Abstract
New Caledonia is an archipelago in the South Pacific with a high prevalence of acute rheumatic fever and rheumatic heart disease. Conducted in 2006, this study aimed at characterizing clinical manifestations and microbial features of isolates obtained from invasive Streptococcus pyogenes disease. Clinical and demographic data were collected prospectively. Isolates were biotyped, T typed, emm sequenced, and tested for antibiotic susceptibility. Detection of the speA, speB, speC, and ssa genes was also carried out. The estimated annual incidence of invasive S. pyogenes disease in 2006 was high at 38 cases/100,000 inhabitants in New Caledonia. Invasive isolates were obtained from 90 patients with necrotizing fasciitis (41 cases), bacteremia with no identified focus (12 cases), myositis (10 cases), septic arthritis (9 cases), erysipelas (8 cases), postpartum infection (4 cases), myelitis and osteomyelitis (3 cases), severe pneumonia (2 cases), and endocarditis (1 case). The most frequent associated comorbidities were skin lesions (71%) and obesity (29%). Thirty-one different emm types were identified, and the following six accounted for 54% of the isolates: emm15 (15.5%), emm92 (12.2%), emm106 (8.9%), emm74 (6.7%), emm89 (5.6%), and emm109 (5.6%). The speA, speC, and ssa genes were expressed at different frequencies in the various emm types. The first epidemiological study of invasive S. pyogenes disease in New Caledonia highlights that emm type distribution is particular and should be taken into account in the development of an appropriate vaccine. These findings support the prevention of pyoderma and other cutaneous lesions in order to limit the development of both invasive disease and poststreptococcal sequelae in the South Pacific.
Streptococcus pyogenes is a major human pathogen causing both mild infections such as pharyngitis and impetigo and severe infections, including sepsis, necrotizing fasciitis (NF), and lethal streptococcal toxic shock syndrome (STSS). Nonsuppurative sequelae such as acute rheumatic fever (ARF) and rheumatic heart disease (RHD) are associated with high morbidity and mortality rates, especially in developing countries (6, 9, 21). emm typing based on sequence analysis of the variable distal part of the gene that encodes the M protein, a major virulence factor, is the “gold standard” method used to characterize S. pyogenes isolates. Currently, there are more than 170 different group A Streptococcus (GAS) emm types (7, 12). Since the late 1980s, there has been an increase in the number of reports of invasive infections caused by S. pyogenes in Europe and the United States, with a significant preponderance of the emm1 and emm3 types (1, 14, 18, 20). A few studies have recently reported a different GAS type distribution in developing countries and in indigenous populations (3, 17, 22). Knowledge of the emm type distribution in a region may shed more light on the pathogenesis of GAS infections and is crucial for selecting appropriate vaccine candidates which would include multiple M protective epitopes. Currently, a 26-valent M protein-based vaccine in the preclinical testing phase is being developed for populations with a high risk of ARF or RHD (16, 19).
We report the first epidemiological study of invasive S. pyogenes disease in New Caledonia, an archipelago in the South Pacific where rheumatic fever is endemic (www.dass.gouv.nc). Our objectives were to characterize the demographic, clinical, and microbiological features of invasive S. pyogenes disease in 2006 in New Caledonia to determine the emm type distribution of GAS strains in the population.
(This study was presented at the XVII Lancefield International Symposium on Streptococci and Streptococcal Diseases, Porto Heli, Greece, 2008.)
MATERIALS AND METHODS
Bacterial isolates and patients.
All isolates of S. pyogenes obtained as pure cultures from infected body sites of patients at the largest hospital in Nouméa, a 285-bed tertiary-care hospital serving most of the New Caledonian population, were sent to the microbiology laboratory of the Pasteur Institute, New Caledonia, between January and December 2006. Only one isolate per patient was analyzed.
Standard patient demographics data, including age, sex, ethnicity, and date and site of isolate collection were recorded. Medical records were also examined prospectively to assess underlying diseases or conditions that might have predisposed patients to invasive disease: skin trauma, injection drug use, varicella infection, alcohol abuse, use of nonsteroidal antiinflammatory drugs (NSAIDs), diabetes, immunosuppressive therapy, malignancy, and HIV infection. The overall fatality rate was assessed 30 days after the date of specimen sampling.
