Abstract
Previous studies using small numbers of serum samples from human patients and experimentally infected animals identified the frequent presence of antibodies recognizing RevA, a borrelial fibronectin-binding outer surface protein. We now demonstrate that most examined Lyme disease spirochetes from North America and Europe contain genes encoding RevA proteins, some with extensive regions of conservation and others with moderate diversity. Line blot analyses using recombinant RevA from two diverse Lyme disease spirochetes of RevA and serum samples from culture-confirmed human Lyme disease patients from the United States (n = 46, mainly with early Lyme disease) and Germany (>500, with early and late manifestations of Lyme disease) were performed. The results indicated that a sizable proportion of patients produced antibodies that recognized recombinant RevA. Overall, RevA-based serological studies were less sensitive and less specific than other assay types, such as the VlsE-based C6 peptide assay. However, sera from patients in the initial stages of Lyme disease contained antibodies against RevA, demonstrating that this protein is expressed early in human infection. Thus, RevA may be a useful target for preventative or curative therapies.
Lyme disease, or Lyme borreliosis, is the most common arthropod-borne disease in the United States and throughout Europe (12, 31, 40). Lyme disease often presents as a nonspecific flu-like illness, with symptoms such as fever and body aches. In the absence of the characteristic erythema migrans (EM) bulls-eye rash (present in 60 to 90% of patients), serology is key to a proper diagnosis (8, 9, 44, 48). With the potential for serious sequelae, such as musculoskeletal, cardiovascular, and neurological damage, early diagnosis is important (6, 33). In the United States, Lyme disease is caused almost exclusively by Borrelia burgdorferi sensu stricto. In Europe, however, several Lyme borreliae are responsible for the disease, including Borrelia burgdorferi sensu stricto, B. afzelii, B. garinii, B. spielmanii, and others (4, 24, 36). Different genospecies of Borrelia are associated with different Lyme disease manifestations (1, 3, 37, 43, 45, 46, 49). The heterogeneity among these isolates complicates diagnosis, as interspecies amino acid identities for commonly used borrelial antigens, such as the decorin-binding protein DbpA, can be as low as 40% (47). A highly sensitive test in the United States involves the C6 invariable domain of the VlsE molecule; this test works well in Europe for B. burgdorferi sensu stricto and B. garinii, but differences in sensitivity for detection of antibodies induced by B. afzelli limit its usefulness (21).
Improved diagnostics for early Lyme disease are needed. Currently, serological tests for Lyme disease are used for confirmation of infection only. The utility of these tests is hindered by their propensity toward false negatives due to lack of antibodies early in infection (2, 17, 25, 29) and false positives due to cross-reactivity with Treponema pallidum, autoimmune, or mononucleosis antibodies (6). The relatively low sensitivity of the available Lyme diagnostics indicates the need to identify additional highly antigenic borrelial proteins.
RevA is a surface-exposed 17-kDa outer membrane protein of B. burgdorferi with no significant homology to any bacterial proteins outside Borrelia spp. (10, 11, 14, 34). RevA is expressed during mammalian infection and repressed in the tick vector (7, 15, 16, 39). Production of RevA can be regulated in vitro, with the protein being produced under temperature and pH conditions that mimic those found in its warm-blooded host (10). The function of RevA was previously unknown, yet its surface location and expression during mammalian infection suggested to us that this protein may be involved in interactions with the Lyme spirochete's host. We recently discovered that RevA binds fibronectin, and its expression early in mammalian infection may be important for the bacterium to establish initial interactions with the host (7). Serum samples from human patients and experimentally infected animals identified the frequent presence of antibodies recognizing RevA (5, 15, 30). Laboratory-infected mice produced antibodies to RevA within 4 weeks postinfection (7), suggesting that RevA might be a useful serological marker of Lyme disease. In this report, we examine serum samples from human Lyme disease patients from the United States and Germany for the presence of antibodies that recognize recombinant RevA proteins.
MATERIALS AND METHODS
Bacteria.
B. burgdorferi B31 MI-16 is an infectious clone of the sequenced type strain (11, 14) which contains all parental plasmids (28). B. garinii PBi is a European isolate from human cerebrospinal fluid (35). All Borrelia strains were grown at 34°C to cell densities of approximately 1 × 107 bacteria/ml in modified Barbour-Stoenner-Kelly (BSK-II) medium supplemented with 6% rabbit serum (50). Total DNA (genomic and plasmids) was isolated using a DNAeasy blood and tissue kit (Qiagen, Valencia, CA).
Recombinant proteins.
