Abstract
Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei is a severe infection in the middle east, resulting in nearly 100% mortality despite the application of combined surgical and antifungal therapy and occurring occasionally in otherwise healthy patients. We report the first case of brain infection in a middle-aged male in India, where R. mackenziei is not endemic.
CASE REPORT
A 50-year-old Indian male who had had type II diabetes mellitus for the last 4 years and suffered from psychiatric illness was admitted to the Department of Neurology, People's College of Medical Sciences, Bhopal, India, and presented with a 1-day history of frontal headache, dizziness, slurred speech, and weakness over the left half of his body. Ten days previously, he had become inattentive due to his uncontrolled diet and irregular antidiabetic treatment. The first computed tomography (CT) scan of the brain demonstrated a mass lesion, and the patient underwent indigenous (Ayurvedic) treatment for 5 to 6 days. The chest X ray was normal. Full laboratory blood analysis revealed a hemoglobin level of 16.8 g/dl; white blood cells, serum electrolytes, and liver function test results were within normal ranges. Blood cultures were sterile, and urine cultures became positive with Escherichia coli. Later, a second CT scan revealed a large (5- to 6-cm-diameter), discrete, irregular, peripheral ring-enhancing necrotic lesion in the right frontoparietal region causing significant mass effects and midline shift with perifocal edema (Fig. 1A and B). Neurosurgical intervention consisted of a right frontoparietal craniotomy for decompression of the space-occupying lesion. The necrotic material was encapsulated, and approximately 8 ml of thick viscous black fluid, which was predominately caseous, was aspirated. The material was analyzed by two mycological and histopathological laboratories. Multiple lesions were ring enhancing, greyish white, and soft, measuring 1.5 by 0.4 cm.
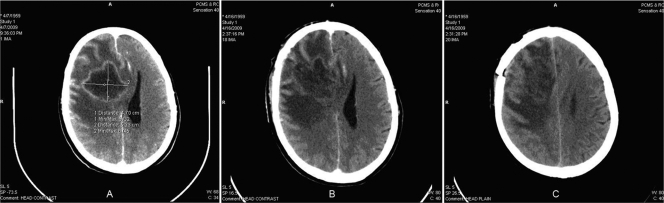
FIG. 1.
(A and B) Initial CT scans showing a discrete, large (approximately 5- to 6-cm-diameter), irregular, peripheral ring-enhancing necrotic mass lesion in the right frontoparietal region with mass effects and midline shift with perifocal edema. (C) Third CT scan showing a decrease in lesion size (to around 4 cm) but increasing parietal edema, enlargement of mass effects, and multiple, coalescing, ring-enhancing cerebral lesions.
Microscopic examination of biopsy sections (pus or necrotic tissue) stained with hematoxylin and eosin revealed necrosis with dense and diffuse mixed inflammatory infiltrates. There were several foreign bodies and Langhans giant cells, with granuloma formation and the presence of numerous septate hyphae surrounded by a dense inflammatory response (Fig. 2A and B). Following this examination, the biopsy specimens were also stained with Ziehl-Neelsen stain, which revealed many moniliform septated hyphal elements (Fig. 2C). The tentative diagnosis of chronic granulomatous inflammation with fungal infection was made. The clinical specimens were cultured for up to 10 days on Sabouraud dextrose agar (SDA; Difco) and SDA supplemented with chloramphenicol (0.5 mg/ml) at 30 to 35°C, as well as on brain heart infusion agar with 5% sheep blood (Oxoid Ltd., Basingstoke, Hampshire, England) at 37°C. Growth of melanized fungi after 1 week was observed, and these fungi were morphologically classified as Rhinocladiella mackenziei (formerly Ramichloridium mackenziei). Stock cultures were maintained on slants of 2% malt extract agar (MEA; Difco) and oatmeal agar (Difco) at 30°C, and a voucher strain was deposited into the CBS-KNAW culture collection and preserved as CBS 125089. Microscopic studies using slide culture techniques with potato dextrose agar were conducted. This medium was selected because it readily induces sporulation and suppresses growth of aerial hyphae (10). After 2 weeks, slides were prepared from these cultures in lactic acid or lactophenol cotton blue under biosafety level 3 regulations, and light micrographs were taken using a Nikon Eclipse 80i microscope with a Nikon digital sight DS-Fi1 camera. After 2 weeks of growth at 27°C on MEA in darkness, the moderately rapidly growing colonies were velvety and olivaceous brown; the reverse side was olivaceous black (Fig. 3A). Conidiophores arose at right angles from creeping hyphae and were stout, thick walled, brown, and 3.0 to 4.5 μm wide, with apical cells 10 to 25 μm long, short cylindrical denticles, and brown conidia which were ellipsoidal, measuring 8.5 to 12.0 by 4 to 5 μm, with prominent, 1-μm-wide basal scars (Fig. 3B and C). Cardinal growth temperatures for strain CBS 125089 were between 9 and >40°C, with an optimum at 30°C and some growth still occurring at 40°C. Morphologically, R. mackenziei resembles Pleurothecium obovoideum (Mats.) Arzanlou and Crous from dead wood, but P. obovoideum has distinct conidiophores and the ascending hyphae are thick walled, with cylindrical denticles up to 1.5 μm long. In contrast, R. mackenziei has only slightly prominent denticles. P. obovoideum clusters in the order Chaetosphaeriales (3).