Case definitions.
Invasive cases were defined as infections associated with the isolation of S. pyogenes from a normally sterile site (blood, cerebrospinal fluid, or other normally sterile fluid/tissue, e.g., peritoneal, pleural, and joint) or as clinical presentation of NF, myositis, or erysipelas associated with monomorphic culture of S. pyogenes from the infected lesion. Cases of STSS were defined according to published criteria (24).
Identification of S. pyogenes.
Identification was based on beta-hemolysis on Columbia sheep blood agar (Oxoid, Dardilly, France), Gram staining, a negative catalase test, a positive pyrrolidonyl arylamidase test (Oxoid), and agglutination with Lancefield group A antiserum (Slidex, Streptokit; bioMérieux, Marcy-l'Etoile, France).
Susceptibility testing.
Antimicrobial susceptibility to penicillin G, amoxicillin, erythromycin, clindamycin, tetracycline, rifampin, streptomycin, kanamycin, gentamicin, and vancomycin was tested by the disk diffusion method on Mueller-Hinton agar with 5% sheep blood, incubated overnight at 37°C in air enriched with 5% CO2 using commercial discs (Bio-Rad) according to the guidelines and interpretation criteria of the antibiogram committee of the French Society for Microbiology (www.sfm.asso.fr).
Typing and detection of toxin or superantigen genes.
Biotypes were obtained on rapid ID 32 Strep strips (bioMérieux) as previously described (5). T serotypes were determined on trypsinated bacteria by slide agglutination with type-specific antisera (Sevapharma, Prague, Czech Republic) (11).
The emm gene was amplified and sequenced as described previously (2). emm type assignments were determined according to the protocol of the Centers for Disease Control and Prevention (Atlanta, GA) available at http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm (7).
A multiplex PCR procedure was performed as previously described to detect the speA, speB, speC, and ssa genes (8, 13).
Data analysis.
Incidence rates by ethnocultural group were calculated by using the 1996 census of the New Caledonian population (http://www.insee.fr). The estimate for the entire population was based on population data for 2004 reevaluated with an increase of 1.9% per annum. With an estimated 239,642 inhabitants in 2006, this Pacific island group is a discrete epidemiological entity with a multicultural population including Melanesians (44% of the total population), Europeans (34%), Polynesians (12%, mainly Wallisian-Futunians and Tahitians), and others (10%, mainly Chinese and Indonesians). Statistical analyses were performed by using the Student t test to compare mean ages and the χ2 test, odds ratios (ORs) with 95% confidence intervals (95% CIs), and the Fisher exact test (for small numbers) to compare the distribution of categorical data (STATA, version 8).
RESULTS
A total of 90 cases of invasive S. pyogenes disease were identified in 2006. Isolates were obtained from skin or soft-tissue samples (n = 64 cases), blood (n = 13), synovial fluid (n = 9), pleural fluid (n = 2), amniotic fluid (n = 1), and spinal fluid (n = 1).
