Abstract
Mitogen-activated protein kinases (MAPKs) are integral to the mechanisms by which cells respond to physiological stimuli and a wide variety of environmental stresses. In Caenorhabditis elegans, the stress response is controlled by a c-Jun N-terminal kinase (JNK)-like MAPK signaling pathway, which is regulated by MLK-1 MAPK kinase kinase (MAPKKK), MEK-1 MAPKK, and KGB-1 JNK-like MAPK. In this study, we identify the max-2 gene encoding a C. elegans Ste20-related protein kinase as a component functioning upstream of the MLK-1-MEK-1-KGB-1 pathway. The max-2 loss-of-function mutation is defective in activation of KGB-1, resulting in hypersensitivity to heavy metals. Biochemical analysis reveals that MAX-2 activates MLK-1 through direct phosphorylation of a specific residue in the activation loop of the MLK-1 kinase domain. Our genetic data presented here also show that MIG-2 small GTPase functions upstream of MAX-2 in the KGB-1 pathway. These results suggest that MAX-2 and MIG-2 play a crucial role in mediating the heavy metal stress response regulated by the KGB-1 pathway.
Mitogen-activated protein kinase (MAPK) signal transduction pathways are evolutionarily conserved in eukaryotic cells and transduce signals in response to a variety of extracellular stimuli. Each pathway is composed of three classes of protein kinases: MAPK, MAPK kinase (MAPKK), and MAPK kinase kinase (MAPKKK) (4, 14). MAPKKK phosphorylates and activates MAPKK, which in turn activates MAPK by dual phosphorylation of threonine and tyrosine residues within a Thr-Xxx-Tyr motif. Three subgroups of MAPKs have been identified: the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 kinases (4, 14). JNK and p38 MAPKs function as key mediators of stress and immune signaling in mammals. The MKK4 and MKK7 MAPKKs have been shown to activate JNK, and the MKK3 and MKK6 MAPKKs serve as the major activators of p38 MAPK (4, 14). The specific MAPKKs are themselves phosphorylated and activated by specific MAPKKKs.
Recent studies of Caenorhabditis elegans have revealed a high degree of conservation of JNK MAPK signaling components between C. elegans and mammals. The C. elegans JNK pathway, composed of an MKK7-type MAPKK JKK-1 and a JNK-type MAPK JNK-1, regulates coordinated movement via type D GABAergic (GABA stands for γ-aminobutyric acid) motor neurons (10) and has a role in synaptic vesicle transport (3). C. elegans also possesses another JNK-like MAPK pathway, composed of MLK-1 MAPKKK, MEK-1 MAPKK, and KGB-1 MAPK, which is homologous to the mammalian MLK-MKK7-JNK MAPK signaling cassette. KGB-1 has a novel activation site, consisting of Ser-Xxx-Tyr rather than Thr-Xxx-Tyr (19, 21). The KGB-1 pathway regulates the stress response to heavy metals (19). We have previously identified the vhp-1 and shc-1 genes as components functioning in the KGB-1 pathway. The vhp-1 and shc-1 genes encode a MAPK phosphatase (MKP) highly homologous to mammalian MKP-7 and a homolog of the mammalian Shc adaptor, respectively (19, 20). VHP-1 plays an important role in the heavy metal stress response in C. elegans by negatively regulating the KGB-1 pathway through dephosphorylation of KGB-1. SHC-1 mediates activation of the KGB-1 pathway by linking MEK-1 MAPKK with MLK-1 MAPKKK. However, it remains unknown what components function upstream of the MLK-1-MEK-1-KGB-1 pathway.
In mammalian cells, the kinase activity of MLK family members is controlled by several different mechanisms, such as dimer formation, autoinhibition mediated by the Src homology 3 (SH3) domain of the MLKs itself, interaction with small GTPases, and phosphorylation by MAPKKK kinase (MAP4K) (6). In this study, we identified MAX-2, a member of the Ste20 group of protein kinases, as a potential component functioning upstream of MLK-1 MAPKKK in the KGB-1 pathway. MAX-2 physically associates with and phosphorylates MLK-1 at a Ser residue in the activation loop located between kinase subdomains VII and VIII of MLK-1, resulting in its activation. Additionally, we found that MIG-2, a member of the Rac family of small GTPases, functions as an upstream regulator of MAX-2. Our results thus identify the in vivo machinery regulating the JNK-mediated stress response pathway via a Ste20-related kinase and Rac-type GTPase.
MATERIALS AND METHODS
Plasmids.
Full-length max-2 cDNAs were amplified by PCR from a C. elegans cDNA library, subcloned, and completely sequenced. The cDNA for mig-2 was isolated by the Y. Kohara expressed sequence tag (EST) project (National Institute of Genetics, Mishima, Japan). The mammalian expression vector for FLAG epitope-tagged MEK-1 (FLAG-MEK-1) was described previously (19). The mammalian expression vectors for hemagglutinin (HA) epitope-tagged MLK-1 (HA-MLK-1) and FLAG epitope-tagged MAX-2 (FLAG-MAX-2) were constructed by inserting each coding sequence into a vector expressing epitope-tagged protein under the control of the cytomegalovirus (CMV) promoter. Each coding sequence was amplified by PCR using primer sets to create restriction sites immediately before the first codon and after the stop codon. Mutated forms of MAX-2, MLK-1, MEK-1, and MIG-2 were made by oligonucleotide-directed PCR, and the mutations were verified by DNA sequencing.
Gateway cloning technology (Invitrogen) was used to construct the max-2p::flag::max-2 plasmids for expression in worms. The max-2p::flag::max-2 plasmids were constructed by fusing three DNA fragments in the following order: a 2.1-kbp genomic fragment containing the max-2 promoter (max-2p), flag, and max-2 coding sequence. The hsp-16p::flag::max-2 plasmids were constructed as follows. A flag::max-2 DNA fragment was obtained by PCR using the mammalian expression vectors for FLAG-MAX-2 as a template. This fragment was subcloned into the EcoRV site of the worm heat-inducible expression vector pPD49.83 and verified by DNA sequencing. This generated the hsp-16p::flag::max-2 plasmid. The dpy-7p::ha::mlk-1 and dpy-30p::venus plasmids were described previously (20).
The Saccharomyces cerevisiae expression vectors for LexA DNA-binding domain (DBD)-fused MIG-2 protein (LexA DBD-MIG-2) and GAL4 activation domain (AD)-fused MAX-2 protein (GAL4 AD-MAX-2) were constructed by inserting each coding sequence into the pBTM116 and pACTII vectors, respectively, after the following modifications. The four C-terminal amino acid residues of MIG-2, Cys-Asp-Ile-Met, responsible for lipid attachment and membrane targeting, were deleted to facilitate the nuclear localization of the LexA DBD-MIG-2 fusion proteins. To create a MIG-2 constitutively in the active, GTP-bound form, Gly-16 was mutated to Val (G16V), and to create a MIG-2 constitutively in the inactive, GDP-bound form, Thr-21 was mutated to Asn (T21N).
Antibodies.
