Abstract
Cattle were inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii to compare the antigen-specific immune responses to various patterns of mycobacterial disease. Disease expression ranged from colonization with associated pathology (M. bovis infection) and colonization without pathology (M. tuberculosis infection) to no colonization or pathology (M. kansasii infection). Delayed-type hypersensitivity and gamma interferon responses were elicited by each mycobacterial inoculation; however, the responses by the M. bovis- and M. tuberculosis-inoculated animals exceeded those of the M. kansasii-inoculated animals. Specific antibody responses were detected in all M. tuberculosis- and M. bovis-inoculated cattle 3 weeks after inoculation. From 6 to 16 weeks after M. tuberculosis inoculation, the antibody responses waned, whereas the responses persisted with M. bovis infection. With M. kansasii inoculation, initial early antibody responses waned by 10 weeks after inoculation and then increased 2 weeks after the injection of purified protein derivative for the skin test at 18 weeks after challenge. These findings indicate that antibody responses are associated with the antigen burden rather than the pathology, cellular immune responses to tuberculin correlate with infection but not necessarily with the pathology or bacterial burden, and exposure to mycobacterial antigens may elicit an antibody response in a presensitized animal.
Tuberculosis (TB) in humans and animals may result from exposure to bacilli within the Mycobacterium tuberculosis complex (i.e., M. tuberculosis, M. bovis, M. africanum, M. pinnipedi, M. microti, M. caprae, or M. canetti [8]). Despite their ∼99.95% sequence identity (12), M. bovis and M. tuberculosis exhibit distinct differences in virulence and host adaptation. Compared to M. tuberculosis, experimental M. bovis infection of mice or rabbits results in a more severe pathology and shorter mean survival times (9, 17, 18). Mycobacterium tuberculosis is primarily a human pathogen that demonstrates a high level of attenuation in cattle (as reviewed by Francis in 1947 [10]), whereas M. bovis has a wider host range and affects many domesticated and free-ranging mammals as well as humans. Prior to the initiation of control and eradication campaigns in the early to mid-1900s, M. bovis infection accounted for up to 30% of human tuberculosis cases, with M. bovis being transmitted to humans primarily by the consumption of unpasteurized dairy products and contact with infected livestock. Control efforts, including slaughter surveillance and test/cull campaigns, have dramatically reduced the prevalence of M. bovis infection in domestic cattle herds, thereby reducing the spread of M. bovis to humans. However, in developing countries, M. bovis infection of humans persists as a serious and relatively common zoonosis (16).
Although Mycobacterium kansasii is not a member of the M. tuberculosis complex, it may cause disease in otherwise healthy humans, albeit infrequently, that is clinically indistinguishable from M. tuberculosis infection (1, 3). As with humans, M. kansasii infection of cattle is uncommon; however, it is occasionally associated with granulomatous lesions within lymph nodes and the respiratory tract of cattle (B. Harris, unpublished observations). Of particular relevance for the diagnosis of tuberculosis, M. kansasii infection/sensitization may elicit responses to antigens generally considered to be tuberculosis specific, such as ESAT-6, CFP-10, and MPB83 (2, 30, 35).
With experimental M. bovis infection of cattle, the levels of MPB83-specific antibody correlate with disease severity, bacterial burden, and specific cell-mediated immune responses (15, 33). With this particular scenario, disease severity (i.e., pathology) and bacterial burden are intimately linked; thus, it is difficult to define a potential correlation of a particular immune response to either readout independently. Prior studies have demonstrated that virulent and attenuated strains of M. bovis induce similar delayed-type hypersensitivity responses in cattle; however, only the virulent M. bovis strain induces a persistent gamma interferon (IFN-γ), interleukin-2 (IL-2), and antibody response (34). The objective of the present study was to compare mycobacterium-specific immune responses to the patterns of mycobacterial disease expression in which the mycobacterial burden is uncoupled from pathological changes. Disease expression patterns included persistent colonization with an associated pathology (i.e., M. bovis infection), colonization without an associated pathology (i.e., M. tuberculosis infection), and no colonization or pathology (i.e., M. kansasii infection). Antigen-specific immune responses were evaluated for their correlation to manifestations of disease expression.
MATERIALS AND METHODS
Calves, challenge inoculum, and necropsy.
