Abstract
Necrotic enteritis (NE) in broiler chickens is caused by Clostridium perfringens, and there is currently no effective vaccine for NE. We previously showed that in broiler chickens protection against NE can be achieved through intramuscular immunization with alpha toxin (AT) and hypothetical protein (HP), and we subsequently identified B-cell epitopes in HP. In the present study, we identified B-cell epitopes in AT recognized by chickens immune to NE. The gene fragments encoding immunodominant epitopes of AT as well as those of HP were codon optimized for Salmonella and cloned into pYA3493, and the resultant plasmid constructs were introduced into an attenuated Salmonella enterica serovar Typhimurium χ9352 vaccine vehicle. The expression of these Clostridium perfringens proteins, alpha toxoid (ATd) and truncated HP (HPt), was confirmed by immunoblotting. The protection of broiler chickens against experimentally induced NE was assessed at both the moderate and the severe levels of challenge. Birds immunized orally with Salmonella expressing ATd were significantly protected against moderate NE, and there was a nonsignificant trend for protection against severe challenge, whereas HPt-immunized birds were significantly protected against both severities of challenge. Immunized birds developed serum IgY and mucosal IgA and IgY antibody responses against Clostridium and Salmonella antigens. In conclusion, this study identified, for the first time, the B-cell epitopes in AT from an NE isolate recognized by chickens and showed the partial protective ability of codon-optimized ATd and HPt against NE in broiler chickens when they were delivered orally by using a Salmonella vaccine vehicle.
Necrotic enteritis (NE) in broiler chickens is caused by Clostridium perfringens. This disease poses a resurging threat to the poultry industry in jurisdictions where there has been a ban on the use of antimicrobials for growth promotion, since broiler chickens reared under intensive conditions in the absence of antimicrobial prophylaxis are at risk of NE (12). There has consequently been an increased effort in recent years to understand the pathogenesis of and immunity to NE. Emerging evidence is that there are distinct NE strains in chickens capable of producing disease characterized by the presence of a novel toxin, NetB (14), and possibly by other features, including intraspecies growth inhibition by C. perfringens (29, 33). The toxin historically implicated in the pathogenesis of NE was alpha toxin (AT), a phospholipase C that was also identified as an important immunogen in protection against NE (2, 3, 22). A recent study has shown that AT is not essential in pathogenesis (15). However, the pathogenesis of NE may be more complex than the involvement of NetB rather than AT, since some non-NE isolates can be netB positive and some NE isolates can be netB negative (5, 23).
Although immunization appears to offer the best alternative to antimicrobials for the control of NE, no effective active vaccine is available for broiler chickens and understanding of the basis of immunity to NE is incomplete. Recent findings reported from our laboratory have indicated that different selected secreted proteins, including AT, have roles in providing protection against NE, with their efficacies varying with the severity of the challenge (17, 18). Two important immunogens, namely, AT and a hypothetical protein (HP), protected birds immunized intramuscularly against severe experimentally induced NE. Subsequently, a region of HP that contained immunoreactive B-cell epitopes was cloned into an attenuated Salmonella vehicle, and birds immunized orally with this vaccine were shown to be significantly but not completely protected against NE (19), and among the clostridial genes expressed in Salmonella, HP gave the best protection. In that study, use of the plasmid expression vector pYA3342 resulted in the relatively poor expression of the cloned genes. There is evidence that AT is an important protective immunogen in immunity to NE (11, 18, 22), although its effect may be indirect (35), when it is delivered as a toxoid subcutaneously (6) or through an attenuated Salmonella oral vaccine expressing the carboxy-terminal domain (35); the choice of the C-terminal domain was based on its successful use as a protective immunogen in mouse model of gas gangrene (31, 35). Although the immunogenic B-cell epitopes of AT for mice have been identified (21), the B-cell epitopes that are recognized by chickens immune to NE and that may be important for protection have not been characterized.
The current study was carried out with two main objectives: (i) to identify the B-cell epitopes in AT by using serum from chickens immune to NE to obtain information about the possible antibody recognition sites and (ii) to clone and express the immunodominant epitopes of AT and of HP (identified in a previous study) in an improved Salmonella antigen expression system and to compare the abilities of these oral vaccine constructs to provide immunity against infection in an experimental NE challenge model at both moderate and severe levels of challenge.
MATERIALS AND METHODS
Epitope mapping of alpha toxin.
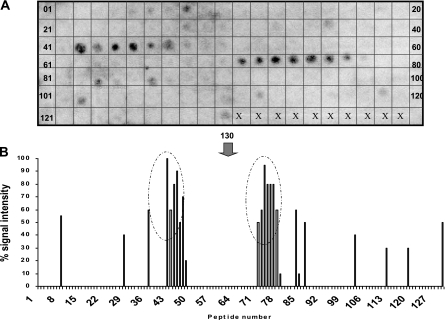
To identify the B-cell epitopes of AT, 130 peptides were synthesized (SPOTs [synthetic peptide arrays on membrane supports] synthesis) on a derivatized cellulose membrane (Sigma-Genosys Biotechnologies, Woodlands, TX). The peptides were identified from the 398-amino-acid sequence of the AT gene of a virulent NE isolate, isolate CP4; these were 12 residues in length and were sequentially offset by 3 amino acid residues (25). The synthesized membranes containing the synthetic peptides either were probed immediately or were stored at −20°C until they were needed. The membrane containing the synthesized peptides was washed briefly with methanol and then three times for 5 min each time with Tris-buffered saline (TBS), before they were blocked overnight with blocking buffer (Sigma-Genosys Biotechnologies) with 5% (wt/vol) sucrose. After the membranes were blocked, they were washed with TBST (50 mM Tris, pH 8.0, 136 mM NaCl, 2.7 mM KCl, 0.05% Tween 20) for 10 min and incubated with serum from birds immune to NE (32) at a dilution of 1:500 for 2 h at room temperature. The membranes were then washed twice with TBST for 10 min and incubated with goat anti-chicken immunoglobulin Y (IgY; heavy and light chains; Cedarlane Laboratories, Hornby, Ontario, Canada) at a 1:2,000 dilution and room temperature for 1 h. After incubation, the membranes were washed with TBST and the bound antibodies were detected by using the chemiluminescent substrate CDP-Star (Applied Biosystems, Foster City, CA) and the enhancer Nitro-Block II (Applied Biosystems), both of which were diluted to 1:100 with 0.1 M Tris-HCl-0.1 M NaCl, pH 9.5. The membrane was visualized with a molecular light imager (Berthold, Bad Wildbad, Germany). The quantified signal of each spot was determined as a relative percentage of a selected spot (peptide) that showed the highest reactivity (which was designated 100% reactivity) by using Win Light software (Berthold), and the corresponding value was given after subtraction of the background reactivity of spots developed in response to the sera from nonimmune chickens (32).
