Abstract
Mycobacterium tuberculosis infection is a major world health issue. The early identification of patients at risk for a poor response to anti-M. tuberculosis therapy would help elucidate the key players in the anti-M. tuberculosis response. The objective of the present study was to correlate the modulation of cytokine expression (interleukin-1 [IL-1], IL-6, IL-8, IL-10, IL-12, gamma interferon [IFN-γ], interferon-inducible protein [IP-10], and monocyte chemotactic protein 1 [MCP-1]) with the clinical response to 2 months of intensive therapy. From January to December 2007, 40 M. tuberculosis-infected patients and 40 healthy patients were recruited. After exclusion for diabetes, 32 patients and 36 controls were analyzed. The clinical responses of the M. tuberculosis-infected patients on the basis of the findings of chest radiography were compared to their plasma cytokine levels measured before and after 2 months of intensive anti-M. tuberculosis therapy and 6 months of therapy with human cytokine antibody arrays. Chest radiographs of 20 of 32 M. tuberculosis-infected patients showed improvement after 2 months of intensive therapy (early responders), while the M. tuberculosis infections in 12 of 32 of the patients resolved after a further 4 months (late responders). The levels of expression of TNF-α, MCP-1, IFN-γ, and IL-1β were decreased; and the level of IL-10 increased in early responders. After adjustment for age, gender, and the result of sputum culture for M. tuberculosis, significant differences in the levels of MCP-1 and IP-10 expression were observed between the early and the late responders after 2 months of intensive anti-M. tuberculosis therapy. Due to the interpatient variability in IP-10 levels, intrapatient monitoring of IP-10 levels may provide more insight into the M. tuberculosis responder status than comparison between patients. Plasma MCP-1 levels were normalized in patients who had resolved their M. tuberculosis infections. Further studies to evaluate the association of the modulation in MCP-1 levels with early and late responses are warranted.
Mycobacterium tuberculosis, the cause of a major world health issue, has evolved mechanisms for eluding or suppressing the host's immune response. M. tuberculosis often festers in a chronic, unresolved state. Cellular immunity plays a major role in the control of the infection. The underlying innate immunity, coupled with cytokine and chemokine secretion, aids with the recruitment, activation, stimulation, or downregulation of the appropriate effectors. It is feasible that some immune mediators such as cytokines and chemokines may correlate with therapeutic efficacy (4). Individuals with M. tuberculosis infection and/or reactivation exhibit the phagocytosis-induced production of tumor necrosis factor alpha (TNF-α) (20, 21), interferon-1β (IL-1β) (16), IL-10 (6, 30), IL-12 (14), and gamma interferon (IFN-γ)-inducing protein (IP-10; CXCL10) (18, 26). Priming, expansion, differentiation, and trafficking of M. tuberculosis antigen-specific CD4+ and CD8+ T cells and NK cells increases the level of production of IFN-γ (24), a cytokine critical for augmenting the microbicidal activity of phagocytes (15). M. tuberculosis reduces the effects of IFN-γ by inhibiting the transcription of IFN-γ-responsive genes and by inducing the secretion of IL-6, which inhibits IFN-γ signaling (23). TNF-α is a pleiotropic cytokine preferentially secreted by monocytes and macrophages, and it plays a prominent role in M. tuberculosis infection (2). TNF or TNF receptor-knockout (KO) mice exhibit significantly increased susceptibility to M. tuberculosis infection (7, 27) and have poorly formed granulomas, mass regions of necrosis, and infiltration of the neutrophilic alveoli. Defective TNF-TNF receptor signaling culminates in massive inflammation and necrosis as a result of an uncontrolled T-helper type 1 immune response to the overproduction of IFN-γ, and IL-12 (33). Chemokines and cytokines (C-X-C chemokines IL-8 and IP-10 in particular) are pivotal in controlling cellular influx into sites of infection, and their multifaceted actions stimulate the focal accumulation of infected macrophages surrounded by T cells, forming the cardinal lung granulomas of M. tuberculosis (5). Defective responses of T cells and/or the inactivation of TNF-α (21) or other effectors of adaptive immunity also contribute to granuloma disruption and the persistence of M. tuberculosis.
While advances in cellular immunology have led to rapid, convenient, antigen-specific T-cell response-based diagnostic tests that evaluate M. tuberculosis-specific IFN-γ expression, no reliable, early immune-based marker of a pulmonary M. tuberculosis relapse currently exists. Clinicians currently rely on evidence of sputum smear conversion after a 2-month intensive phase of anti-TB treatment or later time points (12) and the extent of pulmonary disease noted on chest radiographs (9). The cytokine levels in bronchoalveolar lavage (BAL) fluids (BALFs) of the infected lung region have correlated with the radiologic score in a 15-patient study of active pulmonary M. tuberculosis (11). Active M. tuberculosis was associated with elevated plasma IL-10 and PTX3 levels (4). The modulation of IL-10, IP-10, and IL-12 levels from M. tuberculosis-stimulated whole-blood cells has been associated with disease activity and the response to therapy in recent studies (18, 30). On the basis of the findings of those studies, we hypothesized that the levels of some cytokines in the plasma of M. tuberculosis-infected patients may correlate with the patients' response to anti-TB treatment. The objective of this study was to correlate changes in the levels of cytokine expression (IL-1β, IL-6, IL-8, IL-10, IL-12, IFN-γ, TNF-α, IP-10, and monocyte chemotactic protein 1 [MCP-1]) to the clinical response, monitored by the use of chest radiography, in patients with pulmonary M. tuberculosis infection after 2 months of intensive anti-TB therapy and 6 months of treatment. Although some studies have investigated the release of cytokines in the BALF (11) or from Mycobacterium-stimulated whole-blood cells (18, 30), we chose to measure the cytokine levels present in the plasma, similar to the work of Azzurri et al. (4), to assess potentially rapid, immune-based markers of the therapeutic response.
