Abstract
Based on studies reporting specific antibody titers, it is recommended to vaccinate preterm infants against Bordetella pertussis according to their chronological age. However, as specific T-cell responses also are involved in the protection against B. pertussis, we have determined whether highly preterm infants (<31 weeks) are able to mount these immune responses during vaccination. Forty-eight premature infants were vaccinated at 2, 3, and 4 months of their chronological age with an acellular (Pa; n = 24) or a whole-cell (Pw; n = 24) tetravalent diphtheria-tetanus-pertussis-polio vaccine, and blood samples were collected at 2, 3, and 6 months of age. Most of the Pa- and Pw-vaccinated infants developed at 3 or 6 months of age a gamma interferon (IFN-γ) response to the B. pertussis antigens, accompanied by an interleukin-5 (IL-5) and IL-13 secretion for the Pa-vaccinated infants. No association was found between a very low infant birth weight, the occurrence of severe infections, and corticosteroid treatment or the administration of gammaglobulins with a low level of antigen-induced IFN-γ secretion. We conclude that like full-term infants, most preterm infants are able to mount a specific cellular immune response to the administration of the first doses of an acellular or a whole-cell pertussis vaccine.
Preterm infants are at high risk of Bordetella pertussis infections, and this infection is more severe and is associated with a higher morbidity and mortality than it is in infants born at full term (11, 26). The American Academy of Pediatrics therefore recommends immunizing preterm infants at their chronological age with the same vaccine schedule as that recommended for full-term infants (22). These recommendations initially were based on antibody titers measured in studies performed on small numbers of infants who had received whole-cell pertussis vaccines (Pw) (2, 3, 10). Recent studies with the acellular pertussis vaccines (Pa) have confirmed that preterm infants mount antibody responses to the B. pertussis vaccine antigens, with titers in preterm infants reported to be lower (23) or similar (17, 24) to those obtained in full-term infants. However, these studies assessed only the humoral immune responses, although protection against pertussis relies both on humoral and on cellular Th1-type immune responses (1, 15, 16, 18).
Until recently, doubt existed about the ability of infants to develop a specific Th1-type immune response and therefore adequate immune responses to an early administration of a B. pertussis vaccine. Infants most often are considered relatively deficient in their capacity to secrete gamma interferon (IFN-γ) (19, 25). However, some exceptions have been reported, including the ability to secrete IFN-γ in response to the major B. pertussis antigens during infection (14) or after vaccination (13, 5), indicating that infants are able to mount both antibody-specific and antigen-specific IFN-γ responses upon the infection or administration of pertussis vaccines.
To our knowledge, in contrast to infants born at term, no data on the early cellular immune responses of preterm infants after the administration of the primary series of pertussis vaccines have been reported yet. Therefore, we do not know whether preterm infants are able to mount adequate specific immune responses to an early administration of a B. pertussis vaccine. In the context of the current resurgence of B. pertussis infections, such knowledge should help to offer the best vaccination strategy for preterm infants. We therefore assessed here the specific cellular immune responses, together with the humoral responses, in preterm infants with very low gestational age (VLGA; born at <31 weeks) that have received their first three doses of pertussis vaccines at their chronological ages. Immune responses induced by a Pa vaccine were compared to those induced by a Pw vaccine.
MATERIALS AND METHODS
Infants included in the study.
Forty-eight premature infants were included in this study and were vaccinated against B. pertussis at their chronological age according to the Belgian vaccination recommendations at the time of the study, between 2001 and 2004. They were vaccinated at 2, 3, and 4 months of age with one of two tetravalent diphtheria-tetanus-pertussis-polio vaccines used at that time in Belgium, the Pa vaccine Tetravac or the Pw vaccine Tetracoq (both vaccines from Sanofi Pasteur, Lyon, France), combined with a lyophilized Haemophilus influenzae type b vaccine, as described previously (4). As the recommendations in Belgium to administer Pa vaccines instead of Pw vaccines were implemented in 2001, we had the opportunity to compare two groups of infants receiving for their first three vaccine doses either the Pw (n = 24) or the Pa (n = 24) vaccine. The infants were enrolled at two different hospitals, the Hôpital Erasme and the Centre Hospitalier Inter Régional Edith Cavell. The ethical committees of both hospitals approved the study, and all of the infants' parents gave their informed consent.
