Abstract
The effect of oral administration of β-1,3/1,6-glucans from Saccharomyces cerevisiae on humoral immunity in domestic dogs is not known. In this study, 15 beagle dogs were orally given MacroGard tablets, which contain 150 mg of this β-glucan, daily for 4 weeks. At the end of this period, the total serum immunoglobulin A (IgA) level decreased significantly in the group treated with the glucan compared to that in the control group as well as compared to the concentrations before supplementation. In contrast, the total serum IgM level rose significantly, whereas no effect on the IgG level occurred. Similar changes were seen in Bordetella-specific IgA and IgM titers following vaccination during the supplementation period. The IgA concentration also became significantly lower in the saliva and tears of the glucan group than in the placebo group. The effects disappeared 1 week after the cessation of the supplementation. In conclusion, the results showed a temporary change in the isotype profile during glucan supplementation.
The cell wall glucans of yeasts and fungi have a linear backbone of β-1,3-linked glucosyl units with β-1,6-linked side chains (25, 42). These β-1,3/1,6-glucans have the capacity to act as immunostimulants (23, 30), directly enhancing innate antimicrobial defense mechanisms (11, 20, 27, 39). Furthermore, they can have an adjuvant effect on systemically coadministered vaccines (3, 36, 37), and they tend to increase antigen-specific IgG and IgA levels in serum and secretions when they are given orally or intragastrically (i.g.) to rats (21, 26). β-1,3/1,6-Glucans exert most of these effects by binding to specific receptors on macrophages, neutrophils, monocytes, dendritic cells, and NK cells (6, 28). Dectin 1 has been identified as the major β-glucan receptor. This receptor acts collaboratively with Toll-like receptor 2 (TLR2) for the induction of cytokines (i.e., tumor necrosis factor alpha [TNF-α] and interleukin-12 [IL-12]) and other inflammatory mediators (6, 9). Another less prominent receptor is complement receptor 3. Furthermore, lactosylceramide and scavenger receptors have been proposed to be potential receptors (38). Interestingly, the different β-1,3/1,6-glucans do not all have the same effects (32). Their immunostimulatory activities may vary greatly depending on, among other factors, their origin, the length of the main chain, and the degree of branching (4, 22).
The canine dietary supplement MacroGard (Biotec Pharmacon ASA, Tromsø, Norway) contains β-1,3/1,6-glucans from the cell wall of Saccharomyces cerevisiae. This product has been proven to be beneficial in various animal species, such as fish (1, 15), shellfish (34), mice (11, 14, 41), pigs (33), and horses (19), because it induces greater resistance to infections, better growth, and/or a favorable feed conversion. Whereas a lot is already known about the effects of β-1,3/1,6-glucans on the innate immune system (5, 16), a great deal still needs to be learned about their effects on the adaptive immune system in mammals. Preliminary experiments in the Laboratory of Immunology, Faculty of Veterinary Medicine, University of Ghent, with oral supplementation of β-1,3/1,6-glucans during the systemic immunization of pigs showed a shift in the antigen-specific antibody response toward IgA. Such a shift could be beneficial during systemic vaccination, since most of these types of vaccines aim to protect against pathogens which enter the host via the mucosa. In the present study, we analyzed the effect of oral supplementation with MacroGard on the isotype-specific serum antibody response in healthy dogs against a subcutaneously injected vaccine, Pneumodog, on the total IgA, IgM, and IgG concentrations in serum and the IgA concentration in saliva and tears.
MATERIALS AND METHODS
Animals and experimental procedure.