MATERIALS AND METHODS
Patients.
This laboratory study was approved by the local institutional review board, and informed consent was obtained from all subjects. From January 2007 to December 2007, 32 patients with pulmonary M. tuberculosis infection and 36 healthy participants were recruited from the Tri-Service General Hospital in Taiwan. Pulmonary M. tuberculosis infection was diagnosed according to the following criteria: (i) for primary tuberculosis, middle or lower lung infiltration with or without ipsilateral hilar lymphadenopathy, and (ii) for secondary tuberculosis, unilateral or bilateral upper lung cavitation or infiltration (right upper lobe apical and posterior segments, left lobe apical-posterior segment). The definitive diagnosis of tuberculosis was established by the isolation of tubercle bacilli in culture or the identification of specific nucleic acid sequences (by TB-PCR) from sputum or bronchoalveolar lavage fluid (13). Patients and healthy participants with diabetes, HIV infection, malignancy, uremia, autoimmune disease, or alcoholism were excluded. The exclusion criteria for the healthy participants also included a history of tuberculosis and a pulmonary infection.
Nonsmokers (“never” in Tables 1 and 2) were defined as individuals who had smoked <20 cigarettes in total during their lifetimes, whereas former smokers (“previous” in Tables 1 and 2) had quit smoking at least 6 months before pulmonary tuberculosis was diagnosed. Current smokers (“current” in Tables 1 and 2) had smoked in the previous 6 months, including daily and occasional smoking.
TABLE 1.
Baseline characteristics of healthy participants and TB patientsa
| Characteristic | Control subjects (n = 36) | TB patients (n = 32) | P |
|---|---|---|---|
| Age (yr)b | 40.0 (36.3, 70.3) | 52.5 (40.5, 75.8) | 0.117 |
| Genderc | 0.866 | ||
| Male | 31 (86.1) | 28 (87.5) | |
| Female | 5 (13.9) | 4 (12.5) | |
| Smokingd | 0.652 | ||
| Never | 25 (69.4) | 24 (75.0) | |
| Previous | 3 (8.3) | 1 (3.1) | |
| Current | 8 (22.2) | 7 (21.9) | |
| Severity of lung disease | |||
| Limited | 14 (43.8) | NA | |
| Extended | 18 (56.3) | ||
| Lung pattern | |||
| Coalescent | 21 (65.6) | NA | |
| Infiltrative | 11 (34.4) | ||
| AFB smear result | |||
| Negative | 21 (65.6) | NA | |
| Positive | 11 (34.4) | ||
| Cytokine concn (pg/ml) | |||
| TNF-αb | 28.9 (28.6, 40.3) | 34.7 (32.8, 38.9) | 0.001* |
| IL-6b | 9.3 (9.2, 9.6) | 9.6 (9.2, 11.1) | 0.024* |
| IL-8b | 750.4 (731.6, 763.4) | 901.2 (797.6, 2,267.4) | <0.001* |
| IL-10b | 30.2 (28.9, 35.7) | 30.4 (28.8, 32.4) | 0.610 |
| IL-12b | 183.2 (178.0, 199.4) | 192.9 (189.1, 198.5) | 0.005* |
| MCP-1b | 2,793.2 (2,778.2, 2,853.5) | 3,717.2 (2,991.6, 5,277.3) | <0.001* |
| IFN-γb | 25.8 (24.8, 27.3) | 28.0 (27.4, 30.8) | <0.001* |
| IL-1βb | 28.9 (28.7, 30.5) | 30.8 (30.0, 43.0) | <0.001* |
| IP-10b | 171.5 (167.6, 181.7) | 197.0 (173.1, 251.4) | <0.001* |
Data are for a total of 68 patients and are presented as medians (interquartile ranges) for continuous variables and numbers of individuals (percentages) for categorical variables. AFB, acid-fast bacillus; NA, not applicable; *, significant difference between the two groups (P < 0.05).
P values were calculated by the Mann-Whitney U test for continuous variables.
P values were calculated by the chi-square test for categorical variables.
P values were calculated by Fisher's exact test for categorical variables.
TABLE 2.