All of the infants were born at VLGA (before 31 weeks of gestational age, with a mean gestational age at birth of 28 weeks [range, 25 to 30 weeks]). This age was determined by the known date of the last menstrual period and/or an early ultrasonogram. None of them were HIV positive, and they were not born from HIV-positive mothers. They all were breastfed initially, and 56% of them still were breastfed at discharge from the neonatal intensive care unit. Twenty-three of 48 infants received steroids during the neonatal period (intravenously [i.v.] for 15 of them and by inhalation for 18 of them, with 10 receiving both i.v. and inhaled corticosteroids). For the i.v. route, the last dose was administered at least 2 weeks before their first vaccine dose. Seventeen of 48 infants experienced severe infections during the neonatal period (septicemia on the central catheter, necrotizing enterocolitis, and pneumonia), but they did not receive the vaccines while being infected. Twenty-nine of 48 infants received intravenous immunoglobulins (Sandoglobulin; 400 mg/kg of body weight; Novartis) for severe infection or severe hypo-IgG (less than 200 mg/dl), and the injection was performed at least 21 days before their first vaccine injection, and 16/48 infants received anti-respiratory syncytial virus (RSV) IgG (Synagis; 15 mg/kg/dose; Abbott). Due to the time lapse between the IgG administration and the first vaccination, the IgG administration is not expected to have directly influenced the antibody titers.
Blood samples were collected from the infants at around 2 months of age just before their first vaccine administration, at around 3 months, before their second vaccine injection, and at around 6 months, 2 months after their third vaccine injection.
Cell isolation, culture conditions, and cytokine concentration determinations.
Peripheral blood mononuclear cells (PBMC) were isolated and assayed for cytokine secretion after antigenic stimulation as previously described (4, 13, 14). Because of limitations in the numbers of PBMC harvested, levels of antigen-specific IL-13 and IL-5 could not be assayed for all infants at each time point.
Antibody assays.
Filamentous hemagglutinin (FHA)- and pertussis toxin (PTX)-specific IgG titers were measured by a solid-phase enzyme-linked immunosorbent assay (ELISA). Briefly, plates were coated with 2 μg/ml FHA or PTX (both antigens from Sanofi Pasteur) diluted in phosphate-buffered saline (PBS). Sera serially diluted from 1/50 to 1/4,000 in PBS-Tween 0.05%-casein 0.5% then were incubated for 30 min at room temperature. The antibodies were detected by the addition of peroxidase-labeled goat anti-human IgG (250 ng/ml for 30 min at room temperature; Imtech Diagnostics, NV) and then the extravidine substrate (Sigma). The anti-FHA and anti-PTX IgG concentrations of the samples were evaluated by reference to a standard curve established by testing serial dilutions of a U.S. reference pertussis antiserum (kindly provided by Bruce Meade, FDA, Rockville, MD).
The avidity of the specific IgG was determined by an ELISA elution assay using potassium thiocyanate (KSCN) as a chaotropic agent according to a previously described method (12). Briefly, the serum dilution first was adjusted to a concentration giving ELISA results in the upper portion of the linear part of the standard curve. Seven identical serum dilutions then were incubated on the antigen-coated plates for 30 min at room temperature. After the plates were washed, KSCN was added at final concentrations of 0, 0.25, 0.5, 1, 1.5, 2, and 2.5 M, and the plates were incubated for 15 min at room temperature. After the plates were washed, the antibodies fixed to the plates were detected as described for the classical ELISA. The avidity index was expressed as the molar concentration of KSCN required to cause a 50% reduction in absorbance. Values estimated to be normal were established in pertussis-vaccinated 6-month-old term infants (n = 13). The medians and 25th to 75th percentiles of the values obtained from full-term infants were 1.55 (range, 1.42 to 1.67) for FHA and 1.65 (1.45 to 1.85) for PTX (F. Mascart, unpublished data).