Anti-KGB-1, anti-phospho-KGB-1, and anti-PMK-1 rabbit polyclonal antibodies were described previously (9, 19, 20). Anti-phospho-MLK-1 and anti-phospho-MEK-1 rabbit polyclonal antibodies were raised against synthetic phospho-polypeptides, DANRFpSTAGC and GRLIEpSRAHpSKQAGC (where p stands for phosphorylated), which correspond to the activation loop of MLK-1 and MEK-1, respectively, and affinity purified. Anti-HA monoclonal antibody 16B12 (Covance), anti-FLAG monoclonal antibody M2 (Sigma) and anti-phospho-p38 MAPK monoclonal antibody 28B10 (Cell Signaling) were used.
C. elegans strains.
All C. elegans strains were maintained on nematode growth medium (NGM) plates at 20°C and fed with bacteria of the Escherichia coli OP50 strain as described previously (2). The alleles used in this study were N2 Bristol as the wild type, and kgb-1(km21), mek-1(ks54), mlk-1(km19), pmk-1(km25), sek-1(km4), max-2(nv162), max-2(cy2), pak-1(ok448), pak-2(ok332), and mig-2(mu28). The kgb-1(km21), mek-1(ks54), mlk-1(km19), pmk-1(km25), and sek-1(km4) mutants were described previously (19, 20). The max-2(nv162), max-2(cy2), pak-1(ok448), pak-2(ok332), and mig-2(mu28) mutants were obtained from the Caenorhabditis Genetics Center and backcrossed more than twice to N2 wild-type animals. The muls28 (mig-2p::gfp::mig-2) transgenic strain was also obtained from the Caenorhabditis Genetics Center. To verify the nv162 and cy2 mutations, the max-2 genomic region in each mutation was amplified by PCR and directly sequenced. The nv162 and cy2 mutations are a deletion of 1,468 bp (positions 13024492 to 13025959 of linkage group II) and a single base pair change (position 13018660 of linkage group II), respectively. The pak-1(ok448) and pak-2(ok332) mutations were also verified in the same way. The ok448 allele is a deletion of 1,425 bp (positions 6045214 to 6046638 of linkage group X). The ok332 allele is a deletion of 2,524 bp (positions 11036949 to 11039472 of linkage group V) and an insertion of 866 bp. Strains carrying the max-2p::flag::max-2, hsp-16p::flag::max-2, or dpy-7p::ha::mlk-1 transgene were generated by injecting DNA with the dpy-30p::venus plasmid, which expresses Venus from embryogenesis, as a dominant genetic marker into the gonads of young adult N2 animals as described previously (18).
Stress sensitivity.
The assay for heavy metal toxicity was carried out as follows. Worms were grown and allowed to lay eggs on NGM plates seeded with bacteria of the OP50 strain. Embryos were transferred to NGM plates or NGM plates containing 100 μM copper sulfate. To investigate the effect of transgenes on heavy metal toxicity, embryos expressing Venus were transferred. After incubation for 1 day at 20°C, the numbers of hatched embryos were determined by counting unhatched embryos. The worms that developed into adulthood were counted 4 days after egg laying. The percentage of adults was calculated by multiplying the number of adults by 100 and dividing by the number of hatched worms. Assays for the effect of RNA interference (RNAi) on heavy metal toxicity were carried out as follows. For RNAi by feeding, worms were grown from the L4 larval stage and allowed to lay eggs on NGM plates seeded with bacteria of the E. coli HT115 strain carrying plasmids expressing double-stranded RNA for pak-1 or vhp-1 (Geneservise). Embryos were transferred to NGM plates containing 100 μM copper sulfate and seeded with bacteria of the HT115 strain carrying the corresponding plasmids. For pak-1 RNAi by injection, the double-stranded RNA for pak-1 was prepared by in vitro transcription using plasmids, which were identical to those used for a feeding RNAi method, as a template. Worms from the L4 larval stage to the young adult stage were injected with the double-stranded RNA for pak-1 and then allowed to lay eggs on NGM plates. Embryos were transferred to NGM plates containing 100 μM copper sulfate. The following manipulations and calculations were performed as described above.
To determine the activation of KGB-1 by heavy metal stress, animals grown on NGM plates were transferred to 1.5-ml test tubes in H2O. Worms were then incubated with H2O or 1 mM copper sulfate for 1 h at 20°C and subjected to Western blotting using anti-KGB-1 and anti-phospho-KGB-1 antibodies. To investigate the effect of overexpression of the max-2 gene on the activation of KGB-1, worms carrying the hsp-16p::flag::max-2 transgene were collected at 24 h after heat shock for 3.5 h at 33°C. The assay for MLK-1 phosphorylation in C. elegans was carried out as follows. Animals with the dpy-7p::ha::mlk-1 transgene integrated into their genome and grown on NGM plates were collected and incubated with H2O or 1 mM copper sulfate for 1 h at 20°C. Worms were then washed with H2O and sonicated in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease inhibitors. HA-MLK-1 protein was immunoprecipitated with anti-HA antibodies and subjected to Western blotting with anti-phospho-MLK-1 antibodies.
Yeast two-hybrid assay.
The Saccharomyces cerevisiae reporter strain L40 [MATa trp1 leu2 his3 LYS2::(lexAop)4-HIS3 URA3::(lexAop)8-LacZ] was cotransformed with plasmids expressing the LexA DBD-MIG-2 and GAL4 AD-MAX-2 fusion proteins. The transformants were plated onto synthetic medium lacking histidine and were incubated at 30°C for 48 to 72 h. Interaction of the pairs of fusion proteins will transactivate the HIS3 reporter gene and allow their growth on the plate.
RESULTS
MAX-2 is involved in stress responses regulated by the KGB-1 pathway.
The Ste20 protein was originally found as a key protein kinase in the mating pathway of budding yeast (7). In this pathway, Ste20 activates a yeast MAPKKK by direct phosphorylation to transduce signaling from the mating pheromone receptor to the MAPK pathway. The Ste20-related kinases are a large group of kinases that are evolutionarily highly conserved in eukaryotes from yeast to mammals (5). Mammalian Ste20-related kinases have been shown to phosphorylate MAPKKKs (15). The Ste20-related kinases are further divided into the p21-activated kinase (PAK) and germinal center kinase (GCK) families (5). This study focused on the role of the PAK family in the C. elegans KGB-1 pathway.