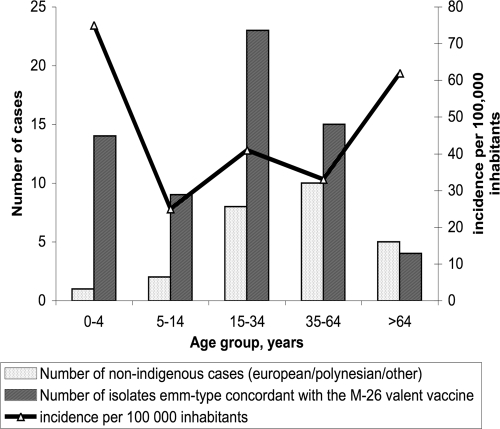
Since all of the patients with severe S. pyogenes infections are usually transferred to the main hospital of the country, we estimated that the annual incidence of invasive S. pyogenes disease in 2006 was 38 cases/100,000 individuals. The incidence by age or by major ethnocultural group showed that three different groups had a higher risk of developing invasive S. pyogenes disease: children under the age of 5 years, adults above the age of 64 years, and Melanesians (Fig. 1 and Table 1). The incidence of S. pyogenes-positive blood culture was estimated to be 10.4 cases/100,000 inhabitants in New Caledonia in 2006. No seasonal or geographical differences in the occurrence of invasive S. pyogenes disease were observed. The median age of patients was 24 years (mean age, 31 ± 24 years; range, birth to 95 years), and the incidence of infection was higher among males (68% of the cases). We observed an overrepresentation of severe S. pyogenes disease in the indigenous Melanesian population (66 cases, 73%) and an underrepresentation among Europeans (13 cases, 14%) compared with the global distribution of ethnic groups in New Caledonia (P ≤ 0.01). The proportions of patients with various underlying diseases, outcomes, and risk factors are shown in Tables 1 and 2. Skin and soft-tissue infections such as NF (41 cases), myositis (10 cases), erysipelas (8 cases), and bacteremia with no identified focus (12 cases) and arthritis (9 cases) were the most frequent clinical manifestations of invasive cases. Other clinical presentations included postpartum infections (four cases, including one case involving a mother and her baby), pleuropneumonia (two cases), osteomyelitis (two cases), myelitis, and endocarditis (one case of each). Puerperal fever cases were related to vaginal delivery in four women 21 to 32 years old. The three STSS cases, associated with one case of bacteremia and two cases of pneumonia, were fatal. Two-thirds of the patients underwent surgery, and 14% required intensive care. Irrespective of skin lesions, at least one comorbid disease was found in 52 (58%) of the 90 patients and was found significantly more frequently in adults up to 30 years old (P ≤ 0.01) and in nonindigenous patients (P ≤ 0.03). The most prevalent underlying conditions included obesity, cardiopulmonary diseases, ethanol abuse, and use of NSAIDs (55 [68%] of 81 studied). There were no cases of HIV infection or intravenous drug use. The presence of skin lesions in 71% of the patients was the predominant local predisposing factor for invasive infection, especially in patients without comorbidities (P ≤ 0.01). They were wounds (40 cases), hematomas (15 cases), furuncles (5 cases), scabies lesions (4 cases), burns (2 cases), stings (2 cases), eczema (2 cases), purpura (2 cases), psoriasis (1 case), and varicella (1 case). NF was more frequent in children and young adults 5 to 29 years old (P ≤ 0.03) and less frequent in Melanesians (P ≤ 0.01). In a univariate analysis, emm15 strains tended to be associated with the indigenous population, but this association was not statistically significant (P ≤ 0.06).
FIG. 1.
Age distribution by ethnocultural group and incidence of invasive S. pyogenes disease in New Caledonia in 2006.
TABLE 1.
Clinical characteristics of 90 cases of invasive S. pyogenes disease in New Caledonia
| Characteristic | No. of cases by major ethnocultural group |
P valueb | OR | 95% CI | No. of cases by age group |
P valueb | OR | 95% CI | No. of cases by sex |
P valueb | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indigenous Melanesian (n = 66) | Nonindigenous European/ Polynesian/ other (n = 25) | ≤30 yr (n = 54) | >30 yr (n = 37) | Male (n = 61) | Female (n = 30) | ||||||||||
| Predominant clinical manifestations | |||||||||||||||
| NF | 24 | 17 | ≤0.01 | 0.3 | 0.1-0.9 | 29 | 12 | ≤0.05 | NS | NS | 31 | 10 | NS | ||
| Bacteremia | 9 | 3 | NS | 5 | 7 | NS | 6 | 6 | NS | ||||||
| Myositis | 9 | 1 | NS | 6 | 4 | NS | 7 | 3 | NS | ||||||
| Arthritis | 6 | 3 | NS | 4 | 5 | NS | 8 | 1 | NS | ||||||
| Erysipelas | 6 | 2 | NS | 1 | 7 | ≤0.01 | 0.1 | 0.1-0.7 | 4 | 4 | NS | ||||
| Pneumonia | 2 | 0 | NS | 0 | 2 | NS | 2 | 0 | NS | ||||||
| At least one comorbidity, except skin lesiona | 33 | 19 | ≤0.03 | 0.3 | 0.1-0.9 | 21 | 31 | ≤0.01 | 0.1 | 0.1-0.4 | 32 | 20 | NS | ||
| Skin lesion | 46 | 18 | NS | 37 | 27 | NS | 48 | 16 | ≤0.01 | 3.2 | 1.1-9.2 | ||||
| Molecular marker emm15 | 13 | 1 | ≤0.06 | NS | NS | 9 | 5 | NS | 7 | 7 | NS | ||||
Comorbidities studied: obesity, ethanol abuse, recent delivery, immunosuppression, dialysis, use of NSAIDs, cardiopulmonary disease, diabetes mellitus, recent (<7 days before) surgery, intravenous drug use, corticotherapy for >6 months, and malignancy treatment.