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains Escherichia coli χ6212 and Salmonella enterica serovar Typhimurium χ9352 and plasmids pYA3493 and pYA3620 were kindly provided by R. Curtiss III (The Biodesign Institute, Arizona State University, Tempe, AZ). The bacteria have a chromosomal deletion of the aspartate β-semialdehyde dehydrogenase (asd) gene, which requires complementation by Asd-positive (Asd+) plasmid pYA expressing heterologous genes (9, 10). Escherichia coli χ6212 is an intermediate host used to clone the genes of interest. Salmonella serovar Typhimurium χ9352, an attenuated strain derived from strain χ3761 (S. Typhimurium UK-1 wild type), has a chromosomal insertion of the lacI gene under the control of the PBAD (ara) promoter that enables the regulated expression of the recombinant protein. Thus, antigen expression can be controlled by addition of arabinose (0.2%) to the culture medium. In the absence of arabinose or in vivo, the trc promoter in the plasmids enables the constitutive expression of the recombinant protein. Escherichia coli χ6212 and S. Typhimurium χ9352 harboring pYA3493 containing C. perfringens genes were grown in Luria-Bertani (LB) medium (Difco, Detroit, MI), and diaminopimelic acid (DAP; 100 μg/ml) was added whenever uncomplemented Δasd strains were grown.
TABLE 1.
Bacterial strains and plasmids
| Bacterial strains and plasmids | Relevant genotype or phenotype | Source |
|---|---|---|
| Bacterial strains | ||
| E. coli χ6212 | φ80d lacZΔM15 deoRΔ(lacZYA-argF)U169 supE44 λ−gyrA96 recA1 relA1 endA1 Δasd Δzhf-2::Tn10 hsdR17(rB− rB+) | R. Curtiss III |
| Salmonella enterica serovar Typhimurium χ9352 | Δpmi-2426 Δ(gmd-fcl)-26 ΔPfur77::TT araC PBADfur ΔPcrp527::TT araC PBADcrp ΔasdA21::TT araC PBADc2 ΔaraE25 ΔaraBAD23 ΔrelA198::araC PBADlacI TT | B. Gunn, R. Curtiss III |
| Clostridium perfringens CP4 | Wild-type, virulent NE isolate | Laboratory |
| Plasmids | ||
| pYA3493 | Asd+, pBR ori | R. Curtiss III |
| pYA3620 | Asd+, pBR ori | R. Curtiss III |
| pYA3493-ATd | Asd+, ATd | This work |
| pYA3493-HPt | Asd+, HPt | This work |
Construction and cloning of C. perfringens genes in E. coli and in S. Typhimurium.
Gene fragments encoding truncated proteins of AT and HP, referred to in this report as alpha toxoid (ATd) and truncated HP (HPt), that included immunodominant B-cell epitopes were codon optimized for expression in Salmonella (DNA2.0, Menlo Park, CA) and cloned into pYA3493. The pYA3493 vector is an improvement over the pYA3342 vector used in an earlier study (19) because it contains a β-lactamase type II signal sequence for better secretion of the cloned heterologous gene products into the periplasm and some proportion (25%) of it is also exported into the culture supernatants (7, 8, 13). In addition to the β-lactamase signal peptide sequence, the other vector plasmid, pYA3620, has the β-lactamase C-terminal protein-coding sequence at the 3′ end of the recombinant gene to facilitate the transport of the recombinant protein across the membranes (13, 16) and was also used in this study. Positive transformants in E. coli χ6212 were selected on LB plates supplemented with tetracycline (50 μg/ml). All constructs were verified by restriction enzyme analysis and by sequencing both strands of the cloned products. Upon confirmation, recombinant plasmid constructs were introduced into Salmonella χ9352 strains by electroporation and Asd+ transformants were selected on LB plates. Plasmid stability was confirmed by the growth of Salmonella χ9352 in LB medium with and without DAP.
Expression of recombinant C. perfringens proteins in S. Typhimurium.
To confirm the expression of ATd (∼20 kDa) and HPt (∼20 kDa) by S. Typhimurium, cells grown in LB to an optical density at 600 nm (OD600) of 0.8 to 0.9 were pelleted and resuspended in 2× SDS sample buffer (20), and the mixture was boiled at 95°C for 5 min. Recombinant protein expression in Salmonella is constitutive, but arabinose (0.2%) was added to the culture medium for the regulation of expression whenever it was required. The expression of these heterologous proteins was first confirmed in E. coli after induction by isopropyl-ß-d-thiogalactopyranoside (IPTG) in the medium at a final concentration of 1 mM. Total proteins were analyzed by standard SDS-PAGE and immunoblot techniques. Proteins that had been separated on a 12% polyacrylamide gel were stained with Coomassie blue or were transferred to a nitrocellulose membrane. The blots were developed with antigen-specific serum, collected from previously immunized birds, as the source of primary antibodies (18), followed by binding with alkaline phosphatase-conjugated goat anti-chicken IgY (heavy and light chains) antibodies (Cedarlane Laboratories). Primary antibodies were absorbed with sonicated E. coli χ6212 and S. Typhimurium χ9352. To determine the extracellular expression of C. perfringens proteins into the culture supernatants of S. Typhimurium, the cells were grown to an OD of 0.8, and the supernatants were concentrated 25-fold with an Amicon filter (cutoff, 10 kDa) and analyzed by SDS-PAGE and Western blotting.
Cloning, expression, and purification of ATd.