Friesian or Friesian cross castrated male calves of ∼6 months of age were obtained from bovine TB-free herds and were housed within either biosafety level 3 facilities at the Veterinary Laboratory Agencies (VLA), Addlestone, United Kingdom (M. bovis- and M. tuberculosis-inoculated animals) or biosafety level 2 facilities at the National Animal Disease Center (NADC), Ames, IA (M. kansasii-inoculated animals). All cattle experiments were cleared by local ethical review, and animal procedures were performed in accordance with either British Home Office requirements [i.e., A(SP)A, 1086; VLA studies] or according to institutional guidelines and approved animal care and use protocols (NADC studies). The treatment groups included M. tuberculosis-inoculated calves (n = 5), M. bovis-inoculated calves (n = 5), and M. kansasii-inoculated calves (n = 4). The inoculation of M. tuberculosis (2.8 × 106 CFU) and M. bovis (1.0 × 106 CFU) was by direct instillation of the challenge inoculum into the tracheas of sedated calves, as described previously (5, 27). The inoculation of M. kansasii (4 × 108 CFU) was by direct instillation of the inoculum into the tonsillar crypts of sedated calves, as described previously (19). In general, these two routes of inoculation of tuberculous mycobacteria to cattle (i.e., intratracheal and intratonsillar) result in similar disease severities and infections (5, 20, 27). Whole blood was collected at 0, 2, 4, 6, 8, 10, 12, and 16 weeks after challenge for IFN-γ assays and placed in tubes with heparin; and serum was collected at 0, 3, 6, 7, 9, 10, 12, 13, 16, and 18 weeks after challenge for antibody detection assays (the slight variations in collection time points were dependent upon the treatment groups and assay). The 100-fold larger dose of M. kansasii was used on the basis of the findings of prior studies (19) and the relatively low virulence of M. kansasii compared to that of M. bovis and M. tuberculosis.
M. bovis field isolate AF2122/97 was grown to mid-log phase in Middlebrook 7H9 medium (Becton, Dickinson and Company [BD], Oxford, United Kingdom) supplemented with 10% (vol/vol) Middlebrook acid-albumin-dextrose-catalase enrichment (BD), 4.16 g/liter sodium pyruvate (Sigma-Aldrich Co., Poole, United Kingdom), and 0.05% (vol/vol) Tween 80 (Sigma-Aldrich); and stocks were stored frozen at −80°C. Stocks of M. tuberculosis H37Rv were prepared in the same way, except that the 7H9 medium was supplemented with 0.2% (vol/vol) glycerol (Sigma-Aldrich) rather than sodium pyruvate. M. kansasii field isolate 03-6931 (subtype 1, based on the 16S-23S rRNA sequence [1, 30]) was grown to mid-log phase in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Becton Dickinson, Franklin Lakes, NJ) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO), and stocks were stored frozen at −80°C. Enumeration of the bacterial stocks (CFU/ml) was by serial dilution on modified Middlebrook 7H11 agar (11). In addition, the virulence of the M. tuberculosis and M. bovis strains was confirmed in guinea pigs prior to the start of the cattle challenge experiment (data not shown). The M. kansasii strain used in the present study was isolated from a tuberculous lesion (i.e., a pyogranuloma) detected within an adult cow upon routine slaughter surveillance. The M. kansasii isolate had 100% identity with the subtype 1 genotype, which is most commonly associated with disease in immunocompetent humans (30).
Postmortem examination.
Approximately 16 to 18 weeks after inoculation, all cattle were euthanized by the intravenous injection of sodium pentobarbital and examined. Specimens of various tissues were collected (tonsil, lung, lung-associated lymph nodes, and head-associated lymph nodes) for bacteriologic culture (described below) and microscopic examination, including Ziehl-Neelsen staining for acid-fast bacilli as well as hematoxylin-eosin staining for morphological assessment of the tissues, as described previously (4, 31). All major visceral organs were visually inspected for lesions, and tissue specimens were collected for further microscopic and bacteriologic assessment, when it was warranted. The lungs were examined externally and were sliced into 0.5- to 1-cm-thick sections for the detection of visible lesions. Lymph node tissues were sliced into thin sections (1 to 2 mm thick) and were examined for the presence of visible lesions. The severity of the gross pathological changes was scored by using a semiquantitative scoring system, as described previously (29). In summary, each of seven lung lobes was scored from 0 to 5, depending on the number of lesions and the extent of the pathology observed, with 0 being no pathology and 5 being extensive gross coalescing lesions. The lymph nodes of the upper and lower respiratory tracts were similarly scored but by use of a score of from 0 to 3. The scores of the individual lung lobes were added to calculate the lung score, and the scores of the individual lymph nodes were added to calculate the lymph node score. Both lymph node and pathology scores were combined to determine the total pathology score for each animal.
Bacterial enumeration.
Tissue sections collected from lymph node and lung samples postmortem were individually homogenized in 5 ml of sterile phosphate-buffered saline (PBS) with a rotating-blade macerator system. Enumeration of the CFU in each sample was done by inoculating modified 7H11 agar plates with 50 μl of tissue homogenate and counting the colonies after incubation at 37°C for 4 to 6 weeks (11). A semiquantitative bacterial burden score of 0 to 4 was determined for each tissue sample, where 0 indicates no colonies, 1 indicates 1 to 10 CFU, 2 indicates 11 to 100 CFU, 3 indicates 101 to 1,000 CFU, and 4 indicates >1,000 CFU per sample. The score for all tissues was combined to provide a total bacteriology burden score per animal. All culture-positive samples from the M. tuberculosis-infected animals and a representative sample from four of the M. bovis-infected cattle were submitted to the VLA Molecular Strain Typing Laboratory to confirm the strain identity by spoligotyping. Strains were spoligotyped by the method of Kamerbeek et al. (14) with minor modifications (6).