Recombinant proteins contained amino-terminal polyhistidine tags, with the RevA or RevB segment beginning with that protein's first amino acid following the cysteine lipidation site. revA genes were PCR amplified from total genomic DNA of B. burgdorferi B31 MI-16 and B. garinii PBi, and revB was amplified from B. burgdorferi B31 MI-16, using the oligonucleotides listed in Table 1. Amplicons were cloned into pET200 (Invitrogen, Carlsbad, CA). Resultant plasmid inserts were entirely sequenced on both strands to ensure that no undesired mutations had occurred during PCR or cloning procedures. Recombinant proteins were expressed in Escherichia coli Rosetta (DE3)pLysS (Novagen, Madison, WI) upon induction with isopropylthiogalactopyranoside. Induced E. coli cultures were harvested and lysed by sonication, and debris was cleared by centrifugation. Recombinant proteins were purified from cleared lysates using either MagneHis nickel-conjugated magnetic beads (Promega, Madison, WI) or His-Trap HP columns and an ÄKTA fast-performance liquid chromatograph (FPLC) equipped with a UPC-900 UV absorbance monitor and a Frac920 fraction collector (GE Healthcare, Piscataway, NJ). Proteins were eluted from FPLC columns by a constantly increasing gradient between the lysis buffer (30 mM imidazole, 0.5 M NaCl, 20 mM NaPO4 [pH 7.4]) and the elution buffer (0.75 M imidazole, 5 M NaCl, 20 mM NaPO4 [pH 7.4]). All recombinant proteins were dialyzed at 4°C overnight against phosphate-buffered saline (PBS) using 3,500 molecular weight cutoff Slide-A-Lyzer cassettes (Pierce, Rockford, IL). Protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by staining with Coomassie brilliant blue. Protein concentrations were determined by bicinchoninic acid protein assays (Pierce).
TABLE 1.
Primer sequences used in this study (5′→3′)
| Primer name | Primer sequence |
|---|---|
| B. burgdorferi B31 revA forward | TGTAAAGCATATGTAGAAGAAAAG |
| B. burgdorferi B31 revA reverse | TTAATTAGTGCCCTCTTCGAGGAA |
| B. garinii PBi revA forward | TGTAAAACATATGTAAAAGAAAAAGAAGAG |
| B. garinii PBi revA reverse | TCAATTAGTACCTTCTTCGAGAAACTTTA |
| B. burgdorferi B31 revB forward | GAACTATTTATAATAAAAAGGAG |
| B. burgdorferi B31 revB reverse | TTAATCTTCTTCAAGATATTTTATTAT |
Serological analyses.
Both enzyme-linked immunosorbent assay (ELISA) and immunoblotting methods were used, essentially as we previously described (20, 26, 27, 41). Preexisting human Lyme disease and control serum samples were kindly provided by Gary Wormser (New York Medical College, Valhalla, NY), the University Hospital of Frankfurt, or the blood bank of Frankfurt, Germany. U.S. serum samples were from clinically diagnosed patients and have been used in previous serological studies (26, 27). German serum samples obtained from Lyme disease patients and from the control groups were pretested for the presence of antiborrelia IgG antibodies using a commercially available line immunoblot assay (Genzyme Virotech, Rüsselsheim, Germany) in which VlsE, BmpA, p83, BBA36, BBO323, BbCRASP-3 (ErpP), and pG (ErpG) are included as target antigens. Additional positive control sera were obtained 4 weeks postinfection from four mice that had been infected with B. burgdorferi B31 MI-16 by infestation with infected Ixodes scapularis ticks.