For this study, 29 healthy female beagle dogs 3 to 4 years old were selected. Two different experiments were performed. In the first experiment, 10 and 9 dogs were allocated at random to a glucan-treated group and a control group, respectively. In the second experiment a glucan-treated group and a control group contained five dogs each, and all dogs were vaccinated. The dogs in each glucan-treated group received orally, at about 8 a.m. daily, 1.5 tablets of MacroGard (150 mg β-1,3/1,6-glucan; 400 mg of liver powder; 37 mg of calcium; and as the remaining ingredients cellulose, lactose, SiO2 and magnesium stearate per tablet; Biotec AS, Tromsø, Norway) and for the 28 subsequent days. At the same time, the control dogs were given 1.5 placebo tablet(s) (49.4% cellulose, 49.4% lactose, 0.2% silicium dioxide, 1% magnesium stearate) to exclude the effects of manipulations on the antibody concentrations.
In the second experiment, the dogs were vaccinated subcutaneously (s.c.) in the neck with 1 ml Pneumodog (Merial, Brussels, Belgium) on days 14 and 28 of the experiment. Pneumodog is a bivalent vaccine containing inactivated Bordetella bronchiseptica and parainfluenza virus type 2 virus and aluminum hydroxide as the adjuvant. All dogs had previously been vaccinated with Pneumodog.
The experiments were approved by the ethical committee of the Faculty of Veterinary Medicine, University of Ghent, Ghent, Belgium.
Sample collection and handling.
Serum, saliva, and tears were sampled at the beginning of the experiment and then weekly for 5 weeks. MacroGard was given for only 4 weeks, so that the last sampling occurred exactly 1 week after the supplementation was ceased.
Sampling was always performed just before administration of the tablet. Blood samples were obtained by puncture of the jugular vein, after which serum was collected and stored at −20°C until analysis. Saliva was collected by gently rolling a sterile plain cotton swab (Copan Italia, Brescia, Italy) over the buccal mucosa, and tears were collected by rolling a swab over the mucosa of the third eyelid until the swab was saturated with fluid. The amount of secretion collected was determined by weighing the swabs before and immediately after sampling. The samples were subsequently diluted (wt/vol) five times in dilution fluid (79.95% phosphate-buffered saline [PBS; pH 7.4], 20% fetal calf serum [Greiner Bio-one, Frickenhausen, Germany], 0.05% Tween 20 [Merck, Darmstadt, Germany]), in which they were stored at −70°C until analysis.
ELISA. (i) Total immunoglobulin concentrations.
The total IgA concentrations in serum, tears, and saliva and the total IgM and IgG concentrations in serum were determined by a sandwich enzyme-linked immunosorbent assay (ELISA), as described by Bethyl Laboratories (Montgomery, TX), with minor modifications. Microtiter plates (Maxisorp; Nunc A/S, Roskilde, Denmark) were coated for 1 h with 10 μg/ml goat anti-dog IgA, IgM, or IgG (Bethyl Laboratories) in PBS, after which nonspecific binding sites were blocked with blocking buffer (0.05 M Tris base, 0.15 M NaCl, 1% bovine serum albumin [BSA], pH 8.0) for 30 min. Twofold serial dilutions of each sample in dilution buffer (0.05 M Tris-base, 0.15 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0) were subsequently added to the wells for 1 h. In addition, serial dilutions of a standard reference serum sample (RS10-105; Bethyl Laboratories) with known isotype-specific Ig concentrations were added to every plate to obtain a calibration curve. Subsequently, horseradish peroxidase (HRP)-conjugated goat anti-dog IgA, IgM, or IgG (1/10,000 in dilution buffer; Bethyl Laboratories) was added to the wells and the plates were incubated for 1 h. All incubations were carried out at room temperature (RT), and between incubations, the plates were washed three times (0.05 M Tris-base, 14 M NaCl, 0.05% Tween 20, pH 8.0). Finally, 50 μl freshly prepared, 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) diammonium salt (ABTS; Roche Diagnostics, Vilvoorde, Belgium) was added. Following 1 h of incubation at 37°C, the optical density at 405 nm (OD405) was read. The concentration of each immunoglobulin in a sample was calculated from the calibration curves.
(ii) Bordetella-specific immunoglobulin concentrations.
