Abstract
Extended-spectrum beta-lactamases (ESBL) of the TEM, SHV, or CTX-M type confer resistance to beta-lactam antibiotics in Gram-negative bacteria. The activity of these enzymes against beta-lactam antibiotics and their resistance against inhibitors can be influenced by genetic variation at the single-nucleotide level. Here, we describe the development and validation of an oligonucleotide microarray for the rapid identification of ESBLs in Gram-negative bacteria by simultaneously genotyping blaTEM, blaSHV, and blaCTX-M. The array consists of 618 probes that cover mutations responsible for 156 amino acid substitutions. As this comprises unprecedented genotyping coverage, the ESBL array has a high potential for epidemiological studies and infection control. With an assay time of 5 h, the ESBL microarray also could be an attractive option for the development of rapid antimicrobial resistance tests in the future. The validity of the DNA microarray was demonstrated with 60 blinded clinical isolates, which were collected during clinical routines. Fifty-eight of them were characterized phenotypically as ESBL producers. The chip was characterized with regard to its resolution, phenotype-genotype correlation, and ability to resolve mixed genotypes. ESBL phenotypes could be correctly ascribed to ESBL variants of blaCTX-M (76%), blaSHV (22%), or both (2%), whereas no ESBL variant of blaTEM was found. The most prevalent ESBLs identified were CTX-M-15 (57%) and SHV-12 (18%).
The resistance of widespread Gram-negative pathogens such as Escherichia coli, Klebsiella species, and other Enterobacteriaceae against broad-spectrum beta-lactam antibiotics is a severe and growing issue in health care (29, 41, 42). In the beginning, the resistance to these antimicrobials, including oxyimino-cephalosporins (e.g., cefotaxime, cefpodoxime, and ceftazidime), was caused mainly by TEM- and SHV-type extended-spectrum beta-lactamases (ESBLs), which evolved via mutation from the classic TEM-1, TEM-2, and SHV-1 enzymes (18). Plasmid-mediated derivatives with an extended substrate spectrum have been known since the early 1980s (24, 44), and other mutational events that affect substrate affinity for beta-lactamase inhibitors, such as clavulanic acid, lead to an inhibitor-resistant (IRT) or complex mutant type (CMT) enzymes (8). During the last 10 years, other ESBL types have gained increased importance, especially the CTX-M enzymes (7, 27, 35, 40). The blaCTX-M genes probably emerged through several mobilization events of their natural homologues in the genus Klyvera (5, 21, 33, 34, 37, 39) and have undergone evolution since that time. They have intrinsic ESBL activity and are clustered in five subgroups, designated CTX-M1, CTX-M2, CTX-M8, CTX-M9, and CTX-M25 (5).
A fast and accurate identification of the antimicrobial resistance of the etiologic pathogen is decisive for the initiation of an appropriate antimicrobial therapy (36, 43). Routine culture-based phenotypic ESBL detection usually requires 2 days or more (48), and the reported sensitivities and specificities of different tests (50) leave room for improvement. In addition, as the required confirmatory tests are based on the synergy effect between antibiotics and inhibitors, the coexistence of the ESBL with other beta-lactamases can lead to misinterpreted results (14, 23). Phenotype-based resistance tests also do not provide gene variant information, which limits their usefulness for epidemiologic surveillance and refined antimicrobial therapy. Molecular ESBL detection has the potential to improve ESBL diagnosis, infection control, and epidemiological surveillance. In addition to standard PCR and gene sequencing, several molecular ESBL identification techniques have been developed during the past few years, such as PCR-restriction fragment length polymorphism (2), PCR-single strand conformation polymorphism (31), real-time PCR (4, 38), or pyrosequencing (22, 32) analysis. However, all of these techniques have an inherently limited multiplexing capacity. The genotyping of TEM, SHV, or CTX-M beta-lactamases, with more than 300 known variants altogether (http://www.lahey.org/studies/), technically would be excessive, very costly, and too time demanding to be used as a diagnostic aid. DNA microarrays, on the other hand, are an increasingly used tool for multiplexed genotyping. Microarrays targeting resistance determinants have been described with focus on certain species (30, 45, 46, 49), specific resistance mechanisms (10, 53), or resistance in Enterobacteriaceae (3, 11, 17). Some examples covered beta-lactam resistance at the gene family level (25) or investigated six point mutations within blaSHV (54), the latter technically pointing toward the refined genotyping of beta-lactamase genes. To cover all known mutations in a beta-lactamase gene, we previously described a DNA microarray for the genotyping of TEM beta-lactamases (19).
In the present study, we describe an advanced DNA microarray that integrates an updated version of the TEM microarray (19) with two new modules for the genotyping of blaSHV and blaCTX-M genes. Because of its unparalleled high coverage of known variants and information depth, combined with its short time to result, this assay can be an enabling tool for epidemiological studies of ESBL outbreaks and infection control, and it offers an attractive way toward the development of the rapid molecular testing of ESBLs in the clinical routine.
MATERIALS AND METHODS
Bacterial strains and antibiotic susceptibility determination.
As reference material for the system setup, we used a panel of reference strains and well-characterized clinical samples (the bla gene of interest was sequenced). Reference strains included E. coli DH5α (Clontech, Heidelberg, Germany) transformed with plasmid pUC19 (blaTEM-116; New England Biolabs, Frankfurt, Germany) or pCCR9 (different strains with several blaSHV genes, including blaSHV-1, blaSHV-2, blaSHV-5, and blaSHV-12; provided by H. Hächler, Institute of Medical Microbiology, University of Zürich), European Antibiotic Resistance Surveillance System reference strain UA 1528 (blaTEM-8), and additional E. coli reference strains provided by W. Witte, Robert Koch Institute, Wernigerode Branch, Wernigerode, Germany (blaTEM-3 and blaTEM-7). Clinical samples additionally harbored blaTEM-1 (several species), blaCTX-M-3 (K. pneumoniae), blaCTX-M-5 (Salmonella enterica serovar Typhimurium), blaCTX-M-9 (Enterobacter sp.), blaCTX-M-14 (E. coli), and blaCTX-M-15 (E. coli) and were provided by our partners in Moscow and Frankfurt am Main.
For the evaluation of the ESBL array, isolates were collected from the Department of Laboratory Medicine at the Robert Bosch Hospital, Stuttgart, Germany (n = 22), and the Institute of Medical Microbiology and Infection Control, J. W. Goethe Universität, Frankfurt am Main, Germany (n = 38). They were recovered from respiratory samples (n = 8), swabs (n = 27), urine (n = 18), and other sites (n = 7). Species identification was carried out with the API 20NE system (bioMérieux, Marcy l'Etoile, France) or the Gram-negative identification card GN on a Vitek 2XL system. All bacterial strains were routinely cultured at 37°C on Columbia blood agar (Heipha Diagnostika) or grown in Schaedler-Bouillon (bioMérieux, Marcy l'Etoile, France). Plasmid DNA from bacteria was extracted with the QIAprep Spin Miniprep kit (Qiagen, Hilden, Germany) according to the protocol provided by the manufacturer. Antibiotic susceptibility was determined by disc diffusion (Kirby-Bauer method) on Mueller-Hinton agar plates according to Clinical and Laboratory Standards Institute standards (13) and by the Etest method (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions. MICs were determined using the Gram-negative susceptibility card AST-N062 on a Vitek 2XL system (bioMérieux, Marcy l'Etoile, France). Susceptibility was determined for amikacin (AMK), amoxicillin (amoxicilline)-clavulanic acid (AMC), ampicillin (AMX), ampicillin-sulbactam (SAM), aztreonam, cefaclor (CEC), cefazolin (CFZ), cefepime (FEP), cefoxitin (FOX), cefotaxime (CTX), cefpodoxime (CPD), ceftazidime (CAZ), ceftriaxone (CRO), cefuroxime (CXM), ciprofloxacin (CIP), colistin, doxycycline (DOX), fosfomycin (FOF), gentamicin (GEN), imipenem (IMP), levofloxacin (LEV), meropenem (MER), mezlocillin (MEZ), moxifloxacin (MXF), nitrofurantion (NIT), piperacillin (PIP), piperacillin-sulbactam (PIP-SUL), piperacillin-tazobactamaztreonam (TZP), tetracycline (TET), tobramycin (TOB), and trimethoprim-sulfamethoxazole (SXT).
Probe design.
