OF BACTERIA TO VITAMINS AND OTHER GROWTH FACTORS. Bacteriol Rev. 1945 Jun;9(2):49–109. doi: 10.1128/br.9.2.49-109.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase W. J., Pun W. T., Withaar J. Lipoquinones of Escherichia coli. Biochim Biophys Acta. 1966 May 5;118(2):425–426. doi: 10.1016/s0926-6593(66)80053-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- Rizza V., Tucker A. N., White D. C. Lipids of Bacteroides melaninogenicus. J Bacteriol. 1970 Jan;101(1):84–91. doi: 10.1128/jb.101.1.84-91.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D. J., Campbell I. M., Bentley R. Glutamate--a precusor for the naphthalene nucleus of bacterial menaquinones. Biochem Biophys Res Commun. 1970;39(6):1081–1086. doi: 10.1016/0006-291x(70)90669-8. [DOI] [PubMed] [Google Scholar]
- Robins D. J., Yee R. B., Bentley R. Biosynthetic precursors of vitamin K as growth promoters for Bacteroides melaninogenicus. J Bacteriol. 1973 Nov;116(2):965–971. doi: 10.1128/jb.116.2.965-971.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Ogura K. Biosynthesis of menaquinones. Enzymatic prenylation of 1,4-dihydroxy-2-naphthoate by Micrococcus luteus membrane fractions. J Biochem. 1981 May;89(5):1445–1452. doi: 10.1093/oxfordjournals.jbchem.a133337. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schmitt M. D. Effects of diphenylamine on carotenoids and menaquinones in bacterial membranes. Biochim Biophys Acta. 1967 May 2;135(2):196–207. doi: 10.1016/0005-2736(67)90114-9. [DOI] [PubMed] [Google Scholar]
- Samuel O., Azerad R. C-methylation of desmethylmenaquinones: Specificity of the enzymatic system of mycobacterium phlei. FEBS Lett. 1969 Mar;2(5):336–338. doi: 10.1016/0014-5793(69)80058-x. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Purvis P., Portelance V. Role of menaquinone in nitrate respiration in Staphylococcus aureus. J Bacteriol. 1974 Feb;117(2):911–913. doi: 10.1128/jb.117.2.911-913.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Surdeanu M., Szabados J., Greceanu V., Horodniceanu T. Menaphthone-requiring mutants of Staphylococcus aureus. Rev Can Biol. 1968 Dec;27(4):333–339. [PubMed] [Google Scholar]
- Schultz G., Ellerbrock B. H., Soll J. Site of prenylation reaction in synthesis of phylloquinone (vitamin K1) by spinach chloroplasts. Eur J Biochem. 1981 Jul;117(2):329–332. doi: 10.1111/j.1432-1033.1981.tb06341.x. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Collins M. D. Fatty acid and isoprenoid quinone composition in the classification of Bacteroides melaninogenicus and related taxa. J Appl Bacteriol. 1980 Feb;48(1):75–87. doi: 10.1111/j.1365-2672.1980.tb05209.x. [DOI] [PubMed] [Google Scholar]
- Shineberg B., Young I. G. Biosynthesis of bacterial menaquinones: the membrane-associated 1,4-dihydroxy-2-naphthoate octaprenyltransferase of Escherichia coli. Biochemistry. 1976 Jun 29;15(13):2754–2758. doi: 10.1021/bi00658a007. [DOI] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder C. D., Rapoport H. Biosynthesis of bacterial menaquinones. Origin of quinone oxygens. Biochemistry. 1970 May 12;9(10):2033–2038. doi: 10.1021/bi00812a001. [DOI] [PubMed] [Google Scholar]