The Bordetella bronchiseptica-specific IgA, IgM, and IgG concentrations in the serum samples were determined by an optimized direct-antibody ELISA. Briefly, microtiter plates (Polysorp) were coated overnight at 4°C with 7.5 μg/ml of Bordetella bronchiseptica antigen (kindly provided by P. Deroose, Merial, Belgium) in carbonate-bicarbonate buffer (6 mM Na2CO3, 43.3 mM NaHCO3, pH 9.4). Residual binding sites were then blocked for 1 h at 37°C with PBS containing 3% BSA. Twofold serial dilutions of the serum samples in dilution buffer (0.05 M Tris-base, 0.15 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0) were subsequently added for 1 h at RT, followed by the addition of HRP-conjugated goat anti-dog IgA, IgM, or IgG (1/10,000 in dilution buffer; Bethyl Laboratories) for 1 h at RT. Between each incubation step, the plates were washed three times with washing buffer (0.05% Tween 20 in PBS). Finally, ABTS (Roche Diagnostics) was added and the OD405 was measured after 45 min of incubation at 37°C. The cutoff values for the Bordetella-specific antibody ELISAs were defined as the mean OD405 value for sera from 15 Bordetella-seronegative dogs increased by three times the standard deviation (the cutoff values for the Bordetella-specific serum IgA, IgM, and IgG assays were 0.116, 0.199, and 0.268, respectively). The antibody titer of the serum samples was the inverse of the highest dilution that still had an OD405 higher than the calculated cutoff value.
Statistical analysis.
The Kolmogorov-Smirnov test (SPSS, version 15.0, for Windows) was used to verify the normal distribution of the data. The total Ig concentrations from day 0 (before treatment) for every dog were subtracted from the concentration on the subsequent days to exclude interference from variations between the dogs. Statistical analysis was performed by a single-factor analysis of variance between groups for every time point in the SPSS (version 15.0) program. The level of significance was set at a P value of <0.05.
RESULTS
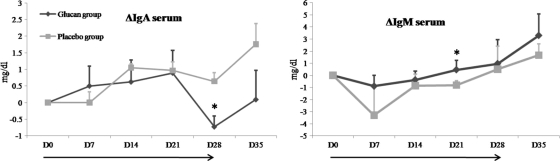
In the first experiment (Fig. 1), as well as in the second experiment (data not shown), glucan administration changed the total IgA and IgM concentrations in serum. In experiment 1, the total IgA concentration declined after day 21 to a level that was significantly lower in the glucan-treated group than in the control group (P = 0.015) (Fig. 1). The same trend in the total IgA concentration was seen in experiment 2 (data not shown). In both experiments, the IgA concentration rose again in the test groups after the cessation of β-glucan administration on day 28. The opposite was seen for total serum IgM. The IgM concentration was significantly higher in the glucan-treated group on day 21 in experiment 1 (P = 0.048) (Fig. 1) and on day 28 in experiment 2 (P = 0.045) (data not shown). In contrast, no effects on the total serum IgG concentration were seen (data not shown).
FIG. 1.
Effect of glucan administration on changes (Δ) in total IgM and IgA concentrations (mg/dl) in serum compared to those on day 0 (D0). The bars indicate the standard errors of the means (for the glucan-treated group, n = 10; for the placebo-treated group, n = 9). *, significant difference (P < 0.05) between groups; →, duration of β-glucan or placebo supplementation.
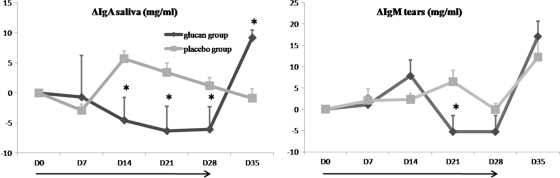
Samples of tears and saliva were taken from all dogs in experiment 1 on a weekly basis and were tested by the capture ELISA for their total IgA concentrations. The results for the IgA concentrations in saliva revealed a significantly lower IgA concentration on days 14, 21, and 28 in dogs treated with β-glucans (P = 0.001, 0.018, and 0.032, respectively) (Fig. 2). As was the case for the concentration in serum, the IgA concentration climbed to a higher level 1 week after the cessation of administration, and the increase was even significant for saliva. For tears, the same effect was seen only from day 21 on (P = 0,029).