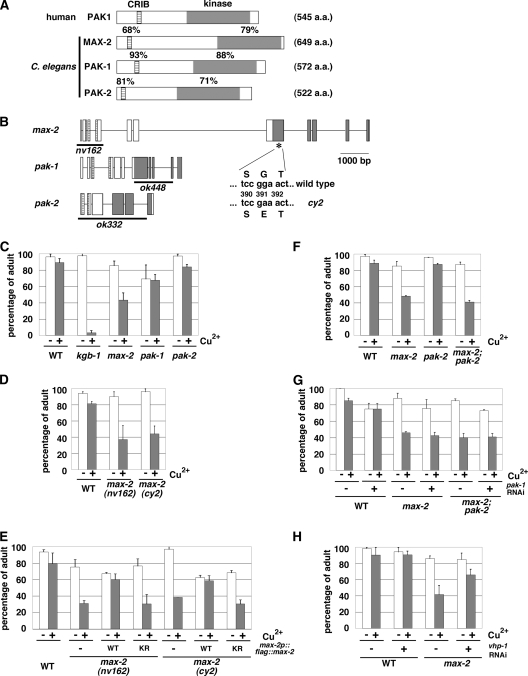
C. elegans contains three genes encoding PAK-like protein kinases, max-2, pak-1, and pak-2 (16). Members of the PAK family of proteins contain an amino-terminal Cdc42/Rac interactive binding (CRIB) motif and a carboxyl-terminal kinase domain (5). The C. elegans PAK proteins have a domain organization similar to that of mammalian PAKs (Fig. 1A). Since the KGB-1 pathway regulates the response to heavy metal stress (19, 20), we tested whether the three C. elegans PAKs regulate the stress response to heavy metals using max-2(nv162), pak-1(ok448), and pak-2(ok332) deletion mutant animals (Fig. 1B). The max-2(nv162) and pak-2(ok332) alleles have a deletion that removes the start codon. The pak-1(ok448) allele has a deletion that removes the majority of the region encoding the kinase domain. These mutations are expected to be null alleles. Animals carrying the max-2(nv162), pak-1(ok448), or pak-2(ok332) deletion mutation were placed on agar plates containing copper (Cu2+) ions, and their development was monitored for any signs of an altered response to heavy metal ions. We found that compared to wild-type animals, max-2(nv162) mutants were partially sensitive to Cu2+. In contrast, neither pak-1(ok448) nor pak-2(ok332) mutants exhibited the heavy metal-sensitive phenotype (Fig. 1C). The max-2 mutants grew poorly on plates containing Cu2+ (100 μM), whereas the N2 wild-type and pak-1 and pak-2 mutant animals grew well, becoming adults by 4 days. In addition, similar phenotypes were observed for the max-2(cy2) mutants (Fig. 1D), which have a missense mutation that alters a conserved glycine residue in the ATP-binding motif of the MAX-2 kinase domain (Fig. 1B). To confirm that the hypersensitivity to copper ions was caused by the max-2 mutation, we constructed a max-2p::flag::max-2 transgene in which the FLAG-tagged MAX-2 is expressed by its own promoter. Introduction of the max-2p::flag::max-2 transgene as an extrachromosomal array significantly rescued the heavy metal-sensitive phenotype associated with the max-2(nv162) and max-2(cy2) mutations (Fig. 1E). To address the biological importance of MAX-2 kinase activity, we generated a catalytically inactive form of MAX-2 [MAX-2(K408R)], in which Lys-408 in the ATP-binding motif was mutated to arginine. When MAX-2(K408R) was expressed in max-2 mutants, the heavy metal sensitivity was not rescued (Fig. 1E). These results indicate that MAX-2 is involved in the regulation of the response to heavy metal stress and that the kinase activity of MAX-2 is crucial to its function.
FIG. 1.
Heavy metal stress sensitivity in C. elegans mutants. (A) Schematic representations of the structures of human PAK1 and C. elegans PAK proteins. Striped boxes and gray boxes represent the CRIB motif and kinase domain, respectively. The percentages of amino acid similarity in the CRIB motif and kinase domain are shown above the boxes. The number of amino acids (a.a.) in the proteins are shown in parentheses to the right of the schematic representation. (B) Schematic representation of the structures of C. elegans PAK genes. Exons and introns are indicated by boxes and lines, respectively. Striped boxes and gray boxes show the CRIB motif and kinase domain, respectively. The thick lines underneath the schematic representation indicate the extent of the deleted region in each deletion mutant. The position of the cy2 mutation is indicated by an asterisk. (C to H) Copper sensitivity. Each animal was cultured from embryogenesis on NGM plates containing 100 μM copper ion (+) and seeded with bacteria of the OP50 strain (C to G) or the HT115 strain expressing the double-stranded RNA for vhp-1 (H). The percentages of worms reaching adulthood 4 days after egg laying are shown with standard errors (error bars). WT, wild type.
The observation that presumptive null mutations in max-2 resulted in only a partial sensitivity to copper raised the possibility that one or more of the other PAKs may play a redundant role with MAX-2 in mediating the heavy metal stress response. To test this idea, we constructed max-2(nv162); pak-1(ok448) and max-2(nv162); pak-2(ok332) double mutants. The pak-2(ok332) deletion did not enhance the heavy metal sensitivity of max-2(nv162) mutants (Fig. 1F). Unfortunately, the max-2(nv162); pak-1(ok448) double mutants could not be tested, because these strains were found to be sterile (data not shown). Therefore, we attempted to examine whether the max-2 mutant phenotype was enhanced by inhibition of pak-1 expression using a feeding RNAi method. The max-2(nv162) or max-2(nv162); pak-2(ok332) mutant animals were placed on agar plates containing Cu2+ ions and fed a bacterial strain expressing the double-stranded RNA for pak-1, and their development was monitored. However, depletion of PAK-1 by RNAi did not enhance stress sensitivity in max-2(nv162) or max-2(nv162); pak-2(ok332) mutants (data not shown). Injection of the double-stranded pak-1 RNA also did not enhance the stress sensitivity in these mutants (Fig. 1G). Therefore, it remains unclear which gene functions redundantly with MAX-2 to mediate the heavy metal stress response.
We have previously shown that the KGB-1 pathway is negatively regulated by the VHP-1 phosphatase as a result of dephosphorylation of KGB-1 (19). If the copper sensitivity of max-2 mutants is due to diminished KGB-1 signaling, then suppression of vhp-1 function should result in the corresponding suppression of the max-2 defect. Indeed, we found that inhibition of vhp-1 expression by RNAi markedly suppressed the stress sensitivity of max-2 mutants (Fig. 1H). These results imply that MAX-2 regulates the response to heavy metal stress in the KGB-1 pathway.
MAX-2 is involved in activation of KGB-1.