NS, not significant; significant, P > 0.05. The chi-square or Student t test was used, depending on the number of samples.
TABLE 2.
Clinical characteristics of invasive S. pyogenes disease in New Caledonia, 2006
| Characteristic | No. (%) of isolatesa |
|---|---|
| Comorbidities | |
| Skin lesion | 64 (71) |
| Obesity | 26 (29) |
| Cardiopulmonary disease | 12 (13) |
| Ethanol abuse | 10 (11) |
| Use of NSAIDs | 7 (8) |
| Diabetes mellitus | 6 (7) |
| Recent delivery | 6 (7) |
| Dialysis | 3 (3) |
| Malignancy | 3 (3) |
| Corticotherapy for >6 month | 2 (2) |
| Surgery <7 days before | 2 (2) |
| Otherb | 4 (4) |
| Outcome | |
| Death | 3 (3) |
| Surgical site drainage | 57 (63) |
| Assisted ventilation | 9 (10) |
| ICU admission | 13 (14) |
Total n = 90.
Other comorbidities studied: no intravenous drug use, one varicella infection, one case of immunosuppressive therapy, two malignancies, and no HIV infection.
Thirty-one different emm types were identified among the 90 isolates distributed as follows: emm15 (14 cases), emm92 (11 cases), emm106 (8 cases), emm74 (6 cases), emm89 and emm109 (5 cases each), emm46 (4 cases), emm49, emm65, emm75, and emm102 (3 cases each), emm71 and emm103 (2 cases each), emm41, emm53, emm54, emm56, emm58, emm77, emm81, emm86, emm87, emm93, emm100, emm101, emm104, emm105, and emm123 (1 case each). One strain belonged to st6735; it was responsible for a postpartum infection and was isolated from the amniotic fluid of the mother, the throat of the baby, and the blood of both. An original strain belonged to a new sequence type, st367 (available at http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm), another belonged to stG1750, a sequence type previously identified from a group G streptococcal isolate, and emm gene amplification was unsuccessful for another strain. The proportions of diseases caused by the major emm types are shown in Table 3. Among the 62 typeable strains (68%), 13 different T types were observed and T3/13/B3264 was predominant (19 strains, 31%). The biotype distribution of strains was dominated by biotype 3 (52 [58%] of 90 cases), followed by biotypes 1 (18 cases), 29 (8 cases), 6 (5 cases), 5 (4 cases), 7 (2 cases), 8, and 13 (1 case each). There was a restricted association between certain emm types, T serotypes, biotypes, and resistance to tetracycline. The chromosomal speB gene was found in all isolates. The speA, speC, and ssa genes, which are associated with prophage elements, were present at different frequencies in various emm types, but the highest frequencies were found in the most prevalent types: emm15 (speA, 93%; speC, 100%), emm92 (speC, 91%), and emm106 (speC, 100%). The analysis revealed that isolates of the same emm type shared a common or predominant toxin gene profile distribution. All of the strains were susceptible to all of the antibiotics tested but tetracycline. Tetracycline resistance, which was detected in nine strains (10%), was distributed among six different emm types, including emm109 (three of the five isolates of this emm type were resistant), emm65 (two cases), emm53 (one case), emm58 (one case), emm104 (one case), and stG1750 (one case).