Recombinant RevA from B. burgdorferi B31 MI-16 (RevAB31) or B. garinii PBi (RevAPBi) and RevB from B. burgdorferi B31 MI-16 were produced based on the sequence of B. burgdorferi B31 or B. garinii PBi (15). For ELISAs, Maxisorp 96-well plates (Nalge-Nunc, Rochester, NY) were coated overnight at 4°C with 10 μg/ml protein: bovine serum albumin (BSA; Millipore, Kankakee, IL) or recombinant RevA or RevB protein in carbonate buffer, pH 9.6. Plates were brought to room temperature and washed once with PBS plus 0.5% (vol/vol) Tween 20 (PBS-T). Wells were blocked for 2 h at room temperature with 2% BSA in PBS-T, washed three times with PBS-T, and then incubated for 1 h at room temperature with a 1:100 dilution of human or murine serum. Plates were washed three times with PBS-T, then wells were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated protein A or anti-mouse IgG, diluted 1:5,000 (GE Healthcare, Little Chalfont, United Kingdom). Wells were again washed three times with PBS-T. Aliquots (100 μl/well) of tetramethylbenzidine substrate (Pierce) were added, and then reactions were stopped by addition of 100 μl/well 2 N H2SO4. Absorbance was read at 450 nm using a Spectramax plate reader and SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
For line blotting, recombinant proteins were transferred to nitrocellulose membranes by a microdispensing method in amounts of 500, 250,125, 62, 31, 16, 8, 4, and 2 ng per stripe. Individual membranes were incubated with human sera (German sera at 1:100 and U.S. sera at 1:200) or mouse sera (1:100). Serum dilutions were based upon serological studies with other borrelial antigens (see above). Binding of specific antibodies was detected by using alkaline phosphatase-conjugated goat anti-human IgG serum (1:100; Genzyme Virotech) or goat anti-mouse Ig antibodies (1:10,000; GE Healthcare), as appropriate. Immunoreactive bands were visualized by addition of 3 ml of diethanolamine buffer supplemented with 5-bromo-4-chloro-3-indolylphosphate (Sigma-Aldrich) at 165 mg/ml and nitroblue tetrazolium (Sigma-Aldrich) at 330 mg/ml as a substrate. Cutoffs were based on a standardized band intensity scale (Genzyme Virotech GmbH, version VI 0803093) of 0 to 5, with 5 being the most intense and 0 being no band visible. Line blots were considered positive if the band intensity was >2.
RESULTS
Conservation of Rev sequences among Lyme disease borreliae.
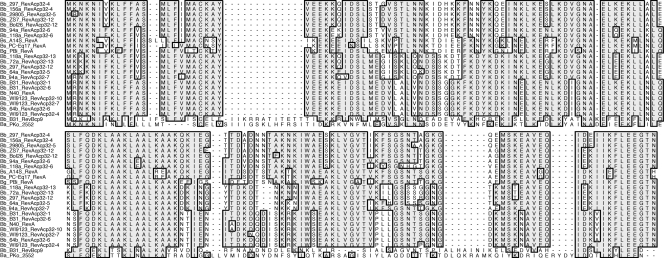
Sequences of revA genes have been published from the Lyme disease spirochete strains B31 and 297 (15, 34). The two revA genes of B. burgdorferi strain B31 are located on its native circular prophages, cp32-1 and cp32-6. B. burgdorferi strain 297 has two copies of revA that share approximately 60 to 70% identity with each other and with the RevA protein of strain B31. B. burgdorferi strain B31 naturally contains a cp32 derivative, cp9-1, which contains a paralogous gene of revA that is designated revB (10, 11, 14). The revB gene shares 47.5% nucleic acid identity with the revA genes, and the RevB protein shares 28% amino acid sequence identity with the two cp32-encoded RevA proteins of strain B31.
Data mining of GenBank sequences (BLAST search of the translated nucleotide database using the RevA protein sequence from strain B31) indicated that many sequenced B. burgdorferi isolates contain genes homologous to revA and revB. Their predicted amino acid sequences were compared with those of strains B31 and 297. B. burgdorferi N40, 156a, 29805, ZS7, Bol26, 72a, 64a, and 64b and B. garinii PBi each carry one revA locus. Two separate revA alleles were identified in two different B. spielmanii strains, A14S and PC-Eq17 (Fig. 1). B. burgdorferi strains 94a and 118a each carry two revA alleles, while strain WI-91-23 has three copies of revA on three different cp32 plasmids. Amino acid sequence identities among predicted RevA proteins were 64 to 100% compared to the sequenced strain B31. No revB loci were identified in other strains with a cp9 prophage (e.g., 94a, WI93-23, 118a). However, a revB-like locus was identified in B. afzelii strain PKo (Fig. 1). The sequenced T. pallidum, relapsing fever Borrelia, and pathogenic Leptospira genomes do not encode proteins with sequence similarities with RevA or RevB.
FIG. 1.
Alignment of predicted amino acid sequences of rev-encoded proteins of B. burgdorferi sensu lato strains. Bb, B. burgdorferi sensu stricto; Ba, B. afzelii; Bg, B. garinii; Bs, B. spielmanii. Designations indicate the genospecies, strain, protein designation, and plasmid on which the gene is located (e.g., Bb_297_RevAcp32-4 indicates B. burgdorferi strain 297 RevA located on cp32-4). Identical amino acids present in the majority of proteins are boxed and shaded in gray.
Serological examination of human Lyme disease patient sera.