Tuberculin skin test procedures.
For the studies at VLA (i.e., for M. bovis- and M. tuberculosis-inoculated animals), skin tests were performed as specified in European Economic Community Directive 80/219EEC and amending directive 64/422/EEC, annex B. For the studies at NADC (i.e., for the M. kansasii-inoculated animals), skin tests were performed as specified in the circular on uniform methods and rules for the eradication of bovine tuberculosis of the Animal and Plant Health Inspection Service (APHIS), USDA (APHIS circular 91-45-011 [24]). At approximately 16 weeks after inoculation, the skin thickness was measured with calipers immediately prior to the administration of M. bovis purified protein derivative (PPD), and the skin thickness was again measured 72 h after injection. The skin tests were performed at the midcervical region and the caudal fold for the studies at VLA and NADC, respectively. Data are presented as the change in the skin thickness (mm) from the preinjection measurements (mean ± standard error of the mean [SEM]).
IFN-γ assay.
Duplicate 250-μl heparinized whole-blood aliquots were distributed in 96-well plates with M. bovis PPD (10 μg/ml; VLA or Prionics Ag, Schlieren, Switzerland) or no antigen and were incubated at 37°C in a 5% CO2 atmosphere for 20 h. The IFN-γ concentrations in stimulated plasma were determined by using a commercial enzyme-linked immunosorbent assay (ELISA)-based kit (Bovigam; Prionics Ag). The absorbances of the standards (recombinant bovine IFN-γ; Endogen, Rockford, IL) and the test samples were read at 450 nm by using an ELISA plate reader (Molecular Devices, Menlo Park, CA). Duplicate samples for individual treatments were analyzed, and the data are presented as the optical densities at 450 nm of the response to M. bovis PPD minus the response to no antigen (mean ± SEM).
Lateral-flow assay.
The VetTB Stat-Pak lateral-flow assay (Chembio Diagnostic Systems, Inc., Medford, NY) was performed as previously described for use with samples from cattle (33). Briefly, the lateral-flow device consists of a plastic cassette containing a strip of nitrocellulose membrane impregnated with test antigen (i.e., MPB83, ESAT-6, and CFP-10) and laminated with several pads made of glass fiber and cellulose. Thirty microliters of serum and 3 drops of sample diluent are sequentially added to the sample pad. As the diluted test sample migrates to the conjugate pad, the latex particles conjugated to antigen bind to antibody (IgM, IgG, and IgA), if antibody is present, thus creating a colored immune complex. This complex flows laterally via capillary forces across the nitrocellulose membrane impregnated with specific antigen and binds to the immobilized antigen, producing a visible blue band. In the absence of specific antibody, no band is visible.
MAPIA.
The multiantigen print immunoassay (MAPIA) was performed as previously described for use with samples from cattle (30, 33). Briefly, M. tuberculosis complex antigens were immobilized on nitrocellulose membrane strips, blocked for 1 h with 1% nonfat skim milk in PBS with 0.05% Tween 20, and then incubated for 1 h with serum samples diluted 1:40 in blocking solution. After the strips were washed, they were incubated overnight with peroxidase-conjugated protein G (Sigma) diluted 1:1,000, washed, and developed with 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD). Responses to individual M. tuberculosis complex antigens were identified by MAPIA.
ELISA for serum IgG to MPB83 and M. bovis culture filtrate.
The amount of antigen-specific IgG in serum from clotted blood was measured as described previously (28). Briefly, Maxisorp ELISA plates (Nunc, Roskilde, Denmark) were coated with either recombinant MPB83 (0.1 μg/ml; Lionex Diagnostics and Therapeutics GmbH, Braunschweig, Germany) or an M. bovis AN5 culture filtrate previously prepared at VLA (1 μg/ml). Serum samples were diluted 1:100 prior to addition to precoated and preblocked assay plates. Wells not coated with antigen were used to ensure the absence of nonspecific responses, and the prechallenge responses were used for comparison to the specific responses after challenge. Total IgG was detected by using a horseradish peroxidase-conjugated sheep anti-bovine IgG (AbD Serotec, Oxford, United Kingdom), with the data being expressed as the optical densities at 450 nm (mean ± SEM).
ELISA for serum Ig to M. bovis LAM.
An ELISA for serum Ig to M. bovis lipoarabinomannan (LAM) was performed as described previously (30, 33). Briefly, Immulon II 96-well microtiter plates (Dynatech, Chantilly, VA) were coated with LAM-enriched mycobacterial antigen (8 μg, prepared as described previously [32]). Serum samples were diluted 1:100 prior to addition to precoated and preblocked assay plates. Total Ig was detected by using horseradish peroxidase-conjugated goat anti-bovine IgG heavy and light chains (Kirkegaard & Perry Laboratories Inc.). Data are presented as sample/positive (S/P) values (mean ± SEM). S/P values of test samples were calculated from absorbency values by using the following formula: sample − negative control/positive control − negative control. Positive and negative control sera with known reactivities were obtained from previous studies.