FIG. 2.
Changes (Δ) in total IgA concentrations (mg/dl) in saliva and tears from the two groups of dogs. Bars indicate the standard errors of the means. *, significant difference (P < 0.05) between the placebo-treated group and the glucan-treated group at the indicated time point; →, duration of β-glucan or placebo supplementation.
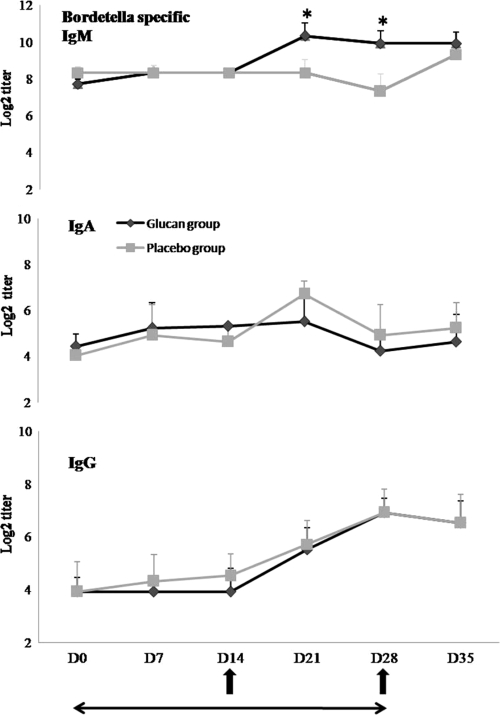
In experiment 2, the animals were vaccinated with Pneumodog on day 14 and received a boost on day 28, which allowed us to determine the effect of the orally administered MacroGard on the antigen-specific response. The animals were tested for their Bordetella-specific IgM, IgA, and IgG titers weekly during the experiment until 1 week after the cessation of glucan administration (Fig. 3). As expected, in the glucan-treated group, an increase in the antigen-specific IgM concentration similar to the increase in the total IgM concentration was seen (in comparison with the concentrations for the control group, P = 0.049 and 0.044 on days 14 and 21, respectively) (Fig. 3). However, the antigen-specific IgA titers, which increased after vaccination of the control dogs, remained nearly unchanged in the glucan-treated dogs.
FIG. 3.
Effect of glucan administration on mean specific IgM, IgA, and IgG titers (log2) to Bordetella bronchiseptica antigen in serum. Bars indicate the standard errors of the means. *, significant difference (P < 0.05) between groups; ↑, time of vaccination; ←→, duration of β-glucan or placebo supplementation.
DISCUSSION
The present study is, to our knowledge, the first to evaluate the effect of orally administered β-1,3/1,6-glucans in dogs. To diminish variation, samples were taken each day at the same hour (13). Furthermore, both experiments were performed with animals of the same breed (17). The oral administration of MacroGard increased the serum IgM concentration and decreased the serum and mucosal IgA concentrations, whereas no effect on IgG concentrations was seen, suggesting that the effect of the β-glucan was induced at the intestinal mucosa. This was further supported by the observation that changes in the IgA concentration were first seen in mucosal secretions (saliva, tears) and were seen only later in serum. The mechanisms involved in this immunomodulating effect of the orally administered MacroGard are unclear. Follicle-associated M cells in the intestinal epithelium have been described to be involved in the uptake of β-glucan particles (2, 10, 18). These cells are specialized in the transport of macromolecules from the intestinal lumen to the underlying lymphoid tissue (31). Glucans may target gastrointestinal macrophages and dendritic cells that express dectin 1 and TLR2 on their surfaces (12, 29). The binding of β-glucan results in tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif of dectin 1, generating intracellular signals that mediate phagocytosis and the production of reactive oxygen species (8). Furthermore, dectin 1 acts synergistically with TLR2 and induces via the TLR pathway the production of cytokines, such as TNF-α and IL-12 (9), directing the immune response toward T-helper 1 (Th1) cells, while a Th2 cell environment facilitates IgA class switching, production, and secretion (7). In the present study, it was remarkable that the IgA concentrations started rising again in serum, saliva, and tears immediately after the cessation of β-glucan administration. This indicates a rapid effect and could suggest that IgA-positive B cells are still present but are perhaps located at the site most in contact with the glucans, namely, the gut. It is unlikely a direct effect on B cells, since such an effect has not yet been described. A change in the cytokine environment more likely influences isotype switching since there is an increase in the IgM concentration and a decrease in the IgA concentration. β-1,3/1,6-Glucans are known to activate macrophages, NK cells, and dendritic cells, with the production of IL-12 resulting (2). Therefore, subsequent experiments should involve analysis of the changes in the cytokine profile.