The Escherichia coli strains were used to clone and express the coding sequence of an immunodominant region of AT identified by epitope mapping; E. coli DH5α recA lacZΔM15 (Stratagene, La Jolla, CA) was the host for plasmid construction, and E. coli BL21-Star(DE3) F− ompT hsdSB (rB− mB−) gal dcm rne-131 (Invitrogen, Carlsbad, CA) was used for overexpression of the histidine-tagged ATd protein. Strains were grown in LB medium at 37°C, and when it was required, kanamycin was added to the medium at a concentration of 50 μg/ml. PCR amplifications were performed with Platinum PCR SuperMix High Fidelity (Invitrogen, Burlington, Ontario, Canada) and specific primers (forward primer, GGCGGAATTCTTCTCAAAGGATAATAGTTG; reverse primer, GGCGCTCGAGGAATCTATAAATATATCCTG). After purification (PCR purification kit; Qiagen, Mississauga, Ontario, Canada), the PCR product was cloned into the pET28a vector (Novagen Inc., Madison, WI) between EcoRI and XhoI restriction sites to generate a protein fused with histidine residues (6-His). This plasmid was introduced into E. coli BL21-Star(DE3), and the nucleotide sequence of the cloned product was verified by sequencing. Expression of the recombinant ATd protein by E. coli was induced by IPTG. Histidine-tagged proteins were purified by affinity chromatography (Qiagen), and the purified recombinant protein fraction was separated by SDS-PAGE and confirmed by Western blotting with antihistidine antibodies. The recombinant protein fractions were concentrated with Amicon filters, and the protein concentration was determined. Loss of the lecithinase and hemolytic activities by ATd was confirmed on 5% egg yolk plates and blood agar plates (32).
Vaccination and challenge procedure.
The vaccine strains of Salmonella were grown in 100 ml of LB broth at 37°C, after inoculation with an overnight culture (2% inoculum) under aeration to an OD600 of 0.8 to 0.9. In the case of Salmonella carrying HPt, arabinose at a 0.2% final concentration was added to the culture. The cells were recovered by centrifugation at 8,000 × g for 15 min at 4°C, and the pellet was resuspended in 1 ml of phosphate-buffered saline (PBS) containing 1% gelatin (brilliant green with sulfa [BGS]). Viable cell counts (the numbers of CFU) were determined by plating serial dilutions onto LB and MacConkey agar plates. The vaccine was prepared fresh on the day of immunization.
The wings of commercial 1-day-old male White Plymouth Rock broiler chicks were tagged. The chicks were then randomly allocated to different groups of about 15 birds each, deprived of food and water for 8 h, and then immunized orally with 100 μl of BSG containing 1.2 × 109 CFU of S. Typhimurium χ9352 carrying either the pYA3493 ATd or the pYA3493 HPt plasmid construct (16, 36). Birds that received S. Typhimurium χ9352 (pYA3493, vector only) were used as negative controls. Feed and water were given after 30 min. The chicks were fed a starter diet containing 20% protein for 13 days, followed by a formulated wheat-based grower diet containing 28% protein (Arkell Research Station, University of Guelph). At day 10 of age, the birds were given a second dose of vaccine (1 × 109 cells/bird). Nine birds not only were immunized twice with S. Typhimurium χ9352 (pYA3493-ATd) but also were immunized with an intramuscular boost of purified recombinant ATd on day 17. At day 23 of age, the birds were challenged with virulent C. perfringens (strain CP4) at a final concentration of 8.1 ± 0.1 log10 CFU/ml. The strain had been grown in fluid thioglycolate medium (FTG; Difco) at 37°C for 24 h. To alter the severity of the challenge, different feeds into which the bacteria were mixed, either turkey starter (28% protein) or wheat-based grower diet (20% protein), were used (18, 32). The C. perfringens cultures were mixed with either feed at a ratio of 2:1 (vol/wt). Infected feed was prepared fresh twice daily and was fed to chickens that were initially fasted for 20 h prior to challenge, and the chickens were challenged for 5 days. The conditions for the use of the chickens were approved by the University of Guelph Animal Care Committee, in accordance with the guidelines of the Canadian Council on Animal Care.
Colonization of chickens with the recombinant Salmonella strain.
To confirm that the chickens were colonized with S. Typhimurium χ9352 harboring C. perfringens genes after oral immunization, the spleens, ceca, and bursas of two birds from each of the immunized groups were collected aseptically on days 4, 8, 12, and 16 postinoculation; and the tissues were homogenized and plated on BGS plates after prior preenrichment in selenite cysteine sulfa broth because the presence of E. coli in high abundance in the samples precluded the growth of the vaccine strain. Pink colonies were plated on MacConkey plates supplemented with 0.5% maltose to confirm the colorless colony phenotype of the vaccine strain, and PCR was performed with representative colonies to confirm the retention of the heterologous genes. Salmonella strains on BGS are pink, but those on MacConkey-maltose are colorless because of the pBAD-crp mutation. Intestinal colonization by the vaccine strain was confirmed by collecting fecal samples from chickens in each of the immunized groups on days 15, 16, and 17; pooling the fecal samples; and then demonstrating colonization as described above.
Protection assessment.
Protection against C. perfringens challenge was assessed on the basis of the gross intestinal lesion scores at necropsy and the body weight gains of birds during the entire study. Birds from each group were weighed individually at weekly intervals. The chickens were euthanized on the day following a 5-day challenge, and at necropsy the small intestine was examined for grossly visible lesions. Any chickens that had reached a predetermined severity of clinical illness prior to necropsy were euthanized and necropsied. Intestinal lesions were scored by the use of two scoring systems, that described by Kulkarni and others (17) and that described by Keyburn et al. (15). Blinded scoring was employed.
Measurement of antibodies in chicken sera and intestinal washings.
Blood was collected from the wing veins of eight birds from each group on day 1 of age, at week 2 (midexperiment), and at week 4 (prechallenge) to assess the serum antibody responses to Salmonella and to C. perfringens antigens. At necropsy, intestinal washings and scrapings from the mucosal side were pooled from five birds from each group and processed as described previously (18).
An enzyme-linked immunosorbent assay (ELISA) was performed to determine the presence of C. perfringens antigen-specific IgY and IgA in immunized birds. For the serum and intestinal washing ELISAs, C. perfringens proteins (AT, HP) purified from E. coli as histidine-tagged recombinant fusion proteins were used as described previously (18). To eliminate the nonspecific reactivities of antibodies to E. coli proteins, serum was absorbed with E. coli cells and their lysates. For the intestinal washing ELISA, intestinal washing samples from five chickens per group were collected as described above, and the total protein content was measured by use of a PlusOne 2-D Quant kit (Amersham Biosciences, San Francisco, CA). Total protein was used as the source of primary antibody after the protein content of initial dilution (1:4) was kept constant. A Salmonella lysate was used in an ELISA to assess the anti-Salmonella responses in the serum and intestines of birds immunized with recombinant S. Typhimurium expressing a C. perfringens antigen, as well as infected control birds treated with the vector only. To make the lysates, S. Typhimurium carrying pYA3493 without an inserted gene was grown in LB medium to an OD600 of ∼0.8, and the cells were then pelleted and resuspended in 0.1 M carbonate buffer, before sonication on ice for 5 to 10 min. The resultant lysate was used as a coating antigen (10 μg protein/ml) in the ELISA as described above, and the Salmonella antibody titers were determined as outlined above.