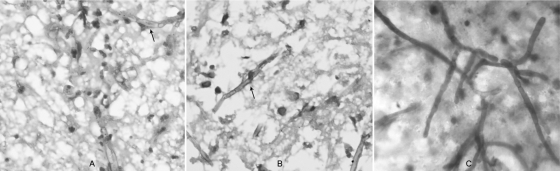
FIG. 2.
(A and B) Hematoxylin and eosin staining revealed necrosis with dense and diffuse mixed inflammatory infiltrates, granuloma formation, and the presence of numerous septate hyphae (arrows) surrounded by a dense inflammatory response. (C) Biopsy specimens were also stained with Ziehl-Neelsen stain, which revealed many moniliform, branched, septated hyphal elements.
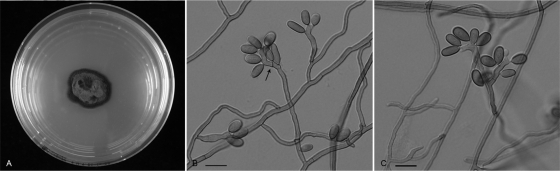
FIG. 3.
R. mackenziei (CBS 125089). (A) Colony on MEA (Difco) at 30°C after 2 weeks in darkness. (B and C) Semimicronematous conidiophores and sympodially proliferating conidiogenous cells (arrow). Scale bars, 10 μm.
DNA was extracted using an ultraclean microbial DNA isolation kit according to the instructions of the manufacturer (Mo Bio, Carlsbad, CA). Internal transcribed spacer (ITS) ribosomal DNA was amplified using primers V9G (5′-TTA CGT CCC TGC CCT TTG TA-3′) and LS266 (5′-GCAT TCC CAAACA ACT CGA CTC-3′) and sequenced with the internal primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′). PCR amplification and sequencing were performed according to the methods of Badali et al. (4). Sequences were compared with entries in GenBank and, by using local blast searching, with entries in a molecular database maintained for research purposes at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. The isolate (CBS 125089) was identified as R. mackenziei, having 99.5% sequence identity to the ex-type isolate of that species (CBS 650.93; accession no. AY857540), which had originally been isolated from a patient in Saudi Arabia with cerebral phaeohyphomycosis. The molecular results confirmed the mycological diagnosis, and histopathological observation led to the diagnosis of cerebral phaeohyphomycosis due to R. mackenziei.
For the management of the case, insulin infusion was given along with intravenous amphotericin B deoxycholate (0.6 mg/kg of body weight/day). Simultaneously, empirical antituberculous and antibacterial therapy, consisting of sequential beta-lactam antibiotics (ceftriaxone, piperacillin, and cefoperazone) combined with metronidazole and amikacin, was started. During follow-up, there was no clinical improvement. Therefore, successive CT scans were obtained after 8 days, showing a decrease in the lesion size (to around 4 cm); however, an increase in parietal edema, enlargement of mass effects, and multiple, coalescing, ring-enhancing cerebral lesions were seen (Fig. 1C). Despite amphotericin B therapy, the patient's condition continued to deteriorate, and he expired 2 weeks after diagnosis of the disease. The in vitro antifungal susceptibilities of this strain (CBS 125089) were determined by broth microdilution according to the guidelines in Clinical and Laboratory Standards Institute document M38-A2 (9). Methods for sporulation and preparation of suspensions were according to Badali et al. (5). Paecilomyces variotii (ATCC 22319), Candida parapsilosis (ATCC 22019), and Candida krusei (ATCC 6258) were used as quality control organisms (9). The MICs of antifungal drugs were as follows: amphotericin B, 16 μg/ml; fluconazole, 32 μg/ml; itraconazole, 0.063 μg/ml; voriconazole, 0.5 μg/ml; posaconazole, 0.031 μg/ml; and isavuconazole, 0.5 μg/ml. The minimum effective concentrations of the two echinocandin agents caspofungin and anidulafungin were 8 and 4 μg/ml, respectively.