Characteristics and cytokine expression of TB patients stratified by treatment outcome at 2 monthsa
| Characteristic | Late responders (n = 12) | Early responders (n = 20) | P |
|---|---|---|---|
| Age (yr)b | 63.5 (43.8, 79.5) | 50 (39.3, 74.3) | 0.255 |
| Genderd | 0.581 | ||
| Male | 10 (83.3) | 18 (90.0) | |
| Female | 2 (16.7) | 2 (10.0) | |
| Smokingd | 0.381 | ||
| Never | 8 (66.7) | 16 (80.0) | |
| Previous | 1 (8.3) | 0 (0.0) | |
| Current | 3 (25.0) | 4 (20.0) | |
| Severity of lung diseasec | 0.854 | ||
| Limited | 5 (41.7) | 9 (45.0) | |
| Extended | 7 (58.3) | 11 (55.0) | |
| Predominant lung patternc | 0.149 | ||
| Coalescent | 6 (50.0) | 15 (75.0) | |
| Disseminated | 6 (50.0) | 5 (25.0) | |
| AFB smear result before therapyc | 0.102 | ||
| Negative | 10 (83.3) | 11 (55.0) | |
| Positive | 2 (16.7) | 9 (45.0) | |
| AFB smear result after 2 mo | 0.114 | ||
| Negative | 7 (58.3) | 14 (70.0) | |
| Positive | 5 (41.7) | 6 (30.0) | |
| Sputum TB culture result after 2 mo | <0.001* | ||
| Negative | 0 (0.0) | 14 (70.0) | |
| Positive | 12 (100.0) | 6 (30.0) | |
| Cytokine concn (pg/ml) before anti-TB therapy | |||
| TNF-αb | 36.5 (33.1, 40) | 34.5 (32.7, 37.8) | 0.239 |
| IL-6b | 10.6 (9.2, 12.5) | 9.4 (9.3, 10.5) | 0.659 |
| IL-8b | 901.2 (762.8, 3,457.9) | 892.1 (827, 2,267.4) | 0.833 |
| IL-10b | 29.3 (28.2, 32.4) | 30.5 (29.8, 32.1) | 0.346 |
| IL-12b | 192.8 (189.3, 198.5) | 192.9 (188.9, 198.4) | 0.833 |
| MCP-1b | 4,470.1 (3,102.9, 6,371.6) | 3,206.6 (2,947.1, 5,134.3) | 0.136 |
| IFN-γb | 28.7 (27.2, 34.5) | 28 (27.4, 30) | 0.803 |
| IL-1βb | 31.5 (29.8, 59.7) | 30.7 (30.1, 35) | 0.578 |
| IP-10b | 236.8 (167.6, 333.5) | 189.1 (175.5, 226.1) | 0.387 |
| Cytokine concn (pg/ml) 2 mo after anti-TB therapy | |||
| TNF-αb | 41.3 (36.4, 52.5) | 30.2 (29.3, 32.6) | <0.001* |
| IL-6b | 10.3 (9.3, 12.3) | 9.4 (9.3, 10) | 0.272 |
| IL-8b | 925.4 (764.6, 1,617.7) | 737.8 (721.7, 783.2) | 0.005* |
| IL-10b | 30.4 (28.8, 33) | 35.7 (32.2, 38.4) | 0.004* |
| IL-12b | 193.5 (186.9, 201.2) | 179.5 (179.1, 184.8) | <0.001* |
| MCP-1b | 4,273.6 (2,979.5, 5,621.1) | 2,922.3 (2,809.7, 3,024.4) | 0.001* |
| IFN-γb | 28.6 (27.2, 33.1) | 25.7 (25.4, 26.2) | <0.001* |
| IL-1βb | 30.9 (30, 65.8) | 29.6 (28.9, 29.9) | <0.001* |
| IP-10b | 226.0 (167.5, 344.6) | 169.8 (167.7, 180.5) | 0.064 |
| Cytokine concn (pg/ml) 5 mo after anti-TB therapy | |||
| TNF-αb | 33.1 (30.6, 34.4) | 30.6 (29.9, 32.1) | 0.032* |
| IL-6b | 9.7 (9.2, 10) | 9.3 (9.3, 9.5) | 0.632 |
| IL-8b | 739.1 (726, 767.7) | 730.2 (718.6, 756) | 0.255 |
| IL-10b | 29.8 (28.3, 31.8) | 35.0 (32.8, 38.9) | 0.007* |
| IL-12b | 189.6 (183.7, 194.7) | 181.2 (179.7, 184.6) | <0.001* |
| MCP-1b | 2,804.0 (2,775.6, 2,862.6) | 2,791.5 (2,780.6, 2,847.1) | 0.863 |
| IFN-γb | 27.4 (25.8, 29.4) | 25.8 (25.4, 26.5) | 0.004* |
| IL-1βb | 30.1 (29, 30.5) | 29.3 (28.9, 29.9) | 0.070 |
| IP-10b | 176.5 (167.4, 188.9) | 170.0 (167.7, 174.6) | 0.209 |
Data are for a total of 32 patients and are presented as medians (interquartile ranges) for continuous variables and numbers of individuals (percentages) for categorical variables. AFB, acid-fast bacillus; *, significant difference between the two groups (P < 0.05).
P values were calculated by the Mann-Whitney U test for continuous variables.
P values were calculated by the chi-square test for categorical variables.
P values were calculated by Fisher's exact test for categorical variables.
Treatment regimen and chest radiography.
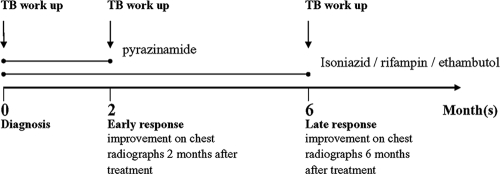
The short-course 6-month anti-TB therapy is depicted in Fig. 1 and consisted of two sequential therapeutic regimens. The 2-month intensive phase involved the administration of isoniazid (5 mg/kg of body weight/day), rifampin (10 mg/kg/day), pyrazinamide (15 to 30 mg/kg/day), and ethambutol (15 to 25 mg/kg/day). Subsequently, the 4-month continuation phase included isoniazid (5 mg/kg/day), rifampin (10 mg/kg/day), and ethambutol (15 to 25 mg/kg/day) administration. To monitor the patients' responses to therapy, conventional chest radiography was performed at three time points: (i) at the baseline or before the intensive phase of anti-TB treatment, (ii) after the 2 months of intensive therapy, and (iii) after the 4-month continuation phase (6 months after the initiation of therapy). Chest radiographs (two views at each of the three time points) were independently scored by two pulmonologists who were blinded to the patients' status. The severity of TB was based on the sizes of the lung lesions in relation to the unilateral lung field detected on chest radiography at pretreatment. The severity was scored as extended disease (lesions in one-third of the lung field or greater) or limited disease (lesions in less than one-third of the lung field). Chest radiographs were also categorized as coalescent-predominant and disseminated-predominant lung patterns.
FIG. 1.
Schematic timeline of therapeutic regimen and clinical assessment. The workup for TB included sputum smear, chest radiographs, and plasma cytokine level determination.
Cytokine detection in plasma.