Statistics.
Data were analyzed by analysis of variance, followed by the Bonferroni multiple comparisons test or Student's t test, using a commercially available statistics program (Prism, version 4.0; GraphPAD Software, La Jolla, CA).
RESULTS AND DISCUSSION
Disease progression.
Granulomatous lesions containing acid-fast bacilli were detected in the lungs as well as the lung- and head-associated lymph nodes from five of five, five of five, and three of five M. bovis-infected animals, respectively (Table 1). Lesions with acid-fast bacilli were not detected in any of the M. tuberculosis- or M. kansasii-inoculated animals (Table 1). Mycobacterium bovis was isolated by culture from five of five animals, whereas M. tuberculosis was isolated from three of five animals inoculated with the respective mycobacteria. For the M. bovis-infected cattle, M. bovis was isolated by culture from lung-associated lymph nodes and lungs from five of five animals and from medial retropharyngeal lymph nodes from three of five cattle. For the M. tuberculosis-infected cattle, M. tuberculosis was isolated by culture from lung-associated lymph nodes from three of five animals and from the medial retropharyngeal lymph nodes from one of five cattle. Analysis of the spoligotype patterns confirmed that the mycobacterial strains isolated from tissues matched the respective challenge strains (i.e., H37Rv for M. tuberculosis and AF2122/97 for M. bovis; data not shown). Mycobacterium spp. were not detected in M. kansasii-inoculated animals. The mycobacterial burden differed (P < 0.05) between challenge species (Table 1), as follows: M. bovis > M. tuberculosis > M. kansasii.
TABLE 1.
Disease expression upon mycobacterial inoculation
| Group | Gross pathologya | Culture scoreb |
|---|---|---|
| M. bovis (n = 5) | All positive | 27.2 ± 7.3 |
| M. tuberculosis (n = 5) | All negative | 13.9 ± 5.5 |
| M. kansasii (n = 4) | All negative | 0 ± 0 |
Gross lesions were confirmed to be tuberculous upon histologic evaluation and culture of lung tissue as well as lung- and head-associated lymph node tissues.
Cumulative score (mean ± SEM) based on a ranking of the total numbers of CFU per plate of lung tissue and lung-associated lymph node tissue homogenates at the highest dilution. Mycobacteria were not detected in any tissues collected from M. kansasii-inoculated animals. Mean scores reflect the results from each animal within the treatment group, including culture-negative animals. The results differ (P < 0.05) according to mycobacterial treatment: M. bovis-inoculated animals > M. tuberculosis-inoculated animals > M. kansasii-inoculated animals.
Cell-mediated responses.
Injection of M. bovis PPD for the skin test (at ∼16 weeks postinoculation) resulted in palpable reactions in all calves, regardless of the mycobacterial treatment; however, differences were detected, with the responses (means ± SEMs) of the M. tuberculosis-inoculated cattle (22.6 ± 5.1) and M. bovis-inoculated cattle (12.8 ± 1.9) exceeding (P < 0.05) the responses of the M. kansasii-inoculated cattle (4.5 ± 0.3). Similarly, IFN-γ responses to M. bovis PPD were elicited in all calves regardless of the mycobacterial treatment. Two weeks after inoculation, the mean IFN-γ responses did not differ (P > 0.05) between treatment groups. From 4 weeks to the end of the study, the mean IFN-γ responses to M. bovis PPD by M. tuberculosis- and M. bovis-inoculated cattle exceeded (P < 0.05) the mean response of the M. kansasii-inoculated cattle (Fig. 1). Throughout the study, the IFN-γ responses to M. bovis PPD did not differ (P > 0.05) between the M. tuberculosis- and the M. bovis-inoculated cattle. The patterns of the IFN-γ responses to the ESAT-6 and CFP10 antigens were similar to the pattern of responses to M. bovis PPD (data not shown) (30). Thus, the delayed-type hypersensitivity and IFN-γ responses did not correlate with the disease progression differences detected between M. tuberculosis- and M. bovis-inoculated cattle. Likewise, transient IFN-γ responses may be elicited to mycobacterial species (e.g., M. kansasii) that are efficiently cleared by cattle. It is interesting to note that the IFN-γ response kinetics toward challenge with all three pathogens developed very similarly up to 2 weeks postinfection, after which time the responses in the M. kansasii-infected calves were curtailed and remained at a significantly lower level throughout the rest of the observation period. One caveat concerning this observation is that M. bovis antigens were used for the assay; thus, the responses may be indicative of early cross-reactive responses. Two weeks postinfection is most likely the time point when a developing cellular immune response becomes effective, and this response appears to be capable of eliminating M. kansasii, while it is unable to fully control M. bovis infection and, to a lesser extent, M. tuberculosis infection. This response kinetic is very similar to that observed after mouse aerosol infection, in which M. tuberculosis is exported to the regional lymph nodes for the development of the initial primary response (7, 21, 36). This delayed immune kinetic in mice is associated with the slow and delayed migration of parenchymal dendritic cells to pulmonary lymph nodes (26). It would be of interest to conduct similar studies with M. kansasii to see if the migration kinetics are identical to those for its more virulent cousins, M. tuberculosis and M. bovis.