Multiple sequence alignments of blaTEM, blaSHV, and blaCTX-M nucleotide sequences were performed to identify sequence variations, mainly single-nucleotide polymorphisms (SNPs), leading to amino acid exchanges (http://www.lahey.org/studies/). Sequence alignments were carried out using ClustalX (version 1.83) (47), the BioEdit sequence alignment editor (version 7.0.5.3) (20), or SeqMan II software (version 5.00; DNAStar). This study includes the integration of previously designed probes, redesigned probes, and de novo-designed probes for additional SNPs to update the ESBL microarray. For the current version of the microarray, 150 probe sets for the characterization of polymorphisms leading to 59 amino acid substitutions known for TEM, 53 known for SHV, and 44 known for the different CTX-M beta-lactamases were developed. Furthermore, four probe sets for CTX-M group identification were included. The subgroups for CTX-M-8-like and CTX-M25-like genes were examined together and labeled group M8/M25. The standard probe design was carried out as described previously (19), with minor modifications. In brief, oligonucleotide probes of various lengths (14 to 27 bases) were designed to detect single-nucleotide substitutions within the blaTEM, blaSHV, and blaCTX-M genes, leading to amino acid exchanges. For each SNP, a probe set of four probes was designed with identical sequences except the investigated mutation site, which was either A, G, C, or T. Ideally, the probes were designed with the mutation at the central position within the probe sequence to maximize perfect match (PM)-to-mismatch (MM) discrimination. The probe length, melting temperature (Tm), and free energies of possible secondary structures (e.g., hairpins [ΔGhairpin] and dimers [ΔGdimer]) were calculated with the online tool OligoAnalyzer (http://eu.idtdna.com/analyzer/Applications/OligoAnalyzer/) by using the default settings for monovalent ions and DNA concentration (50 mM and 0.25 μM). The following values and thresholds were regarded as optimal: Tm = 53 ± 3.5°C; ΔGhairpin ≥ 0 kcal/mol; and ΔGdimer ≥ −6 kcal/mol. All probes were designed as sense probes. The probe sets are named after the position of the amino acid substitution within the blaTEM, blaSHV, or blaCTX-M sequence. Unless otherwise noted, amino acid residue numbering followed the standard numbering scheme for the class A beta-lactamases described by Ambler et al. (1). For 10 amino acid substitution positions there are two polymorphisms within one triplet leading to different amino acids. For these, two probe sets (four probes each) were designed (e.g., TEM 164.1 and 164.2). Six amino acid substitutions occur at the same position but in different CTX-M beta-lactamase groups. For these, two probe sets, each based on the sequence specific for the CTX-M group (e.g., CTX-M 167.M1 and CTX-M 167.M9), were designed. In several cases an alternative probe design was applied. (i) The probe set for positions 238 and 240 within the SHV amino acid sequence, which covers the ESBL-relevant substitutions G238S, G238A, and E240K (18), includes one perfect-match probe for each different naturally occurring SHV sequence. As multiple combinations of amino acid exchanges occur, a set of nine probes was designed and named according to the amino acids at positions 238/240 (GE, SE, SK, AE, and AK) and the last nucleotide within the codon for amino acid 240 (GEa or GEg). (ii) At or around several amino acid positions, the mutant sequence differs in more than a single nucleotide from the wild-type sequence (e.g., TEM position 35 [GA to CC]; SHV position 140 [GGCC to CCGG], 192/193 [GC to CG], or 167 [insertion of 15 nucleotides—DRWET, or Asp-Arg-Trp-Glu-Thr]). Here, one probe each was designed matching the wild-type (WT) and the mutant (MUT) sequence and designated, for example, 140WT and 140MUT. For the wild-type sequence, the sequences of the parental genes TEM-1, SHV-1, and CTX-M-1 (or CTX-M-2 or CTX-M-9 for other CTX-M groups) were used. (iii) The sequences of several probe sets (e.g., probe set SHV 61, TEM 224, etc.) contain artificial mismatches to the target sequence at certain positions involved in hairpin or duplex formation to decrease the stability of such secondary structures, which hinder the hybridization event. To compensate for the loss of hybridization efficiency associated with such a method, the probe length usually had to be elongated to match a Tm mismatch (also calculated with OligoAnalyzer) within the desired Tm range (as described above). Where required, different versions of a probe set were tested, and the one showing the best PM-MM discrimination and hybridization efficiency using the applied hybridization conditions was selected. Sequences of all probes included on the chip are shown in Table S1 in the supplemental material.
Oligonucleotide array fabrication.
Oligonucleotide arrays consisted of three stripes of subarrays, each representing a chip module for the detection of TEM, SHV, or CTX-M beta-lactamases. Every array was manufactured with 618 different oligonucleotide capture and control probes. The oligonucleotides were purchased from Metabion (Planegg-Martinsried, Germany) in desalted purity and quality controlled by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Each oligonucleotide was equipped with a 13-thymidine spacer and an amino modification at the 5′ end. Each beta-lactamase gene-specific probe was spotted onto the microarray in triplicate. The array production process and the subsequent washing steps were carried out as described previously (26), with modifications. In brief, a BioRobotics MicroGrid II microarrayer (Genomic Solutions Ltd., Huntingdon, United Kingdom) with eight tungsten split pins (PT3000; Point Technologies, Boulder, CO) was used to print the oligonucleotide probes, which were dissolved in spotting buffer (Nexterion Spot with 0.0075% Triton X-100 [final concentration]; Schott, Jena, Germany) to a final concentration of 20 μM (except for the spotting control [5 μM] and 11 different capture probes of the CTX-M chip module [12 to 50 μM]) onto epoxy-coated glass slides (Nexterion Slide E; Schott, Jena, Germany). For covalent immobilization, the oligonucleotide array was incubated at 60°C for 30 min in a drying compartment (Memmert, Schwabach, Germany). After being spotted, the slides were rinsed for 5 min in 0.1% (vol/vol) Triton X-100 in double-distilled H2O (ddH2O), 4 min in 0.5 μl of concentrated HCl per ml of ddH2O, and 10 min in a 100 mM KCl solution with constant stirring. Subsequently, the slides were incubated in blocking solution (25% [vol/vol] ethylene glycol in ddH2O, 0.5 μl of concentrated HCl per ml of blocking solution) with the spotted side upwards at 50°C in a heating compartment (OV5; Biometra, Göttingen, Germany), rinsed in ddH2O for 1 min, and finally dried under a flow of nitrogen. The average spot size was 130 μm, and the spot-to-spot distance was 320 μm. The processed slides were stored dry at room temperature in the dark for a maximum of 20 days until further use.
Controls.
In addition to the beta-lactamase gene-specific probes, each array included several controls: a prelabeled spotting control (5′-TTTTTTTTTTTTTTCTAGACAGCCACTCATA-cyanine3 [Cy3]-3′), a positive hybridization control (5′-TTTTTTTTTTTTTGATTGGACGAGTCAGGAGC-3′) complementary to a labeled oligonucleotide target (5′-Cy3-GCTCCTGACTCGTCCAATC-3′), which was spiked during hybridization, and a negative hybridization control (5′-TTTTTTTTTTTTTTCTAGACAGCCACTCATA-3′). All of these control sequences are unrelated to sequences found in bacterial species. The spotting controls were set at the corner positions of each of the 14 subarrays. The positive and negative hybridization controls were spotted alternately at the side borders of each subarray. The sequences of the process controls (TEM, 5′-TTTTTTTTTTTTTAGAAACGCTGGTGAAAGT-3′; SHV, 5′-TTTTTTTTTTTTTTTTAAAGTAGTGCTCTGCGGC-3′; CTX-M, 5′-TTTTTTTTTTTTTTATCGCGGTGATCTGGC-3′) corresponded to sequences conserved within the blaTEM, blaSHV, and blaCTX-M gene families. Within the subarrays belonging to a certain chip module, the corresponding process control was spotted in two lines bordering the central probe sets (Fig. 1; also see Fig. S1 in the supplemental material).
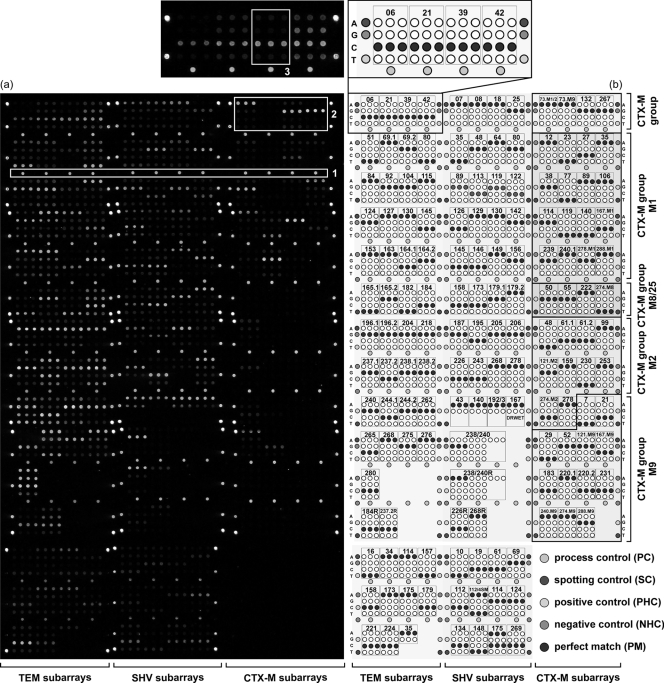
FIG. 1.