Anti-human cytokine monoclonal antibodies for TNF-α, IL-6, IL-8, IL-10, MCP-1, IFN-γ, IL-1β, IP-10, and IL-12 (R&D Systems, Inc.) were dissolved in sterile phosphate-buffered saline (PBS) to concentrations of 500 μg/ml and printed on an array by use of a Microsys 5100 microarray system (Cartesiam Technology). Acetaldehyde was spread on the microarrays (4 by 7; CSS-100 silylated glass slides; CEL Associates, Houston, TX) along with PBS containing 1% bovine serum albumin and 0.05% Tween 20 (PBSBT; negative control) and biotinylated anti-human cytokine antibody (positive control). The cytokine arrays were placed at 4°C for 24 h. After the cytokine arrays were blocked with PBS containing 1% bovine serum albumin (PBSB; 2%) blocking buffer at room temperature for 30 min, they were washed with PBS-Tween 20 (PBST; 0.025%) for 1 min and with PBS for 2 min and were then dried by centrifugation.
One-milliliter blood samples were collected and placed in tubes with the anticoagulant EDTA, and the tubes were centrifuged at 3,000 rpm for 10 min. The supernatant or plasma (10 μl) was added to the corresponding blocks of the cytokine arrays at room temperature and was removed after 30 min. The cytokine arrays were washed in PBST (0.025%) for 3 min and dried by centrifugation. A mixture of antibodies for the detection of nine biotinylated anti-human cytokine antibodies (R&D Systems, Inc.) were diluted with PBSBT at 40 μg/ml (1:200) and added to the cytokine arrays, which were kept at room temperature for 30 min. The arrays were then removed and washed with PBST (0.025%) for 3 min and with PBS wash buffer for 3 min, before they were dried by centrifugation. Anti-human IgG Cy5-conjugated streptavidin (Jackson ImmunoResearch laboratories, Inc.) diluted 1:1,000 with PBSBT was then added to the corresponding blocks of the cytokine array at room temperature for 20 min, removed and washed as described above, and dried by centrifugation. The cytokine arrays were examined with a microarray scanner (Genepix 4000B); and the fluorescence intensity at an absorbance of 635 nm was measured, analyzed by the use of GenePix Pro (version 6.0) software, and transformed to the cytokine concentrations (pg/ml) by the use of standard curves.
Statistical analysis.
Because normality could not be assumed, all continuous variables were represented as the median (interquartile range) and the Mann-Whitney U test was used to examine the differences between the control and the TB groups. A linear mixed model was used to evaluate the differences in the levels of cytokine expression after adjustment for covariates. The linear mixed model was also used to test the time effect for the group that improved; Tukey's test was utilized for post-hoc tests when the time effect was significant. The corresponding results are presented as means ± standard deviations (SDs) for the mixed model. Categorical variables were presented as the number (percent) and the chi-square test or the Fisher test was used, where appropriate. A P value of less than 0.05 was defined as a significant difference. All statistics were two-sided, and statistical analyses were performed by using SPSS software (version 15.0; SPSS Inc., Chicago, IL).
RESULTS
Differential cytokine expression between control subjects and TB patients.
The baseline characteristics of the 68 subjects included in this study are described in Table 1. The samples from the M. tuberculosis-infected patients showed significantly higher levels of most inflammatory cytokines compared with the levels for the control participants, including TNF-α (34.7 versus 28.9 pg/ml; P = 0.001), IL-8 (901.2 versus 750.4 pg/ml; P < 0.001), MCP-1 (3,717.2 versus 2,793.2 pg/ml; P < 0.001), IFN-γ (28.0 versus 25.8 pg/ml; P < 0.001), IL-1β (30.8 versus 28.9 pg/ml; P < 0.001), and IP-10 (197.0 versus 171.5 pg/ml; P < 0.001), followed by IL-12 and IL-6 (P < 0.05). However, the plasma IL-10 levels (30.4 versus 30.2 pg/ml; P = 0.610) were not significantly different between the TB patients and the healthy controls.
Differential cytokine expression between early and late TB responders.
Clinical improvements based on chest radiographs were observed after 2 months of intensive anti-TB therapy in 20 of 32 M. tuberculosis participants (62.5%), who were called “early responders.” After 6 months, the chest radiographs and clinical characteristics indicated that all patients had improved. The remaining 12 of the 32 patients were named “late responders.” A majority of the early responders presented negative sputum smear culture results, whereas all of the late responders displayed positive sputum smear culture results at 2 months (P < 0.001; Table 2). Before treatment, the baseline level of cytokine expression was not significantly different between the early- and the late-responding M. tuberculosis patients, although the MCP-1 level appeared to be decreased in the early responders (3,206.6 pg/ml for the early responders versus 4,470.1 pg/ml for the late responders; P = 0.136). In contrast, after 2 months of intensive anti-TB therapy, the levels of most cytokines in the early responder group were significantly different from those in the late responder group. Notably, the early responders exhibited significantly decreased levels of expression of TNF-α, IL-8, IL-12, IFN-γ, IL-1β, and MCP-1 and significantly increased levels of IL-10 expression (35.7 pg/ml for the early responders versus 30.4 pg/ml for the late responders; P = 0.004). The cytokine levels at 6 months were compared between the two groups to assess whether the cytokine levels in the late responder group had normalized within the subsequent 4 months. At 6 months, the early responders still exhibited higher IL-10 levels and lower levels of expression of TNF-α, IL-12, and IFN-γ (all P < 0.05) than the late responders. In contrast, the differences in IL-8, MCP-1, and IL-1β levels at 6 months were no longer significant (Table 2). Although the patient sample was small, the plasma MCP-1 concentration was significantly associated with improvement in the TB patients. Due to interpatient variability, the absolute levels of cytokines often overlapped between the two groups. Thus, we examined whether the change in the cytokine levels over time correlated with the early or the late responder status of the M. tuberculosis-infected patients.