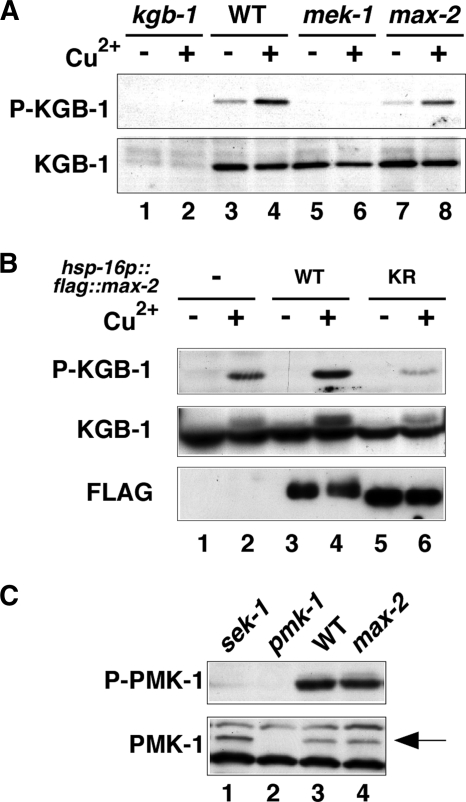
We investigated whether MAX-2 is involved in the activation of KGB-1 in C. elegans. KGB-1 is activated by MEK-1-mediated phosphorylation at the Ser-198 and Tyr-200 residues located between kinase subdomains VII and VIII of KGB-1 (19). Anti-phospho-KGB-1 antibodies that recognize the phosphorylated form of KGB-1 were used to monitor KGB-1 activation in C. elegans (20). Western blot analysis with anti-phospho-KGB-1 antibody clearly detected the phosphorylated form of KGB-1 in wild-type animals and its expression was upregulated by treatment of animals with Cu2+ ion (Fig. 2A, lanes 3 and 4). Animals harboring the kgb-1(km21) deletion mutation exhibited diminished levels of KGB-1 protein and KGB-1 activation (Fig. 2A, lanes 1 and 2). In mek-1(ks54) deletion mutants, KGB-1 activity was markedly reduced compared with wild-type animals (Fig. 2A, lanes 5 and 6). To examine the role of MAX-2 in the activation of KGB-1, we examined animals expressing a deletion mutant allele (nv162) of the max-2 gene. We found that phosphorylated KGB-1 levels were partially decreased in max-2(nv162) mutants both in the presence or absence of Cu2+ treatment (Fig. 2A, lanes 7 and 8). These results indicate that MAX-2 is required for the full activation of KGB-1. The partial decrease of KGB-1 activity could be due to redundancy in the system.
FIG. 2.
Effects of the max-2 mutation on KGB-1 and PMK-1 activities. (A) Effects of the max-2 mutation on KGB-1 activity. N2 (wild type [WT]), kgb-1(km21), mek-1(ks54), and max-2(nv162) animals were treated with copper ion (+) or not treated with copper ion (−). Extracts prepared from each animal were immunoblotted with anti-phospho-KGB-1 and anti-KGB-1 antibodies. P-KGB-1, phospho-KGB-1. (B) Effects of max-2 overexpression on KGB-1 activity. N2 wild-type animals and N2 overexpressing either FLAG-MAX-2 (wild type [WT]) or FLAG-MAX-2(K408R) (KR) were treated with copper ion or left alone. Extracts prepared from each animal were immunoblotted with anti-phospho-KGB-1, anti-KGB-1 and anti-FLAG antibodies. (C) Effects of the max-2 mutation on PMK-1 activity. Extracts prepared from each animal were immunoblotted with anti-phospho-p38 MAPK and anti-PMK-1 antibodies. The position of PMK-1 is indicated by an arrow. P-PMK-1, phospho-PMK-1.
To confirm that MAX-2 is involved in the KGB-1 activation pathway, we examined the effect of max-2 overexpression on KGB-1 activation. For this purpose, we constructed a hsp-16p::flag::max-2 transgene expressing FLAG-MAX-2 under the control of the heat shock promoter. In transgenic animals harboring the hsp-16p::flag::max-2 transgene as an extrachromosomal array, we observed that KGB-1 activity was increased by Cu2+ treatment compared with animals not overexpressing MAX-2 (Fig. 2B, lanes 2 and 4). In contrast, overexpression of a kinase-negative MAX-2(K408R) inhibited Cu2+-induced activation of KGB-1 (Fig. 2B, lane 6), suggesting that MAX-2(K408R) acts as a dominant-negative mutant. These results suggest that MAX-2 overexpression is not sufficient for constitutive activation of KGB-1 but rather potentiates the upregulation of KGB-1 activity by heavy metal stress.
To examine whether MAX-2 is specifically involved in the KGB-1 pathway, we asked whether the max-2 mutation affected activation of PMK-1, the C. elegans p38 homolog involved in stress response and innate immunity (9, 12, 19). We first examined a sek-1 deletion mutation, which is defective in SEK-1 MAPKK activity upstream of PMK-1. Western blot analysis using an anti-phospho-p38 antibody (12) that specifically recognizes the phosphorylated, activated form of p38 MAPK revealed that the sek-1 mutant was defective in the activation of PMK-1 (Fig. 2C, lane 1). In contrast, the max-2 deletion mutation was found to have no effect on PMK-1 activation (Fig. 2C, lane 4). Taken together, these results suggest that MAX-2 specifically participates in the KGB-1 pathway.
MAX-2 interacts with and phosphorylates MLK-1 MAPKKK.
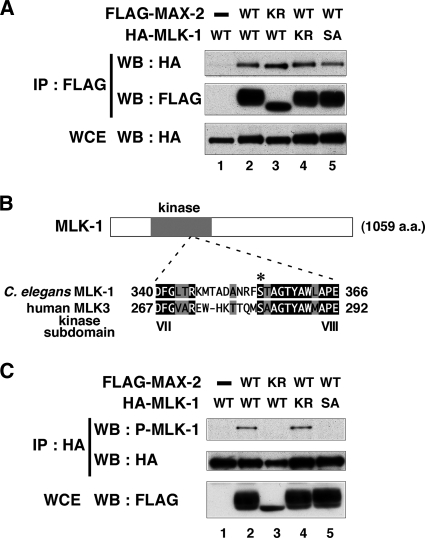
It has been shown that a Ste20-related kinase, HPK1, directly interacts with MLK3 MAPKKK in mammals (11). To determine whether MAX-2 similarly functions upstream of MLK-1 MAPKKK in the KGB-1 pathway, we tested the interaction between MAX-2 and MLK-1. We conducted immunoprecipitation assays using mammalian COS-7 cells. FLAG-tagged MAX-2 and HA-tagged MLK-1 were coexpressed in COS-7 cells. We immunoprecipitated FLAG-MAX-2 with FLAG antibodies and probed for HA-MLK-1 on a Western blot with anti-HA antibodies. We found that MAX-2 coimmunoprecipitated with MLK-1 (Fig. 3A, lane 2), indicating that MAX-2 can physically associate with MLK-1. We then asked whether the catalytic activity of MAX-2 affects this interaction. A catalytically inactive form of MAX-2 [MAX-2(K408R)] was coexpressed with HA-MLK-1 in COS-7 cells. We found that FLAG-MAX-2(K408R) coimmunoprecipitated with HA-MLK-1 (Fig. 3A, lane 3). We also tested whether MAX-2 interacts with a catalytically inactive form of MLK-1 [MLK-1(K193R)] and found that HA-MLK-1(K193R) also coimmunoprecipitated with FLAG-MAX-2 (Fig. 3A, lane 4). Therefore, MAX-2 association with MLK-1 does not require the catalytic activity of either protein.
FIG. 3.