Statistical analysis.
The statistical analyses were performed using the GraphPad Prism 4.0b program (GraphPad Software, San Diego, CA). The comparison of two groups of data was performed with the nonparametric Wilcoxon match pair test. Categorical variables were compared using the two-tailed Fisher's exact test. Values of P < 0.05 were considered significant. Correlations were analyzed by the nonparametric Spearman test.
RESULTS
Antigen-specific IFN-γ responses in Pa- and in Pw-vaccinated infants.
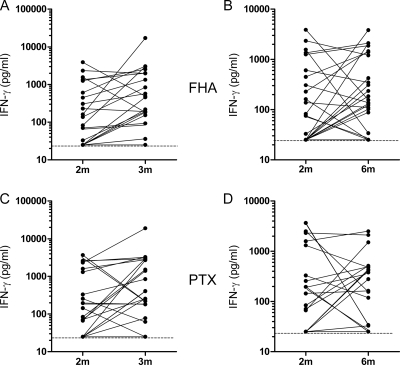
The FHA- and PTX-induced IFN-γ responses were measured in cell culture supernatants collected after 72 h of in vitro culture of PBMC in the presence of FHA or PTX. The concentrations of the IFN-γ released were compared for each infant between blood samples collected at 2 months, before any vaccination, and at 3 and 6 months of age, respectively, 1 month after the first vaccine injection and 2 months after the administration of the first three vaccine doses. The IFN-γ response was considered positive when the IFN-γ concentration released at 3 or 6 months was higher than the sensitivity limit of the ELISA (25 pg/ml) and at least three times higher than the value detected for the sample collected at 2 months of age. These criteria were chosen because, for unknown reasons, a significant antigen-specific IFN-γ response already was detected in some blood samples collected before vaccination. Among the Pa-vaccinated infants, results from one of them were not considered due to a technical problem, and one infant dropped out of the study at 6 months. A specific IFN-γ response of the PBMC from the Pa-vaccinated infants was noted for nearly half of the 3-month-old and of the 6-month-old infants in response to both FHA and PTX (Fig. 1 and Table 1). Only some of the responders were the same at 3 and 6 months of age. Combining the responses at 3 and 6 months provided 68.2% of response to FHA and 50% of response to PTX (Table 1).
FIG. 1.
FHA- and PTX-induced IFN-γ secretion in Pa-vaccinated preterm infants. The IFN-γ concentrations were measured in 72-h cell culture supernatants from PBMC stimulated in vitro with FHA or PTX. (A and B) FHA-induced IFN-γ secretion at 3 months compared to that at 2 months of age (A) and at 6 months compared to that at 2 months of age (B). (C and D) PTX-induced IFN-γ secretion at 3 months compared to that at 2 months of age (C) and at 6 months compared to that at 2 months of age (D). Filled lines link the results obtained for PBMC from the same infants. Broken lines indicate the limit of the sensitivity of IFN-γ detection (25 pg/ml).
TABLE 1.