In the second experiment, the vaccination of dogs that did not receive the glucan supplement induced a Bordetella-specific IgA and IgG response and no IgM response, which is the normal response for animals who have been primed by one or more previous immunizations, as was the case in the present study. Since MacroGard is promoted as an optimizer of the immune system and may be administered to dogs on a daily basis, we aimed to know if this administration enhanced immunity to a booster vaccination. Since the interval between the previous and the present vaccination varied for all dogs, we decided to immunize the dogs twice at an interval of 14 days with the intention of obtaining a more homogeneous response in the group. We performed the first immunization 14 days after the start of glucan administration, since we expected to see a strong glucan effect at that time. Indeed, in a previous experiment with pigs, MacroGard (β-1,3/1,6-glucans) was administered orally for 21 days and the immune response of these pigs to a systemically injected antigen was determined (unpublished results). The pigs were immunized twice. A first immunization occurred during β-glucan intake, while the second one occurred 3 weeks later and after the cessation of the supplementation. A higher antigen-specific IgM and IgA response was induced and lasted until the end of the experiment, 3 weeks after the cessation of β-glucan administration, so the effect remained after the end of the glucan supplementation. Therefore, the second immunization in the present study was done at the time that glucan administration was ceased, 14 days after the first immunization, and was expected to increase the effect of the first immunization, as we observed in pigs.
The oral glucan administration did change the antigen-specific isotype response. The glucan-treated animals showed a normal IgG response but a significantly increased Bordetella-specific IgM response, whereas the IgA titers slowly decreased, suggesting diminished IgA class switching. The effect on antigen-specific IgA was less obvious than that on total IgA, which could have been due to the systemic administration of Bordetella, whereas the glucan effect is probably the most pronounced at the mucosa. We therefore checked for changes in total IgA concentrations in feces but could not demonstrate a consistent effect due to a large variation in the titers within each group (data not shown).
An increase in the total serum IgM concentration has also been seen in immunosuppressed mice during β-glucan supplementation (40). However, that study used a β-1,3/1,4-glucan and i.g. or s.c. administration to these mice, which increased not only the total IgM concentration but also the total IgG and IgA concentrations, whereas only the antisporozoite- and antimerozoite-specific IgG concentrations were higher in the sera of the β-1,3/1,4-glucan-treated groups. Higher antigen-specific serum IgG concentrations and higher antiamebic copro-IgG and -IgA concentrations were also observed in rats following the i.g. administration of a β-1,3-glucan (26). However, those authors did not mention whether the β-1,3-glucan had 1,6 or 1,4 branches. In contrast to our study with dogs, the oral administration of soluble β-1,3/1,6-glucans to humans significantly increased the salivary IgA concentration when the glucans were administered at a concentration of 400 mg/day, whereas no effect on serum IgA or IgG was seen (24). In that particular study, they administered the β-glucans for only 4 days, and sampling occurred only before supplementation and at day 5.
