Abstract
We evaluated a novel psoralen-inactivated dengue virus type 1 (DENV-1) vaccine candidate in Mus musculus mice. Mice received intradermal alum or 5 to 10 ng of psoralen-inactivated virus. Anti-DENV-1 neutralizing antibody was detectable in 10/11 mice receiving a 10-ng dose at 90 days. Psoralen-inactivated DENV-1 is immunogenic in mice.
Dengue viruses consist of four distinct RNA viruses of the genus Flaviviridae and are transmitted to humans primarily through the bite of the Aedes aegypti mosquito. Prior dengue virus infection is a major risk factor for the subsequent development of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) following reinfection with a heterotypic dengue virus serotype. Dengue virus vaccine development has thus been hindered by the competing needs to develop a vaccine that provides lasting and uniform protection against all four serotypes without predisposing recipients to an increased risk of DHF and DSS.
Psoralens are photoreactive compounds that freely permeate phospholipid membranes and intercalate between nucleic acids. Following exposure to UV-A radiation, the intercalated psoralen covalently cross-links pyrimidine residues, leading to viral inactivation through the inhibition of genome replication. The interaction of psoralen with viral nucleic acids leaves immunogenic surface epitopes intact (4).
In this study, we photoinactivated the dengue virus type 1 (DENV-1) Western Pacific 74 strain with three different psoralens: 4′-aminomethyltrioxsalen hydrochloride (AMT; product number A4330, CAS number 62442-61-9; Sigma-Aldrich), 8-methoxypsoralen (8-MOP; catalog number 214150010, CAS number 298-81-7; Acros Organics), and 4,5′,8-trimethylpsoralen (TMP; catalog number 229881000, CAS number 3902-71-4; Acros Organics). We then determined the immunogenicity of AMT-inactivated DENV-1 in Mus musculus mice.
(The data provided here were presented in part as a poster presentation at the Annual Meeting of the American Society of Tropical Medicine and Hygiene, New Orleans, LA, 7 to 11 December 2008.)
DENV-1 inactivation.
Five-milliliter aliquots of DENV-1 culture supernatant at a concentration of 3.4 × 105 PFU/ml were transferred into petri dishes 60 by 15 mm. Four test groups of dishes were made; a single psoralen compound was added to each dish in the first three groups, while the fourth group served as a control. The dishes were exposed to UV-A radiation (365 nm; UVAB-18 lamp; UltraLum, Inc., Claremont, CA) for 0, 1, 5, 10, or 20 min at an intensity of 200 or 1,000 μW/cm2 and purified on a Centri-sep column. Photoinactivation was measured by redetermining the titer in BHK cell culture (Table 1). Control specimens unexposed to UV-A produced a titer of 5.63 × 105 PFU/ml. No detectable PFU was noted following 10 min of exposure of the AMT-containing DENV-1 supernatant to 200 μW/cm2 of UV-A or following 5 min of exposure to 1,000 μW/cm2. The former intensity and duration of UV-A exposure were chosen as the candidate for in vitro testing on the basis of its lower overall energy exposure.
TABLE 1.
Psoralen inactivation of DENV-1a
| Time of UV-A irradiation (min) | DENV-1 titer (no. of PFU/ml) after treatment with the following drug and UV-A dose: |
|||||||
|---|---|---|---|---|---|---|---|---|
| AMT |
8-MOP |
TMP |
No psoralen |
|||||
| 1,000 μW/cm2 | 200 μW/cm2 | 1,000 μW/cm2 | 200 μW/cm2 | 1,000 μW/cm2 | 200 μW/cm2 | 1,000 μW/cm2 | 200 μW/cm2 | |
| 0 | 1.92 × 105 | 1.92 × 105 | 6.14 × 105 | 6.14 × 105 | 4.86 × 105 | 4.86 × 105 | 5.63 × 105 | 5.63 × 105 |
| 1 | 90 | 4.32 × 104 | 3.46 × 105 | 4.35 × 105 | 1.54 × 105 | 3.33 × 105 | 4.90 × 105 | 3.58 × 105 |
| 5 | 0 | 1.70 × 102 | 1.09 × 105 | 2.94 × 105 | 4.60 × 103 | 1.47 × 105 | 2.05 × 105 | 3.33 × 105 |
| 10 | 0 | 0 | 5.12 × 104 | 2.18 × 105 | 40 | 8.64 × 104 | 2.05 × 105 | 3.33 × 105 |
| 20 | 0 | 0 | 6.00 × 103 | 1.66 × 105 | 0 | 2.24 × 104 | 1.86 × 105 | 2.94 × 105 |
DENV-1 was inactivated by exposure to UV-A at two intensities and three different psoralen compounds. Inactivation was most efficient with UV-A at 1,000 μW/cm2 in combination with 10 μg/ml of AMT. DENV-1 Western Pacific 74 was used at a titer of 3.4 × 105 PFU/ml to infect Vero-7/C6/36-1 06/01/06 cells. The volume of DENV-1 and the compound was 5 ml in a 50-mm dish. Psoralen was used at a final concentration of 10 μg/ml. Centri-sep columns (catalog number CS-901; Princeton Separation) were used for DENV-1 purification (for each treatment).