Antigen-specific IFN-γ responses in Pa- and Pw-vaccinated preterm infants
| Vaccine and antigen | No. of IFN-γ responders/no. of infants tested (%) at: |
||
|---|---|---|---|
| 3 mo | 6 mo | 3 or 6 mo | |
| Pa | |||
| FHA | 10/23 (43.4) | 12/22 (54.5) | 15/22 (68.2) |
| PTX | 10/21 (47.6) | 6/18 (33.3) | 9/18 (50) |
| Pw | |||
| FHA | 7/24 (29.1) | 10/24 (41.6) | 14/24 (58.3) |
| PTX | 7/24 (29.1) | 11/24 (45.8) | 11/24 (45.8) |
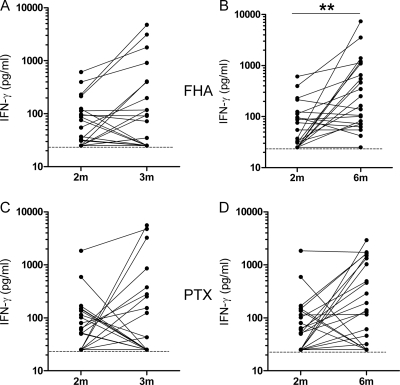
Among the Pw-vaccinated infants, the PBMC from only one-third of them secreted IFN-γ in response to FHA or PTX at 3 months of age, compared to a response for more than 40% of them at 6 months (Fig. 2 and Table 1). Combining both responses at 3 and 6 months provided 58.3 and 45.8% of the response to FHA and PTX, respectively (Table 1).
FIG. 2.
FHA- and PTX-induced IFN-γ secretion in Pw-vaccinated preterm infants. The IFN-γ concentrations were measured in 72-h cell culture supernatants from PBMC stimulated in vitro with FHA or PTX. (A and B) FHA-induced IFN-γ secretion at 3 months compared to that at 2 months of age (A) and at 6 months compared to that at 2 months of age (B). (C and D) PTX-induced IFN-γ secretion at 3 months compared to that at 2 months of age (C) and at 6 months compared to that at 2 months of age (D). Filled lines link the results obtained for PBMC from the same infants. Broken lines indicate the limit of sensitivity of the IFN-γ detection (25 pg/ml). **, P < 0.01.
In response to the two different pertussis vaccines, the IFN-γ responses to FHA and to PTX were correlated (r = 0.88 and P < 0.001 for Pa; r = 0.59 and P < 0.001 for Pw; Spearman correlation), indicating that most of the nonresponding infants were nonresponders to both antigens.
Influence of clinical parameters on the specific IFN-γ response.
As some infants had received i.v. immunoglobulins and/or corticosteroids, and as some had experienced early severe infections, as defined in the patient's description, we analyzed whether the absence of the IFN-γ response to FHA or PTX in some infants was related to these clinical parameters, and no association was found (Fisher's exact test). Similarly, even though all of the included infants were born before 31 weeks of gestation, their birth weight varied between 700 and 1,630 g (median, 1,080 g). However, there was no significant difference between the birth weights of the two groups of infants, the Pa- and Pw-vaccinated infants, and no correlation was noted between infant birth weight and antigen-induced IFN-γ secretion.
Antigen-specific Th2 cytokine responses in Pa- and Pw-vaccinated preterm infants.
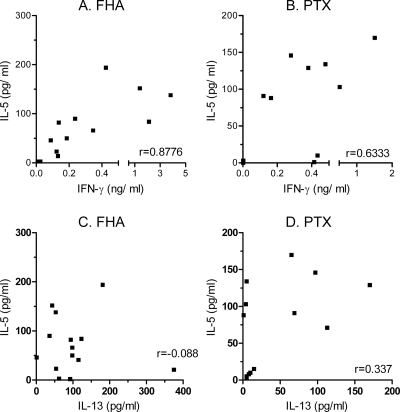
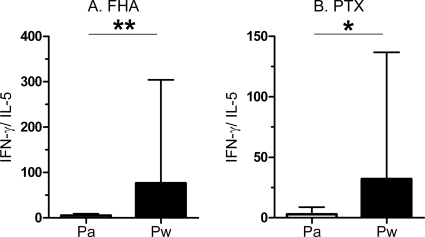
As previously described for term infants (13), the PBMC from preterm infants who received the Pa vaccine also secreted Th2-type cytokines in response to FHA and PTX and the in vitro antigen-induced IFN-γ and IL-5 concentrations were well correlated (FHA, r = 0.8776 and P < 0.0001; PTX, r = 0.633 and P = 0.027) (Fig. 3A and B). They also secreted IL-13 in response to FHA and PTX, as shown in Fig. 3C and D, but the IL-5 and IL-13 concentrations were only poorly correlated. In contrast, the Pw-vaccinated infants only rarely secreted IL-5 (2/22 infants in response to FHA and 1/14 infant in response to PTX) (data not shown). As a consequence, at 6 months, the ratios between antigen-specific IFN-γ and IL-5 were significantly lower in the Pa recipients than in the Pw recipients (Fig. 4). Similar results were found for the ratios of the antigen-specific IFN-γ to IL-13 concentrations (data not shown). However, no association was found between a low antigen-specific Th1/Th2 ratio and the occurrence of bronchial hyperreactivity or nocturnal cough during the first 12 months of the life of the infants (Fisher's exact test).