Our data show that the oral administration of the β-1,3/1,6-glucan MacroGard to dogs influences the humoral immune response, possibly by the initial interaction of the β-glucan particles with macrophages and dendritic cells in the gut, thereby initializing the cascade of immunological events (35). The oral administration of glucans had an effect on systemically induced IgA and IgM responses but not on the IgG response, which suggests that the glucans can reach not only Peyer's patches but also mucosa-draining lymph nodes, such as mesenteric lymph nodes, as these represent the transition between gut-associated lymphoid tissue and the systemic immune system.
In summary, this study is the first to evaluate the adjuvant effect of orally administered β-1,3/1,6-glucans in healthy dogs on the total IgM, IgA, and IgG concentrations in serum; the IgA concentrations in tears and saliva; and the isotype-specific antibody response against Bordetella. There seems to be a temporary decrease in the switch from IgM toward IgA due to oral MacroGard supplementation in dogs, so that serum and mucosal IgA levels decrease. The same effect on a systemically induced serum antibody response against a Bordetella vaccine was seen.
Acknowledgments
We thank Biotec for providing the MacroGard tablets and P. Deroose from Merial for the Bordetella antigen used in the specific ELISA.
Footnotes
Published ahead of print on 23 December 2009.
REFERENCES
- 1.Bagni, M., N. Romano, M. G. Finoia, L. Abelli, G. Scapigliati, P. G. Tiscar, M. Sarti, and G. Marino. 2005. Short- and long-term effects of a dietary yeast beta-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 18:311-325. [DOI] [PubMed] [Google Scholar]
- 2.Baran, J., D. J. Allendorf, F. Hong, and G. D. Ross. 2007. Oral beta-glucan adjuvant therapy converts nonprotective Th2 response to protective Th1 cell-mediated immune response in mammary tumor-bearing mice. Folia Histochem. Cytobiol. 45:107-114. [PubMed] [Google Scholar]
- 3.Benach, J. L., G. S. Habicht, T. W. Holbrook, and J. A. Cook. 1982. Glucan as an adjuvant for a murine Babesia microti immunization trial. Infect. Immun. 35:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn, J. A., and J. N. BeMiller. 1995. (1→3)-beta-d-glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr. Polymers 28:3-14. [Google Scholar]
- 5.Brown, G. D., and S. Gordon. 2003. Fungal beta-glucans and mammalian immunity. Immunity 19:311-315. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. D., J. Herre, D. L. Williams, J. A. Willment, A. S. Marshall, and S. Gordon. 2003. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerutti, A., and M. Rescigno. 2008. The biology of intestinal immunoglobulin A responses. Immunity 28:740-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, Y. K., B. A. Fallert, M. A. Murphey-Corb, and T. A. Reinhart. 2003. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101:1684-1691. [DOI] [PubMed] [Google Scholar]
- 9.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto, K., I. Suzuki, and T. Yadomae. 1991. Oral administration of SSG, a beta-glucan obtained from Sclerotinia sclerotiorum, affects the function of Peyer's patch cells. Int. J. Immunopharmacol. 13:437-442. [DOI] [PubMed] [Google Scholar]
- 11.Hetland, G., and P. Sandven. 2002. Beta-1,3-glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol. Med. Microbiol. 33:41-45. [DOI] [PubMed] [Google Scholar]
- 12.Hong, F., J. Yan, J. Baran, D. J. Allendorf, R. D. Hansen, G. Ostroff, P. X. Xing, N. K. Cheung, and G. D. Ross. 2004. Mechanism by which orally administered β-1,3-glucans enhance the tumouricidal activity of antitumour monoclonal antibodies in murine tumour models. J. Immunol. 173:797-806. [DOI] [PubMed] [Google Scholar]