TABLE 3.
Correlation between predominant clinical manifestations and emm types of invasive S. pyogenes strains
| Clinical manifestation(s) or parameter | Total no. of cases | No. with following emm type: |
||||||
|---|---|---|---|---|---|---|---|---|
| emm15 | emm92 | emm106 | emm74 | emm89 | emm109 | Other | ||
| NF | 41 | 8 | 5 | 4 | 3 | 1 | 2 | 18 |
| Bacteremia | ||||||||
| No identified focus | 12 | 2 | 2 | 1 | 2 | 0 | 0 | 5 |
| STSSa | 1 | 1 | ||||||
| Myositis | 10 | 1 | 1 | 1 | 0 | 2 | 1 | 4 |
| Septic arthritis | 9 | 0 | 1 | 0 | 0 | 2 | 1 | 5 |
| Erysipelas | 8 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| Postpartum infectionb | 4 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| Osteomyelitis | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pneumonia + STSSa | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Endocarditis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Myelitis | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (% of overall total) | 90 | 14 (15.5) | 11 (12.2) | 8 (8.9) | 6 (6.7) | 5 (5.6) | 5 (5.6) | 41 (45.5) |
Fatal.
Four cases, including one mother-and-baby case due to an st6735 strain.
DISCUSSION
This study constitutes a first approach regarding the burden caused by S. pyogenes disease in New Caledonia, which is a French overseas archipelago located in the South Pacific, about 2,000 km northeast of Sydney, Australia. The Department and Agency of Health and Social Affairs in New Caledonia (http://www.dass.gouv.nc/) annually updates the number of cases of ARF. In 2002, the incidence of ARF, which was estimated at 9.8 cases/100,000 inhabitants among the whole population of New Caledonia, reached 85.6 cases/100,000 children 5 to 19 years old, about 200 cases/100,000 Melanesians. This information is consistent with published data from neighboring countries where active surveillance is established; the median estimated incidence of ARF cases in children 5 to 14 years old has been estimated to reach 374 cases/100,000 inhabitants in the Pacific islands and indigenous populations of Australia and New Zealand versus 0.5 case/100,000 people in developed countries (6). In temperate regions, rheumatic fever is usually associated with throat infection but data suggest that S. pyogenes skin infections might play a greater role in the tropics (4, 15). Interestingly, in the present study conducted in New Caledonia, two cases of ARF were detected among the 90 patients with invasive GAS disease. Indeed, the original S. pyogenes emm type distribution observed among invasive S. pyogenes diseases might be related to the local environmental and host risk factors for infection, but it seems to contribute weakly to the high prevalence of ARF in New Caledonia, as previously described in indigenous children in New Zealand (6).
Our study confirmed that invasive S. pyogenes disease occurs at greater rates in New Caledonia than in Western countries and that these rates are similar to those reported for other countries in the Pacific, such as Fiji, and for the indigenous populations of Australia and New Zealand (6, 15, 17, 22, 23). Thus, the incidence of S. pyogenes-positive blood culture was five times higher in New Caledonia than in metropolitan France in 2006 (report of the French National Institute of Health Surveillance) but was similar to that in neighboring countries such as Fiji (22). Invasive GAS disease predominantly affected the elderly, over 64 years old, with coexisting medical conditions, as in most industrialized and developing countries (6). The high incidence among children in New Caledonia, at 75 cases/100,000 young children less than 5 years old, was similar to those reported in other developing countries and even twice as high as that in Fiji (22).
NF, other skin or soft-tissue infections, and bacteremias of cutaneous origin were more frequently reported in patients less than 30 years old. Interestingly, indigenous Melanesian children (under 15 years old) are overrepresented (23 out of 26 cases, P = 0.03). The high proportion of young male Melanesians hospitalized for invasive S. pyogenes disease associated with skin lesions is in accordance with a previous study on the strong role of traditional behavior and environmental conditions in the development of impetigo in the tropics (4). In contrast, comorbidities, bacteremia, intensive care unit (ICU) admission, and being nonindigenous accounted for an increasing proportion of severe S. pyogenes cases as age increased. The low case fatality rate in our study (3.3%), compared to the rate of 32% reported in Fiji, remains unexplained (22).