Chip layout. (a) Fluorescence image after hybridization with 200 ng labeled TEM-1/SHV-1 (NT/F = 71) and 150 ng CTX-M-15 (NT/F = 97) target DNA (Iso-37; PMT, 60%). Box 1, fluorescence signals of some process controls; box 2, probe sets for CTX-M group determination; box 3, signal pattern of probe set TEM 39. The signal intensity is encoded in the 65,636 gray scales of the 16-bit TIF image. (b) Schematic representation of the modular layout of the oligonucleotide microarray. The areas specific for the different beta-lactamase genes and the different subgroups of the CTX-M type genes are labeled. The enlarged part describes in detail the arrangement of the A, G, C, and T probes of each probe set. The probe sets are named as described in Materials and Methods, and numbers represent interrogated amino acid positions. Theoretical perfect matches after hybridization with TEM-1, SHV-1, CTX-M-1, CTX-M-2, CTX-M-8, and CTX-M-9 are highlighted in black. An enlarged version of this figure is shown in Fig. S1 in the supplemental material.
Amplification, labeling, and purification of target DNA.
The target DNA used for hybridization on the oligonucleotide arrays was amplified by PCR. The blaTEM and blaSHV genes were amplified simultaneously in a dual-plex PCR using the primers TEM-forw (5′-TGAGTATTCAACATTTCCGTGT-3′), TEM-rev (5′-TTACCAATGCTTAATCAGTGA-3′), SHV-forw (5′-CAAAACGCCGGGTTATTC-3′), and SHV-rev (5′-TTAGCGTTGCCAGTGCT-3′), with expected amplicon lengths of 861 and 937 bp, respectively. For amplification and labeling, 50 ng plasmid DNA was supplemented with 0.4 μM (each) primers; 100 μM (each) dATP, dGTP, and dTTP; 60 μM dCTP; 40 μM Cy3-dCTP (ratio of unlabeled/labeled dCTP, 3:2) (GE Healthcare, Munich, Germany); 1× PCR buffer containing 2.5 mM Mg(OAc)2 (Eppendorf, Hamburg, Germany); 2% dimethyl sulfoxide (Fluka Chemie, Buchs, Switzerland); and 5 U of Taq DNA polymerase (Eppendorf, Hamburg, Germany) in a total volume of 50 μl. An initial denaturation at 94°C for 1 min was followed by 30 cycles of 94°C for 30 s, 54°C for 30 s, and 68°C for 1 min, with a final extension step at 68°C for 4 min. For the detection of blaCTX-M genes, a multiplex PCR was carried out using primers described by Woodford et al. (52), which leads to different amplicon sizes for the different blaCTX-M groups. For the amplification, 50 ng template DNA was supplemented with primers (each at 0.2 μM, except for 0.4 μM for the M9 group primer pair) and Qiagen multiplex PCR master mix (Qiagen, Hilden, Germany) containing optimized concentrations of HotStarTaq DNA polymerase, MgCl2, deoxynucleoside triphosphates, and reaction buffer in a total volume of 50 μl. An activation step at 95°C for 15 min was followed by 35 cycles of 94°C for 30 s, 60°C for 90 s, and 72°C for 1 min, and a final extension step at 72°C for 10 min. Detected blaCTX-M genes were amplified and labeled to produce target DNA using the corresponding CTX-M group-specific primer pair. The primers were described previously by Eckert et al. (16). The expected amplicon lengths were 864 bp for CTX-M1 and 870 bp for the CTX-M9 group. The labeling PCR protocol described for the amplification of blaTEM and blaSHV genes was used with the following modifications: primer concentration, 0.2 μM; annealing temperature, 60°C. To screen for blaCMY-2-like AmpC genes originating from Citrobacter freundii, the primers CIT-forw (5′-CGGACACCTTTTTGCTTTTAATT-3′) and CIT-rev (5′-GAAAAAAAGAGAAAGAAAGGAGGC-3′) were used by applying the TEM/SHV dual-plex PCR protocol. Amplifications were performed in a Mastercycler gradient or gradient S (Eppendorf, Hamburg, Germany). A no-template negative control was included in each PCR run. After the purification of the PCR product with the QIAquick Spin PCR purification kit (Qiagen, Hilden, Germany), the DNA concentration and the rate of the incorporation of Cy3-dCTP, expressed as the number of nucleotides/number of incorporated fluorescent dyes (NT/F), was determined by the measurement of the optical density (ND-1000 spectrophotometer; NanoDrop Technologies, Rockland, ME).
DNA sequencing.
For DNA sequencing, the blaTEM, blaSHV, and blaCTX-M genes detected in clinical samples subsequently were amplified in separate reactions. Amplifications were carried out using the same primer pairs (TEM-forw and TEM-rev, SHV-forw and SHV-rev, and CTX-M group-specific primer pairs) and the same PCR conditions as those described for the production of target DNA but without applying Cy3-labeled dCTP; 100 μM dCTP was used instead. A no-template negative control was included in each PCR run. The PCR product was purified with the QIAquick spin PCR purification kit (Qiagen, Hilden, Germany) according to the protocol of the manufacturer. The DNA was eluted in 30 μl of ddH2O. The purified PCR products were sequenced with the following primers: blaTEM genes, TEM-forw, TEM-rev, TEMseq-forw (5′-GCCAACTTACTTCTGACAAC-3′), and TEMseq-rev (5′-GCCAACTTACTTCTGACAAC-3′); blaSHV genes, SHV-forw, SHV-rev, SHVseq-forw (5′-GGATTGACTGCCTTTTTGC-3′), and SHVseq-rev (5′-GCAAAAAGGCAGTCAATCC-3′); blaCTX-M group M1 genes, CTX-M1-forw (5′-GGTTAAAAAATCACTGCGTC-3′), CTX-M1-rev (5′-TTGGTGACGATTTTAGCCGC-3′), CTX-M1seq-forw (5′-GCGATGTGCAGCACCAGTAA-3′), and CTX-M1seq-rev (5′-AGCTTATTCATCGCCACGTT-3′); blaCTX-M group M9 genes, CTX-M9-forw (5′-ATGGTGACAAAGAGAGTGCA-3′), CTX-M9-rev (5′-CCCTTCGGCGATGATTCTCG-3′), CTX-M9seq-forw (5′-CCAATGTGCAGTACCAGTAA-3′), and CTX-M9seq-rev (5′-ATTGGAAAGCGTTCATCACC-3′). Sequencing was provided by GATC-Biotech (Konstanz, Germany). All bla genes detected were sequenced in both directions with two primers each, resulting in four overlapping fragments. The sequence data were assembled by using SeqMan II software (version 5.00; DNAStar, Madison, WI).
Fragmentation.
Target DNA for the hybridization on two chips (duplicate) was fragmented with 0.4 mU DNase I (Promega, Mannheim, Germany) per ng labeled target DNA to increase the efficiency of hybridization and avoid denaturation. All target DNAs amplified from one isolate were included in the same reaction. The fragmentation was carried out in 1× reaction buffer (40 mM Tris-HCl [pH 8], 10 mM MgSO4, 1 mM CaCl2) at room temperature for 5 min in a total volume of 20 to 30 μl depending on the concentrations of the purified PCR products. The reaction was stopped by the addition of 2 mM EGTA and incubation at 65°C for 10 min. Fragment sizes were estimated to be in the range of 15 to 150 bp using lab-on-a-chip electrophoresis (Bioanalyzer 2100, DNA 1000 LabChip kit; Agilent, Böblingen, Germany).
Hybridization.
Depending on the presence of either one or both genes, 150 or 200 ng TEM/SHV target DNA was hybridized. For CTX-M, 150 ng target DNA was used. As standard conditions, the fragmented target DNA for one slide together with DNA complementary to the positive control (Cy3-GCTCCTGACTCGTCCAATC; 0.05 pmol) was hybridized in 65 μl of 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) containing 0.1% sodium dodecyl sulfate (SDS). For hybridization, an HS400 automated hybridization station (Tecan, Crailsheim, Germany) was used with an initial wash step at 47°C for 30 s with 2× SSPE (the same concentration as that used for the hybridization solution) followed by the injection of 65 μl hybridization solution and hybridization at 47°C for 1 h using a high agitation frequency. After hybridization, the slides were washed two times at room temperature: 1.5 min with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS, and 1.5 min with 0.2× SSC. The slides subsequently were dried with N2 at 30°C for 4 min. The duration of the whole procedure was 90 min. Different SSPE and SDS concentrations or hybridization temperatures were tested during assay optimization.
Data acquisition and processing.
After the hybridization reaction, the data from the oligonucleotide arrays were read out by the acquisition of the fluorescence signals using a ScanArray Express microarray scanner (Perkin-Elmer, Waltham, MA). The laser power setting was 100%. Each slide was scanned with multiple photomultiplier tube (PMT) gain settings, including 55, 60, 65, and 70% and a 10-μm resolution. The image processing and calculation of signal intensities were performed with ScanArray Express analysis software, version 3.0 (Perkin-Elmer, Waltham, MA). Individual net signal intensities were calculated by the subtraction of the local background from the raw spot intensity value. Further data processing was performed using Microsoft Excel (Microsoft, Redmond, WA). Unless otherwise noted, the standard data processing procedure as follows. Net signal intensities were extracted from the PMT 60% scans. For the calculation of the mean net signal intensities, referred to as signal intensity, the six spot replicates of a certain oligonucleotide probe on two identically processed microarrays (three replicates each) were used. Standard deviations also were calculated.