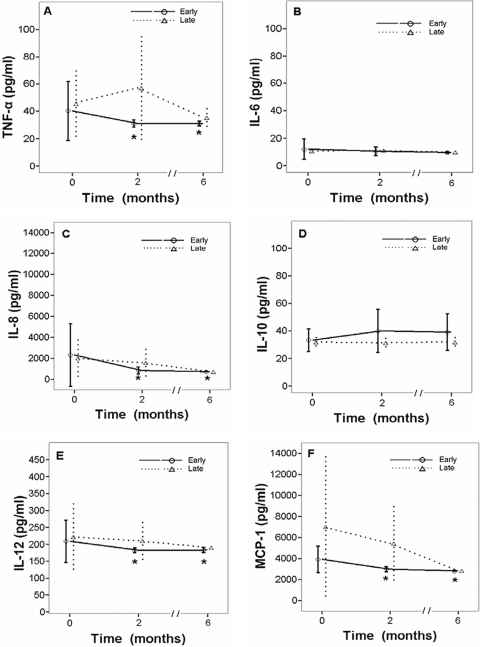
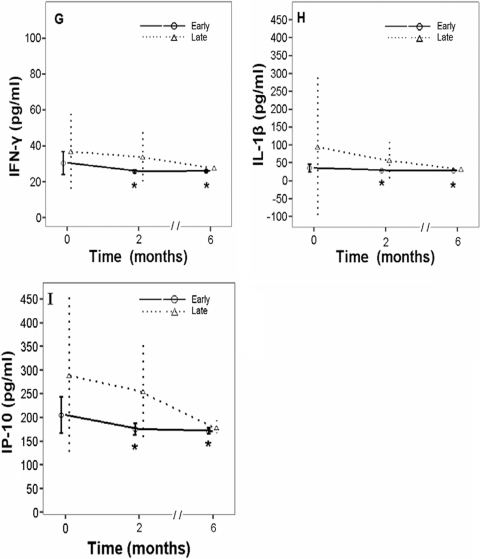
The plasma concentrations for each cytokine at pretreatment, after 2 months of intensive therapy, and after 6 months of anti-TB therapy were measured; and the changes in the levels of expression were calculated for each patient. The results for TNF-α, IL-6, IL-8, IL-10, IL-12, MCP-1, IFN-γ, IL-1β, and IP-10 were pooled for the early and the late responders (Fig. 2). Significant differences in the levels of cytokine expression relative to the baseline values were observed for TNF-α (P = 0.037), IL-8 (P = 0.008), IL-12 (P = 0.043), MCP-1 (P < 0.001), IFN-γ (P < 0.001), IL-1β (P = 0.003), and IP-10 (P < 0.001) in the early responders (Fig. 2). After adjustment for age, gender, and the result of the sputum smear, significant differences in the levels of MCP-1 and IP-10 expression between the early and the late responders were observed after 2 months of intensive anti-TB therapy (Table 3). Due to the interpatient variability in the IP-10 levels, intrapatient monitoring of IP-10 may provide more insight into the M. tuberculosis responder status than a comparison of patients.
FIG. 2.
Effects of 2 months and 6 months of intensive anti-TB therapy on the levels of expression of TNF-α (A), IL-6 (B), IL-8 (C), IL-10 (D), IL-12 (E), MCP-1 (F), IFN-γ (G), IL-1β (H), and IP-10 (I) in relationship to the initial levels of expression in 32 patients with TB. As the results were grouped by the outcome for each patient, the linear mixed model was established separately for the early and the late responder groups on the basis of the findings of chest radiography. Tukey's test was used for post-hoc tests of the effect of time. Asterisks, level significantly different from the baseline level for early responders (P < 0.05). Data are presented as the means ± SDs.
TABLE 3.
Kinetics of cytokine expression for 32 TB patients stratified by treatment outcome at 2 monthsa
| Cytokine concn (pg/ml) and time of measurement | Late responders (n = 12) | Early responders (n = 20) | P for late responders |
|---|---|---|---|
| TNF-α | 0.076 | ||
| Before | 45.5 ± 24.8 | 40.2 ± 21.6 | |
| After 2 mo | 57 ± 38.8 | 31 ± 2.7 | |
| After 6 mo | 35.4 ± 7.9 | 31 ± 1.6 | |
| IL-6 | 0.442 | ||
| Before | 10.9 ± 1.7 | 12 ± 7.4 | |
| After 2 mo | 11 ± 2 | 10.5 ± 3.1 | |
| After 6 mo | 9.8 ± 0.8 | 9.6 ± 0.6 | |
| IL-8 | 0.914 | ||
| Before | 2,012.1 ± 2,124.5 | 2,323.8 ± 2,980.8 | |
| After 2 mo | 1,574.4 ± 1,395.5 | 834.9 ± 339.8 | |
| After 6 mo | 747.2 ± 28.6 | 736 ± 19.7 | |
| IL-10 | 0.821 | ||
| Before | 31.9 ± 6.1 | 33.2 ± 8.3 | |
| After 2 mo | 31.4 ± 3.6 | 40 ± 15.7 | |
| After 6 mo | 32 ± 5.6 | 39 ± 13.3 | |
| IL-12 | 0.136 | ||
| Before | 222.5 ± 99.7 | 208.9 ± 62.5 | |
| After 2 mo | 210.3 ± 59.8 | 182.7 ± 7.2 | |
| After 6 mo | 190.8 ± 8.5 | 183.2 ± 7.4 | |
| MCP-1 | 0.041* | ||
| Before | 7,042.5 ± 6,901.2 | 3,921.6 ± 1,247.5 | |
| After 2 mo | 5,431.9 ± 3,628.8 | 2,977.9 ± 239.9 | |
| After 6 mo | 2,827.6 ± 69.4 | 2,822.4 ± 67 | |
| IFN-γ | 0.061 | ||
| Before | 37 ± 23.1 | 30.5 ± 6.3 | |
| After 2 mo | 33.9 ± 14.4 | 25.9 ± 0.9 | |
| After 6 mo | 27.7 ± 1.9 | 26.0 ± 0.8 | |
| IL-1β | 0.247 | ||
| Before | 96.5 ± 198.3 | 35.6 ± 10.6 | |
| After 2 mo | 57.5 ± 52 | 29.5 ± 0.6 | |
| After 6 mo | 32.6 ± 7.8 | 29.4 ± 0.6 | |
| IP-10 | 0.026* | ||
| Before | 290 ± 175.3 | 205.1 ± 37.9 | |
| After 2 mo | 255.4 ± 105.7 | 175.5 ± 12.5 | |
| After 6 mo | 179.5 ± 13.7 | 171.7 ± 5.8 |
A linear mixed model with adjustment for age, gender, and sputum smear result was used. Data are presented as the means ± standard deviations. *, significant difference between the two groups (P < 0.05).