Alpha toxin neutralization assay.
Purified C. perfringens alpha toxin (Sigma Laboratories, St. Louis, MO) reconstituted to 0.5 μg/μl of protein was diluted to different concentrations in PBS (pH 7.2), and 100 μl of each dilution was used to check the lecithinase activity as well as the hemolytic activity of alpha toxin on 5% egg yolk agar (Difco) and blood agar plates, respectively, as described previously (17).
Statistical analysis.
Data were analyzed to determine whether there was a significant difference between the number of birds with lesions for birds from the recombinant Salmonella-immunized groups and the control group treated with the Salmonella vector only. A two-tailed Fisher's exact test was used to determine whether the birds in the two groups differed in the proportion with which they fell into the two classification of either lesions or no lesions, under the null hypothesis that the proportions would be the same. Data were analyzed in a two-by-two contingency table, with the data for the control group treated with the vector only being in one column and the data for the immunized groups being in the other column. Lesion scores were ranked from 0 to 5+, with a “protective” response given to birds with lesion scores of 0 to 1+. The null hypothesis was rejected at an α level of 0.05. For the serum ELISA, a one-way analysis of variance was used to determine significant (P ≤ 0.05) differences in the antibody titers between the preimmunized and the immunized birds across all groups. Statistical analysis could not be performed for the intestinal washing ELISA, since the intestinal washings were collected only at necropsy.
RESULTS
Identification of B-cell epitopes in alpha toxin.
The AT nucleotide sequence obtained from the AT gene of strain CP4 had 99.5% identity to that of strain 13 (GenBank gene identifier 988262) and ATCC 13124 (GenBank gene identifier 4201274). Figure 1 shows the reactivities of overlapping AT peptides with serum from birds immune to NE. Two distinct regions (amino acid positions 96 to 122 and 183 to 212), each of which comprised two to three linear 12-mer peptides were identified as immunodominant epitopes. On the basis of these reactivities of the peptides, a region of 162 amino acid residues (486 bp) that included two sections of immunodominant epitopes as well as regions of weak reactivity was cloned and expressed as a truncated nontoxic AT, named ATd. A hydropathy profile of the ATd amino acid sequence obtained by using the Hopp-Woods scale (http://www.vivo.colostate.edu/molkit/hydropathy/index.html) predicted several potentially antigenic (hydrophilic) regions that are likely to be exposed on the surface of ATd upon conformation for recognition by B-cell receptors.
FIG. 1.
Epitope mapping of alpha toxin. On the basis of the primary sequence of alpha toxin, a total of 130 peptides, each of which was 12 amino acids in length, offset by 3 residues were synthesized as spots on a cellulose-derived matrix and reacted with polyclonal chicken immune serum (A). The membrane was visualized under a molecular light imager, and each black spot represents a reactive peptide. The quantified signal of each spot was obtained by using Win Light software, and the value was expressed as a relative percentage of the signal intensity (B). Each vertical bar in panel B represent a peptide, and those inside an oval circle represent the immunodominant epitope regions of the N terminus of alpha toxin.
Expression of C. perfringens gene products in S. Typhimurium χ9352.
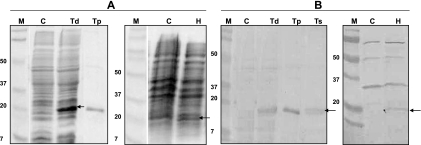
Figure 2 shows the expression of codon-optimized ATd and HPt by S. Typhimurium χ9352 (pYA3493 constructs) and their immunoreactivities to the antigen-specific antibodies from immunized chickens. The level of intracellular ATd expression by S. Typhimurium was estimated to be ∼5% of the total proteins expressed by the vector, and the level of ATd secreted in the culture supernatants was estimated to be ∼1 to 2% of the total proteins expressed by the vector. However, the expression of HPt by S. Typhimurium was poor and required the addition of arabinose to the culture medium to prevent the lysis of cells grown without arabinose. The level of intracellular expression of HPt in S. Typhimurium was ∼1 to 3% of the total proteins expressed by the vector, but the secreted HPt was found only in trace amounts. Use of the pYA3620 plasmid vector to clone the gene sequence for ATd resulted in reduced protein expression compared to that achieved with pYA3493, and cloning of the gene for HPt into pYA3620 was unsuccessful. Therefore, the constructs consisting of the attenuated Salmonella strain carrying pYA3493 were used to immunize the chickens.
FIG. 2.
Expression of C. perfringens genes by the S. Typhimurium χ9352 vaccine vehicle. Total proteins expressed by recombinant Salmonella were separated on a 12% SDS-polyacrylamide gel and stained with Coomassie blue (A), followed by transfer onto nitrocellulose membranes, and the proteins were reacted with immune serum collected from chickens that were previously intramuscularly immunized with purified recombinant proteins (B). Lanes C, controls administered S. Typhimurium χ9352(pYA3493); lanes Td, intracellular expression of ATd by S. Typhimurium χ9352(pYA3493 Atd); lane Ts, extracellular expression of ATd in the culture supernatants of S. Typhimurium χ9352(pYA3493 Atd); lanes Tp, recombinant histidine-tagged ATd purified from E. coli as a positive control; lanes H, intracellular expression of HPt by S. Typhimurium χ9352(pYA3493 HPt); lanes M, molecular weight standards; arrows, protein expressed by recombinant Salmonella.
In vivo colonization of S. Typhimurium χ9352 carrying C. perfringens genes.
Birds to which S. Typhimurium χ9352 carrying pYA3493 ATd, pYA3493 HPt, or the pYA3493 vector only was orally administered (109 CFU) on the day of hatching were checked for the colonization and tissue distribution of the vaccine strain in the spleens, ceca, and bursas (data not shown). Colonization was observed in the ceca at all times, whereas infection was inconsistent in the bursas. No infection of the spleen was evident. The level of colonization by S. Typhimurium χ9352 carrying pYA3493 ATd in both the cecum and the bursa was, however, poor compared to that by S. Typhimurium χ9352 carrying pYA3493 HPt or the vector only control, since ∼30% of the birds immunized with these constructs did not show the presence of the vaccine strain, despite the use of preenrichment. This trend of poor colonization was also confirmed when the intestinal colonization by the vaccine strain was evaluated by examination of pooled fecal samples from each of the immunized groups (data not shown).