Within each oligonucleotide probe set related to a certain mutation, the probe showing the highest signal intensity was considered the potential perfect match (PM). The remaining ones with lower signal intensities were considered mismatches (MM). The quality of the discrimination between single nucleotides within the target using such a probe set is reflected by the difference between the perfect match signal and mismatch signals. Therefore, the ratio between the individual net signal intensity of each spot replicate of a certain probe within the probe set (NISpot) and the mean signal intensity of the perfect match signal (IPM) was calculated (NIspot/IPM). The mean of those values is referred to as the relative intensity (RI or RIMEAN) of a certain oligonucleotide probe. Accordingly, the perfect matches always had RI values of 1 (RIPM = 1), and the relative intensities of the mismatches have values below 1 (RIMM < 1). Standard deviations also were calculated.
For the evaluation of the performance of each probe set, the median of the relative intensities (NIspot/IPM) of the six technical replicates (three spots on two arrays each) were calculated and referred to as RIMEDIAN. The means and standard deviations from the RIMEDIAN values of repeated experiments (biological replicates) were calculated, and the means again were referred to as relative intensity (RI). The probe sets of the TEM module were analyzed using data of all clinical isolates harboring a TEM-1 gene (n = 38). For the SHV module, all SHV-1-positive isolates (n = 10) were used, and for the CTX-M module, all CTX-M-15 (n = 34)- or CTX-M-14/M-9 (n = 3 to 8)-positive isolates were used. Signals obtained from mixed genotypes were evaluated calculating the 95% confidence intervals in addition to standard deviations. Standard t testing was applied where necessary.
Nucleotide sequence accession numbers.
The sequences obtained by sequencing have been submitted to GenBank under accession numbers FJ668714 to FJ668818.
RESULTS
In the present study, we describe a diagnostic oligonucleotide microarray for the rapid identification of ESBLs in Enterobacteriaceae by the simultaneous genotyping of TEM, SHV, and CTX-M beta-lactamase genes. The performance of this integrated chip and its applicability as an identification tool was validated using 60 blinded clinical samples collected in two clinical laboratories. The current version of the chip includes probe sets for the identification of 99% of all TEM variants and 89% of all SHV variants published (http://www.lahey.org/studies/; as of 2008). All positions known to be associated with ESBL activity (18) and inhibitor resistance (8) were included. Probes within the CTX-M chip module cover CTX-M group determination and the identification of 82% of all known CTX-M group M1 beta-lactamases as well as 83, 50, and 50% of all group M2, group M9, and group M8/M25 beta-lactamases.
ESBL chip development.
The development of the described chip included the integration of previously designed chips for different beta-lactamase genes as well as the integration of newly designed and redesigned probe sets. An easily expandable modular organization was chosen as the layout (Fig. 1; also see Fig. S1 in the supplemental material). During the integration process, reference strains with known gene variants were used and several adjustments to hybridization parameters were made compared to those of our previous studies (19). As standard conditions, the hybridization of fragmented target DNA was carried out for 1 h at 47°C in a solution containing 2× SSPE and 0.1% SDS. The application of the automated hybridization station resulted in a hybridization procedure of only 90 min, including washing and drying steps. The data acquisition was possible immediately afterwards.
For the production of target DNA, plasmid DNA isolated from clinical samples or from reference material was amplified by PCR. For the simultaneous genotyping of different gene targets, a multiplexed amplification and labeling strategy is desirable. TEM and SHV genes were amplified and labeled with Cy3 using a robust dual-plex PCR. Amplification product sizes were 867 and 927 bp, respectively. The integration of the CTX-M amplification into a multiplexed format, which would simultaneously amplify and label all of the TEM, SHV, and CTX-M genes, was dropped due to the formation of heterodimers between primers specific for the different CTX-M groups. Hence, CTX-M target DNA was amplified with group-specific primers in a singleplex PCR after clarifying the allocation to a certain CTX-M group by using a multiplex screening PCR. The PCR product sizes for CTX-M1 and CTX-M9 group members were 864 and 870 bp, respectively. The Cy3 incorporation rates were similar for all labeling PCRs applied. The mean NT/F ratios (variation depending on template quality) of the TEM/SHV PCR products were 84 (45 to 170) and 97 (56 to 173), respectively, for the CTX-M group-specific PCR products.
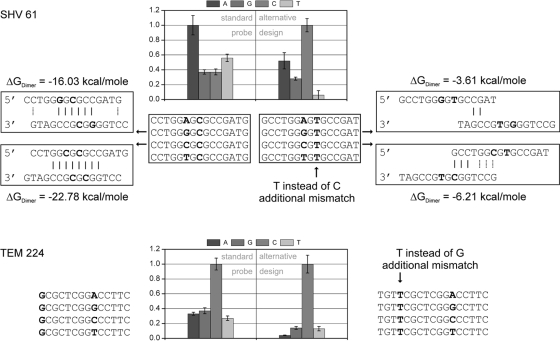
The performance of an individual probe set was assessed with regard to its discrimination power for various DNA targets and expressed as the mismatch-to-perfect match ratio (MM/PM). For allele-specific hybridization, which was chosen as a format in the presented microarray, the probe position relative to that of the target is virtually fixed if single-nucleotide polymorphisms are targeted. However, at certain positions it was impossible to select probes, because stable secondary structures would have formed within the probe themselves, rendering a meaningful detection impossible. We therefore chose to incorporate artificial mismatches at positions involved in the undesired secondary structure formation at difficult target positions. Figure 2 compares the standard and alternative probe designs for probe sets SHV 61 and TEM 224. In both cases, the probe containing a cytosine represents the perfect match when hybridizing SHV-1 and TEM-116 target DNA. For SHV 61, the replacement of a cytosine by thymine at a position significantly involved in dimer formation shifted the highest hybridization signal from the wrong A probe to the correct C probe. The highest mismatch signal of the resulting probe set showed only 52% of the perfect match signal. In another case, the performance of the already correct working probe set TEM 224 was further improved by the same method (Fig. 2). Here, the average RIMM value was decreased from 0.32 to 0.11. For a complete list of all probes used for the current version of the chip, see Table S1 in the supplemental material.
FIG. 2.
Alternative probe design. Shown is the effect of the incorporation of an artificial mismatch at a position involved in dimer formation on the identification capability of probe set SHV 61. The improvement of the performance of probe set TEM 224 also is shown. Standard and alternative probe sets were printed within the same array and hybridized simultaneously. Data are based on hybridization experiments under standard conditions with TEM-3 (NT/F = 72) and SHV-1 (NT/F = 30) target DNA.
Testing of clinical isolates.
The performance of the array was validated using 60 blinded samples containing DNA isolated from clinical isolates collected in clinical routines of the Department of Clinical Chemistry and Laboratory Medicine at the Robert Bosch Hospital (RBK) in Stuttgart and the Institute of Medical Microbiology and Infection Control of the J. W. Goethe-Universität in Frankfurt am Main. Fifty-eight were phenotypically characterized as ESBL positive using standard laboratory procedures for susceptibility testing (agar diffusion using CLSI breakpoints; Vitek 2). Two samples showed an ambiguous or ESBL-negative phenotype (Iso-23 and Iso-62). The identified species were mainly E. coli and different Klebsiella species, but also one isolate each of Enterobacter sakazakii, E. intermedius, Proteus vulgaris, C. freundii, and Rahnella aquatilis (Table 1).
TABLE 1.