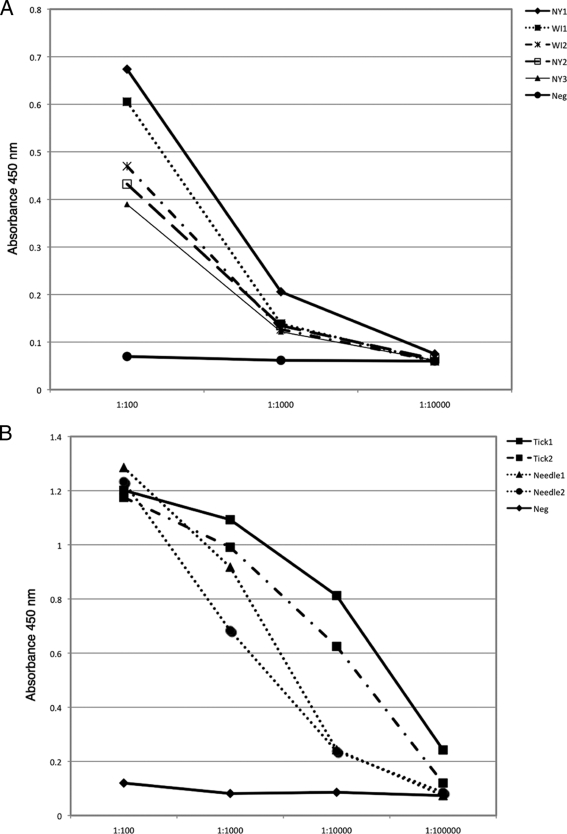
We previously established that B. burgdorferi produces elevated levels of revA transcript within 2 weeks of infecting mice and that such animals produce RevA-directed antibodies within 4 weeks of infection (7). ELISA formats are particularly well suited to serodiagnosis due to their adaptability and potential for automation. Therefore, we tested sera from Lyme disease patients and from infected mice for reactivity to B. burgdorferi strain B31 RevA (RevAB31) by ELISA. Antibodies to RevAB31 were readily detected at dilutions up to 1:1,000 for human sera (n = 5) and 1:10,000 for mouse sera (n = 4) in 100% of samples tested (Fig. 2).
FIG. 2.
ELISA analyses of human Lyme disease patient sera (A) or murine sera (B) for antibodies recognizing RevA. Plates were coated with recombinant B. burgdorferi RevAB31 (10 μg/ml). Sera were diluted serially 10-fold. Antibodies to RevA were detected using HRP-conjugated protein A (A) or HRP-conjugated anti-mouse IgG (B). (A) NY, patient serum from New York; WI, patient serum from Wisconsin; Neg, confirmed Lyme disease-negative human serum. (B) Tick, serum from mouse infected via tick infestation; Needle, serum from mouse infected via needle inoculation; Neg, uninfected mouse serum.
To expand upon these results and ascertain if a majority of humans are exposed to RevA during the early stages of Lyme disease, we examined serum samples from patients with Lyme disease from Germany and the United States. The patients exhibited a wide range of symptoms, including erythema migrans and neuroborreliosis.
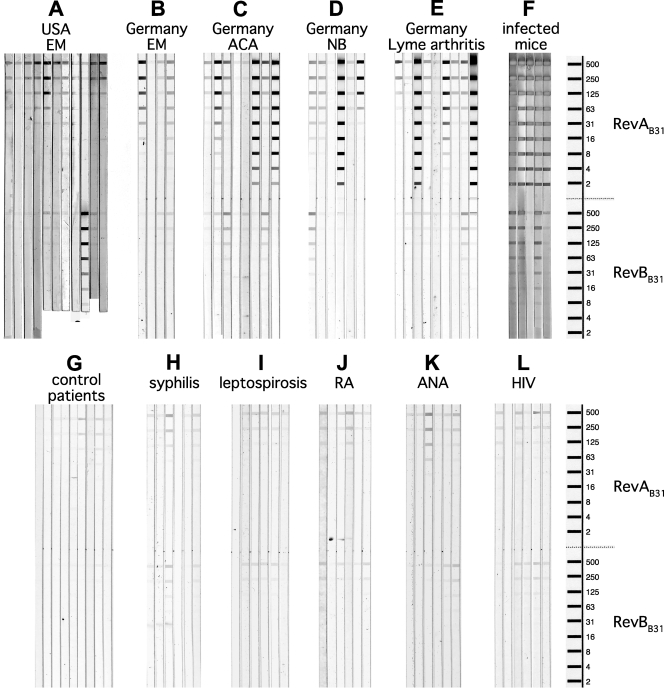
Human serum samples were examined by line immunoblot assays containing various amounts of recombinant RevAB31 or B. garinii strain PBi RevA (RevAPBi). A total of 35% of confirmed U.S. Lyme patient sera recognized RevAB31, while only 12% of German patient sera recognized RevAB31; however, 29% of the German patient sera recognized RevAPBi. Strikingly, 50% of German patients with EM yielded immunoblot signals to RevAB31. Mice infected with B. burgdorferi B31 MI-16 via tick bite or needle inoculation all produced antibodies directed to RevAB31 (Fig. 3), while uninfected mice did not produce anti-RevA antibodies (Fig. 2 and data not shown). The sensitivity of U.S. patient sera for RevAB31 was 61%, and the sensitivity of German patient sera for RevAB31 was 56%.