Protection of immunized birds against experimentally induced necrotic enteritis.
Birds immunized with S. Typhimurium χ9352(pYA3493 ATd) or χ9352(pYA3493 HPt) were significantly protected against a moderate challenge compared to the protection achieved for the controls treated with the vector only (Table 2). Birds immunized with Salmonella χ9352(pYA3493 HPt) were significantly protected against a more severe challenge, but birds immunized with S. Typhimurium χ9352 (pYA3493 ATd) either alone or followed by an intramuscular booster with purified recombinant ATd were not significantly protected.
TABLE 2.
Intestinal (duodenum to ileum) lesion scores of birds immunized with recombinant S. Typhimurium χ9352 carrying C. perfringens genes orally and then infected with virulent C. perfringens CP4
| Challenge preparation and group | Total no. of chickens | No. of chickens with the indicated lesion score by use of system of: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Keyburn et al. (15) |
Kulkarni and others (17) |
|||||||||||||||
| 0 | 1+ | 2+ | 3+ | 4+ | 5+ | 6+ | Mean | 0 | 1+ | 2+ | 3+ | 4+ | 5+ | Mean | ||
| Challenge prepared in turkey starter diet | ||||||||||||||||
| Controls treated with Salmonella vector only | 17 | 0 | 0 | 1 | 1 | 6 | 6 | 3 | 4.52 | 0 | 0 | 8 | 6 | 2 | 1 | 2.76 |
| Treated with Salmonella ATd | 12 | 1 | 2 | 3 | 1 | 4 | 0 | 1 | 2.75 | 1 | 2 | 8 | 0 | 1 | 0 | 1.83 |
| Treated with Salmonella HPt | 17 | 0 | 10 | 2 | 1 | 2 | 1 | 1 | 2.11a | 0 | 10 | 5 | 1 | 1 | 0 | 1.58a |
| Challenge prepared in wheat-based diet | ||||||||||||||||
| Controls treated with Salmonella vector only | 17 | 0 | 2 | 3 | 1 | 8 | 3 | 0 | 3.41 | 0 | 2 | 12 | 3 | 0 | 0 | 2.25 |
| Treated with Salmonella ATd | 13 | 0 | 7 | 3 | 1 | 2 | 0 | 0 | 1.84a | 0 | 7 | 6 | 0 | 0 | 0 | 1.46a |
| Treated with Salmonella ATd + i.m.b booster | 9 | 0 | 0 | 1 | 0 | 8 | 0 | 0 | 3.77 | 0 | 0 | 9 | 0 | 0 | 0 | 2.00 |
| Treated with Salmonella HPt | 18 | 1 | 9 | 2 | 2 | 2 | 1 | 1 | 2.05a | 1 | 9 | 6 | 1 | 1 | 1.55a | |
Immunized groups that had significantly fewer chickens with lesions compared to vector-only controls (Fisher's exact test, P ≤ 0.05).
i.m., intramuscular.
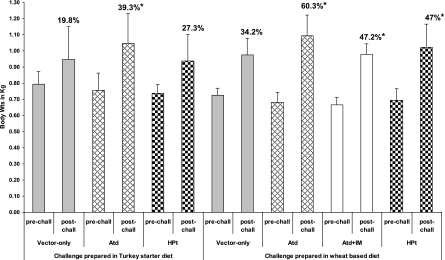
The body weight gains of birds in all the groups, determined at weekly intervals, were consistent until the challenge (Fig. 3). During the challenge period, significant body weight gains were observed in birds immunized with χ9352(pYA3493 ATd) or χ9352(pYA3493 HPt) and challenged by use of the moderate-challenge NE model compared to those observed in birds immunized with χ9352(pYA3493) (controls) (Table 2). Birds immunized with χ9352(pYA3493 ATd) in the severe-challenge NE model and those immunized with S. Typhimurium χ9352(pYA3493 ATd) followed by an intramuscular booster in the moderate-challenge NE model showed greater weight gains than the controls treated with the vector only, even though these birds were not significantly protected. In contrast, birds that were immunized with S. Typhimurium χ9352(pYA3493 HPt) in the severe-challenge NE model and that were significantly protected did not show significant weight gain. Birds immunized with S. Typhimurium χ9352(pYA3493 ATd) and challenged with the moderate-challenge infection had the highest weight gains compared to the weight gain for the controls and subjectively had the most uniform appearance at time of euthanasia.
FIG. 3.
Body weight (Wt) gains of birds immunized with recombinant Salmonella χ9352 carrying C. perfringens antigens. Birds immunized twice orally with recombinant Salmonella expressing C. perfringens proteins were challenged at two levels of severity of infection in an experimental NE model. Birds were weighed individually on day 20 (prechallenge [pre-chall]) and day 27 (postchallenge[post-chall]), and the group mean weight gain during the challenge is expressed in percent. The vertical bars represent absolute body weights. Atd, birds immunized with recombinant Salmonella expressing truncated alpha toxoid; HPt, birds immunized with recombinant Salmonella expressing HPt; and vector only, controls immunized with Salmonella only. IM, intramuscular; *, significant weight gain values compared to the prechallenge status (P ≤ 0.05).
Antibody responses to C. perfringens antigens delivered by recombinant S. Typhimurium χ9352.