Discussion.
Cerebral phaeohyphomycosis caused by melanized fungi is a rare but highly significant disease due to the regional prevalence and high mortality of up to 70% despite combined surgical and antifungal therapy (11). Previously, the majority of reported central nervous system (CNS) infections caused by dematiaceous fungi were found to be brain abscesses in patients with no predisposing factors or immunodeficiency; symptoms included headache, seizures, cerebral irritation, fever, and neurological deficits (13). Binford et al. (6) described one of the first cases of brain abscess due to Cladosporium trichoides (Cladophialophora bantiana), and Campbell and Al-Hedaithy (7) reported similar cases caused by R. mackenziei.
The latter review listed eight cases of this type of infection, all occurring in countries of the middle east. Exophiala dermatitidis (8), Cladophialophora bantiana (12), and R. mackenziei (7, 16), all members of the order Chaetothyriales, are notorious agents of cerebral infections. One of the most striking features of these organisms is their neurotropism in humans.
Arzanlou et al. studied the phylogenetic and morphotaxonomic revision of the genus Ramichloridium and allied genera, because Rhinocladiella was in the past frequently confused with the genus Ramichloridium. Rhinocladiella species were shown to cluster in the Chaetothyriales, while Ramichloridium species cluster in the order Capnodiales (3).
R. mackenziei affects only the CNS and the integuments, with nearly 100% mortality in both immunocompetent and immunocompromised individuals.
This species is the most common agent of brain infection and was thought to be restricted to the middle east and the Persian Gulf region (7). Cases identified in the United Kingdom or the United States concerned immigrants from Saudi Arabia and Kuwait (16). Until now, R. mackenziei has never been isolated from the environment. The natural niche of this organism thus remains unknown. To the best of our knowledge, this patient represents the first case of R. mackenziei infection outside the arid climate zone of the middle east. In contrast to the strains involved in all cases reported thus far, this strain (CBS 125089) originated from a humid subtropical climate, infecting a patient who claimed he had never traveled outside India.
Cerebral infections due to R. mackenziei have thus far been diagnosed after CT-guided needle aspiration and were proven by positive histopathology and culture results. The mortality is almost 100% for all reported cases of infection, despite surgical resection and antifungal therapy. There is no standard therapy for this disease. Treatment as presented in the literature has involved mostly (high-dose lipid) amphotericin B, itraconazole, and flucytosine or a combination of these drugs (14). However, strains of R. mackenziei isolated from infected patients are in vitro resistant to amphotericin B, which is often used as the gold standard of treatment. Only a single patient is reported to have survived an R. mackenziei cerebral infection, with pronounced radiological and clinical improvement after switching therapy from a combination of liposomal amphotericin B, flucytosine, and itraconazole to posaconazole (2). This account is supported by in vitro results and data from a murine infection model (1), in which posaconazole prolonged the survival of mice and reduced the brain fungal burdens compared to the survival of and burdens in mice treated with itraconazole and amphotericin B.
Previous in vitro antifungal susceptibility testing of 10 strains of R. mackenziei (5) has shown that the widest range of MICs (2 to 16 μg/ml) and the highest MICs (MIC50, 8 μg/ml, and MIC90, 16 μg/ml) were those of amphotericin B. In contrast, quite uniform patterns of susceptibility to itraconazole, posaconazole, and isavuconazole were obtained. Our results were in line with animal test data, but clinical experience and animal experiments with the newer antifungal drugs have not been reported. Animal studies have suggested that no benefit from amphotericin B is achieved in experimental infection due to poor penetration into the CNS (1). Although liposomal amphotericin B likely has better CNS penetration, liposomal amphotericin therapy failed for the only patient reported to have survived an R. mackenziei cerebral infection (2), who improved only on posaconazole, an agent with good CNS penetration (15) and a low MIC for the pathogen (2) Delay in diagnosis, misidentification, poor standard therapy, and limited data on effective alternative drugs are the main factors promoting the development of cerebral phaeohyphomycosis. With early diagnosis, when the lesion is singular, and with effective therapy including complete surgical excision of brain abscesses, the patient's outcome may be improved.