FIG. 3.
Line blot analyses of human patient sera for antibodies recognizing RevAB31 or RevBB31. Nitrocellulose membranes contained stripes of recombinant RevAB31 and RevBB31 in amounts of 500, 250, 125, 62, 31, 16, 8, 4, or 2 ng. (A) Acute-phase serum samples from patients diagnosed with EM at various locations in the United States. (B) Serum samples from German patients diagnosed with EM with positive Lyme disease serology. (C) Serum samples from German patients diagnosed with acrodermatitis chronica atrophicans (ACA). (D) Serum samples from German patients diagnosed with facial palsy, meningitis, or Bannwarth's syndrome (neuroborreliosis [NB], stage II). (E) Serum samples from German patients diagnosed with Lyme arthritis. (F) Sera collected from mice 4 weeks postinfestation with ticks infected with B. burgdorferi B31 MI-16. (G) Serum samples from blood donors provided by the blood bank in Frankfurt, Germany. (H) Serum samples from German patients demonstrating a positive syphilis serology. (I) Serum samples from German patients diagnosed with leptospirosis. (J) Serum samples from German patients diagnosed with rheumatoid arthritis (RA). (K) Serum samples from German patients exhibiting antinuclear antibodies (ANA). (L) Serum samples from German patients diagnosed with HIV.
Serum samples from control patients, which included negative healthy blood donors and patients with syphilis, leptospirosis, HIV, antinuclear antibodies, or rheumatoid arthritis, gave mixed results. While very few negative samples from healthy blood donors produced immunoblot signals, some samples from patients with syphilis and other diseases contained antibodies that recognized higher concentrations of RevA (Fig. 3 and Table 2). The specificity of U.S. patient sera for RevAB31 was 100%, while the sensitivity of German patient sera for RevAB31 was only 88%.
TABLE 2.
RevAB31 (B. burgdorferi sensu stricto), RevAPBi (B. garinii), and RevBB31 (B. burgdorferi sensu stricto) line blot analyses results for human Lyme disease patient and control serum samples
| Sample seta | No. of positive blots/total no. of samples (%) |
||
|---|---|---|---|
| RevAB31 | RevAPBi | RevBB31 | |
| EM, Germany | 7/14 (50) | NDb | 5/14 (36) |
| NB, Germany | 32/123 (26) | 32/82 (39) | 5/41 (12) |
| ACA, Germany | 31/169 (18) | 19/59 (32) | 2/63 (3) |
| LA, Germany | 37/144 (26) | 25/97 (26) | 7/47 (15) |
| Lyme positive, Germany | 14/116 (12) | 5/17 (29) | 6/116 (5) |
| Lyme negative, Germany | 2/107 (2) | 0/54 (0) | 2/43 (5) |
| Syphilis, Germany | 3/10 (30) | ND | 2/10 (20) |
| Leptospirosis, Germany | 3/14 (21) | ND | 3/14 (21) |
| RA, Germany | 7/16 (44) | ND | 3/16 (19) |
| ANA, Germany | 4/20 (20) | ND | 4/20 (20) |
| HIV, Germany | 3/10 (30) | ND | 3/10 (30) |
| Lyme negative, United States | 0/5 (0) | 0/2 (0) | 0/5 (0) |
| Lyme positive, United States | 16/46 (35) | 6/10 (60) | 3/46 (7) |
EM, erythema migrans; NB, neuroborreliosis; ACA, acrodermatitis chronica atrophicans; LA, Lyme arthritis; RA, rheumatoid arthritis; ANA, antinuclear antibodies.
ND, not done.
Although RevBB31 shares limited amino acid identity with RevAB31, both proteins bind fibronectin (7). Nothing is yet known about revB expression during human or mouse infection. Therefore, human and mouse sera tested for antibodies to RevAB31 were also examined for reactivity to RevBB31 by line immunoblot assay. Sera from all mice infected with B. burgdorferi B31 MI-16 produced antibodies to RevBB31 (Fig. 3 and data not shown). However, very few sera obtained from Lyme disease patients recognized this antigen (Fig. 3 and Table 2).