MAX-2 interacts with and phosphorylates MLK-1. (A) Interaction of MAX-2 with MLK-1. COS-7 cells were transfected with expression vectors encoding FLAG-MAX-2 (wild type [WT]), FLAG-MAX-2(K408R) (KR), HA-MLK-1 (WT), HA-MLK-1(K193R) (KR), and HA-MLK-1(S355A) (SA) as indicated. Whole-cell extracts (WCE) and immunoprecipitated complexes obtained with anti-FLAG antibodies (IP : FLAG) were analyzed by Western blotting (WB). (B) Comparison of kinase subdomains VII and VIII between C. elegans MLK-1 and human MLK3. Identical residues (black background) and similar residues (gray background) are indicated. The phosphorylated residue of human MLK3 is indicated by an asterisk. (C) Phosphorylation of MLK-1 by MAX-2. COS-7 cells were transfected with expression vectors encoding FLAG-MAX-2 (WT), FLAG-MAX-2(K408R) (KR), HA-MLK-1 (WT), HA-MLK-1(K193R) (KR), and HA-MLK-1(S355A) (SA) as indicated. Whole-cell extracts and immunoprecipitated complexes obtained with anti-HA antibodies were analyzed by Western blotting.
Next, we investigated whether MAX-2 phosphorylates MLK-1. Previous studies of mammalian cell lines have revealed that mammalian MLK3 is phosphorylated by HPK1 at the Ser-281 residue in the activation loop located between kinase subdomains VII and VIII (11, 15). Sequence comparison revealed that the amino acid sequence of the activation loop is highly conserved between mammalian MLK3 and C. elegans MLK-1. The Ser-281 residue of mammalian MLK3 corresponds to the Ser-355 residue of C. elegans MLK-1 (Fig. 3B) and might serve as a potential phosphorylation site. We therefore asked whether MAX-2 phosphorylates MLK-1 at the Ser-355 residue. To assay phosphorylation of MLK-1, we prepared anti-phospho-MLK-1 antibodies that specifically recognize the phosphorylated Ser-355 form of MLK-1. HA-MLK-1 was coexpressed with FLAG-MAX-2 in COS-7 cells, immunoprecipitated from cell lysates, and then analyzed by Western blotting using anti-phospho-MLK-1 antibodies. Transfection with MAX-2 resulted in strong phosphorylation of MLK-1 (Fig. 3C, lanes 1 and 2). Transfection with a catalytically inactive form of MAX-2 abrogated MLK-1 phosphorylation (Fig. 3C, lane 3). This indicates that the kinase activity of MAX-2 is required for phosphorylation of MLK-1. We then asked whether the catalytic activity of MLK-1 is required for Ser-355 phosphorylation. A catalytically inactive form of MLK-1 [MLK-1(K193R)] was coexpressed with FLAG-MAX-2 in COS-7 cells. We found that MLK-1(K193R) was still phosphorylated at Ser-355 in cells coexpressing MAX-2 (Fig. 3C, lane 4), excluding the possibility that the Ser-355 residue in MLK-1 is autophosphorylated. To confirm that anti-phospho-MLK-1 antibodies could specifically recognize MLK-1 phosphorylated at Ser-355, we generated a mutant form of MLK-1 [MLK-1(S355A)], in which Ser-355 had been mutated to alanine. The MLK-1(S355A) mutated form was able to associate with MAX-2 (Fig. 3A, lane 5). Even when coexpressed with FLAG-MAX-2, MLK-1(S355A) was not detected by anti-phospho-MLK-1 antibodies (Fig. 3C, lane 5). Taken together, these results suggest that MAX-2 directly phosphorylates MLK-1 at the Ser-355 residue.
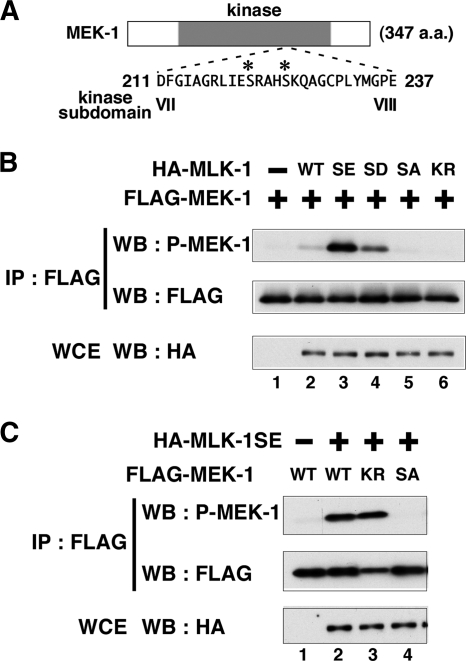
Phosphorylation of MLK-1 at Ser-355 is required for its kinase activity.
Since many kinases are activated by phosphorylation of their activation loop (1), we asked whether the kinase activity of MLK-1 is affected by phosphorylation at Ser-355. Previous studies showed that MLK-1 phosphorylates MEK-1 at the Ser-221 and Ser-225 residues in the kinase domain (13, 19). To monitor the kinase activity of MLK-1, we prepared anti-phospho-MEK-1 antibodies that specifically recognize MEK-1 phosphorylated by MLK-1 (Fig. 4A). FLAG-MEK-1 was coexpressed with HA-MLK-1 in COS-7 cells, immunoprecipitated from cell lysates, and then analyzed by Western blotting using anti-phospho-MEK-1 antibodies. Transfection with MLK-1 resulted in subtle phosphorylation of MEK-1 (Fig. 4B, lanes 1 and 2). The effects of phosphorylation on protein function can often be mimicked by replacement of phosphorylated amino acids with glutamate or aspartate residues. To characterize the functional role of Ser-355 in the regulation of MLK-1, we replaced it with glutamate or aspartate and assayed phosphorylation of MEK-1. When coexpressed with a phospho-mimetic of MLK-1 [MLK-1(S355E) or MLK-1(S355D)], MEK-1 was strongly phosphorylated (Fig. 4B, lanes 3 and 4). In contrast, transfection with a phospho-defective form of MLK-1 [MLK-1(S355A)] abrogated MEK-1 phosphorylation, similar to a catalytically inactive form of MLK-1 [MLK-1(K193R)] (Fig. 4B, lanes 5 and 6). These data imply that the kinase activity of MLK-1 is upregulated by phosphorylation at Ser-355. The observation that a catalytically inactive form of MEK-1 [MEK-1(K99R)] was sufficiently phosphorylated by HA-MLK-1(S355E) indicates that MEK-1 phosphorylation is not caused by autophosphorylation (Fig. 4C, lane 3). To confirm that anti-phospho-MEK-1 antibodies could specifically recognize the phosphorylated form of MEK-1, we generated a mutant form of MEK-1 [MEK-1(S221A S225A)], in which both phosphorylation sites had been changed to alanines, and found that this mutant could not be detected by the anti-phospho-MEK-1 antibodies (Fig. 4C, lane 4). These results suggest that phosphorylation of MLK-1 at Ser-355 is important for regulation of MLK-1 kinase activity.
FIG. 4.