Genotyping of clinical isolates
| No.a | Isolateb | Array resultc |
MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|---|
| blaTEM | blaSHV | blaCTX-M | CTX | CAZ | CTX/CAZ | ESBL testd | ||
| 24* | E. sakazakii | TEM-1 | SHV-12 | 4 | 32 | <1 | ||
| 55* | K. oxytoca | TEM-1 | SHV-12 | 4 | 8 | <1 | Pos | |
| 25* | Klebsiella sp. | SHV-12 | 16 | ≥64 | <1 | |||
| 2+ | K. pneumoniae | TEM-1 | SHV-12 (+SHV-1) | 16 | ≥64 | <1 | Pos | |
| 12+ | K. pneumoniae | TEM-1 | SHV-12 (+SHV-1) | ≥64 | ≥64 | Pos | ||
| 36+ | K. pneumoniae | TEM-1 | SHV-12 (+SHV-1) | 4 | ≥64 | <1 | Pos | |
| 1+ | K. terrigena | TEM-1 | SHV-12 (+SHV-1) | 32 | ≥64 | <1 | ||
| 15+ | K. terrigena | TEM-1 | SHV-12 (+SHV-1) | 32 | ≥64 | <1 | ||
| 17+ | K. terrigena | TEM-1 | SHV-12 (+SHV-1) | ≥64 | ≥64 | |||
| 52* | Klebsiella sp. | TEM-1 | SHV-12 (+SHV-1) | 32 | ≥64 | <1 | Pos | |
| 5+ | E. coli | SHV-5 | 1 | ≥64 | <1 | Pos | ||
| 19+ | K. pneumoniae | SHV-5 | ≥64 | ≥64 | Pos | |||
| 49* | Klebsiella sp. | TEM-1 | SHV-12 | CTX-M-15 | ≥64 | ≥64 | ||
| 13+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 18+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 20* | E. coli | TEM-1 | CTX-M-15 | ≥64 | 32 | >1 | Pos | |
| 26+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 33* | E. coli | TEM-1 | CTX-M-15 | ≥64 | 32 | >1 | Neg | |
| 38+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 40+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 48* | E. coli | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 60+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 61+ | E. coli | TEM-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 6+ | E. coli | CTX-M-15 | ≥64 | 16 | >1 | Pos | ||
| 9+ | E. coli | CTX-M-15 | ≥64 | ≥64 | Pos | |||
| 30* | E. coli | CTX-M-15 | ≥64 | 16 | >1 | Pos | ||
| 32* | E. coli | CTX-M-15 | ≥64 | 16 | >1 | Pos | ||
| 34+ | E. coli | CTX-M-15 | ≥64 | 16 | >1 | Pos | ||
| 57+ | E. coli | CTX-M-15 | ≥64 | 16 | >1 | Pos | ||
| 3+ | E. intermedius | CTX-M-15 | ≥64 | ≥64 | ||||
| 29+ | K. oxytoca | SHV-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 7+ | K. pneumoniae | TEM-1 | SHV-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos |
| 16+ | K. pneumoniae | TEM-1 | SHV-1 | CTX-M-15 | ≥64 | ≥64 | Pos | |
| 37+ | K. pneumoniae | TEM-1 | SHV-1 | CTX-M-15 | ≥64 | ≥64 | Pos | |
| 10+ | K. pneumoniae | SHV-1 | CTX-M-15 | Pos | ||||
| 35+ | K. pneumoniae | SHV-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 42+ | K. pneumoniae | SHV-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 11+ | K. pneumoniae | TEM-1 | CTX-M-15 | ≥64 | 16 | >1 | Pos | |
| 22* | K. pneumoniae | TEM-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 50* | K. pneumoniae | TEM-1 | CTX-M-15 | ≥64 | ≥64 | Pos | ||
| 14+ | K. terrigena | TEM-1 | SHV-1 | CTX-M-15 | ≥64 | ≥64 | ||
| 28+ | K. terrigena | SHV-1 | CTX-M-15 | ≥64 | ≥64 | |||
| 21* | K. terrigena | TEM-1 | CTX-M-15 | ≥64 | ≥64 | |||
| 51* | Klebsiella sp. | TEM-1 | CTX-M-15 | ≥64 | ≥64 | |||
| 8+ | E. coli | TEM-1 | CTX-M-3 | 32 | 4 | >1 | Pos | |
| 56* | E. coli | CTX-M-3 | 16 | ≤1 | >1 | Pos | ||
| 59+ | E. coli | TEM-1 | CTX-M-15 + CTX-M-14b | ≥64 | ≥64 | Pos | ||
| 27+ | E. coli | TEM-1 | CTX-M-15 + CTX-M-14b | ≥64 | ≥64 | Pos | ||
| 4+ | E. coli | TEM-1 | CTX-M-14 | ≥64 | 1 | >1 | Pos | |
| 39+ | E. coli | TEM-1 | CTX-M-14 | ≥64 | 1 | >1 | Pos | |
| 58+ | E. coli | CTX-M-14 | ≥64 | 1 | >1 | Pos | ||
| 46* | E. coli | TEM-1 | CTX-M-9 | 4 | ≥64 | <1 | Pos | |
| 41+ | E. coli | CTX-M-9 | ≥64 | 4 | >1 | Pos | ||
| 47+ | P. vulgaris | CTX-M-9 | ||||||
| 44* | E. coli | TEM-1 | 32 | ≥64 | <1 | Pos | ||
| 53* | E. coli | TEM-1 | ≥64 | 16 | >1 | Pos | ||
| 31* | E. coli | TEM-1 | 32 | ≥64 | <1 | Pos | ||
| 54* | Citrobacter freundii | 32 | ≥64 | <1 | ||||
| 62* | Klebsiella pnemoniae | SHV-1 | ||||||
| 23* | Rahnella aquatilis | ≤1 | ≤1 | |||||
Data were sorted first by the different genotypes detected and afterwards were clustered by species. Isolate numbers were used for labeling the blinded plasmid isolates: *, samples originally collected in Stuttgart; +, samples originally collected in Frankfurt. Samples Iso-27 and Iso-59 originated from the same patient but different specimens (Iso-27, urine; Iso-59, respiratory sample).
Species were identified as described in Materials and Methods.
Array results were validated by standard the DNA sequencing of the PCR product received.
The Vitek ESBL test is FDA approved only for E. coli, K. pneumoniae, and K. oxytoca. Pos, positive; Neg, negative.
Prior to the genotyping with the integrated ESBL chip, all samples were screened by the described amplification procedures for the presence of the target genes. As a result, 63% of all 60 isolates were positive for blaTEM, 38% for blaSHV, and 70% for blaCTX-M; 3% were negative for all targets. From all isolates found to be E. coli, 55% harbored blaTEM, 3% blaSHV, and 94% blaCTX-M. For Klebsiella spp., 69% were positive for blaTEM, 81% for blaSHV, and 58% for blaCTX-M. With the applied multiplex screening PCR for blaCTX-M it was possible to distinguish between different CTX-M groups. The highest prevalence within the CTX-M-positive isolates was found for group M1 (81%), followed by 14% for group M9 and 4% for group M1 and M9 together.
The further identification of the isolates' beta-lactamase genotype was based on a hierarchical readout of the DNA microarray signals after labeling PCR and hybridization to the ESBL chip. As an example, Fig. 1a shows the hybridization result for Iso-37. (i) The gene family was determined by the process controls (PC), which act as gene family-specific probes. For Iso-37, the PCs detected the presence of a TEM, SHV, and CTX-M gene (box 1). (ii) The classification of detected CTX-M genes into subgroups (M1, M2, M9, and M8/25) was done by four probe sets (see the layout section labeled CTX-M group in Fig. 1b). The Iso-37 CTX-M gene showed the characteristic hybridization signal pattern for a blaCTX-M-1-like gene as indicated by the identified adenosine (A) for probe set 73.M1/2, the absent signal for probe set 73.M9, and the identified guanosines (G) for probe sets 132 and 267 (box 2). (iii) Finally, the beta-lactamase gene variant was identified by signal patterns exhibited by the individual probe sets corresponding to the different interrogated mutation positions. For example, when hybridizing the Iso-37 TEM gene, the signal pattern of probe set 39 (box 3) identified the presence of cytosine (C) and therefore a TEM-1 (wild-type) codon at this position (box3). None of the other TEM probe sets identified a point mutation. Hence, the gene was identified as blaTEM-1. The SHV gene also was identified as the parental blaSHV-1 gene, as no mutation was identified. The CTX-M gene was identified as blaCTX-M-15 due to the specific mutations identified with the probe sets 77, 114, 140, 240, and 288 (compare theoretical perfect matches after hybridization with blaCTX-M-1 and the actual hybridization signals for Iso-37). The results for all 60 isolates are summarized in Table 1. In 54 isolates an ESBL gene variant was detected, whereas 6 isolates contained no TEM-, SHV-, or CTX-M-derived ESBL. All blaTEM genes coded for TEM-1, meaning that no ESBL phenotype could be assigned to TEM. For SHV, a range of gene variants was detected covering SHV-1, SHV-12, and SHV-5. CTX-M-15 and CTX-M-3 were found as members of CTX-M group M1 and CTX-M-9, CTX-M-14, and CTX-M-14b as members of the group M9. SHV- and CTX-M ESBLs were detected in the following combinations: CTX-M (72.2%, 39/54), SHV (22.2%, 12/54), 2× CTX-M (3.7%, 2/54), and SHV plus CTX-M (1.9%, 1/54). SHV-12 was the most prevalent SHV-ESBL (84.6%, 11/13), CTX-M-15 the most frequently detected CTX-M-ESBL (81%, 34/42). SHV-ESBLs were found mainly in Klebsiella species (84%), CTX-M group M9 ESBLs in E. coli (87%), and CTX-M group M1 ESBLs in E. coli and Klebsiella at similar frequencies (55 and 41%). All DNA array results were confirmed by Sanger sequencing. The three E. coli isolates Iso-34, Iso-44, and Iso-53 did not harbor a TEM-, SHV-, or CTX-M ESBL gene and also were found to be negative in a separate PCR screening for the presence of a blaCMY-2-like AmpC gene.