The high genetic diversity of our S. pyogenes collection (31 different emm types were identified among 90 strains) confirms previous findings that S. pyogenes isolates from the tropics show considerable emm type variability. Furthermore, emm1, emm3, and emm4, the most prevalent types reported in the western world, were absent from our findings (14, 19). Moreover, the emm type distribution identified in this study was original and characterized by six predominant emm types (emm15, emm92, emm106, emm74, emm89, and emm109), accounting for 54% of the 90 strains. This distribution differed significantly from that in neighboring areas such as Hawaii, Fiji, and North Queensland, Australia (10, 22, 17). In contrast to these previous studies, in which no previous dominant emm type or clone has been demonstrated, we described the first predominance of the emm15 type among indigenous Melanesians. These emm type findings could be used to estimate the potential benefits of the proposed 26-valent M protein-based vaccine (which has successfully completed a phase 2 trial) for age groups at the highest risk of developing invasive S. pyogenes disease (children <5 years old and adults ≥65 years old) by checking whether vaccine types M1, -1.2, -2, -3, -5, -6, -11, -12, -14, -18, -19, -22, -24, -28, -29, -33, -43, -59, -75, -76, -77, -89, -92, -94, -101, and -114 would be effective against the isolates reported in this study (16, 19). The number of children <5 years old diagnosed with invasive GAS disease due to the types represented by the 26-valent vaccine was 5 (33%) of 15 cases, and the number of adults ≥65 years old diagnosed was 2 (22%) out of 9. Despite the absence of pharyngitis S. pyogenes isolates in our study, the absence of known causative rheumatogenic strains in our S. pyogenes collection (M1, -3, -5, -6, -14, -18, -19, and -24) and the unusual distribution of emm types highlight the difficulties in building a universal M valence vaccine that is effective within our borders.
This is the first epidemiological study of invasive S. pyogenes disease in New Caledonia, a region with a high incidence of ARF. This study highlights an emm type distribution that is particular to New Caledonia and should be taken into account in the development of an appropriate vaccine. The reported incidence is far greater in indigenous communities than in other communities. Soft tissues were the most common sites of infection. These findings call for better prevention of pyoderma and other cutaneous lesions to limit the development of severe invasive disease. Further studies are ongoing to specify the role of the predominant emm types in ARF or other poststreptococcal sequelae in the South Pacific.
Acknowledgments
We thank Gislène Collobert and Françoise Charavay for excellent technical assistance. We gratefully acknowledge Paul Martin for lending support to the development of molecular epidemiology in New Caledonia. We also thank all of the medical department of the Nouméa Hospital for participating in this project.
We have no conflict of interest in relation to this work.
Footnotes
Published ahead of print on 2 December 2009.
REFERENCES
- 1.Aziz, R. K., and M. Kotb. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkley, J. A., B. S. Lowe, I. Mwangi, T. Williams, E. Bauni, S. Mwarumba, C. Ngetsa, M. P. Slack, S. Njenga, C. A. Hart, K. Maitland, M. English, K. Marsh, and J. A. Scott. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39-47. [DOI] [PubMed] [Google Scholar]
- 4.Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 182:1109-1116. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet, A., P. Geslin, P. Kriz-Kuzemenska, V. Blanc, C. Devine, and F. Grimont. 1994. Restricted association between biotypes and serotypes within group A streptococci. J. Clin. Microbiol. 32:1312-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685-694. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2006. Streptococcus pyogenes emm sequence database. http://www.cdc.gov/ncidod/biotech/strep/emmtypes.htm.