Effects of mutations at the phosphorylation site on MLK-1 activity. (A) Amino acid sequence of kinase subdomains VII and VIII in MEK-1. The phosphorylated residues are indicated by asterisks. (B and C) Phosphorylation of MEK-1 by MLK-1. COS-7 cells were transfected with expression vectors encoding HA-MLK-1 (wild type [WT]), HA-MLK-1(S355E) (SE), HA-MLK-1(S355D) (SD), HA-MLK-1(S355A) (SA), HA-MLK-1(K193R) (KR), FLAG-MEK-1 (WT), FLAG-MEK-1(K99R) (KR), and FLAG-MEK-1(S221A S225A) (SA) as indicated. Whole-cell extracts (WCE) and immunoprecipitated complexes obtained with anti-FLAG antibodies (IP : FLAG) were analyzed by Western blotting (WB).
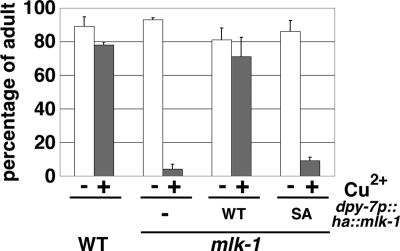
We next examined whether Ser-355 of MLK-1 is functionally important in animals. Wild-type MLK-1 and MLK-1(S355A) were expressed under the control of the dpy-7 promoter in mlk-1(km19) mutants. As observed previously (20), the dpy-7p::ha::mlk-1 fusion gene fully rescued the heavy metal-sensitive phenotype of mlk-1 mutants (Fig. 5). However, expression of MLK-1(S355A) in mlk-1 mutants had little effect on rescuing the sensitivity to heavy metal compared to wild-type MLK-1 (Fig. 5). This demonstrates that Ser-355 is important for MLK-1 function in conferring resistance to heavy metal stress.
FIG. 5.
MLK-1 Ser-355 is essential for heavy metal stress response. Each animal was cultured from embryogenesis on NGM plates containing 100 μM copper ion. The percentages of worms reaching adulthood 4 days after egg laying are shown with standard errors (error bars).
MIG-2 functions upstream of MAX-2 in the KGB-1 pathway.
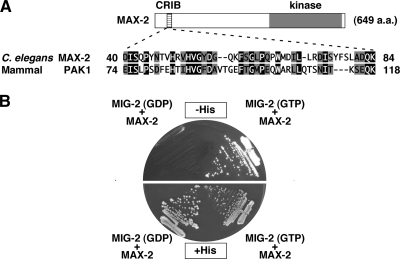
PAKs are kinases that are regulated by small GTPases (5). These GTPases act as molecular switches that interconvert between two states, the active, GTP-bound state and the inactive, GDP-bound state. PAKs have been shown to bind the GTP-bound form of Cdc42/Rac family GTPases through a CRIB domain (5, 8). As MAX-2 contains a CRIB motif (Fig. 6A), it was expected to be regulated by Cdc42/Rac-type small GTPases. C. elegans has one Cdc42 protein and three Rac proteins, designated CDC-42, CED-10, MIG-2, and RAC-2, respectively. Of these, we found that MIG-2 was able to bind to MAX-2. The yeast two-hybrid assay revealed that MIG-2 interacted with the activated, GTP-bound form of MIG-2, but not with the GDP-bound form (Fig. 6B).
FIG. 6.
MIG-2 interacts with MAX-2. (A) Comparisons of the CRIB motifs between C. elegans MAX-2 and human PAK1. Identical residues (black background) and similar residues (gray background) are indicated. (B) A two-hybrid assay for the interaction of MAX-2 with MIG-2. The reporter strain L40 was cotransformed with expression vectors encoding LexA DBD-MIG-2(T21N) (GDP), LexA DBD-MIG-2(G16V) (GTP), and GAL4 AD-MAX-2 (MAX-2) as indicated.
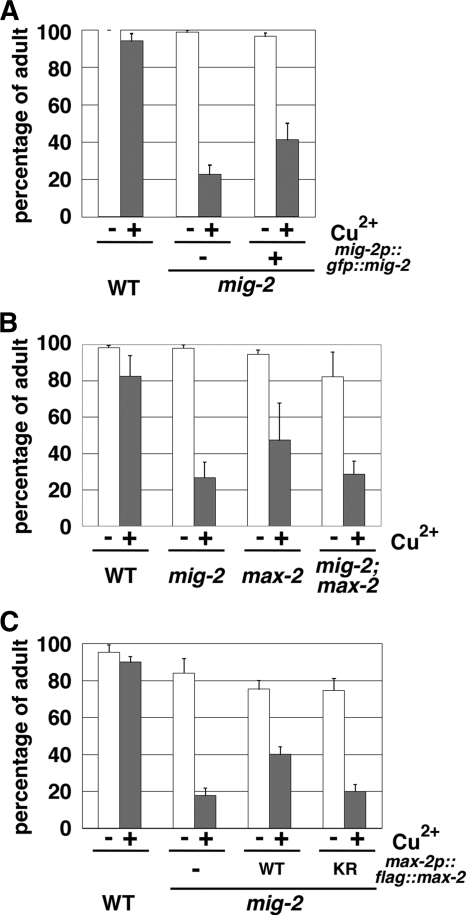
We next asked whether MIG-2 functions in the KGB-1 pathway. We examined the effects of the mig-2(mu28) null allele on Cu2+ sensitivity, MLK-1 phosphorylation, and KGB-1 activation. Animals harboring the mig-2(mu28) mutation exhibited hypersensitivity to heavy metals (Fig. 7A). The heavy metal-sensitive phenotype associated with mig-2(mu28) could be partially rescued by introduction of the integrated mig-2p::gfp::mig-2 transgene, which expresses MIG-2 tagged with green fluorescent protein (GFP) at the amino terminus under the control of its own promoter (Fig. 7A). To further address whether mig-2 and max-2 function in the same pathway, we constructed mig-2(mu28); max-2(nv162) double mutants. The max-2(nv162) mutation did not enhance the Cu2+ sensitivity of mig-2(mu28) mutants (Fig. 7B), suggesting that MIG-2 and MAX-2 function in the same pathway. Furthermore, overexpression of the max-2 gene in mig-2(mu28) mutants partially suppressed the Cu2+ sensitivity in a manner dependent on MAX-2 kinase activity (Fig. 7C), suggesting that MAX-2 functions downstream of MIG-2.
FIG. 7.
Heavy metal stress sensitivity in mig-2 mutants. (A to C) Each animal was cultured from embryogenesis on NGM plates containing 100 μM copper ion. The percentages of worms reaching adulthood 4 days after egg laying are shown with standard errors (error bars).