Table 1 also includes the ceftazidime (CAZ) and cefotaxime (CTX) MICs for the different clinical samples obtained by measurements with the Vitek 2 Gram-negative susceptibility card (AST-N062). SHV-5 and SHV-12 both feature the Glu240Lys mutation in combination with Gly238Ser, which is known to shift the substrate specificity toward ceftazidime (18). Generally, a CTX/CAZ MIC ratio of <1 was observed for the samples harboring those ESBLs. Typically, CTX-M enzymes favor cefotaxime over ceftazidime as a substrate. This leads to the observed CTX/CAZ ratio of >1 for the CTX-M ESBLs. CTX-M-15 features the Asp240Gly mutation, which is thought to increase activity against ceftazidime compared to that of CTX-M-3, -M-9, or -M-14 (4, 5). This trend also was observed. Only sample Iso-46 showed a deviant behavior with a CTX-M-9 gene present and a CTX/CAZ ratio of <1. For the full Vitek data set as well as standard susceptibility testing results, see Table S2 in the supplemental material.
Chip performance.
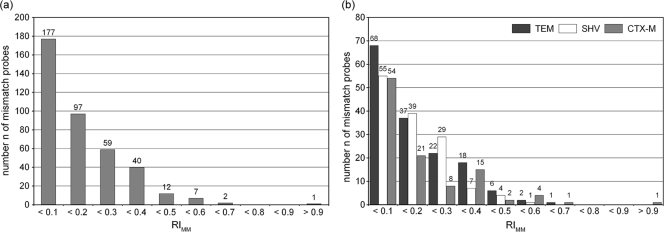
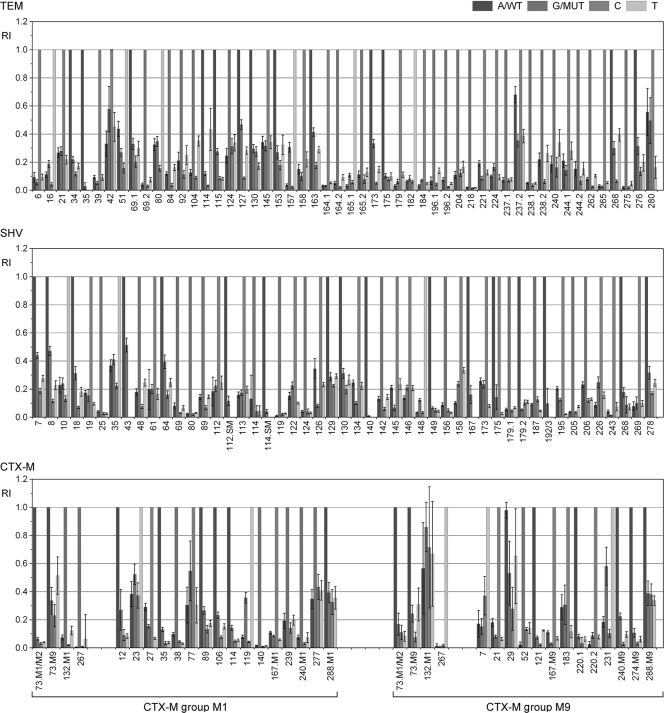
The performance of the ESBL chip was assessed according to the distribution of RIMM values across defined RIMM ranges, as displayed in Fig. 3. For the performance analysis, all experiments leading to the same perfect match signals were subsumed as replications. For the whole ESBL chip, 94% of all MM signals had an RIMM of <0.4, which demonstrated the excellent discrimination capacity of the setup. The three chip modules showed similar distributions of mismatch signals, with the SHV chip being the most specific (Fig. 3b). The performances of individual probe sets relevant for the genotyping of blaTEM, blaSHV, and group M1 and M9 blaCTX-M genes are displayed in Fig. 4. The vast majority of the 135 probe sets identified the present nucleotide without ambiguity. Intermediate RIMM values (0.5 to 0.7) were found for TEM 42, TEM 280, TEM 237.2, SHV 43, CTX-M-231, CTX-M-23, and CTX-M-77. These probe sets are potential candidates for improvement by redesign. The only probe set that did not work correctly in all relevant hybridizations was CTX-M-29. However, this behavior could be assigned to an artifact of the array printing process and not probe properties.
FIG. 3.
Performance of the whole chip (a) and individual chip modules (b). The distribution of RIMM values across different RIMM ranges is displayed. The total amount of mismatch probes is 394 for the whole chip and 154, 135, and 105 for the TEM, SHV, and CTX-M chip modules, respectively. Relative intensities of the probe sets are averaged from data of all clinical isolates harboring the TEM-1 (n = 38), SHV-1 (n = 10), CTX-M-15 (n = 34), and CTX-M-14/M-9 (n = 3 to 8) genes.
FIG. 4.
Performance of 135 individual probe sets on the basis of the analysis of clinical isolates. Relative intensities and standard deviations of the TEM, SHV, and CTX-M probe sets were calculated from data of all clinical isolates harboring the TEM-1 (n = 38), SHV-1 (n = 10), CTX-M-15 (n = 34), and CTX-M-14/M-9 (n = 3 to 8) gene. Values of the four probes (A, G, C, and T) of each probe set are displayed in different shades of gray as indicated in the legend. The CTX-M chip module figure is divided into CTX-M group M1 and M9 performance. Probe sets for group and variant identification are separated.
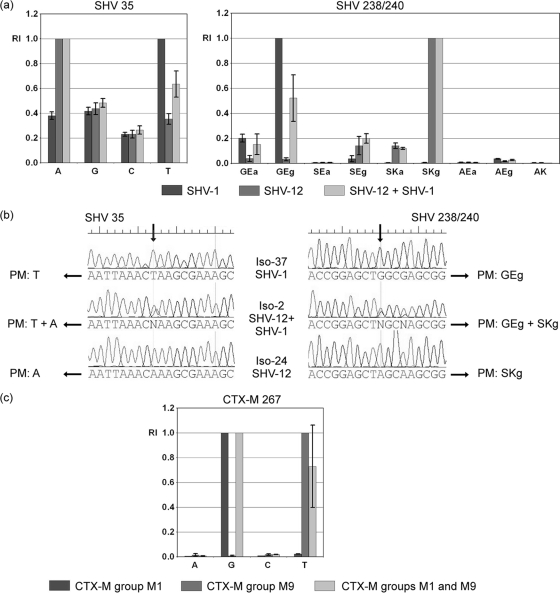
One remarkable property of the ESBL chip is its ability to detect mixed genotypes (alleles) of the same beta-lactamase family. Our study samples Iso-1, Iso-2, Iso-12, Iso-15, Iso-17, Iso-36, and Iso-52 were reliably positive by the chip for SHV-12 in the presence of SHV-1. The signals obtained at the relevant positions 35 and 238/240 for isolates containing either SHV-1, SHV-12, or a mixture of both are displayed in Fig. 5a. The chip clearly could discriminate between the different situations, as the signals of the T probe at position 35 and the GEg probe of probe set 238/240 were significantly different (nonoverlapping confidence intervals; P < 0.05 by t test). For confirmation, Fig. 5b includes the Sanger sequencing results for the Iso-24, Iso-37, and Iso-2 SHV-PCR products. At the mutation sites, clear double peaks were apparent. For samples Iso-59 and Iso-27, two CTX-M ESBLs were detected simultaneously, namely, CTX-M-15 and CTX-M-14b. The group discrimination probe set 267 is relevant for such a mixture. The signal at the T probe of this probe set was significantly different when a CTX-M group M9 gene (CTX-M-14b) was hybridized in addition to a group M1 gene (CTX-M-15) compared to a group M1 gene alone (Fig. 5c).
FIG. 5.
Detection of mixed SHV and CTX-M genotypes. (a) Relative signals for probe sets SHV 35 and SHV 238/240. The displayed intensities were calculated by analyzing data for all isolates harboring SHV-1 (n = 7), SHV-12 (n = 4), or both (n = 7). Error bars represent the 95% confidence interval (CI) scores calculated. (b) Sequencing results at the relevant nucleotide positions for isolates containing blaSHV-1 (Iso-37), blaSHV-12 (Iso-24), or a mixture of both (Iso-2). Depending on the different sequences, the potential perfect match probes are indicated (PM). (c) Relative signals for group discrimination probe set CTX-M 267. The displayed intensities were calculated by analyzing data for all isolates harboring a CTX-M group M1 gene (n = 35), a CTX-M group M9 gene (n = 6), or both (n = 2). Error bars also represent the 95% confidence interval scores calculated.