Mouse inoculation.
Three groups of seven study-naïve, adult Swiss-Webster outbred Mus musculus mice and one group of four mice were selected from the Naval Medical Research Center Detachment (NMRCD) mouse colony. All mice were 30 g or greater in mass. All procedures were conducted in accordance with protocols approved by the NMRCD Institutional Animal Care and Use Committee. Injections and blood sampling were performed by trained personnel who injected ketamine (100 mg/ml), acepromazine (5 mg/ml), and xylazine (20 mg/ml) intraperitoneally at a starting dose of 0.1 ml/100 g body mass. Group A (seven mice) received 0.05 ml of AMT-DENV-1 (5 ng), which was injected intradermally into the tail on days 0, 14, and 28. Group B (seven mice) received 0.1 ml of AMT-DENV-1 (10 ng) injected on days 0, 14, and 28. Group C (four mice) received 0.1 ml of AMT-DENV-1 (10 ng) injected on days 0, 28, and 60. Control animals (seven mice) received injections of 10% Alhydrogel and phosphate-buffered saline on days 0, 14, and 28. Blood samples were obtained from the retro-orbital sinus of the mice while they were under anesthesia on days 0, 14, 28, 60, and 90. Sera were assayed for anti-DENV antibodies by an IgG enzyme-linked immunosorbent assay (ELISA) and a 50% plaque reduction neutralization test (PRNT50) (9).
All mice were seronegative for anti-DENV-1 antibodies by ELISA and PRNT50 at the baseline. Anti-DENV-1 IgG was detectable in all vaccinated mice following the administration of two doses (on day 28 for groups A and B, on day 60 for group C). IgG remained detectable in seven of seven mice in group B and four of four mice in group C at day 90 (30 days after the administration of the third vaccine dose in this group), but only five of seven mice in group A had detectable IgG at day 60 and five of six mice in group A had detectable IgG at day 90 (following the death of one mouse between days 60 and 90).
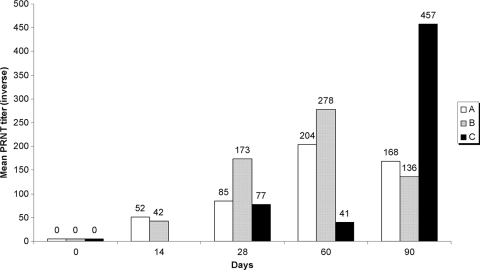
The PRNT50 results are shown in Fig. 1. Although IgG was detectable by the anti-DENV-1 ELISA in more group B mice than group A mice at 90 days, the geometric mean PRNT50 titers were slightly higher in group A mice. The PRNT50 titers were slower to rise in the mice in group C but were higher than those in the other groups at any other time point at 90 days (30 days after the last vaccine dose for group C versus 60 days for groups A and B).
FIG. 1.
Geometric mean PRNT50 titers against DENV-1 at 14, 28, 60, and 90 days. The mice in group A received 5-ng vaccine doses on study days 0, 14, and 28. The mice in group B mice received 10-ng vaccine doses on study days 0, 14, and 28. The mice in group C received 10-ng vaccine doses on study days 0, 28, and 60.
Anti-DENV-1 IgG was undetectable in all control mice throughout the experiment, with the PRNT50 titers being <1:40 in all seven study mice at all time points.
Conclusions.