FIG. 1.
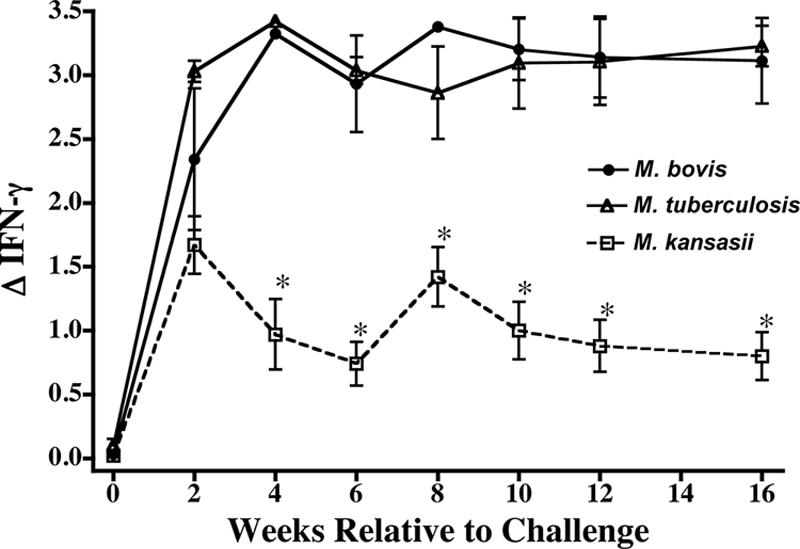
IFN-γ responses upon experimental inoculation of calves with M. bovis (n = 5), M. tuberculosis (n = 5), or M. kansasii (n = 4). Data are presented as optical densities (means ± SEMs) at 450 nm of the response to M. bovis PPD minus the response to no antigen (ΔIFN-γ). *, the response was significantly different (P < 0.5) from the responses by the M. bovis- and M. tuberculosis-inoculated animals at the same time point.
Antibody responses.
Specific antibody responses were detected in all M. tuberculosis- and M. bovis-inoculated cattle 3 weeks after inoculation (Table 2). At 12 and 16 weeks after inoculation, the IgG responses to MPB83 and M. bovis culture filtrate by M. bovis-inoculated cattle exceeded (P < 0.05) the respective responses by the M. tuberculosis-inoculated cattle (Fig. 2). Antibody levels to MPB83 persisted from 3 to 16 weeks after challenge with the M. bovis group, whereas the responses to MPB83 by the M. tuberculosis-inoculated cattle waned from 6 to 16 weeks after challenge (Fig. 2). Using a nonquantitative assay (i.e., MAPIA), three of four M. bovis-inoculated cattle responded to MPB83 by 3 weeks after challenge and four of four cattle responded at all other subsequent time points, whereas three of five, four of five, four of five, and three of five M. tuberculosis-inoculated cattle responded to MPB83 at 3, 6, 9, and 12 weeks after challenge, respectively. Mycobacterium kansasii inoculation elicited LAM-specific IgG: the preinoculation response (given as the mean ± SEM S/P ratio) was 0.12 ± 0.13, whereas the response was 1.01 ± 0.28 at 7 weeks after inoculation (P < 0.05). The initial early responses waned by 10 weeks after challenge to 0.50 ± 0.20 and then increased (P < 0.05) 2 weeks after injection of PPD for the skin test at 18 weeks after challenge to 1.47 ± 0.26. Prior to the skin test, only two of four M. kansasii-inoculated calves had antibody specific to the MPB83, ESAT-6, and CFP10 antigen cocktail (i.e., as determined by the VetTB Stat-Pak lateral-flow assay), whereas after the skin test, all four animals had responses to this antigen cocktail (Table 2).
TABLE 2.
Kinetics of serum antibody responses to MPB83, ESAT-6, and CFP-10 in cattle during experimental mycobacterial infection detected by VetTB Stat-Pak lateral-flow assay
| Group | No. of animals positive/no. of animals tested at the following wk after inoculation: |
|||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 18a | |
| M. bovisb | 0/4 | 4/4 | 4/4 | 3/4 | 3/4 | NAc |
| M. tuberculosis | 0/5 | 5/5 | 4/5 | 2/5 | 0/5 | NA |
| M. kansasii | 0/4 | 0/4 | 0/4 | 2/4 | 1/4 | 4/4 |
For the M. kansasii-inoculated cattle, the responses at 18 weeks were 2 weeks after the injection of PPD for the skin test.
One of the M. bovis-infected cattle was euthanized at week 6, and therefore, kinetics data were available for only four of the five original study animals.