DISCUSSION
With an increased prevalence of ESBL-producing pathogens, the fast and accurate identification of such isolates becomes increasingly clinically important. Despite a variety of available methods, the identification of ESBLs by conventional phenotypic methods remains difficult in practice due to the large number of beta-lactamase variants, the association of ESBLs with overproduced or plasmid-borne cephalosporinases (AmpC) or metallo-beta-lactamases (MBL) (15), and the change of outer-membrane permeability (28). Hence, molecular methods that have the potential to disentangle this complex resistance etiology and translate it into clinically useful information have the potential to improve the current diagnostic practice. Multiplexing is a key requisite to cover this task, and microarray platforms have been used repeatedly for such purposes. Several studies described DNA microarrays for the detection of various resistance gene panels, including some beta-lactam resistance genes (11, 12, 25). These publications used PCR products (200 to 800 bp) or long oligonucleotide (70mer) probes (6, 17), which are not suitable for gene variant discrimination at the single-nucleotide level. A more refined chip was described by Batchelor and colleagues, who developed an oligonucleotide microarray that covered beta-lactam resistance genes with 14 probes (3). Most of their targets, including blaTEM and blaSHV, were detected on a family level using group-specific probes. The blaCTX-M genes were detected on a subgroup level with three probes specific for CTX-M-1-like, CTX-M-2-like, and CTX-M-9-like genes. Another similar study recently reported a microarray-based method to detect blaTEM, blaSHV, and blaCTX-M in combination with plasmid-encoded AmpC beta-lactamase genes (54). The level of detection was also on the gene family level, plus six point mutations in the blaSHV gene. These studies all demonstrate strategies to set up diagnostic systems that quickly give a deeper insight into the genetic background of the beta-lactam resistance of a clinical isolate. In this study, we aimed to further extend these activities and cover all known gene variants of the three selected beta-lactamase families. As a result, the DNA microarray was able to genotype mutations within blaTEM, blaSHV, and blaCTX-M genes, which account for their classification into different variants and define their resistance spectra. As an example, the array was able to detect SHV-12 and SHV-5 in different Klebsiella and E. coli isolates. In a study that retrospectively correlated ESBL types, including SHV-12 and SHV-5, with treatment outcomes in bloodstream infections, it was found that the therapy failed when ESBL-producing strains were treated empirically with ceftazidime (51). If the respective clinician had had the right information before choosing this extended-spectrum cephalosporin, i.e., from a hospital infection control, knowing that this type of organism was present in the hospital, the type of empirical therapy could have been based on the molecular surveillance data obtained by the hospital, leading to alternative drug choices (e.g., imipenem [36]). With its short assay time, the ESBL array described in this study has the potential to provide such information faster than conventional susceptibility tests. At the time of design, the ESBL chip could identify all known mutations in the target genes. Naturally, such a DNA microarray would have to be updated as new mutations emerge and are described, but as we have experienced since our first study (19), this can be done with a reasonable amount of time and effort. With regard to the published literature, this progression currently is faster with the CTX-M type-beta-lactamases than the TEM- and SHV-type beta-lactamases. CTX-M-type enzymes are now the most prevalent beta-lactamase species worldwide (40).
The performance of the new integrated chip was tested with 60 blinded clinical samples, which were phenotypically precharacterized mainly as ESBL positive (58 isolates). Using the phenotypic characterization as the gold standard, our assay showed 93% sensitivity (54 of 58) for the detection of ESBLs. DNA microarray results and DNA sequencing were 100% concordant. For four isolates, the resistance phenotype could not be explained by the presence of a TEM, SHV, or CTX-M ESBL gene. When calculating the sensitivity, it has to be considered that the phenotypic gold standard is contentious and also error prone for ESBL detection. For example, the MICs for CTX-M-15-positive E. coli isolate 33 were identical to those for other CTX-M-15-positive E. coli isolates (see Table S2 in the supplemental material), but a synergy-based Vitek ESBL test was negative. Additionally, CLSI recommendations and the Vitek ESBL test are valid only for E. coli, K. pneumoniae, K. oxytoca, and Proteus mirabilis, but not for other Gram-negative species like C. freundii or R. aquatilis. As a consequence, the sensitivity could be underestimated. Molecular methods like the described DNA microarray assay can be used for a wide range of species, including AmpC producers like C. freundii. As possible explanations for the phenotypic data from the three E. coli isolates 31, 44, and 53, we anticipate the presence of a plasmid-carried ampC gene (other than C. freundii-derived blaCMY genes, as they could be experimentally excluded), promoter mutations within the chromosomal ampC gene, metallo-beta-lactamases, alterations of the membrane composition, or rare beta-lactamases. The fourth false-negative sample contained a C. freundii strain that could harbor a derepressed chromosomal ampC gene. For all positive isolates, the substrate spectra connected to the identified gene variants and the phenotypically measured MIC data correlated very well. The increased hydrolytic activity against ceftazidime of the CTX-M-15 variant was especially apparent compared to that of most other CTX-M variants, such as CTX-M-3, which differs in only one point mutation from CTX-M-15. Since only a small number of ESBL-negative isolates were included, the DNA array achieved a theoretic specificity of 100%. However, this value needs to be seen in context and the performance to be confirmed in future studies including a larger number of negative isolates.
A comparison of the prevalence and spread of ESBL-producing Enterobacteriaceae in Europe (8) with the ESBL genes identified in this study revealed a high concordance with the current resistance situation. CTX-M enzymes have become more and more predominant, and the blaCTX-M genes also were by far the most prevalent ESBL genes found among the tested isolates. CTX-M-15 has risen to prominence all over Europe (27) and also was the most frequent CTX-M ESBL in our study. CTX-M-15 was detected in E. coli as well as in Klebsiella isolates. Within the SHV family, SHV-12 is one of the most prevalent enzymes and has been reported all over the world for K. pneumoniae (9). In our study, SHV-12 also was the most prevalent SHV ESBL and was detected exclusively in Klebsiella isolates. Interestingly, no TEM ESBL was identified. More than 60% of the tested isolates harbored a blaTEM gene, but all were identified as TEM-1 and thus not an ESBL. These findings for TEM and SHV indicate that the detection of a resistance gene family alone is not sufficient as a diagnostic parameter.
Conclusions.
We described an integrated oligonucleotide microarray for the genotyping of TEM, SHV, and CTX-M beta-lactamase variants at the single-nucleotide level within 5 h and with a sensitivity of 93%. As it is, the DNA microarray provides an interesting tool for epidemiological studies or surveillance with unparalleled coverage. The integration of other resistance genes is under way and will further increase its information value. In addition, this study provides a key building block for the development of advanced antibiotic resistance diagnostics, such as microfluidic sample-to-answer systems, which integrate the microarray analysis with preanalytics and readout. Their introduction into the clinical routine could contribute to the prudent use of antibiotics and optimal treatment outcome.
Supplementary Material
Acknowledgments
This work was financed by the German Federal Ministry of Education and Research (BMBF) within the GenoMik (Genome Research on Microorganisms) project.
We also thank our scientific partners within the project for helpful discussions, W. Witte and H. Hächler for supply of isolates, and our industry partner Eppendorf AG (Hamburg, Germany) for technical support and equipment.
Footnotes
Published ahead of print on 9 December 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet, G., G. Brami, D. Decre, A. Flippo, O. Gaillot, P. H. Lagrange, and A. Philippon. 1995. Molecular characterization by PCR-restriction fragment length polymorphism of TEM beta-lactamases. FEMS Microbiol. Lett. 134:203-208. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor, M., K. L. Hopkins, E. Liebana, P. Slickers, R. Ehricht, M. Mafura, F. Aarestrup, D. Mevius, F. A. Clifton-Hadley, M. J. Woodward, R. H. Davies, E. J. Threlfall, and M. F. Anjum. 2008. Development of a miniaturised microarray-based assay for the rapid identification of antimicrobial resistance genes in Gram-negative bacteria. Int. J. Antimicrob. Agents. 31:440-451. [DOI] [PubMed] [Google Scholar]
- 4.Birkett, C. I., H. A. Ludlam, N. Woodford, D. F. Brown, N. M. Brown, M. T. Roberts, N. Milner, and M. D. Curran. 2007. Real-time TaqMan PCR for rapid detection and typing of genes encoding CTX-M extended-spectrum beta-lactamases. J. Med. Microbiol. 56:52-55. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet, R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruant, G., C. Maynard, S. Bekal, I. Gaucher, L. Masson, R. Brousseau, and J. Harel. 2006. Development and validation of an oligonucleotide microarray for detection of multiple virulence and antimicrobial resistance genes in Escherichia coli. Appl. Environ. Microbiol. 72:3780-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantón, R., and T. M. Coque. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 8.Cantón, R., M. I. Morosini, O. Martin, S. de la Maza, and E. G. G. De la Pedrosa. 2008. IRT and CMT beta-lactamases and inhibitor resistance. Clin. Microbiol. Infect. 14:53-62. [DOI] [PubMed] [Google Scholar]
- 9.Canton, R., A. Novais, A. Valverde, E. Machado, L. Peixe, F. Baquero, and T. M. Coque. 2008. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl. 1):144-153. [DOI] [PubMed] [Google Scholar]
- 10.Cassone, M., M. M. D'Andrea, F. Iannelli, M. R. Oggioni, G. M. Rossolini, and G. Pozzi. 2006. DNA microarray for detection of macrolide resistance genes. Antimicrob. Agents Chemother. 50:2038-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, S., S. Zhao, P. F. McDermott, C. M. Schroeder, D. G. White, and J. Meng. 2005. A DNA microarray for identification of virulence and antimicrobial resistance genes in Salmonella serovars and Escherichia coli. Mol. Cell Probes 19:195-201. [DOI] [PubMed] [Google Scholar]
- 12.Cleven, B. E., M. Palka-Santini, J. Gielen, S. Meembor, M. Kronke, and O. Krut. 2006. Identification and characterization of bacterial pathogens causing bloodstream infections by DNA microarray. J. Clin. Microbiol. 44:2389-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. 2009. Performance standards for antimicrobial susceptibility testing, M100-S19, 19th informational supplement, vol. 29. Wayne, PA.