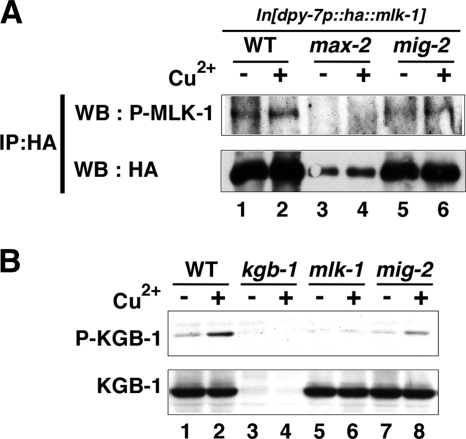
To assay phosphorylation of MLK-1 in animals, we constructed a strain integrated with the dpy-7p::ha::mlk-1 transgene, which expresses HA-tagged MLK-1 under the control of the dpy-7 promoter and is able to fully rescue the sensitivity to heavy metal stress in mlk-1 deletion mutants (Fig. 5) (20). HA-MLK-1 was immunoprecipitated from worm lysates and analyzed by Western blotting using anti-phospho-MLK-1 antibodies that recognize the phosphorylated Ser-355 form of MLK-1. In wild-type animals expressing HA-MLK-1, we could detect phosphorylation of MLK-1 at Ser-355 (Fig. 8A, lane 1). Its phosphorylation was slightly increased by treatment of animals with Cu2+ ion (Fig. 8A, lane 2). When the integrated dpy-7p::ha::mlk-1 transgene was transferred to max-2(nv162) mutants, the transgene was unexpectedly found to be silenced (Fig. 8A, lanes 3 and 4); therefore, we could not test the effect of the max-2 mutation on MLK-1 phosphorylation. We found that the mig-2(mu28) mutation caused a decrease in the phosphorylation of MLK-1 compared to wild-type animals (Fig. 8A, lanes 1, 2, 5, and 6). Furthermore, the mig-2 mutant was also defective in the activation of KGB-1 (Fig. 8B, lanes 7 and 8). Taken together, these results suggest that MIG-2 functions in the KGB-1 pathway.
FIG. 8.
Effects of the mig-2 mutation on MLK-1 phosphorylation and KGB-1 activity. (A) Phosphorylation of MLK-1 at Ser-355 in animals. N2 (wild-type [WT]), max-2(nv162), and mig-2(mu28) animals integrated with the dpy-7p::ha::mlk-1 transgene were treated with copper ion (+) or not treated with copper ion (−). Extracts prepared from each animal were subjected to immunoprecipitation with anti-HA antibodies (IP:HA). The immunoprecipitates were immunoblotted with anti-phospho-MLK-1 and anti-HA antibodies. In max-2(nv162) mutants, expression of HA-MLK-1 was significantly decreased, presumably by silencing of the transgene (lanes 3 and 4). P-MLK-1, phospho-MLK-1. (B) Effects of the mig-2 mutation on KGB-1 activity. N2 (wild-type), kgb-1(km21), mlk-1(km19), and mig-2(mu28) animals were treated with copper ion or left alone. Extracts prepared from each animal were immunoblotted with anti-phospho-KGB-1 and anti-KGB-1 antibodies. P-KGB-1, phospho-KGB-1.
DISCUSSION
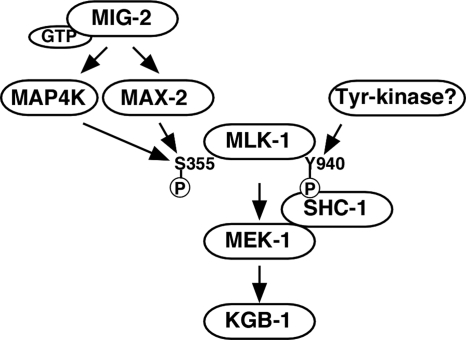
JNK MAPK cascades are pivotal signaling modules controlling diverse signal transduction pathways in eukaryotes. Little is understood, however, about the components functioning upstream of the JNK pathway in the context of whole organisms. In this study, we present functional evidence showing that the Ste20-related kinase MAX-2 and the Rac-type small GTPase MIG-2 are crucial components functioning upstream in the KGB-1 JNK-mediated stress response pathway (Fig. 9).
FIG. 9.
Proposed model for KGB-1 JNK signaling pathway. The circled P stands for phosphate.
Relationships among MLK-1, MAX-2, and MIG-2 in the KGB-1 pathway.
The results of biochemical analyses using mammalian cell lines have suggested several different mechanisms for the regulation of MLKs (6). However, the regulation of MLKs in vivo is not well understood. Genetic studies using the insect Drosophila melanogaster have revealed several physiological regulators of MLK. Drosophila MLK plays a critical role in the dorsal closure process in mid-embryonic development (17). Analysis of mutants defective in dorsal closure led to the identification of the slipper (slpr) gene, which encodes a Drosophila homolog of MLK (22). In the absence of Slpr function, lateral epithelial cells fail to stretch, and the embryo develops a hole in its dorsal cuticle. Studies of the process of dorsal closure in the fly have also shown that Misshapen (Msn), belonging to a germinal center kinase (GCK) family of the Ste20 group, operates upstream of Slpr (23). However, the mechanism by which Msn regulates Slpr remains to be defined, as there are no biochemical data. The genetic and biochemical data presented here clearly demonstrate that MAX-2, a member of the PAK family of Ste20-related kinases, phosphorylates MLK-1 at an evolutionarily conserved serine residue located in the activation loop of the MLK kinase domain, resulting in its activation.
Previously, it has been shown that the Rac-type small GTPase, termed D-Rac1, acts upstream of Slpr in the Drosophila JNK pathway (22). The homology between Drosophila Slpr and mammalian MLKs is found not only in their kinase domains but also in their noncatalytic domains (22). Drosophila Slpr contains an amino-terminal SH3 domain, followed sequentially by a kinase domain and a CRIB motif, common to the MLK subfamily. It is possible that D-Rac1 directly binds to and activates Slpr because the CRIB motif is found in Slpr. As we demonstrate here, the Rac-type small GTPase acts upstream of the JNK pathway in C. elegans. One interesting observation is that C. elegans MLK-1 is highly homologous to mammalian MLKs only in the SH3 and kinase domains and contains amino acid sequences related to but quite divergent from the CRIB motif in MLK-1. Consistent with this observation, we were unable to detect binding of MIG-2 to MLK-1 by yeast two-hybrid assays (data not shown). Therefore, it is unlikely that MIG-2 regulates MLK-1 directly. The finding that MIG-2 interacts with MAX-2 suggests that MAX-2 mediates the signaling from MIG-2 to MLK-1. This model is supported by genetic analysis showing that the heavy metal-sensitive phenotype observed for mig-2 mutants was not enhanced by the max-2 mutation but was suppressed by overexpression of the max-2 gene. Thus, MIG-2 and MAX-2 appear to act in the same pathway and function to activate the KGB-1 pathway (Fig. 9).
Our results presented in this study strongly suggest that MAX-2 functions upstream of MLK-1 MAPKKK in the KGB-1 pathway. However, presumptive null mutations in max-2, unlike mutations in kgb-1, mek-1, or mlk-1, resulted in a partial sensitivity to heavy metal stress. Consistent with this, the decrease in KGB-1 activity caused by the max-2 mutation was weaker than that observed with mek-1 and mlk-1 mutations. Furthermore, mig-2 mutants were more sensitive to heavy metal stress than max-2 mutants. These observations raise the possibility that another component may play a redundant role with MAX-2 in mediating the signaling from MIG-2 to MLK-1 (Fig. 9). C. elegans possesses two other PAK-like protein kinases, PAK-1 and PAK-2, which may be good candidates for such a component(s). In fact, it has been reported that MAX-2 and PAK-1 are redundantly required for cell migration (16). However, the stress-sensitive phenotype observed for max-2 mutants was not enhanced by inhibition of both pak-1 and pak-2. Therefore, it is at present unclear which components function redundantly with MAX-2 in the heavy metal stress response. It is possible that one or more of Ste20-related kinases play a redundant role with MAX-2 in the KGB-1 pathway.