DISCUSSION
The current study demonstrates that human Lyme disease patients are exposed to the borrelial fibronectin-binding protein RevA. A number of serum samples from early infection (e.g., patients manifesting the early-stage erythema migrans rash, which usually appears within days to weeks of initial infection) contained antibodies against RevA. Consistent with our previous studies with experimentally infected mice, the current results indicate that Lyme disease spirochetes produce RevA surface protein during early stages of human infection. Since RevA is an adhesin that promotes bacterial interactions with host tissue, this protein appears to be a good target for development of therapies to prevent and treat Lyme disease.
RevA sensitivity for patient sera varied, likely due to antigenic differences between RevA from different genospecies and strains of Lyme disease spirochetes. RevA from B. garinii, for example, shares 64% amino acid identity with the RevA from B. burgdorferi B31, while the two alleles of revA encoded by strain 297 share only 72% amino acid identity with each other. The diversity of Lyme disease borreliae in Europe prompted us to examine the reactivity of patient sera to RevA from different borrelia genospecies. Surveys of Ixodes ricinus ticks in Germany suggest that either B. garinii (13) or B. afzelii (32) is the most common B. burgdorferi sensu lato species detected. However, we were unable to identify or amplify a revA paralogous gene in the sequenced strain of B. afzelii PKo, so we examined patient sera responses to RevA from B. burgdorferi sensu stricto and B. garinii. In general, serum samples from German patients were more likely to contain antibodies that reacted with B. garinii RevA than with B. burgdorferi RevA. These data suggest that serological assays may need to be adapted to account for regional and continent-specific strain differences.
Some of the serum samples examined from syphilis and leptospirosis patients contained antibodies that bound recombinant RevA. The sequenced T. pallidum, relapsing fever Borrelia, and pathogenic Leptospira genomes do not encode proteins with sequence similarities with RevA. Likely, T. pallidum and Leptospira species may possess proteins with conformational similarities to RevA and/or RevB. We are currently examining this possibility by Western blot assays of spirochetal lysates with anti-RevA antiserum.
The related protein RevB was not a good serological indicator of infection. Although mice infected by tick bite produced antibodies against RevB, very few human sera reacted to it. RevB shares only 28% amino acid identity with RevA, but it retains the ability to bind fibronectin (7). Many B. burgdorferi strains lack the plasmid cp9, which in strain B31 carries the revB allele. Of the other genospecies examined, only B. afzelii appears to have a revB homolog.
B. burgdorferi has another fibronectin-binding protein, BBK32, that has also been investigated as a diagnostic marker. Differences in antibody levels due to sequence heterogeneity among BBK32 proteins from different species suggest that variant antigens are needed to cover all areas, as is the case with RevA (22). In addition, studies with BBK32 indicate that protein fragments may be better suited for early Lyme disease serology. We are currently investigating that approach for RevA, using various protein fragments to eliminate possible cross-reactive epitopes and increase the specificity of this serological test (23).
In conclusion, RevA is a surface-associated fibronectin adhesin that is expressed early in mammalian infection and is recognized by both mouse and human sera. Our studies indicated that while animals infected with B. burgdorferi strain B31 produced significant levels of antibodies directed against RevA, Lyme disease patients produced antibodies at varying levels, possibly due to differences in RevA sequences among the infectious borrelial genospecies. Other early antigens of B. burgdorferi, such as OspC, Erp proteins, BBK32, and DbpAB, also exhibit extensive sequence variation (18, 19, 38, 42). RevA is a widely distributed antigen among Lyme disease borreliae, but its interspecies heterogeneity may preclude RevA from being broadly applicable for serodiagnosis of human Lyme disease. Its expression early in infection, however, suggests that RevA may still be a useful target for preventative or curative therapies.
Acknowledgments
This work was supported by exploratory funds provided by the University of Kentucky College of Medicine to B. Stevenson, by Deutsche Forschungsgemeinschaft grant Kr3383/1-2 to P. Kraiczy, and by NIH Ruth L. Kirschstein Individual National Research Service Award F32-AI081480 to C. A. Brissette.
We thank Logan Burns, Brandon Jutras, and Ashutosh Verma for helpful comments and technical advice.
Footnotes
Published ahead of print on 23 December 2009.