FIG. 3.
FHA- and PTX-induced IFN-γ, IL-5, and IL-13 secretions in 6-month-old Pa-vaccinated preterm infants. The cytokine concentrations were measured in 72-h cell culture supernatants from PBMC stimulated in vitro with FHA or PTX. The correlations between IFN-γ and IL-5 secretions in response to FHA (A) and PTX (B) are shown in the upper panels, and those between IL-5 and IL-13 secretions are shown in the lower panels for FHA (C) and PTX (D), respectively.
FIG. 4.
Ratios of antigen-specific IFN-γ to IL-5 secretion in 6-month-old Pa- or Pw-vaccinated preterm infants. IFN-γ and IL-5 concentrations were measured in 72-h cell culture supernatants from PBMC stimulated in vitro with FHA (A) or PTX (B). The ratios between the IFN-γ and IL-5 concentrations are reported as medians and 75th percentiles. *, P < 0.05; **, P < 0.01.
Antigen-specific IgG titers and affinities.
As the previously reported studies of the immune responses of preterm infants to the administration of pertussis vaccines assessed only the humoral immune responses, we completed our study by measuring the anti-FHA and anti-PTX IgG responses in the sera. In addition, the avidity indexes of these antibodies were measured when they were detectable.
No significant anti-FHA or anti-PTX IgG concentrations were detected in sera from prevaccinated 2-month-old infants and in sera from 3-month-old vaccinated infants, regardless of the type of vaccine administered (Table 2). In contrast, anti-FHA and anti-PTX IgG concentrations were significantly higher in sera from 6-month-old than in those from 3-month-old infants vaccinated with the Pa vaccine (P < 0.0001), and they were significantly higher than the antibody concentrations detected in 6-month-old Pw-vaccinated infants (P < 0.0001) (Table 2), confirming previously reported data.
TABLE 2.
Anti-FHA and anti-PTX antibody titers in Pa- and Pw-vaccinated preterm infants
| Vaccine and antigen | Antibody titera (U/ml) |
||
|---|---|---|---|
| Prevaccination (2 mo) | After first dose (3 mo) | After third dose (6 mo) | |
| Pa | |||
| FHA | 7.0 (4.0-9.5) | 5.0 (3.0-7.5)b | 65.0 (35.0-90.0) |
| PTX | 5.0 (2.0-6.5) | 4.5 (3.0-7.5)b | 38.0 (33.0-52.0) |
| Pw | |||
| FHA | 3.0 (3.0-6.0) | 6.5 (4.0-8.5) | 3.0 (2.5-7.0) |
| PTX | 2.5 (2.0-3.0) | 3.0 (1.0-3.0) | 3.0 (2.0-5.5) |
Results are medians and 25th to 75th percentiles.
P < 0.0001.
For Pa-vaccinated infants, a significant correlation was found between the FHA IgG concentrations and the in vitro FHA-induced IL-5 and IFN-γ concentrations (IL-5, r = 0.73 and P = 0.0064; IFN-γ, r = 0.60 and P = 0.01).