DISCUSSION
While comparison of chest radiographs provides an accepted strategy for monitoring the efficacy of therapy, alternative immune-based assays would provide insights into the effectiveness of the antibacterial regimen and the host's immune response. The results of this study demonstrate that the levels of expression of most cytokines after 2 months of intensive anti-TB therapy were significantly different between the early and the late responder groups. Specifically, the levels of expression of TNF-α, MCP-1, IFN-γ, and IL-1β were decreased and the level of expression of IL-10 was increased in the early responders. In contrast, the levels of expression of IL-6 were not significantly altered by intensive anti-TB therapy in either group, despite the possible inhibitory activity of IL-6 on macrophages and dendritic cells (23). After we controlled for the confounding factors of the initial levels of expression, age, gender, and the findings on the initial chest radiograph, the levels of expression of IP-10 and MCP-1 were found to significantly decline in the early responders. These findings indicate that there is an association between the suppressed levels of expression of TNF-α, MCP-1, IFN-γ, and IL-1β and the increased level of expression of IL-10 in the TB patients who were early responders.
Despite the key role of TNF-α in the response to M. tuberculosis infection (2, 7, 21, 27), the plasma TNF-α concentrations did not distinguish between the groups, after we controlled for age, gender, and the sputum culture result. The C-C chemokines (two adjacent cysteine residues proximal to the amino terminus, such as MCP-1 and macrophage-inhibitory protein-1α) and the C-X-C chemokines (such as IL-8, IP-10, and IL-1β) are putatively involved in the orchestrated recruitment of inflammatory cells conducive to granuloma formation (1, 5). MCP-1 expression has correlated with potent nitric oxide generation and the subsequent reduction in the M. tuberculosis loads in murine lungs (19). Similarly, our data provide a correlation between normalized MCP-1 levels and the resolution of the M. tuberculosis infection in patients, as detected by chest radiography. These data suggest that MCP-1 levels may decline as the M. tuberculosis infection resolves. The potential utility of the levels of MCP-1, MCP-2, and IP-10 secreted by M. tuberculosis-stimulated whole blood as a biomarker of M. tuberculosis infection was recently observed in a small study of eight patients (28). Our study suggests that plasma MCP-1 levels may also provide a novel biomarker that can be coupled with M. tuberculosis diagnostic parameters. Similar to Ruhwald et al. (28), Lighter et al. (18) observed a strong correlation between the IP-10 levels released by M. tuberculosis-stimulated whole blood from M. tuberculosis-infected children and a positive result by the IFN-γ release assay. In contrast, the interpatient variability of the IP-10 plasma levels in our study suggested that the intrapatient determination of IP-10 expression kinetics may provide a stronger correlation to the anti-M. tuberculosis therapeutic response than the threshold IP-10 level. The wide intrapatient variation in plasma IL-10 levels raises the possibility that a host genetic factor(s) contributes to its regulation. Furthermore, the different sources of the cytokine samples may contribute to the disparate findings because plasma samples reflect the circulating cytokine level at a given point in time, but cytokine-laden supernatants from M. tuberculosis-stimulated cultures (18, 28) reflect the potential M. tuberculosis reactivity of circulating mononuclear cells.
IL-1β secreted by infected macrophages, in particular, is involved in the signaling of granuloma formation, and when IL-1β levels in mice are deficient, enhanced mycobacterial growth at the site of infection is induced (17). However, the majority (56.2%) of late-responding TB patients who exhibited infiltrative lesions on chest radiographs also expressed significantly elevated MCP-1, IL-1β, and TNF-α levels compared to the baseline values and the levels for the early responder group. Although our study, which had a reduced sample size due to the exclusion of individuals with diabetes mellitus, found that the differences in the levels of IL-1β and TNF-α expression but not those of MCP-1 expression were reduced by normalization by age, gender, and sputum smear result, the higher levels of these inflammatory cytokines may be indicative of tissue necrosis and cavitation in the lungs and the unimpaired production of proinflammatory cytokines (3, 32). TB patients with elevated TNF-α, IL-1β, and IL-6 levels in BALF were associated with significantly larger cavities (32). The relative amount of TNF-α at the site of infection rather than the level in serum modulates its effects, ranging from protective to harmful, and may contribute to severe inflammatory diseases and acute-phase events, such as fever, cachexia, hemorrhagic necrosis, and septic shock (8, 31). Local cytokine expression could not be determined in the present study, as lung biopsy, sputum, and BALF specimens were not examined for comparative cytokine detection.