MLK-1 is regulated by double phosphorylation.
The carboxyl termini of MLKs diverge, suggesting that these regions might serve various regulatory functions. We have previously shown that the C. elegans Shc adaptor protein SHC-1 is an essential component of the KGB-1-mediated stress response pathway that acts as an adaptor to link MEK-1 MAPKK to MLK-1 MAPKKK (20). SHC-1 constitutively forms a complex with MEK-1, whereas the interaction between SHC-1 and MLK-1 depends on phosphorylation of the Tyr-940 residue located in the carboxyl terminus of MLK-1. Mutation of Tyr-940 in MLK-1 abrogates its ability to confer resistance to heavy metal stress (20). These results suggest that phosphorylation of MLK-1 at Tyr-940 creates a binding site for SHC-1 and results in the recruitment of the SHC-1-MEK-1 complex to MLK-1. This further raises the possibility that C. elegans may contain a tyrosine kinase that is involved in the stress signaling pathway by phosphorylating MLK-1 Tyr-940. Here we demonstrate that phosphorylation of MLK-1 Ser-355 is also important for its ability to confer resistance to heavy metal stress and results in upregulation of its kinase activity. Taken together, the following results suggest that MLK-1 is regulated by double phosphorylation. (i) Phosphorylation of Ser-355 regulates its kinase activity. (ii) Phosphorylation of Tyr-940 regulates its association with SHC-1, which in turn modulates its interaction with MEK-1 (Fig. 9). Thus, regulation of MLK-1 in the KGB-1 pathway by double phosphorylation can modulate the amplitude and duration of the stress response signal. Does heavy metal stress lead to phosphorylation of MLK-1 at Ser-355 and/or Tyr-940? We observed that KGB-1 was much more strongly phosphorylated as a result of stress treatment than was MLK-1 Ser-355. Furthermore, although max-2 overexpression was able to enhance the activation of KGB-1, this activation was not constitutive but was still dependent on stress treatment. These results suggest that the stress signal results predominantly in the phosphorylation of Tyr-940 rather than Ser-355 in MLK-1. Therefore, identification of other components upstream of MLK-1, such as a tyrosine kinase responsible for MLK-1 Tyr-940 phosphorylation, will undoubtedly provide valuable insights into the signaling pathway regulating the response to heavy metal stress.
Acknowledgments
We thank Y. Kohara, the National Bioresource Project for the Nematode (NBRP) (Tokyo Women's Medical University School of Medicine, Tokyo, Japan) and the Caenorhabditis Genetics Center for materials.
This work was supported by Nakajima Science Foundation (N.H.) and the Grants-in-Aids for Scientific Research programs in Japan (T.M., N.H., and K.M.).
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Bossemeyer, D. 1995. Protein kinases—structure and function. FEBS Lett. 369:57-61. [DOI] [PubMed] [Google Scholar]
- 2.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd, D. T., M. Kawasaki, M. Walcoff, N. Hisamoto, K. Matsumoto, and Y. Jin. 2001. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32:787-800. [DOI] [PubMed] [Google Scholar]
- 4.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 5.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell. Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 6.Gallo, K. A., and G. L. Johnson. 2002. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3:663-672. [DOI] [PubMed] [Google Scholar]
- 7.Herskowitz, I. 1995. MAP kinase pathways in yeast: for mating and more. Cell 80:187-197. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman, G. R., and R. A. Cerione. 2000. Flipping the switch: the structural basis for signaling through the CRIB motif. Cell 102:403-406. [DOI] [PubMed] [Google Scholar]
- 9.Inoue, H., N. Hisamoto, J. H. An, R. P. Oliveira, E. Nishida, T. K. Blackwell, and K. Matsumoto. 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19:2278-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasaki, M., N. Hisamoto, Y. Iino, M. Yamamoto, J. Ninomiya-Tsuji, and K. Matsumoto. 1999. A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J. 18:3604-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiefer, F., L. A. Tibbles, M. Anafi, A. Janssen, B. W. Zanke, N. Lassam, T. Pawson, J. R. Woodgett, and N. N. Iscove. 1996. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 15:7013-7025. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, D. H., R. Feinbaum, G. Alloing, F. E. Emerson, D. A. Garsin, H. Inoue, M. Tanaka-Hino, N. Hisamoto, K. Matsumoto, M. W. Tan, and F. M. Ausubel. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623-626. [DOI] [PubMed] [Google Scholar]
- 13.Koga, M., R. Zwaal, K. L. Guan, L. Avery, and Y. Ohshima. 2000. A Caenorhabditis elegans MAP kinase kinase, MEK-1, is involved in stress responses. EMBO J. 19:5148-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 15.Leung, I. W., and N. Lassam. 2001. The kinase activation loop is the key to mixed lineage kinase-3 activation via both autophosphorylation and hematopoietic progenitor kinase 1 phosphorylation. J. Biol. Chem. 276:1961-1967. [DOI] [PubMed] [Google Scholar]
- 16.Lucanic, M., M. Kiley, N. Ashcroft, N. L'etoile, and H. J. Cheng. 2006. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development 133:4549-4559. [DOI] [PubMed] [Google Scholar]
- 17.Martin, P., and W. Wood. 2002. Epithelial fusions in the embryo. Curr. Opin. Cell Biol. 14:569-574. [DOI] [PubMed] [Google Scholar]
- 18.Mello, C. C., J. M. Kramer, D. Stinchcomb, and V. Ambros. 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10:3959-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno, T., N. Hisamoto, T. Terada, T. Kondo, M. Adachi, E. Nishida, D. H. Kim, F. M. Ausubel, and K. Matsumoto. 2004. The C. elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J. 23:2226-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno, T., K. Fujiki, A. Sasakawa, N. Hisamoto, and K. Matsumoto. 2008. Role of the Caenorhabditis elegans Shc adaptor protein in the c-Jun N-terminal kinase signaling pathway. Mol. Cell. Biol. 28:7041-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, P., W. M. Leung-Chiu, R. Montgomery, A. Orsborn, K. Kuznicki, E. Gressman-Coberly, L. Mutapcic, and K. Bennett. 2002. The GLH proteins, Caenorhabditis elegans P granule components, associate with CSN-5 and KGB-1, proteins necessary for fertility, and with ZYX-1, a predicted cytoskeletal protein. Dev. Biol. 251:333-347. [DOI] [PubMed] [Google Scholar]
- 22.Stronach, B., and N. Perrimon. 2002. Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev. 16:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su, Y. C., J. E. Treisman, and E. Y. Skolnik. 1998. The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev. 12:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]