- 13.Hucklebridge, F., A. Clow, and P. Evans. 1998. The relationship between salivary secretory immunoglobulin A and cortisol: neuroendocrine response to awakening and the diurnal cycle. Int. J. Psychophysiol. 31:69-76. [DOI] [PubMed] [Google Scholar]
- 14.Instanes, C., H. Ormstad, B. Rydjord, H. G. Wiker, and G. Hetland. 2004. Mould extracts increase the allergic response to ovalbumin in mice. Clin. Exp. Allergy 34:1634-1641. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen, J. B., and B. Robertsen. 1995. Yeast beta-glucan stimulates respiratory burst activity of Atlantic salmon (Salmo salar L.) macrophages. Dev. Comp. Immunol. 19:43-57. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka, K., T. Muta, S. Yamazaki, and K. Takeshige. 2002. Activation of macrophages by linear (1→3)-beta-d-glucans—implications for the recognition of fungi by innate immunity. J. Biol. Chem. 277:36825-36831. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy, L. J., A. Barnes, G. M. Happ, R. J. Quinnell, D. Bennett, J. M. Angles, M. J. Day, N. Carmichael, J. F. Innes, D. Isherwood, S. D. Carter, W. Thomson, and W. E. Ollier. 2002. Extensive interbreed, but minimal intrabreed, variation of DLA class II alleles and haplotypes in dogs. Tissue Antigens 59:194-204. [DOI] [PubMed] [Google Scholar]
- 18.Kournikakis, B., R. Mandeville, P. Brousseau, and G. Ostroff. 2003. Anthrax-protective effects of yeast beta 1,3 glucans. MedGenMed 5:1. [PubMed] [Google Scholar]
- 19.Krakowski, L., J. Krzyzanowski, Z. Wrona, and A. K. Siwicki. 1999. The effect of nonspecific immunostimulation of pregnant mares with 1,3/1,6 glucan and levamisole on the immunoglobulin levels in colostrum, selected indices of nonspecific cellular and humoral immunity in foals in neonatal and postnatal period. Vet. Immunol. Immunopathol. 68:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Kruse, D., and G. T. Cole. 1992. A seroreactive 120-kilodalton beta-1,3-glucanase of Coccidioides immitis which may participate in spherule morphogenesis. Infect. Immun. 60:4350-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudoh, K., J. Shimizu, A. Ishiyama, M. Wada, T. Takita, Y. Kanke, and S. Innami. 1999. Secretion and excretion of immunoglobulin A to cecum and feces differ with type of indigestible saccharides. J. Nutr. Sci. Vitaminol. (Tokyo) 45:173-181. [DOI] [PubMed] [Google Scholar]
- 22.Kulicke, W. M., A. I. Lettau, and H. Thielking. 1997. Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-beta-d-glucans. Carbohydr. Res. 297:135-143. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J. N., D. Y. Lee, I. H. Ji, G. E. Kim, H. N. Kim, J. Sohn, S. Kim, and C. W. Kim. 2001. Purification of soluble beta-glucan with immune-enhancing activity from the cell wall of yeast. Biosci. Biotechnol. Biochem. 65:837-841. [DOI] [PubMed] [Google Scholar]
- 24.Lehne, G., B. Haneberg, P. Gaustad, P. W. Johansen, H. Preus, and T. G. Abrahamsen. 2006. Oral administration of a new soluble branched beta-1,3-d-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 143:65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manners, D. J., A. J. Masson, J. C. Patterson, H. Bjorndal, and B. Lindberg. 1973. The structure of a beta-(1-6)-d-glucan from yeast cell walls. Biochem. J. 135:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-Garcia, F., M. Pedroso, and R. Lopez-Revilla. 2000. Immunomodulation of rat serum and mucosal antibody responses to Entamoeba histolytica trophozoites by beta-1,3-glucan and cholera toxin. Clin. Immunol. 97:182-188. [DOI] [PubMed] [Google Scholar]