NA, animals in the M. tuberculosis- and M. bovis-inoculated groups were euthanized 16 weeks after inoculation; thus, samples were not available for analysis at that time point.
FIG. 2.
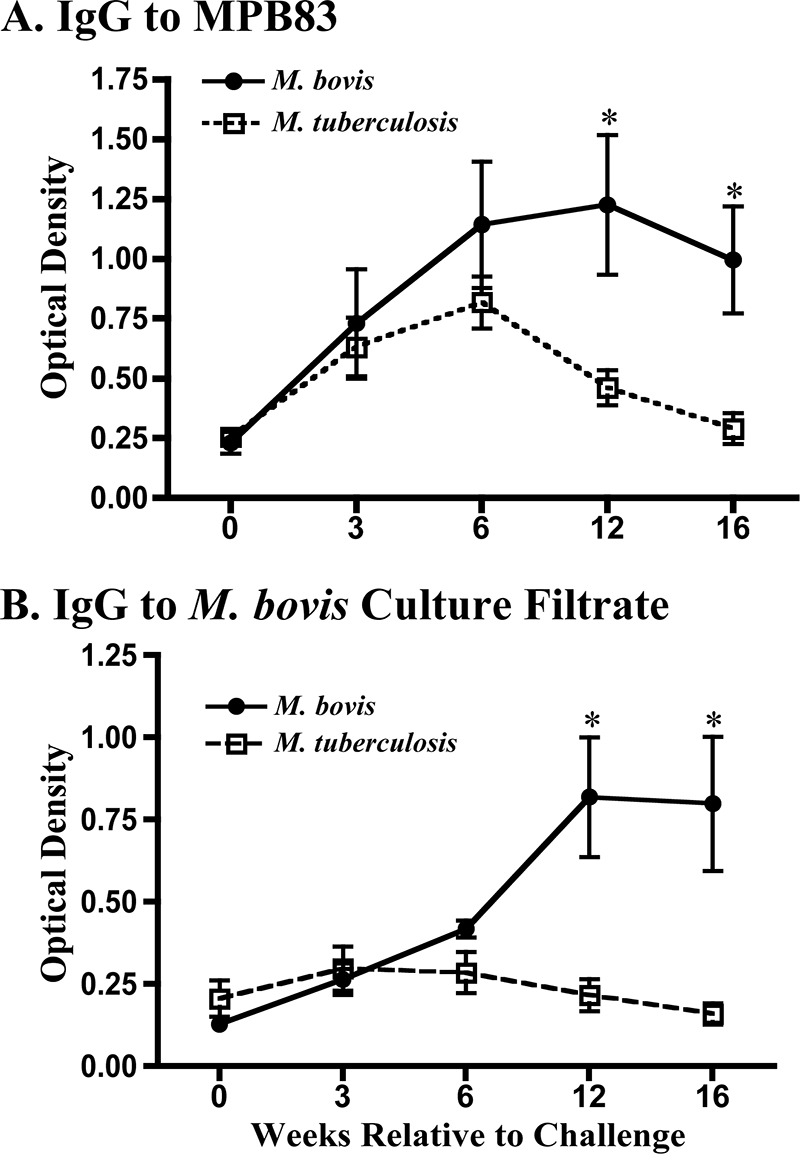
Antibody responses upon experimental inoculation of calves with M. bovis (n = 5) or M. tuberculosis (n = 5). Data are presented as the optical densities (means ± SEMs) at 450 nm of the response to MPB83 (A) or M. bovis culture filtrate (B). *, the response was significantly different (P < 0.5) from the response by M. tuberculosis-inoculated animals at the same time point.
The strong and persistent generation of MPB83-specific IgG following infection with M. tuberculosis is an interesting observation, given that in vitro M. tuberculosis expresses very small amounts of MPB83 compared with the amount expressed by M. bovis (35). Potent immune responses to MPB83 have previously been observed in M. tuberculosis-infected mice (13), and the response is also upregulated in M. tuberculosis-infected macrophages (i.e., in vitro) (22, 23). Together with the present findings of MPB83-specific responses by M. tuberculosis-inoculated cattle, these data support the notion that MPB83 expression is upregulated by M. tuberculosis in vivo.
Immune response-disease progression associations.
As indicated in the early 1900s (25), the administration of culture-derived M. tuberculosis (in this case, H37Rv) to healthy cattle elicits an immune response without the expression of disease (i.e., pathology), thus providing the rationale for early 20th century studies evaluating live M. tuberculosis as a vaccine for bovine tuberculosis. In the present study, M. tuberculosis was detected in three of five inoculated animals upon necropsy, which was at ∼16 weeks after inoculation. MPB83-specific antibody was detectable early after challenge in all five M. tuberculosis-inoculated animals, yet these responses waned to preinoculation levels by the end of the study (Fig. 2A). Thus, with experimental M. tuberculosis H37Rv infection of cattle, antibody responses are associated with mycobacterial burden rather than pathology. A wane in the early MPB83-specific antibody response likely followed the clearance of M. tuberculosis from the host. In contrast, persistent M. bovis infection resulted in lesions and sustainable specific antibody responses (Fig. 2). Together, these findings indicate that antibody responses upon mycobacterial infection are indicative of the antigen load rather than the mycobacterium-induced pathology.