- Soliman A. G., Harper A. E. Effect of protein content of diet on lysine oxidation by the rat. Biochim Biophys Acta. 1971 Jul 20;244(1):146–154. doi: 10.1016/0304-4165(71)90131-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R. The biosynthesis of anthranilate from [3,4-'+C]glucose in Escherichia coli. Biochemistry. 1965 Dec;4(12):2860–2865. doi: 10.1021/bi00888a043. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Suttie J. W. Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–172. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Portelance V., Dobardzic R., Sonea S. Classification of vitamin K-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1971 Feb;65(2):125–130. doi: 10.1099/00221287-65-2-125. [DOI] [PubMed] [Google Scholar]
- Taber H. W., Dellers E. A., Lombardo L. R. Menaquinone biosynthesis in Bacillus subtilis: isolation of men mutants and evidence for clustering of men genes. J Bacteriol. 1981 Jan;145(1):321–327. doi: 10.1128/jb.145.1.321-327.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. W., Sugarman B. J., Halfenger G. M. Involvement of menaquinone in the active accumulation of aminoglycosides by Bacillus subtilis. J Gen Microbiol. 1981 Mar;123(1):143–149. doi: 10.1099/00221287-123-1-143. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Ogura K. Farnesyl pyrophosphate synthetase from Bacillus subtilis. J Biochem. 1981 May;89(5):1581–1587. doi: 10.1093/oxfordjournals.jbchem.a133352. [DOI] [PubMed] [Google Scholar]
- Tamir H., Srinivasan P. R. Studies of the mechanism of anthranilate synthase reaction. Proc Natl Acad Sci U S A. 1970 Jun;66(2):547–551. doi: 10.1073/pnas.66.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R., Griffiths W. T., Goodwin T. W. Biosynthesis of the prenyl side chains of plastoquinone and related compounds in maize and barley shoots. Biochem J. 1967 Jun;103(3):831–851. doi: 10.1042/bj1030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R. Incorporation of L-(Me- 2 H 3 )methionine into isoprenoid quinones and related compounds by Euglena gracilis. Biochim Biophys Acta. 1972 Nov 30;280(3):472–480. doi: 10.1016/0005-2760(72)90255-x. [DOI] [PubMed] [Google Scholar]
- Walker N., Janes N. F., Spokes J. R., van Berkum P. Degradation of 1-naphthol by a soil pseudomonad. J Appl Bacteriol. 1975 Dec;39(3):281–286. doi: 10.1111/j.1365-2672.1975.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977 Jul 7;461(1):84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Brown B. S., Threlfall D. R. Biosynthesis of ubiquinone in non-photosynthetic gram-negative bacteria. Biochem J. 1970 Mar;117(1):119–128. doi: 10.1042/bj1170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Effect of anaerobiosis on the concentrations of demethylmenaquinone, menaquinone and ubiquinone in Escherichia freundii, Proteus mirabilis and Aeromonas punctata. Biochem J. 1968 Jul;108(3):505–507. doi: 10.1042/bj1080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G. Biosynthesis of bacterial menaquinones. Menaquinone mutants of Escherichia coli. Biochemistry. 1975 Jan 28;14(2):399–406. doi: 10.1021/bi00673a029. [DOI] [PubMed] [Google Scholar]
- Young I. G., Gibson F., MacDonald C. G. Enzymic and nonenzymic transformations of chorismic acid and related cyclohexadienes. Biochim Biophys Acta. 1969 Oct 7;192(1):62–72. doi: 10.1016/0304-4165(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Young I. G., Leppik R. A., Hamilton J. A., Gibson F. Biochemical and genetic studies on ubiquinone biosynthesis in Escherichia coli K-12:4-hydroxybenzoate octaprenyltransferase. J Bacteriol. 1972 Apr;110(1):18–25. doi: 10.1128/jb.110.1.18-25.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk M. H., Leistner E. On the mode of incorporation of shikimic acid into 2-hydroxy-1,4-naphthoquinone (lawsone). Z Naturforsch B. 1967 Apr;22(4):460–460. doi: 10.1515/znb-1967-0425. [DOI] [PubMed] [Google Scholar]