REFERENCES
- 1.Anthonissen, F. M., M. De Kesel, P. P. Hoet, and G. H. Bigaignon. 1994. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res. Microbiol. 145:327-331. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmelli, T., and J. C. Piffaretti. 1995. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146:329-340. [DOI] [PubMed] [Google Scholar]
- 4.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J.-C. Piffaretti, M. Assous, and P. A. D. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G., A. Jasinskas, M. A. Kayala, D. H. Davies, A. C. Steere, P. Baldi, and P. L. Felgner. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 76:3374-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratton, R. L., J. W. Whiteside, M. J. Hovan, R. L. Engle, and F. D. Edwards. 2008. Diagnosis and treatment of Lyme disease. Mayo Clin. Proc. 83:566-571. [DOI] [PubMed] [Google Scholar]
- 7.Brissette, C. A., T. Bykowski, A. E. Cooley, A. Bowman, and B. Stevenson. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 77:2802-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, S. L., S. L. Hansen, and J. J. Langone. 1999. Role of serology in the diagnosis of Lyme disease. JAMA 282:62-66. [DOI] [PubMed] [Google Scholar]
- 9.Brown, S. L., S. L. Hansen, J. J. Langone, N. Lowe, and N. Pressly. 1999. Lyme disease test kits: potential for misdiagnosis. FDA Med. Bull., Summer 1999. http://www.fda.gov/medbull/summer99/Lyme.html.
- 10.Carroll, J. A., N. El-Hage, J. C. Miller, K. Babb, and B. Stevenson. 2001. Borrelia burgdorferi RevA antigen is a surface-exposed outer membrane protein whose expression is regulated in response to environmental temperature and pH. Infect. Immun. 69:5286-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2007. Lyme disease—United States, 2003-2005. MMWR Morb. Mortal. Wkly. Rep. 56:573-576. [PubMed] [Google Scholar]
- 13.Fingerle, V., U. C. Schulte-Spechtel, E. Ruzic-Sabljic, S. Leonhard, H. Hofmann, K. Weber, K. Pfister, F. Strle, and B. Wilske. 2008. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 298:279-290. [DOI] [PubMed] [Google Scholar]
- 14.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore, R. D., Jr., and M. L. Mbow. 1998. A monoclonal antibody generated by antigen inoculation via tick bite is reactive to the Borrelia burgdorferi Rev protein, a member of the 2.9 gene family locus. Infect. Immun. 66:980-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Solecki, M. J., G. P. Wormser, D. H. Persing, B. W. Berger, J. D. Glass, X. Yang, and R. J. Dattwyler. 2001. A first-tier rapid assay for the serodiagnosis of Borrelia burgdorferi infection. Arch. Intern. Med. 161:2015-2020. [DOI] [PubMed] [Google Scholar]
- 18.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, M. Peltomaa, T. Julin, S. A. Carlsson, and P. Lahdenne. 2002. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 40:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanova, L., I. Christova, V. Neves, M. Aroso, L. Meirelles, D. Brisson, and M. Gomes-Solecki. 2009. Comprehensive seroprofiling of sixteen B. burgdorferi OspC: implications for Lyme disease diagnostics design. Clin. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraiczy, P., A. Seling, C. A. Brissette, E. Rossmann, K. P. Hunfeld, T. Bykowski, L. H. Burns, M. J. Troese, A. E. Cooley, J. C. Miller, V. Brade, R. Wallich, S. Casjens, and B. Stevenson. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin. Vaccine Immunol. 15:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupka, I., J. Knauer, L. Lorentzen, T. P. O'Connor, J. Saucier, and R. K. Straubinger. 2009. Borrelia burgdorferi sensu lato species in Europe induce diverse immune responses against C6 peptides in infected mice. Clin. Vaccine Immunol. 16:1546-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahdenne, P., J. Panelius, H. Saxen, T. Heikkila, H. Sillanpaa, M. Peltomaa, M. Arnez, H. I. Huppertz, and I. J. Seppala. 2003. Improved serodiagnosis of erythema migrans using novel recombinant borrelial BBK32 antigens. J. Med. Microbiol. 52:563-567. [DOI] [PubMed] [Google Scholar]
- 23.Lahdenne, P., H. Sarvas, R. Kajanus, M. Eholuoto, H. Sillanpaa, and I. Seppala. 2006. Antigenicity of borrelial protein BBK32 fragments in early Lyme borreliosis. J. Med. Microbiol. 55:1499-1504. [DOI] [PubMed] [Google Scholar]