- 27.Onderdonk, A. B., R. L. Cisneros, P. Hinkson, and G. Ostroff. 1992. Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo. Infect. Immun. 60:1642-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid, D. M., M. Montoya, P. R. Taylor, P. Borrow, S. Gordon, G. D. Brown, and S. Y. Wong. 2004. Expression of the beta-glucan receptor, dectin-1, on murine leukocytes in situ correlates with its function in pathogen recognition and reveals potential roles in leukocyte interactions. J. Leukoc. Biol. 76:86-94. [DOI] [PubMed] [Google Scholar]
- 29.Rice, P. J., E. L. Adams, T. Ozment-Skelton, A. J. Gonzalez, M. P. Goldman, B. E. Lockhart, L. A. Barker, K. F. Breuel, W. K. Deponti, J. H. Kalbfleisch, H. E. Ensley, G. D. Brown, S. Gordon, and D. L. Williams. 2005. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 314:1079-1086. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai, T., N. Ohno, and T. Yadomae. 1996. Effects of fungal beta-glucan and interferon-gamma on the secretory functions of murine alveolar macrophages. J. Leukoc. Biol. 60:118-124. [PubMed] [Google Scholar]
- 31.Sanderson, I. R., and W. A. Walker. 1993. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update). Gastroenterology 104:622-639. [DOI] [PubMed] [Google Scholar]
- 32.Soltanian, S., E. Stuyven, E. Cox, P. Sorgeloos, and P. Bossier. 2009. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 35:109-138. [DOI] [PubMed] [Google Scholar]
- 33.Stuyven, E., E. Cox, S. Vancaeneghem, S. Arnouts, P. Deprez, and B. M. Goddeeris. 2009. Effect of beta-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 128:60-66. [DOI] [PubMed] [Google Scholar]
- 34.Supamattaya, K., J. Pongmaneerat, and T. Klowklieng. 2000. The effect of β-glucan (MacroGard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon Fabricius. J. Sci. Technol. 22:677-688. [Google Scholar]
- 35.Tsukada, C., H. Yokoyama, C. Miyaji, Y. Ishimoto, H. Kawamura, and T. Abo. 2003. Immunopotentiation of intraepithelial lymphocytes in the intestine by oral administrations of beta-glucan. Cell. Immunol. 221:1-5. [DOI] [PubMed] [Google Scholar]
- 36.Williams, D. L. 1997. Overview of (1→3)-beta-d-glucan immunobiology. Mediators Inflamm. 6:247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams, D. L., R. G. Yaeger, H. A. Pretus, I. W. Browder, R. B. McNamee, and E. L. Jones. 1989. Immunization against Trypanosoma cruzi: adjuvant effect of glucan. Int. J. Immunopharmacol. 11:403-410. [DOI] [PubMed] [Google Scholar]
- 38.Willment, J. A., S. Gordon, and G. D. Brown. 2001. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276:43818-43823. [DOI] [PubMed] [Google Scholar]
- 39.Xiao, Z., C. A. Trincado, and M. P. Murtaugh. 2004. Beta-glucan enhancement of T cell IFNgamma response in swine. Vet. Immunol. Immunopathol. 102:315-320. [DOI] [PubMed] [Google Scholar]
- 40.Yun, C. H., A. Estrada, A. Van Kessel, A. A. Gajadhar, M. J. Redmond, and B. Laarveld. 1997. Beta-(1→3, 1→4) oat glucan enhances resistance to Eimeria vermiformis infection in immunosuppressed mice. Int. J. Parasitol. 27:329-337. [DOI] [PubMed] [Google Scholar]
- 41.Yun, C. H., A. Estrada, A. Van Kessel, B. C. Park, and B. Laarveld. 2003. Beta-glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections. FEMS Immunol. Med. Microbiol. 35:67-75. [DOI] [PubMed] [Google Scholar]
- 42.Zekovic, D. B., S. Kwiatkowski, M. M. Vrvic, D. Jakovljevic, and C. A. Moran. 2005. Natural and modified (1→3)-beta-d-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 25:205-230. [DOI] [PubMed] [Google Scholar]