The antibody avidity indexes could not be determined for Pw-vaccinated children, as the antibody levels were too low. For Pa-vaccinated children, the avidity indexes for anti-FHA IgG were slightly lower than those found for full-term infants (medians and 25th to 75th percentiles were 1.10 and 1.02 to 1.42 in preterm infants and 1.55 and 1.42 to 1.67 in full-term infants, respectively; P < 0.05). In contrast, the avidity indexes for anti-PTX IgG were similar in preterm infants (median and 25th to 75th percentiles, 1.50 and 1.15 to 1.80, respectively) and in full-term infants (median and 25th to 75th percentiles, 1.65 and 1.45 to 1.85, respectively).
DISCUSSION
Even though the scientific basis for the recommendation of the pertussis immunization of preterm infants still is limited, the American Academy of Pediatrics recommends immunizing preterm infants at their chronological age with the same vaccine schedules as those for full-term infants (22). A correct vaccination attitude is particularly important to protect preterm infants against B. pertussis, as they are especially vulnerable to B. pertussis infection (11, 26). Furthermore, the incidence of pertussis is so far the only one to increase among all communicable diseases for which vaccines are available, even in countries with high vaccination coverage (9). However, this recommendation requires that pertussis vaccines are both safe and immunogenic in preterm infants.
The specific immune responses induced by the priming doses of a pertussis vaccine in preterm infants have been analyzed only in a limited number of studies, and all have focused exclusively on the humoral immune responses. Results from these studies are difficult to compare, as different vaccines and different administration schedules of these vaccines were used, as well as different nonstandardized antibody assays. Globally, these studies reported the appearance in preterm infants of significant vaccine-induced specific antibody titers, albeit sometimes at lower levels than those found in vaccinated full-term infants (23). Two studies reported similar antibody titers in preterm and full-term infants (17, 24). We confirmed here that the Pa vaccine induces the appearance of significant antibody levels in 6-month-old infants, and that the Pa vaccine is more effective than the Pw vaccine for the induction of antibodies against FHA and PTX (8). In addition, we report for the first time for preterm infants that the avidity indexes of these antibodies were similar to those of age-matched full-term infants for PTX but were slightly lower than those of full-term infants for FHA. The antibody avidity is one of the important functional parameters involved in antibody quality, and it has been proposed as a surrogate marker for the induction of successful priming with conjugated polysaccharide vaccines (7), but so far no data were available on the antibody avidities induced by pertussis vaccines. In view of the recent indications that antibody titers are not the only effectors of protection against pertussis, and that other parameters of the immune response also are involved (1, 15, 16, 18), we have focused this study on the specific cellular immune responses induced in VLGA preterm infants by a vaccination with two different pertussis vaccines, a Pa and a Pw vaccine, administrated at their chronological age. Among the investigated parameters, we concentrated especially on antigen-specific IFN-γ secretion, which is known to participate in protection against B. pertussis infection (1, 14, 15, 16, 18, 20). For a few infants, we already detected an antigen-specific IFN-γ secretion when we tested blood samples collected at 2 months of age, just before the first administration of the vaccine. Although this may be surprising, it already was noted to sometimes occur in full-term infants (21). These infants most often failed to develop a further antigen-specific IFN-γ response after the vaccine administrations (Fig. 1 and 2). However, when all of the infants included in the study are considered, a significant FHA- and PTX-induced IFN-γ secretion by the PBMC was noted for more than half of the vaccinated infants, indicating that the immune system of most preterm infants is mature enough to mount T-cell responses. Interestingly, these responses already were detectable in some infants very early, i.e., at 3 months of age, in contrast to the humoral immune responses that were detectable only in 6-month-old infants who received the Pa vaccine. However, the cellular immune responses probably are only very transiently present among circulating PBMC, as they were not always detected at both 3 and 6 months of age for the same infants. The antigen-specific IFN-γ-producing cells most probably rapidly reach the lymph nodes. The IFN-γ responses to FHA and PTX were well correlated, so that infants most often responded either to both antigens or to neither of them. Importantly, we found no influence of the treatment often administered to preterm infants (corticosteroids and immunoglobulins) on their capacity to develop an antigen-specific IFN-γ response to the vaccine administration. We also found no association between the cellular immune response to the vaccine and the birth weight of the infant or the early occurrence of severe infections.