- 24.LeFleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 25.Liang, F. T., A. C. Steere, A. R. Marques, B. J. B. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodomonant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. C., N. El-Hage, K. Babb, and B. Stevenson. 2000. Borrelia burgdorferi B31 Erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J. Clin. Microbiol. 38:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, J. C., and B. Stevenson. 2003. Immunological and genetic characterization of Borrelia burgdorferi BapA and EppA proteins. Microbiology 149:1113-1125. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowakowski, J., I. Schwartz, D. Liveris, G. Wang, M. E. Aguero-Rosenfeld, G. Girao, D. McKenna, R. B. Nadelman, L. F. Cavaliere, and G. P. Wormser. 2001. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 33:2023-2027. [DOI] [PubMed] [Google Scholar]
- 30.Nowalk, A. J., R. D. Gilmore, Jr., and J. A. Carroll. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 74:3864-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connell, S., M. Granstrom, J. S. Gray, and G. Stanek. 1998. Epidemiology of European Lyme borreliosis. Zentralbl. Bakteriol. 287:229-240. [DOI] [PubMed] [Google Scholar]
- 32.Pichon, B., O. Kahl, B. Hammer, and J. S. Gray. 2006. Pathogens and host DNA in Ixodes ricinus nymphal ticks from a German forest. Vector Borne Zoonotic Dis. 6:382-387. [DOI] [PubMed] [Google Scholar]
- 33.Plorer, A., N. Sepp, E. Schmutzhard, S. Krabichler, S. Trobos, G. Schauer, C. Pahl, G. Stoffler, and P. Fritsch. 1993. Effects of adequate versus inadequate treatment of cutaneous manifestations of Lyme borreliosis on the incidence of late complications and late serologic status. J. Invest. Dermatol. 100:103-109. [DOI] [PubMed] [Google Scholar]
- 34.Porcella, S. F., T. G. Popova, D. R. Akins, M. Li, J. D. Radolf, and M. V. Norgard. 1996. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J. Bacteriol. 178:3293-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preac-Mursic, V., B. Wilske, and G. Schierz. 1986. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl. Bakteriol. Hyg. A 263:112-118. [DOI] [PubMed] [Google Scholar]
- 36.Richter, D., S. Endepols, A. Ohlenbusch, H. Eiffert, A. Spielman, and F.-R. Matuschka. 1999. Genopspecies diversity of Lyme disease spirochetes in rodent reservoirs. Emerg. Infect. Dis. 5:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryffel, K., O. Péter, B. Rutti, A. Suard, and E. Dayer. 1999. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J. Clin. Microbiol. 37:4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte-Spechtel, U., V. Fingerle, G. Goettner, S. Rogge, and B. Wilske. 2006. Molecular analysis of decorin-binding protein A (DbpA) reveals five major groups among European Borrelia burgdorferi sensu lato strains with impact for the development of serological assays and indicates lateral gene transfer of the dbpA gene. Int. J. Med. Microbiol. 296(Suppl. 40):250-266. [DOI] [PubMed] [Google Scholar]
- 39.Skare, J. T., D. M. Foley, S. R. Hernandez, D. C. Moore, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1999. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect. Immun. 67:4407-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanek, G., and F. Strle. 2003. Lyme borreliosis. Lancet 362:1639-1647. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson, B., and J. C. Miller. 2003. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57:309-324. [DOI] [PubMed] [Google Scholar]
- 43.Terekhova, D., R. Iyer, G. P. Wormser, and I. Schwartz. 2006. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 188:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tugwell, P., D. T. Dennis, A. Weinstein, G. Wells, B. Shea, G. Nichol, R. Hayward, R. Lightfoot, P. Baker, and A. C. Steere. 1997. Laboratory evaluation in the diagnosis of Lyme disease. Ann. Intern. Med. 127:1109-1123. [DOI] [PubMed] [Google Scholar]
- 45.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. P. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 46.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. A. Schwartz. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilske, B. 2003. Diagnosis of Lyme borreliosis in Europe. Vector Borne Zoonotic Dis. 3:215-227. [DOI] [PubMed] [Google Scholar]
- 48.Wormser, G. P., M. E. Aguero-Rosenfeld, and R. B. Nadelman. 1999. Lyme disease serology: problems and opportunities. JAMA 282:79-80. [DOI] [PubMed] [Google Scholar]
- 49.Wormser, G. P., D. Liveris, J. Nowakowski, R. B. Nadelman, L. F. Cavaliere, D. McKenna, D. Holmgren, and I. Schwartz. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]
- 50.Zückert, W. R. 2007. Laboratory maintenance of Borrelia burgdorferi, p. 12C.1.1-12C.1.10. In R. T. Coico, T. F. Kowalik, J. Quarles, B. Stevenson, and R. Taylor (ed.), Current protocols in microbiology. J. Wiley & Sons, Hoboken, NJ. [DOI] [PubMed]