Higher levels of IL-10, as well as higher levels of IL-8 and IL-6, in BALF were associated with active pulmonary TB in HIV-infected patients (22). In the present study, the levels of IL-6 (13.7 to 12.5 pg/ml) and IFN-γ (20.2 to 22.5 pg/ml) remained elevated in the late responder group following anti-TB therapy, whereas the IL-6 level remained unchanged (7.9 to 7.1 pg/ml) and the IFN-γ level decreased from 21.3 to 8.8 pg/ml (P < 0.001) in the early responder group. The paradoxical decrease in the level of IFN-γ expression observed in the early responder group appears to be a non sequitur to the microbicidal activating functions of IFN-γ. More recently, Sahiratmadja et al. (29) evaluated the cytokine profiles for 93 TB patients before and after curative treatment and found that IFN-γ was strongly depressed in patients with active TB before treatment but that the IFN-γ level increased after treatment, whereas our study observed elevated IFN-γ levels pretreatment in comparison to those in the controls and a significant decline in IFN-γ levels after the intensive 2-month anti-TB therapy. The mechanisms for these diametrically opposed results remains unclear. Regardless, the conflicting nature of these cytokine markers renders prompt and convenient extrapolation difficult in the clinical setting.
One notable aspect of our findings was the singular increase in IL-10 levels observed in the early responders, which is in contrast to the findings described in previous reports. The IL-10 levels found in the serum and BALF of TB patients have directly correlated with susceptibility to M. tuberculosis by deactivating macrophages, monocytes, dendritic cells, CD4 T cells, and CD8 T cells (10, 25). IL-10-deficient mice also exhibited an increased early resistance to infection with M. tuberculosis, and bacterial growth and exacerbation of disease were promoted in a susceptible CBA/J mouse model of infection (6). Although the reason for the paradoxical increase in the IL-10 level in the present study was unclear, it may correlate with the resolution of inflammation after the bacterial load is reduced. Similarly, the decline in the levels of proinflammatory cytokines may reflect the contraction of M. tuberculosis-specific pools as the bacterial load drops and tissue inflammation resolves.
In summary, the results presented here indicate that increased levels of expression of TNF-α, MCP-1, and IL-1β after 2 months of intensive anti-TB therapy were significantly associated with poorer outcomes on chest radiography. Plasma MCP-1 levels were normalized in patients with resolution of their M. tuberculosis infections. The roles of IL-10, MCP-1, IP-10, and IFN-γ with respect to treatment outcomes warrant additional research with larger patient cohorts and additional time points and by the use of diagnostic criteria more objective for the evaluation of improvements on chest radiographs. However, further studies are warranted before implementation of a cytokine-based predictive scale for the clinical setting.
Acknowledgments
This work was supported by the Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan (grants TSGH-C99-076 and TSGH IRB 095-05-104).
None of us has a conflict of interest to disclose.
We declare that all authors listed have actively participated in the study and that all authors meet the requirements of the authorship. Wen-Lin Su and Jenn-Han Chen designed the study and wrote the protocol; Ching-Hui Huang and Cheng-Yu Yang managed the literature searches and analyses; Wen-Lin Su undertook the statistical analysis; Wen-Lin Su, Jenn-Han Chen, and Chin-Pyng Wu wrote the first draft of the manuscript; and Chin-Pyng Wu and Wann-Cherng Perng participated in the preparation of the manuscript.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Algood, H. M., J. Chan, and J. L. Flynn. 2003. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 14:467-477. [DOI] [PubMed] [Google Scholar]
- 2.Algood, H. M., P. L. Lin, and J. L. Flynn. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41(Suppl. 3):S189-S193. [DOI] [PubMed] [Google Scholar]
- 3.Antas, P. R., F. L. Cardoso, K. C. Pereira, K. L. Franken, K. S. Cunha, P. Klatser, E. N. Sarno, T. H. Ottenhoff, and E. P. Sampaio. 2005. T cell immune responses to mycobacterial antigens in Brazilian tuberculosis patients and controls. Trans. R. Soc. Trop. Med. Hyg. 99:699-707. [DOI] [PubMed] [Google Scholar]
- 4.Azzurri, A., O. Y. Sow, A. Amedei, B. Bah, S. Diallo, G. Peri, M. Benagiano, M. M. D'Elios, A. Mantovani, and G. Del Prete. 2005. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15:675-705. [DOI] [PubMed] [Google Scholar]
- 6.Beamer, G. L., D. K. Flaherty, B. D. Assogba, P. Stromberg, M. Gonzalez-Juarrero, R. de Waal Malefyt, B. Vesosky, and J. Turner. 2008. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J. mice. J. Immunol. 181:5545-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 8.Bekker, L. G., A. L. Moreira, A. Bergtold, S. Freeman, B. Ryffel, and G. Kaplan. 2000. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 68:6954-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benator, D., M. Bhattacharya, L. Bozeman, W. Burman, A. Cantazaro, R. Chaisson, F. Gordin, C. R. Horsburgh, J. Horton, A. Khan, C. Lahart, B. Metchock, C. Pachucki, L. Stanton, A. Vernon, M. E. Villarino, Y. C. Wang, M. Weiner, and S. Weis. 2002. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 360:528-534. [DOI] [PubMed] [Google Scholar]