On the basis of our findings, AMT-inactivated DENV-1 is immunogenic in Mus musculus mice. Psoralen-inactivated viruses should retain their three-dimensional structure, permitting the development of antibodies to multiple epitopes that may participate in immunity. Psoralens freely permeate through lipid bilayers and do not appear to interact with proteins. Additionally, they induce the cross-linking of pyrimidines only following UV exposure (5, 6). This feature of psoralens has made them attractive in transfusion medicine for pathogen inactivation (1, 7, 11), particularly the use of amotosalen as an alternative to traditional leukocyte reduction methods for the prevention of parvovirus (1) and cytomegalovirus (10) transmission.
Psoralens may have advantages over formalin as a mode of viral inactivation. Formalin modifies viral epitopes through the addition of carbonyl groups, which has been suggested to be a mechanism for the hypersensitivity to natural infection seen after the administration of a formalin-inactivated respiratory syncytial virus vaccine (8). Disease outbreaks due to incomplete formalin inactivation have also been reported (2, 3).
Several questions remain following this pilot study. The waning of antibody titers in treated mice over 90 days suggests that this particular formulation may benefit from strategies to augment its response (e.g., through the use of different adjuvants, higher vaccine doses, or alternate routes of administration). The efficacy of our strategy against DENV-2, DENV-3, and DENV-4 remains to be demonstrated, as does the immunogenicity of a tetravalent formulation. Testing with nonhuman primates will provide additional data about the necessary dose and duration of the serological response, as well as immunity following in vivo dengue virus challenge. Further study of psoralen inactivation is warranted for dengue vaccine development.
Acknowledgments
We are employees of the United States Government. This work was prepared as part of our official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of the Navy, the U.S. Department of Defense, or the U.S. Government.
This work was funded by work unit number S0082_06_NM, Military Infectious Diseases Research Program.
The experiments reported herein were reviewed and approved by the Institutional Animal Care and Use Committee at the United States Naval Medical Research Center Detachment and were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals (9a).
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Allain, J. P., J. Hsu, M. Pranmeth, D. Hanson, A. Stassinopoulos, L. Fischetti, L. Corash, and L. Lin. 2006. Quantification of viral inactivation by photochemical treatment with amotosalen and UV A light, using a novel polymerase chain reaction inhibition method with preamplification. J. Infect. Dis. 194:1737-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, F. 1991. An overview of the inactivation of FMDV and the implications when residual virus is present in vaccines. Dev. Biol. Stand. 75:37-41. [PubMed] [Google Scholar]
- 3.Brown, F. 1993. Review of accidents caused by incomplete inactivation of viruses. Dev. Biol. Stand. 81:103-107. [PubMed] [Google Scholar]
- 4.Groene, W. S., and R. D. Shaw. 1992. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J. Virol. Methods 38:93-102. [DOI] [PubMed] [Google Scholar]
- 5.Hanson, C. V., J. L. Riggs, and E. H. Lennette. 1978. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J. Gen. Virol. 40:345-358. [DOI] [PubMed] [Google Scholar]
- 6.Hearst, J. E. 1981. Psoralen photochemistry and nucleic acid structure. J. Invest. Dermatol. 77:39-44. [DOI] [PubMed] [Google Scholar]
- 7.Lin, L., D. N. Cook, G. P. Wiesehahn, R. Alfonso, B. Behrman, G. D. Cimino, L. Corten, P. B. Damonte, R. Dikeman, K. Dupuis, Y. M. Fang, C. V. Hanson, J. E. Hearst, C. Y. Lin, H. F. Londe, K. Metchette, A. T. Nerio, J. T. Pu, A. A. Reames, M. Rheinschmidt, J. Tessman, S. T. Isaacs, S. Wollowitz, and L. Corash. 1997. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion 37:423-435. [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam, A., W. Olszewska, B. Wang, J. S. Tregoning, R. Helson, Q. J. Sattentau, and P. J. Openshaw. 2006. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 12:905-907. [DOI] [PubMed] [Google Scholar]
- 9.Morens, D. M., S. B. Halstead, P. M. Repik, R. Putvatana, and N. Raybourne. 1985. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 22:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.National Research Council, Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 10.Roback, J. D., M. Conlan, W. L. Drew, P. Ljungman, W. G. Nichols, and J. K. Preiksaitis. 2006. The role of photochemical treatment with amotosalen and UV-A light in the prevention of transfusion-transmitted cytomegalovirus infections. Transfus. Med. Rev. 20:45-56. [DOI] [PubMed] [Google Scholar]
- 11.Singh, Y., L. S. Sawyer, L. S. Pinkoski, K. W. Dupuis, J. C. Hsu, L. Lin, and L. Corash. 2006. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion 46:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]