Inoculation of M. kansasii elicited LAM-specific responses in four of four cattle and MPB83-specific responses in two of four cattle, as detected by MAPIA. Injection of PPD for the skin test boosted both the LAM- and the MPB83-specific responses in all four animals, as previously detected with M. bovis infection of cattle (33). Mycobacteria were not isolated upon necropsy, and lesions were not detected. These findings demonstrate the potential for a correlation of the specific antibody responses to a boosting effect resulting from exposure to cross-reactive antigens, even when the mycobacteria have likely been cleared weeks prior to subsequent exposure.
Conclusions.
In this study, cellular immune responses correlated with mycobacterial infection but not necessarily with the pathology or bacterial burden. The findings of the present study highlight several scenarios for the interpretation of antibody responses by cattle to mycobacterial exposure. (i) The first scenario is the classic model, in which infection (e.g., M. bovis infection of cattle) is not controlled by the host, resulting in significant pathology and mycobacterial burden. With this scenario, the association of the antibody responses to pathology (15) is likely coincident with the antigen burden. (ii) The second scenario is an antigen load model in which the antibody responses are positively correlated to the antigen burden. With M. tuberculosis H37Rv infection of cattle, as the mycobacterium is cleared, the antibody responses wane. With M. bovis infection of cattle, as the infection progresses and the mycobacteria persist, the antibody responses also persist or increase. (iii) The final scenario is the sensitization model, in which exposure to a mycobacterium (e.g., M. kansasii inoculation) induces an antibody response which wanes over time, yet the response may be boosted significantly by reexposure to mycobacterial antigens (e.g., PPD) or other live mycobacteria.
Good and reliable models of human latent tuberculosis are not readily available for support of both applied tuberculosis research (e.g., vaccinology and diagnostic studies) and basic tuberculosis research (e.g., immunopathogenesis and bacterial pathogenesis). An animal model in which different infection outcomes can be modeled with organisms with overlapping antigen repertoires, such as the three organisms studied here, would be of advantage. Mycobacterium bovis infection of cattle could be considered a model of clinical tuberculosis, while M. tuberculosis H37Rv infection could be akin to latent, subclinical tuberculosis. Infection with M. kansasii, on the other hand, might be a model for a human tuberculous infection that has successfully been cleared from its human host. Therefore, we propose that these three infection systems be developed into useful models mimicking different stages of human M. tuberculosis infection.
Acknowledgments
We thank Mike Howard, Peter Lasley, Jessica Pollock, Todd Stuber, and Shelly Zimmerman for excellent technical support. We also appreciate the combined efforts of the animal care staff at NADC and VLA.
The studies performed at VLA were funded by the Department for Environment, Food and Rural Affairs (DEFRA), United Kingdom.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Alcaide, F., I. Richter, C. Bernasconi, B. Springer, C. Hagenau, R. Schulze-Robbecke, E. Tortoli, R. Martin, E. C. Bottger, and A. Telenti. 1997. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J. Clin. Microbiol. 35:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend, S. M., P. de Haas, E. Leyten, I. Rosenkrands, L. Rigouts, P. Andersen, W. Mijs, J. T. van Dissel, and D. van Soolingen. 2005. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J. Infect. Dis. 191:1301-1310. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., E. Cerda de Palou, P. de Haas, R. Janssen, M. A. Hoeve, E. M. Verhard, T. H. Ottenhoff, D. van Soolingen, and J. T. van Dissel. 2004. Pneumonia caused by Mycobacterium kansasii in a series of patients without recognized immune defect. Clin. Microbiol. Infect. 10:738-748. [DOI] [PubMed] [Google Scholar]
- 4.Bolin, C. A., D. L. Whipple, K. V. Khanna, J. M. Risdahl, P. K. Peterson, and T. W. Molitor. 1997. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. J. Infect. Dis. 176:1559-1566. [DOI] [PubMed] [Google Scholar]
- 5.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 6.Cadmus, S., S. Palmer, M. Okker, J. Dale, N. Smith, K. Jahans, R. G. Hewinson, and S. V. Gordon. 2006. Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J. Clin. Microbiol. 44:996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chackerian, A., J. Alt, T. Perera, C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousins, D. V., R. Bastida, A. Cataldi, V. Quse, S. Redrobe, S. Dow, P. Duignan, A. Murray, C. Dupont, N. Ahmed, D. M. Collins, W. R. Butler, D. Dawson, D. Rodríguez, J. Loureiro, M. I. Romano, A. Alito, M. Zumarraga, and A. Bernardelli. 2003. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 53:1305-1314. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, P. L., and R. J. North. 1995. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce pathology, and cause mortality in mice. Infect. Immun. 63:3428-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, J. 1947. Bovine tuberculosis, including a contrast with human tuberculosis, p. 86-87. Staples Press Ltd., London, United Kingdom.