- 10.Bonecini-Almeida, M. G., J. L. Ho, N. Boechat, R. C. Huard, S. Chitale, H. Doo, J. Geng, L. Rego, L. C. Lazzarini, A. L. Kritski, W. D. Johnson, Jr., T. A. McCaffrey, and J. R. Silva. 2004. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect. Immun. 72:2628-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casarini, M., F. Ameglio, L. Alemanno, P. Zangrilli, P. Mattia, G. Paone, A. Bisetti, and S. Giosue. 1999. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 159:143-148. [DOI] [PubMed] [Google Scholar]
- 12.Chang, K. C., C. C. Leung, W. W. Yew, S. C. Ho, and C. M. Tam. 2004. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am. J. Respir. Crit. Care Med. 170:1124-1130. [DOI] [PubMed] [Google Scholar]
- 13.Drosten, C., M. Panning, and S. Kramme. 2003. Detection of Mycobacterium tuberculosis by real-time PCR using pan-mycobacterial primers and a pair of fluorescence resonance energy transfer probes specific for the M. tuberculosis complex. Clin. Chem. 49:1659-1661. [DOI] [PubMed] [Google Scholar]
- 14.Feng, C. G., D. Jankovic, M. Kullberg, A. Cheever, C. A. Scanga, S. Hieny, P. Caspar, G. S. Yap, and A. Sher. 2005. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J. Immunol. 174:4185-4192. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garlanda, C., D. Di Liberto, A. Vecchi, M. P. La Manna, C. Buracchi, N. Caccamo, A. Salerno, F. Dieli, and A. Mantovani. 2007. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 179:3119-3125. [DOI] [PubMed] [Google Scholar]
- 17.Juffermans, N. P., S. Florquin, L. Camoglio, A. Verbon, A. H. Kolk, P. Speelman, S. J. van Deventer, and T. van Der Poll. 2000. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182:902-908. [DOI] [PubMed] [Google Scholar]
- 18.Lighter, J., M. Rigaud, M. Huie, C. H. Peng, and H. Pollack. 2009. Chemokine IP-10: an adjunct marker for latent tuberculosis infection in children. Int. J. Tuberc. Lung Dis. 13:731-736. [PubMed] [Google Scholar]
- 19.Majumder, N., S. Bhattacharjee, S. Bhattacharyya Majumdar, R. Dey, P. Guha, N. K. Pal, and S. Majumdar. 2008. Restoration of impaired free radical generation and proinflammatory cytokines by MCP-1 in mycobacterial pathogenesis. Scand. J. Immunol. 67:329-339. [DOI] [PubMed] [Google Scholar]
- 20.Mohan, V. P., C. A. Scanga, K. Yu, H. M. Scott, K. E. Tanaka, E. Tsang, M. M. Tsai, J. L. Flynn, and J. Chan. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mootoo, A., E. Stylianou, M. A. Arias, and R. Reljic. 2009. TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm. Allergy Drug Targets 8:53-62. [DOI] [PubMed] [Google Scholar]
- 22.Mwandumba, H. C., S. Bertel Squire, S. A. White, M. H. Nyirenda, S. D. Kampondeni, E. R. Rhoades, E. E. Zijlstra, M. E. Molyneux, and D. G. Russell. 2008. Association between sputum smear status and local immune responses at the site of disease in HIV-infected patients with pulmonary tuberculosis. Tuberculosis (Edinb.) 88:58-63. [DOI] [PubMed] [Google Scholar]
- 23.Nagabhushanam, V., A. Solache, L. M. Ting, C. J. Escaron, J. Y. Zhang, and J. D. Ernst. 2003. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J. Immunol. 171:4750-4757. [DOI] [PubMed] [Google Scholar]
- 24.North, R. J., and Y. J. Jung. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599-623. [DOI] [PubMed] [Google Scholar]
- 25.Olobo, J. O., M. Geletu, A. Demissie, T. Eguale, K. Hiwot, G. Aderaye, and S. Britton. 2001. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand. J. Immunol. 53:85-91. [DOI] [PubMed] [Google Scholar]
- 26.Qiu, L., D. Huang, C. Y. Chen, R. Wang, L. Shen, Y. Shen, R. Hunt, J. Estep, B. F. Haynes, W. R. Jacobs, Jr., N. Letvin, G. Du, and Z. W. Chen. 2008. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J. Infect. Dis. 198:1514-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 28.Ruhwald, M., M. Bjerregaard-Andersen, P. Rabna, J. Eugen-Olsen, and P. Ravn. 2009. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res. Notes 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahiratmadja, E., B. Alisjahbana, T. de Boer, I. Adnan, A. Maya, H. Danusantoso, R. H. Nelwan, S. Marzuki, J. W. van der Meer, R. van Crevel, E. van de Vosse, and T. H. Ottenhoff. 2007. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect. Immun. 75:820-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sai Priya, V. H., B. Anuradha, S. Latha Gaddam, S. E. Hasnain, K. J. Murthy, and V. L. Valluri. 2009. In vitro levels of interleukin 10 (IL-10) and IL-12 in response to a recombinant 32-kilodalton antigen of Mycobacterium bovis BCG after treatment for tuberculosis. Clin. Vaccine Immunol. 16:111-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strieter, R. M., S. L. Kunkel, and R. C. Bone. 1993. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit. Care Med. 21:S447-S463. [DOI] [PubMed] [Google Scholar]
- 32.Tsao, T. C., J. Hong, C. Huang, P. Yang, S. K. Liao, and K. S. Chang. 1999. Increased TNF-alpha, IL-1 beta and IL-6 levels in the bronchoalveolar lavage fluid with the upregulation of their mRNA in macrophages lavaged from patients with active pulmonary tuberculosis. Tuber. Lung Dis. 79:279-285. [DOI] [PubMed] [Google Scholar]
- 33.Zganiacz, A., M. Santosuosso, J. Wang, T. Yang, L. Chen, M. Anzulovic, S. Alexander, B. Gicquel, Y. Wan, J. Bramson, M. Inman, and Z. Xing. 2004. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J. Clin. Invest. 113:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]