- 8.Chatellier, S., N. Ihendyane, R. G. Kansal, F. Khambaty, H. Basma, A. Norrby-Teglund, D. E. Low, A. McGeer, and M. Kotb. 2000. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68:3523-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdem, G., C. Mizumoto, D. Esaki, L. Abe, V. Reddy, and P. V. Effler. 2009. Streptococcal emm types in Hawaii: a region with high incidence of acute rheumatic fever. Pediatr. Infect. Dis. J. 28:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, D. R., E. L. Kaplan, J. Sramek, R. Bicova, J. Havlicek, H. Havlickova, J. Motlova, and P. Kriz. 1996. Determination of T-protein agglutination patterns, p. 37-41. In D. R. Johnson, E. L. Kaplan, J. Sramek, R. Bicova, J. Havlicek, H. Havlickova, J. Motlova, and P. Kriz (ed.), Laboratory diagnosis of group A streptococcal infections. World Health Organization, Geneva, Switzerland.
- 12.Johnson, D. R., D. L. Stevens, and E. L. Kaplan. 1992. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 166:374-382. [DOI] [PubMed] [Google Scholar]
- 13.Kotb, M. 1995. Bacterial pyrogenic exotoxins as superantigens. Clin. Microbiol. Rev. 8:411-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luca-Harari, B., J. Darenberg, S. Neal, T. Siljander, L. Strakova, A. Tanna, R. Creti, K. Ekelund, M. Koliou, P. T. Tassios, M. van der Linden, M. Straut, J. Vuopio-Varkila, A. Bouvet, A. Efstratiou, C. Schalén, B. Henriques-Normark, the Strep-EURO Study Group, and A. Jasir. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, D. R., L. M. Voss, S. J. Walker, and D. Lennon. 1994. Acute rheumatic fever in Auckland, New Zealand: spectrum of associated group A streptococci different from expected. Pediatr. Infect. Dis. J. 13:264-269. [DOI] [PubMed] [Google Scholar]
- 16.McNeil, S. A., S. A. Halperin, J. M. Langley, B. Smith, A. Warren, G. P. Sharratt, D. M. Baxendale, M. A. Reddish, M. C. Hu, S. D. Stroop, J. Linden, L. F. Fries, P. E. Vink, and J. B. Dale. 2005. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114-1122. [DOI] [PubMed] [Google Scholar]
- 17.Norton, R., H. V. Smith, N. Wood, E. Siegbrecht, A. Ross, and N. Ketheesan. 2004. Invasive group A streptococcal disease in North Queensland (1996-2001). Indian J. Med. Res. 119(Suppl.):148-151. [PubMed] [Google Scholar]
- 18.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35:268-276. [DOI] [PubMed] [Google Scholar]
- 19.O'Loughlin, R. E., A. Roberson, P. R. Cieslak, R. Lynfield, K. Gershman, A. Craig, B. A. Albanese, M. M. Farley, N. L. Barrett, N. L. Spina, B. Beall, L. H. Harrison, A. Reingold, and C. Van Beneden for the Active Bacterial Core Surveillance Team. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853-862. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz, B., R. R. Facklam, and R. F. Breiman. 1990. Changing epidemiology of group A streptococcal infection in the USA. Lancet 336:1167-1171. [DOI] [PubMed] [Google Scholar]
- 21.Steer, A. C., N. Curtis, and J. R. Carapetis. 2007. Diagnosis and treatment of invasive group A streptococcal infections. Expert Opin. Med. Diagnostics 2:289-302. [DOI] [PubMed] [Google Scholar]
- 22.Steer, A. C., A. Jenney, J. Kado, M. F. Good, M. Batzloff, L. Waqatakirewa, E. K. Mullholland, and J. R. Carapetis. 2009. Prospective surveillance of invasive group A streptococcal disease, Fiji, 2005-2007. Emerg. Infect. Dis. 15:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steer, A. C., A. J. Jenney, F. Oppedisano, M. R. Batzloff, J. Hartas, J. Passmore, F. M. Russell, J. H. Kado, and J. R. Carapetis. 2008. High burden of invasive β-haemolytic streptococcal infections in Fiji. Epidemiol. Infect. 136:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA 269:390-391. [PubMed] [Google Scholar]