- 11.Gallagher, J., and D. M. Horwill. 1977. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. J. Hyg. 79:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewinson, R. G., S. L. Michell, W. P. Russell, R. A. McAdam, and W. R. Jacobs, Jr. 1996. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand. J. Immunol. 43:490-499. [DOI] [PubMed] [Google Scholar]
- 14.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Sooliingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcotty, T., F. Matthys, J. Godfroid, L. Rigouts, G. Ameni, N. G. van Pittius, R. Kazwala, J. Muma, P. van Helden, K. Walravens, L. M. de Klerk, C. Geoghegan, D. Mbotha, M. Otte, K. Amenu, N. Abu Samra, C. Botha, M. Ekron, A. Jenkins, F. Jori, N. Kriek, C. McCrindle, A. Michel, D. Morar, F. Roger, E. Thys, and P. van den Bossche. 2009. Zoonotic tuberculosis and brucellosis in Africa: neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Ann. Trop. Med. Parasitol. 103:401-411. [DOI] [PubMed] [Google Scholar]
- 17.Medina, E., L. Ryan, R. LaCourse, and R. J. North. 2006. Superior virulence of Mycobacterium bovis over Mycobacterium tuberculosis (Mtb) for Mtb-resistant and Mtb-susceptible mice is manifest as an ability to cause extrapulmonary disease. Tuberculosis (Edinb.) 86:20-27. [DOI] [PubMed] [Google Scholar]
- 18.Nedeltchev, G. G., T. R. Raghunand, M. S. Jassal, S. Lun, Q. J. Cheng, and W. R. Bishai. 2009. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect. Immun. 77:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, M. V., and W. R. Waters. 2006. Advances in bovine tuberculosis diagnosis and pathogenesis: what policy makers need to know. Vet. Microbiol. 112:181-190. [DOI] [PubMed] [Google Scholar]
- 21.Reiley, W. W., M. D. Calayag, S. T. Wittmer, J. L. Huntington, J. E. Pearl, J. J. Fountain, C. A. Martino, A. D. Roberts, A. M. Cooper, G. M. Winslow, and D. L. Woodland. 2008. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in mediastinal lymph nodes. Proc. Natl. Acad. Sci. U. S. A. 105:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saïd-Salim, B., S. Mostowy, A. S. Kristof, and M. A. Behr. 2006. Mutations in Mycobacterium tuberculosis Rv0444c, the gene encoding anti-SigK, explain high level expression of MPB70 and MPB83 in Mycobacterium bovis. Mol. Microbiol. 62:1251-1263. [DOI] [PubMed] [Google Scholar]
- 23.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.USDA, APHIS. 2005. Bovine tuberculosis eradication uniform methods and rules, p. 1-29. Circular APHIS 91-45-011. U.S. Government Printing Office, Washington, DC.
- 25.Von Behring, E. 1901. Nobel lecture: serum therapy in therapeutics and medical science, 12 December 1901. http://nobelprize.org/nobel_prizes/medicine/laureates/1901/behring-lecture.html.
- 26.von Garnier, C., L. Filgueira, M. Wikstrom, M. Smith, J. Thomas, D. H. Strickland, P. G. Holt, and P. A. Stumbles. 2005. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 175:1609-1618. [DOI] [PubMed] [Google Scholar]
- 27.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, M. A. Chambers, D. Clifford, K. Huygen, R. Tascon, D. Lowrie, M. J. Colston, and R. G. Hewinson. 2000. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:1246-1255. [DOI] [PubMed] [Google Scholar]
- 29.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters, W. R., M. V. Palmer, T. C. Thacker, J. B. Payeur, N. B. Harris, F. C. Minion, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2006. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13:611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters, W. R., M. V. Palmer, and D. L. Whipple. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diagn. Invest. 14:470-475. [DOI] [PubMed] [Google Scholar]
- 33.Waters, W. R., M. V. Palmer, T. C. Thacker, J. P. Bannantine, H. M. Vordermeier, R. G. Hewinson, R. Greenwald, J. Esfandiari, J. McNair, J. M. Pollock, P. Andersen, and K. P. Lyashchenko. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wedlock, D. N., F. E. Aldwell, D. M. Collins, G. W. de Lisle, T. Wilson, and B. M. Buddle. 1999. Immune response induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed type hypersensitivity with ability of strains to grow in macrophages. Infect. Immun. 67:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiker, H. G. 2009. MPB70 and MPB83—major antigens of Mycobacterium bovis. Scand. J. Immunol. 69:492-499. [DOI] [PubMed] [Google Scholar]
- 36.Wolf, A., L. Desvignes, B. Linas, N. Banaiee, T. Tamura, K. Takatsu, and J. D. Ernst. 2008. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]