Pa-vaccinated infants also produced antigen-specific Th2-type cytokines, similarly to previously reported data for vaccinated full-term infants (13), resulting in a lower antigen-specific IFN-γ/IL-5 balance in the Pa-vaccinated compared to the Pw-vaccinated infants. In Pa-vaccinated infants, the IL-5 and IFN-γ secretions were well correlated, indicating that nonresponder infants did not secrete any of these two cytokines in response to FHA or PTX. We found no association between this nonresponder status and any of the treatments received by the infants (such as corticosteroids or immunoglobulins) or their birth weight, supporting the recommendation that preterm infants should be vaccinated at their chronological age even if they require special medical treatments.
We conclude that the administration of the first three doses of either a Pa or a Pw vaccine in preterm infants at their chronological age is able to induce in more than 50% of infants a strong cellular response to B. pertussis antigens. In addition, preterm infants vaccinated with a Pa vaccine mount PTX- and FHA-specific antibody responses that are not seen in Pw-vaccinated preterm infants. However, we cannot exclude that the development and persistence of memory immune responses in these infants is impaired, requiring earlier booster administrations in these infants, as suggested by Esposito et al. (6). Therefore, the follow-up of these children is under way to determine whether B. pertussis-specific cellular immune responses still will be detectable in 13- and 15-month-old children before and after the administration of a vaccine booster dose.
Acknowledgments
This work was supported by the European Union (Project Improving Vaccination in Early Life—Neonatal Vaccination, contract QLK2-CT-0429).
We are grateful to G. Del Giudice (Chiron Vaccines, Sienna, Italy) and to E. Trannoy (Sanofi Pasteur, Lyon, France), who were members of the EU consortium and who provided us with the antigens for in vitro studies. We also thank B. Meade for the U.S. reference pertussis antiserum and the staff of the two neonatal care units involved in this study for their important help in collecting blood samples from the infants.
Footnotes
Published ahead of print on 16 December 2009.
REFERENCES
- 1.Barbic, J., M. F. Leef, D. L. Burns, and R. D. Shahin. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 65:4904-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernbaum, J. C., A. Daft, R. Anolik, J. Samuelson, R. Barkin, S. Douglas, and R. Polin. 1985. Response of preterm infants to diphteria-tetanus-pertussis immunizations. J. Pediatr. 107:184-188. [DOI] [PubMed] [Google Scholar]
- 3.D'Angio, C. T., W. M. Maniscalo, and M. E. Pichichero. 1995. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics 96:18-22. [PubMed] [Google Scholar]
- 4.Dirix, V., V. Verscheure, T. Goetghebuer, M. Hainaut, A. S. Debrie, C. Locht, and F. Mascart. 2009. Cytokine and antibody profiles in one-year-old children vaccinated with either acellular or whole-cell pertussis vaccine during infancy. Vaccine 27:6042-6047. [DOI] [PubMed]
- 5.Dirix, V., V. Verscheure, T. Goetghebuer, M. Hainaut, A. S. Debrie, C. Locht, and F. Mascart. 2009. Monocyte-derived interleukin-10 depresses the Bordetella pertussis-specific gamma interferon response in vaccinated infants. Clin. Vaccine Immunol. 16:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito, S., G. Faldella, A. Giammanco, S. Bosis, O. Friscia, M. Clerici, and N. Principi. 2002. Long-term pertussis-specific immune responses to a combined diphtheria, tetanus, tricomponent acellular pertussis and hepatitis B vaccine in pre-term infants. Vaccine 20:2928-2932. [DOI] [PubMed] [Google Scholar]
- 7.Goldblatt, D., A. R. Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 77:1112-1115. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334:349-355. [DOI] [PubMed] [Google Scholar]