- 14.Doi, Y., and D. L. Paterson. 2007. Detection of plasmid-mediated class C beta-lactamases. Int. J. Infect. Dis. 11:191-197. [DOI] [PubMed] [Google Scholar]
- 15.Drieux, L., F. Brossier, W. Sougakoff, and V. Jarlier. 2008. Phenotypic detection of extended-spectrum beta-lactamase production in Enterobacteriaceae: review and bench guide. Clini. Microbiol. Infect. 14:90-103. [DOI] [PubMed] [Google Scholar]
- 16.Eckert, C., V. Gautier, and G. Arlet. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 57:14-23. [DOI] [PubMed] [Google Scholar]
- 17.Frye, J. G., T. Jesse, F. Long, G. Rondeau, S. Porwollik, M. McClelland, C. R. Jackson, M. Englen, and P. J. Fedorka-Cray. 2006. DNA microarray detection of antimicrobial resistance genes in diverse bacteria. Int. J. Antimicrob. Agents 27:138-151. [DOI] [PubMed] [Google Scholar]
- 18.Gniadkowski, M. 2008. Evolution of extended-spectrum beta-lactamases by mutation. Clin. Microbiol. Infect. 14:11-32. [DOI] [PubMed] [Google Scholar]
- 19.Grimm, V., S. Ezaki, M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Use of DNA microarrays for rapid genotyping of TEM beta-lactamases that confer resistance. J. Clin. Microbiol. 42:3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41:95-98. [Google Scholar]
- 21.Humeniuk, C., G. Arlet, V. Gautier, P. Grimont, R. Labia, and A. Philippon. 2002. Beta-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob. Agents Chemother. 46:3045-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, C. H., A. Ruzin, M. Tuckman, M. A. Visalli, P. J. Petersen, and P. A. Bradford. 2009. Pyrosequencing using the single-nucleotide polymorphism protocol for rapid determination of TEM- and SHV-type extended-spectrum beta-lactamases in clinical isolates and identification of the novel beta-lactamase genes blaSHV-48, blaSHV-105, and blaTEM-155. Antimicrob. Agents Chemother. 53:977-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye, K. S., H. S. Gold, M. J. Schwaber, L. Venkataraman, Y. Qi, P. C. De Girolami, M. H. Samore, G. Anderson, J. K. Rasheed, and F. C. Tenover. 2004. Variety of beta-lactamases produced by amoxicillin-clavulanate-resistant Escherichia coli isolated in the northeastern United States. Antimicrob. Agents Chemother. 48:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliebe, C., B. A. Nies, J. F. Meyer, R. M. Tolxdorff-Neutzling, and B. Wiedemann. 1985. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 28:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, Y., C. S. Lee, Y. J. Kim, S. Chun, S. Park, Y. S. Kim, and B. D. Han. 2002. Development of DNA chip for the simultaneous detection of various beta-lactam antibiotic-resistant genes. Mol. Cells 14:192-197. [PubMed] [Google Scholar]
- 26.Leinberger, D. M., U. Schumacher, I. B. Autenrieth, and T. T. Bachmann. 2005. Development of a DNA microarray for detection and identification of fungal pathogens involved in invasive mycoses. J. Clin. Microbiol. 43:4943-4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Martinez, L. 2008. Extended-spectrum beta-lactamases and the permeability barrier. Clin. Microbiol. Infect. 14:82-89. [DOI] [PubMed] [Google Scholar]
- 29.Melzer, M., and I. Petersen. 2007. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J. Infect. 55:254-259. [DOI] [PubMed] [Google Scholar]
- 30.Monecke, S., and R. Ehricht. 2005. Rapid genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolates using miniaturised oligonucleotide arrays. Clin. Microbiol. Infect. 11:825-833. [DOI] [PubMed] [Google Scholar]
- 31.M'Zali, F. H., J. Heritage, D. M. Gascoyne-Binzi, A. M. Snelling, and P. M. Hawkey. 1998. PCR single strand conformational polymorphism can be used to detect the gene encoding SHV-7 extended-spectrum beta-lactamase and to identify different SHV genes within the same strain. J. Antimicrob. Chemother. [DOI] [PubMed]
- 32.Naas, T., C. Oxacelay, and P. Nordmann. 2007. Identification of CTX-M-type extended-spectrum-beta-lactamase genes using real-time PCR and pyrosequencing. Antimicrob. Agents Chemother. 51:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver, A., J. C. Perez-Diaz, T. M. Coque, F. Baquero, and R. Canton. 2001. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-10) isolated in Spain. Antimicrob. Agents Chemother. 45:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson, A. B., M. Silverman, D. A. Boyd, A. McGeer, B. M. Willey, V. Pong-Porter, N. Daneman, and M. R. Mulvey. 2005. Identification of a progenitor of the CTX-M-9 group of extended-spectrum beta-lactamases from Kluyvera georgiana isolated in Guyana. Antimicrob. Agents Chemother. 49:2112-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson, D. L., W. C. Ko, A. Von Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugman, R. A. Bonomo, L. B. Rice, J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poirel, L., P. Kampfer, and P. Nordmann. 2002. Chromosome-encoded Ambler class A beta-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 46:4038-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randegger, C. C., and H. Hächler. 2001. Real-time PCR and melting curve analysis for reliable and rapid detection of SHV extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 45:1730-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez, M. M., P. Power, M. Radice, C. Vay, A. Famiglietti, M. Galleni, J. A. Ayala, and G. Gutkind. 2004. Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob. Agents Chemother. 48:4895-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossolini, G. M., M. M. D'Andrea, and C. Mugnaioli. 2008. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):33-41. [DOI] [PubMed] [Google Scholar]
- 41.Schwaber, M. J., and Y. Carmeli. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J. Antimicrob. Chemother. 60:913-920. [DOI] [PubMed] [Google Scholar]
- 42.Schwaber, M. J., S. Navon-Venezia, K. S. Kaye, R. Ben Ami, D. Schwartz, and Y. Carmeli. 2006. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 50:1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, W., E. S. Moland, N. D. Hanson, J. S. Lewis, J. H. Jorgensen, and K. S. Thomson. 2005. Failure of cefepime therapy in treatment of Klebsiella pneumoniae bacteremia. J. Clin. Microbiol. 43:4891-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sougakoff, W., S. Goussard, G. Gerbaud, and P. Courvalin. 1988. Plasmid-mediated resistance to third-generation cephalosporins caused by point mutations in TEM-type penicillinase genes. Rev. Infect. Dis. 10:879-884. [DOI] [PubMed] [Google Scholar]
- 45.Strommenger, B., C. Schmidt, G. Werner, B. Roessle-Lorch, T. T. Bachmann, and W. Witte. 2007. DNA microarray for the detection of therapeutically relevant antibiotic resistance determinants in clinical isolates of Staphylococcus aureus. Mol. Cell Probes 21:161-170. [DOI] [PubMed] [Google Scholar]
- 46.Tang, X., S. L. Morris, J. J. Langone, and L. E. Bockstahler. 2005. Microarray and allele specific PCR detection of point mutations in Mycobacterium tuberculosis genes associated with drug resistance. J. Microbiol. Methods 63:318-330. [DOI] [PubMed] [Google Scholar]
- 47.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weile, J., and C. Knabbe. 2009. Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal. Bioanal. Chem. 394:731-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weile, J., R. D. Schmid, T. T. Bachmann, M. Susa, and C. Knabbe. 2007. DNA microarray for genotyping multidrug-resistant Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 59:325-338. [DOI] [PubMed] [Google Scholar]
- 50.Wiegand, I., H. K. Geiss, D. Mack, E. Sturenburg, and H. Seifert. 2007. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J. Clin. Microbiol. 45:1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong-Beringer, A., J. Hindler, M. Loeloff, A. M. Queenan, N. Lee, D. A. Pegues, J. P. Quinn, and K. Bush. 2002. Molecular correlation for the treatment outcomes in bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae with reduced susceptibility to ceftazidime. Clin. Infect. Dis. 34:135-146. [DOI] [PubMed] [Google Scholar]
- 52.Woodford, N., E. J. Fagan, and M. J. Ellington. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 57:154-155. [DOI] [PubMed] [Google Scholar]
- 53.Yu, X., M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Development and validation of a diagnostic DNA microarray to detect quinolone-resistant Escherichia coli among clinical isolates. J. Clin. Microbiol. 42:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, L. X., Z. W. Zhang, D. Liang, D. Jiang, C. Wang, N. Du, Q. Zhang, K. Mitchelson, and J. Cheng. 2007. Multiplex asymmetric PCR-based oligonucleotide microarray for detection of drug resistance genes containing single mutations in Enterobacteriaceae. Antimicrob. Agents Chemother. 51:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.