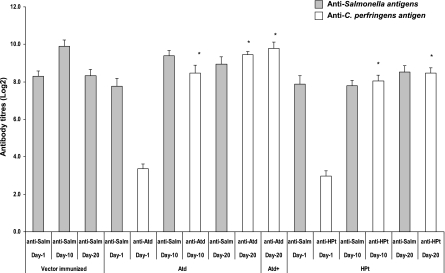
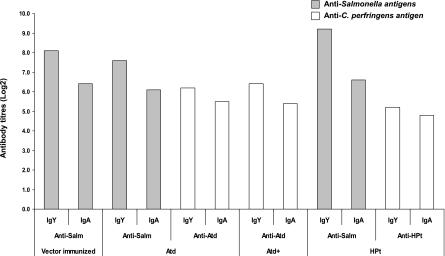
All birds immunized orally with recombinant S. Typhimurium carrying C. perfringens antigens, including controls treated with the vector only, had Salmonella antibodies (IgA, IgY) in their serum and intestinal washes (Fig. 4 and 5). The anti-Salmonella antibody titers in the birds that received S. Typhimurium χ9352(pYA3493 ATd) showed a slight decline over time. Birds immunized with χ9352(pYA3493 ATd) or χ9352(pYA3493 HPt) had significant clostridial antigen-specific serum antibody titers following immunization (Fig. 4 and 5), with the birds immunized with S. Typhimurium χ9352(pYA3493 HPt) having lower titers than the birds immunized with S. Typhimurium χ9352 (pYA3493 ATd). Intramuscular boosting with purified ATd did not result in higher titers of anti-ATd antibodies. The immunized birds developed intestinal antigen-specific IgY and IgA responses to the C. perfringens antigens (Fig. 5). However, the birds immunized with S. Typhimurium χ9352(pYA3493 HPt) had slightly lower intestinal titers than the birds immunized with S. Typhimurium χ9352(pYA3493 ATd). The intestinal IgY titers to Salmonella antigens were slightly higher than those to C. perfringens antigens. In the toxin neutralization assay, the partial inhibition of AT hemolytic activity (serum dilution, 1:5) but no inhibition of lecithinase activity was observed.
FIG. 4.
Serum IgY responses of broiler chickens immunized with recombinant Salmonella χ9352 carrying pYA3493, pYA3493 ATd, or pYA3493 HPt to Salmonella and C. perfringens antigens, as determined by ELISA. The birds were immunized orally on days 1 and 10; and serum samples were collected at days 1, 10, and 20. Whole-cell lysates and purified antigens were used as coating antigens to assess the anti-Salmonella (anti-Salm) and the anti-C. perfringens responses, respectively. ATd, birds immunized with recombinant Salmonella expressing alpha toxoid; Atd+, birds immunized with recombinant Salmonella expressing Atd and then administered an intramuscular booster of recombinant, purified histidine-tagged Atd; HPt, birds immunized with recombinant Salmonella expressing HPt; vector immunized, controls immunized with Salmonella only; *, significantly different titers compared to the titers on day 1 (P ≤ 0.05).
FIG. 5.
Intestinal IgY and IgA responses of broiler chickens antigens immunized with recombinant Salmonella χ9352 carrying pYA3493, pYA3493 ATd, or pYA3493 HPt to Salmonella and C. perfringens, as determined by ELISA. The birds were immunized orally on days 1 and 10, and pooled intestinal scrapings and washings were collected at necropsy (day 27). Whole-cell lysates and purified antigens were used as coating antigens to assess the anti-Salmonella and anti-C. perfringens responses, respectively. ATd, birds immunized with recombinant Salmonella expressing alpha toxin; Atd+, birds immunized with recombinant Salmonella expressing Atd and then administered an intramuscular booster of recombinant, purified histidine-tagged Atd; HPt, birds immunized with recombinant Salmonella expressing HPt; vector immunized, controls immunized with Salmonella only.
DISCUSSION
The study described here shows that two truncated, immunogenic C. perfringens proteins, AT and HP, provide partial protective efficacy against an experimental NE model in broiler chickens when they are delivered orally through an attenuated Salmonella strain as a vaccine vehicle. The improvements in the attenuated Salmonella strains used for the delivery of antigens from mucosal pathogens include regulated delayed attenuation and regulated antigen synthesis, and these strains have been designed to express protective antigens at high levels and to stimulate strong primary and lasting memory immune responses without much tissue or other damage (9, 16, 28). The choice of plasmid vector and the Salmonella host strain greatly influence the outcome of protection after oral immunization. An earlier study that used pYA3342 as the vector and S. Typhimurium χ9241 as the vehicle for the delivery of C. perfringens antigens, including HP, resulted in partial protection but was associated with poor antigen expression in vitro (19). The current study employed a better antigen expression plasmid vector, pYA3493, that utilizes the type II secretion system for the expression of heterologous proteins (13, 36), as well as a more attenuated vehicle, S. Typhimurium χ9352 (Table 1), which has multiple mutations that promote both regulated delayed attenuation and regulated antigen synthesis (7-9).
The level of colonization by the vaccine strain (S. Typhimurium χ9352) was found to be poor, in general, since some proportion (∼30%) of the immunized birds, in particular, birds that received S. Typhimurium χ9352(pYA3493 ATd), did not show the vaccine strain in their tissues, despite preenrichment in selenite cysteine broth (data not shown). Although the current study did not use mannose to grow these vaccine constructs, a subsequent study that used one of these vehicles grown in mannose colonized the chicken tissues poorly. Failure to colonize well in vivo induces insufficient immune responses against the heterologous proteins and is the most likely reason for the poor protection efficacy against NE in the current study, since AT and HP, when they were delivered intramuscularly in a previous study (18), showed protection superior to that achieved in the present study. The codon optimization of genes is a useful strategy for better expression by attenuated vaccine vehicles (8, 30). The present study also employed codon-optimized gene sequences for AT and HP for expression by Salmonella, although no comparison with non-codon-optimized genes was made. In the context of the cloning of HP, we found continued difficulties that were previously suspected of being associated with toxicity. In the present study, only a short fragment of HP (450 bp) that included previously identified epitopes was cloned into Salmonella, since a 1-kb fragment that was successfully cloned previously (19) could not be cloned into either of the two plasmid vectors used in this study. In addition, because of its apparent toxicity, its expression required addition of arabinose, which caused reduced expression in vitro by bringing it under the control of the araC promoter (8, 16). In the present study, HPt had both a lower antibody response and a lower protective response than those observed in an earlier study in which a larger HP fragment was delivered by strain χ9241 (19). This reduced immunizing efficacy was likely in part the result of poor colonization by the Salmonella vehicle, but it may also have been because of both the poor expression of HPt and the possible absence of important antigenic sites due to its smaller size.