- 9.Hewlett, E. L., and K. M. Edwards. 2005. Pertussis—not just for kids. N. Engl. J. Med. 352:1215-1222. [DOI] [PubMed] [Google Scholar]
- 10.Koblin, B. A., T. R. Townsend, A. Muñoz, I. Onorato, M. Wilson, and B. F. Polk. 1988. Response of preterm infants to diphtheria-tetanus-pertussis vaccine. Pediatr. Infect. Dis. J. 7:704-711. [DOI] [PubMed] [Google Scholar]
- 11.Langkamp, D. L., and J. P. Davis. 1996. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J. Pediatr. 128:654-659. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald, R. A., C. S. Hosking, and C. L. Jones. 1988. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J. Immunol. Methods 106:191-194. [DOI] [PubMed] [Google Scholar]
- 13.Mascart, F., M. Hainaut, A. Peltier, V. Verscheure, J. Levy, and C. Locht. 2007. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 25:391-398. [DOI] [PubMed] [Google Scholar]
- 14.Mascart, F., V. Verscheure, A. Malfroot, M. Hainaut, D. Piérard, S. Temerman, A. Peltier, A. S. Debrie, J. Levy, G. Del Giudice, and C. Locht. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170:1504-1509. [DOI] [PubMed] [Google Scholar]
- 15.Mills, K. H. G., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis infection: role of Th1 cells in bacterial clearance in a murine respiratory model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills, K. H. G. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 17.Omeñaca, F., J. García-Sicilia, P. Garcia-Corbeira, R. Boceta, A. Romero, G. Lopez, and R. Dal-Ré. 2005. Response of preterm newborns to immunization with a hexavalent diphteria-tetanus-acellular pertussis+hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics 116:1292-1298. [DOI] [PubMed] [Google Scholar]
- 18.Redhead, K., J. Watkins, A. Barnard, and K. H. G. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 61:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe, J., C. Macaubas, T. M. Monger, B. J. Holt, J. Harvey, J. T. Poolman, P. D. Sly, and P. G. Holt. 2000. Antigen-specific responses to diphteria-tetanus-acellular pertussis vaccine in human infants are initially Th2-polarized. Infect. Immun. 68:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan, M., G. Murphy, L. Gothefors, L. Nilsson, J. Storsaeter, and K. H. Mills. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246-1250. [DOI] [PubMed] [Google Scholar]
- 21.Ryan, M., G. Murphy, E. Ryan, L. Nilsson, F. Shackley, L. Gothefors, K. Oymar, E. Miller, J. Storsaeter, and K. H. G. Mills. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saari, T. N., and Committee on Infectious Diseases. 2003. Immunization of preterm and low birth weight infants. Pediatrics 112:193-198. [DOI] [PubMed] [Google Scholar]
- 23.Schloesser, R. L., D. Fischer, W. Otto, W. Rettwitz-Volk, P. Herden, and S. Zielen. 1999. Safety and immunogenicity of an acellular pertussis vaccine in premature infants. Pediatrics 103:e60. [DOI] [PubMed] [Google Scholar]
- 24.Slack, M. H., S. Cade, D. Schapira, R. J. Thwaites, A. Crowley-Luke, J. Southern, R. Borrow, and E. Miller. 2005. DT5aP-Hib-IPV and MCC vaccines: preterm infants' response to accelerated immunization. Arch. Dis. Child. 90:338-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vekemans, J., M. O. Ota, E. C. Wang, M. Kidd, L. K. Borysiewicz, H. Whittle, K. P. McAdam, G. Morgan, and A. Marchant. 2002. T cell responses to vaccines in infants: defective IFN gamma production after oral polio vaccination. Clin. Exp. Immunol. 127:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wortis, N., P. M. Strebel, M. Wharton, B. Bardenheier, and I. R. Hardy. 1996. Pertussis deaths: report of 23 cases in the United States, 1992 and 1993. Pediatrics 97:607-612. [PubMed] [Google Scholar]