Brain abscesses incited by R. mackenziei in patients from areas where the fungus is not endemic have not been reported previously. Now that the fungus has been observed outside its area of endemicity, R. mackenziei, along with other neurotropic agents, should always be considered a potential etiologic agent of cerebral phaeohyphomycosis in all patients, regardless of their area of residence.
Nucleotide sequence accession number.
The sequence from isolate CBS 125089 determined in this study has been deposited in GenBank under accession number GQ863214.
Acknowledgments
This work was supported by a grant (no. 13081) to H. Badali from the Ministry of Health and Medical Education of the Islamic Republic of Iran and the School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. We report no conflicts of interest.
We thank Vichal Rastogi and V. K. Ramnani, Department of Microbiology; Sushil Jindal, Department of Medicine; and Mridul Shai, Department of Surgery, People's College of Medical Sciences & Research Centre, Bhopal, Madhya Pradesh, India, for their contributions. Hena Rani, Department of Microbiology, Government Medical College Hospital, Chandigarh, India, is acknowledged for help in building up part of the microbiological work.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Al-Abdely, H. M., L. Najvar, R. Bocanegra, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and J. R. Graybill. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Abdely, H. M., A. M. Alkhunaizi, J. A. Al-Tawfiq, M. Hassounah, M. G. Rinaldi, and D. A. Sutton. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91-95. [DOI] [PubMed] [Google Scholar]
- 3.Arzanlou, M., J. Z. Groenewald, W. Gams, U. Braun, H. D. Shin, and P. W. Crous. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud. Mycol. 58:57-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badali, H., V. O. Carvalho, V. Vicente, D. Attili-Angelis, I. B. Kwiatkowski, A. H. Gerrits Van Den Ende, and G. S. De Hoog. 2009. Cladophialophora saturnica sp. nov., a new opportunistic species of Chaetothyriales revealed using molecular data. Med. Mycol. 47:51-62. [DOI] [PubMed] [Google Scholar]
- 5.Badali, H., G. S. De Hoog, I. Curfs-Breuker, and J. F. Meis. 2010. In vitro activities of antifungal drugs against Rhinocladiella mackenziei, an agent of fatal brain infection. J. Antimicrob. Chemother. 65:175-177. [DOI] [PubMed] [Google Scholar]
- 6.Binford, C. H., R. K. Thompson, and M. E. Gorhan. 1952. Mycotic brain abscess due to Cladosporium trichoides, a new species; report of a case. Am. J. Clin. Pathol. 22:535-542. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, C. L., and S. S. Al-Hedaithy. 1993. Phaeohyphomycosis of the brain caused by Ramichloridium mackenziei sp. nov. in Middle Eastern countries. J. Med. Vet. Mycol. 31:325-332. [Google Scholar]
- 8.Chang, C. L., D. S. Kim, D. J. Park, H. J. Kim, C. H. Lee, and J. H. Shin. 2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. J. Clin. Microbiol. 38:1965-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 11.Horré, R., and G. S. De Hoog. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176-193. [Google Scholar]
- 12.Levin, T. P., D. E. Baty, T. Fekete, A. L. Truant, and B. Suh. 2004. Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: case report and review of the literature. J. Clin. Microbiol. 42:4374-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, D. M., and G. S. De Hoog. 2009. Cerebral phaeohyphomycosis—a cure at what lengths? Lancet Infect. Dis. 9:376-383. [DOI] [PubMed] [Google Scholar]
- 14.Revankar, S. G., D. A. Sutton, and M. G. Rinaldi. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206-216. [DOI] [PubMed] [Google Scholar]
- 15.Ruping, M. J. G. T., N. Albermann, F. Ebinger, I. Burckhardt, C. Beisel, C. Müller, J. J. Vehreschild, M. Kochanek, G. Fätkenheuer, C. Bangard, A. J. Ullmann, W. Herr, K. Kolbe, M. Hallek, and O. A. Cornely. 2008. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 62:1468-1470. [DOI] [PubMed] [Google Scholar]
- 16.Sutton, D. A., M. Slifkin, R. Yakulis, and M. G. Rinaldi. 1998. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J. Clin. Microbiol. 36:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]