This study identified, for the first time, the B-cell epitopes of AT recognized by antibodies from birds immune to NE. An earlier report described B-cell epitopes of AT recognized by a panel of mouse monoclonal antibodies in the context of gas gangrene (21). The present study revealed two distinct immunodominant regions at amino acid positions 96 to 122 and 183 to 212, both of which are regions at the N terminus of the protein. In contrast, Zekarias et al. (36) recently expressed amino acids 248 to 370 of the C-terminal domain of AT in S. Typhimurium χ8914 that contained defined attenuating deletions of the pabA and pabB genes and found that it partially protected broiler birds against NE. The choice for the use of the C terminus in this study was based on the successful use of this domain as an immunogen to achieve complete protection against AT in a murine myonecrosis model (4, 31, 34). Interestingly, in the context of the murine gas gangrene model, a monoclonal antibody identified by Logan et al. (21) that specifically bound to an octapeptide (YARGFAKT; positions 193 to 198) capable of neutralizing AT activity in vitro and in vivo was identified in the present study as an immunodominant epitope within the second antigenic region (positions 183 to 212). However, no further investigation has evaluated the role of the N-terminal region in providing immunity to gas gangrene in the mouse model. Two of the membrane-interacting regions of alpha toxin (positions 204 to 205 and 208 to 211), as revealed by its crystal structure (1, 24), were also found within the second immunodominant region of AT identified here. It is apparent that antibodies capable of binding and neutralizing the critical epitopes of the catalytic domain (N terminus) are important in AT-mediated NE immunity. These findings should improve the design of a suitable vaccine against NE. Further work is required to demonstrate that the N-terminal immunodominant region of AT is an immunoprotective region, since the boosting of birds immunized with the Salmonella vector pYA3493 ATd failed to provide improved protection against NE (Table 2). This failure might, however, be attributed to the apparently poor priming by the Salmonella-vectored antigen pYA3493 ATd (Fig. 4). Nevertheless, the current study expanded on the findings of Zekarias et al. (36) by using a different expression vector and by examining a larger number of birds and two severities of challenge, as well as by using a different and codon-optimized fragment of AT on the basis of epitope mapping. However, presentation of these epitopes for immune recognition through a vaccine construct may be problematic since the information on linear epitopes obtained in vitro may not be completely relevant in vivo when the protein is folded into its native conformation, thus missing some critical conformational epitopes.
Analysis of the protection provided against NE was assessed on the basis of the lesion scores and the body weight gains of birds challenged at two levels of severity of infection (moderate and severe), since the outcome of protection depends on the severity of NE (18). How the protection afforded by this immunization-challenge model relates to the extent of protection that might be observed in naturally occurring disease is unknown, because of the variables affecting the outcome of naturally occurring disease. However, use of a range of challenge severities will indicate the efficacy of a vaccine. Birds immunized with either construct were significantly protected against a moderate challenge, with the level of protection induced by S. Typhimurium χ9352(pYA3493 ATd) being apparently higher than that induced by S. Typhimurium χ9352(pYA3493 HPt), as assessed on the basis of the greater weight gains (Fig. 3) and fewer lesions (Table 2). Although S. Typhimurium χ9352 (pYA3493 ATd) failed to protect birds significantly against a more severe challenge, in contrast to S. Typhimurium χ9352(pYA3493 HPt), the ATd-immunized birds had greater weight gains than the HPt-immunized birds. The birds that were primed with orally administered S. Typhimurium χ9352(pYA3493 ATd) and that then received an additional intramuscular booster with purified ATd were also not significantly protected, although these birds had significantly greater weight gains than the control birds (Table 2; Fig. 3). The hypothetical protein, a possible zinc metalloprotease consistently identified as a protective immunogen in our previous studies (17-19), offered significant protection against both severities of challenge, despite the poor expression in Salmonella in vitro relative to the level of ATd expression (Fig. 2). Since this expression was deliberately downregulated in vitro by the addition of arabinose to the medium, there should have been better expression in vivo since the host tissues contain very less arabinose (8, 13). However, the apparent toxicity observed in vitro in the absence of arabinose may have occurred in vivo. The anti-HP antibody titers in these birds were lower than those in a previous study that delivered a 37-kDa HP through another Salmonella vector (19), an observation consistent with the earlier suggestion of poor delivery by the vaccine vehicle perhaps associated with poor colonization. An alternative explanation is that the protein expressed did not contain relevant conformational epitopes that are important in protection. There was considerable difficulty in cloning HP into pYA3493 and pYA3620, which appeared to relate to the size of the fragment being cloned, but successful cloning required E. coli χ6212 (the intermediate host), which had the tetracycline resistance gene to favor the selection of positive colonies. The partial protective efficacy of truncated HP further supports the involvement of this protease in the development of NE (26).
The immunogenicity of the carrier vaccine strain is an essential requirement in eliciting effective immune responses to heterologous antigens (7, 13). Immunized birds showed Salmonella as well as C. perfringens antigen-specific IgY and IgA antibodies in their sera and intestines (Fig. 4 and 5). The presence of intestinal IgY antibodies to C. perfringens antigens strengthens previous observations (17-19) that mucosal IgY has an important contribution to protection against NE. This is suggestive of the access of circulating antigen-specific IgY in the intestinal mucosa under the inflammatory conditions caused by C. perfringens during the initial period of C. perfringens challenge. The presence of C. perfringens antigen-specific IgY and IgA antibodies in the intestine suggests some colonization of the carrier vaccine strain in the gut-associated lymphoid tissues and their use for the delivery of C. perfringens antigens.
Two scoring systems were used for the evaluation of the gross lesions of NE. The narrower system that we have used in the past (18, 19, 32) does not indicate the extent of the gross lesions, particularly of moderate NE, as encompassed by lesions with scores of 2+, so that the expanded system described by Keyburn et al. (14, 15) was also used. One problem with that scoring system is that immunization may modify the lesions produced in the small intestine, so that vaccine-modified lesions appear atypical, particularly lesions that scored 1+ in the earlier system.
In summary, the findings of the present study strengthen previous findings that AT and HP are important proteins in immunity to NE. The findings also indicate that the B-cell epitopes of AT and that the oral delivery of the immunodominant B-cell epitopes of these proteins through attenuated Salmonella to chickens produce at least partial protection against NE. There is, however, an element of empiricism in the choice of improved attenuated vehicles. There are also differences in the abilities of vector plasmids to clone and express clostridial antigens, and these are possibly affected by the vehicle. Further work is required to optimize the expression of the HPt and ATd antigens and identify suitable Salmonella vaccine strains.
Acknowledgments
This work was supported by the Ontario Ministry of Agriculture, Food, and Rural Affairs and by the Poultry Industry Council, Ontario, Canada. We gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada, and the Canadian Poultry Research Council of Canada for funding.
We thank Prithy Babu for her help with epitope mapping and the staff of the OMAFRA Isolation Facility, University of Guelph, for the housing and care of the broiler chickens. We also thank Roy Curtiss III for supplying the Salmonella vaccine vehicle and associated plasmids and Ken Roland for advice during the course of this work.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Alape-Giron, A., M. Flores-Diaz, I. Guillouard, C. E. Naylor, R. W. Titball, A. Rucavado, B. Lomonte, A. K. Basak, J. M. Gutierrez, S. T. Cole, and M. Thelestam. 2000. Identification of residues critical for toxicity in Clostridium perfringens phospholipase C, the key toxin in gas gangrene. Eur. J. Biochem. 267:5191-5197. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sheikhly, F., and R. B. Truscott. 1977. The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens. Avian Dis. 21:256-263. [PubMed] [Google Scholar]
- 3.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum. Avian Dis. 21:241-255. [PubMed] [Google Scholar]
- 4.Bennett, A. M., T. Lescott, R. J. Phillpotts, M. Mackett, and R. W. Titball. 1999. Recombinant vaccinia viruses protect against Clostridium perfringens alpha-toxin. Viral Immunol. 12:97-105. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, K. K., and J. G. Songer. 2009. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 15:55-60. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, K. K., H. T. Trinh, and J. G. Songer. 2009. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet. Microbiol. 133:92-97. [DOI] [PubMed] [Google Scholar]
- 7.Curtiss, R., III, X. Zang, S. Wanda, H. Kang, V. Konjufca, and Y. Li. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p. 297-313. In K. Brodgen, F. Minion, N. Cornick, T. Stanton, Q. Zang, L. Nolan, and M. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC.
- 8.Curtiss, R., III. 2002. Bacterial infectious disease control by vaccine development. J. Clin. Invest. 110:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtiss, R., III, J. E. Galan, K. Nakayama, and S. M. Kelly. 1990. Stabilization of recombinant avirulent vaccine strains in vivo. Res. Microbiol. 141:797-805. [DOI] [PubMed] [Google Scholar]
- 10.Galan, J. E., K. Nakayama, and R. Curtiss III. 1990. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 94:29-35. [DOI] [PubMed] [Google Scholar]
- 11.Hoang, T. H., H. A. Hong, G. C. Clark, R. W. Titball, and S. M. Cutting. 2008. Recombinant Bacillus subtilis expressing the Clostridium perfringens alpha toxoid is a candidate orally delivered vaccine against necrotic enteritis. Infect. Immun. 76:5257-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaldhusdal, M., and A. Lovland. 2000. Necrotic enteritis (4): the economical impact of Clostridium perfringens is greater than anticipated. World Poultry 16:50-51. [Google Scholar]
- 13.Kang, H. Y., and R. Curtiss III. 2003. Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol. Med. Microbiol. 37:99-104. [DOI] [PubMed] [Google Scholar]
- 14.Keyburn, A. L., J. D. Boyce, P. Vaz, T. L. Bannam, M. E. Ford, D. Parker, A. Di Rubbo, J. I. Rood, and R. J. Moore. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad, J. I. Rood, and R. J. Moore. 2006. The alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74:6496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konjufca, V., S. Y. Wanda, M. C. Jenkins, and R. Curtiss III. 2006. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect. Immun. 74:6785-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2006. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin. Vaccine Immunol. 13:1358-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2007. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 14:1070-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2008. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine 26:4194-4203. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Logan, A. J., E. D. Williamson, R. W. Titball, D. A. Percival, A. D. Shuttleworth, J. W. Conlan, and D. C. Kelly. 1991. Epitope mapping of the alpha-toxin of Clostridium perfringens. Infect. Immun. 59:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovland, A., M. Kaldhusdal, K. Redhead, E. Skjerve, and A. Lillehaug. 2004. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 33:83-92. [DOI] [PubMed] [Google Scholar]
- 23.Martin, T. G., and J. A. Smyth. 2009. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet. Microbiol. 136:202-205. [DOI] [PubMed] [Google Scholar]
- 24.Naylor, C. E., J. T. Eaton, A. Howells, N. Justin, D. S. Moss, R. W. Titball, and A. K. Basak. 1998. Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 5:738-746. [DOI] [PubMed] [Google Scholar]
- 25.Nisbet, A. D., R. H. Saundry, A. J. Moir, L. A. Fothergill, and J. E. Fothergill. 1981. The complete amino-acid sequence of hen ovalbumin. Eur. J. Biochem. 115:335-345. [DOI] [PubMed] [Google Scholar]
- 26.Olkowski, A. A., C. Wojnarowicz, M. Chirino-Trejo, B. Laarveld, and G. Sawicki. 2008. Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res. Vet. Sci. 85:543-553. [DOI] [PubMed] [Google Scholar]
- 27.Prescott, J. F. 1979. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian Dis. 23:1072-1074. [PubMed] [Google Scholar]
- 28.Roland, K., K. Karaca, and D. Sizemore. 2004. Expression of Escherichia coli antigens in Salmonella typhimurium as a vaccine to prevent airsacculitis in chickens. Avian Dis. 48:595-605. [DOI] [PubMed] [Google Scholar]
- 29.Sawires, Y. S., and J. G. Songer. 2006. Clostridium perfringens: insight into virulence evolution and population structure. Anaerobe 12:23-43. [DOI] [PubMed] [Google Scholar]
- 30.Sharp, P. M., E. Bailes, R. J. Grocock, J. F. Peden, and R. E. Sockett. 2005. Variation in the strength of selected codon usage bias among bacteria. Nucleic Acids Res. 33:1141-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-Schroer, and A. E. Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 190:767-773. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, D. R., V. R. Parreira, R. R. Kulkarni, and J. F. Prescott. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 113:25-34. [DOI] [PubMed] [Google Scholar]
- 33.Timbermont, L., A. Lanckriet, F. Pasmans, F. Haesebrouck, R. Ducatelle, and F. Van Immerseel. 2009. Intra-species growth-inhibition by Clostridium perfringens is a possible virulence trait in necrotic enteritis in broilers. Vet. Microbiol. 137:388-391. [DOI] [PubMed] [Google Scholar]
- 34.Titball, R. W., C. E. Naylor, and A. K. Basak. 1999. The Clostridium perfringens alpha-toxin. Anaerobe 5:51-64. [DOI] [PubMed] [Google Scholar]
- 35.Van Immerseel, F., J. I. Rood, R. J. Moore, and R. W. Titball. 2009. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 36.Zekarias, B., H. Mo, and R. Curtiss III. 2008. Recombinant attenuated Salmonella expressing the carboxy-terminal domain of alpha-toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin. Vaccine Immunol. 15:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]