Abstract
Utah had a high rate of pediatric pneumococcal empyema (PPE) prior to licensure of the pneumococcal conjugate vaccine (PCV-7) in 2000. The majority (62%) of PPE cases was due to nonvaccine serotypes, primarily Streptococcus pneumoniae serotype 1, multilocus sequence type (MLST) 227. PPE in Utah children has increased over the last decade. It is unclear whether the increase was due to serotype replacement or switch. In this study, we describe the incidence and molecular epidemiology of PPE by MLST in Utah children after the licensure of PCV-7. Empyema rates increased from 8.5/100,000 children in the state of Utah in 2001 to 12.5/100,000 children in 2007 (P = 0.006). Ninety-eight percent was due to nonvaccine serotypes (P < 0.001 when compared to the pre-PCV-7 period). PPE was primarily due to serotypes 1, 3, 19A, and 7F, with MLST demonstrating sequence types (ST) that were commonly present in the United States prior to licensure of PCV-7. Serotype switch was not documented. Replacement disease with common ST of serotypes 1,3, 7F, and 19A rather than serotype switch was responsible for the increase in PPE in Utah children.
The prevalence of parapneumonic empyema (PPE), once a rare complication of pediatric pneumonia (17), has been increasing worldwide over the last decade (10, 11, 13, 14, 19, 23, 26, 28, 30, 34, 37, 46). In Utah, rates of pediatric empyema were increasing prior to the introduction of the seven-valent pneumococcal conjugate vaccine (PCV-7) in 2000 (2, 3). In 1993, we documented empyema in 1/100,000 Utah children, and this rate increased 5-fold to 5/100,000 by 1999 (13). Children with empyema and a positive bacterial culture from blood or pleural fluid were most likely to have Streptococcus pneumoniae serotype 1 infection (13). Furthermore, we documented that all serotype 1 strains isolated in our population between 1996 and 2002 were multilocus sequence type (MLST) 227 (12).
Following the licensure of PCV-7, the Utah pediatric population demonstrated immunization rates similar to those demonstrated by other regions of the United States but lower than those in the Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance (ABCs) sites (http://www.cdc.gov/abcs/index.htm). By 2003, 55% of Utah children had received three doses of PCV-7 by 35 months, compared to 59% nationally and 62% in states with ABCs sites (16). In 2007, 88% of Utah children had received three doses of PCV-7 by 35 months, compared to 90% nationally and 92% in states with ABCs sites (15).
In spite of vaccine uptake that was similar to the rest of the United States, children in Utah did not realize the same degree of decrease in invasive pneumococcal disease (IPD) reported in other parts of the United States (27, 47). The limited effectiveness of the PCV-7 in Utah was in part a result of the rapid emergence of IPD, particularly empyema, due to nonvaccine serotypes (11, 12).
Other investigators have identified nonvaccine serotypes, particularly 19A, as important causes of IPD following the licensure of PCV-7 (33, 39-41, 45). Some isolates of 19A from children younger than 5 years old from the ABCs sites demonstrated serotype switching within certain vaccine serotypes (7, 40). Capsular switching or transformation in pneumococci is a well-documented mechanism for the evasion of the host immune response (18). Strain replacement (in part facilitated by capsular switching) in response to the selective pressure exerted by a successful pneumococcal vaccine program that targets a limited number of serotypes could limit the utility of PCV-7.
MLST is a nucleotide sequence-based typing system for bacteria and other organisms (32). MLST is based on sequence variation within seven conserved “housekeeping genes” with essential functions. Changes in these gene sequences occur slowly, reflect evolutionary phylogeny, and are not affected by selection pressure. Different sequences at each locus are assigned distinct allele numbers, and “sequence types” (ST) are defined by the combination of alleles at the seven loci interrogated. In the case of S. pneumoniae, genes involved in capsular biosynthesis are not closely linked to MLST targets on the pneumococcal chromosome, so ST are generally unaffected by recombinational replacements that affect serotype switch events. Therefore, screening for new capsular associations found within established ST is an effective method to detect serotype switch events (22).
The objectives of this study were to (i) describe the incidence of empyema in Utah children after the licensure of PCV-7, (ii) describe the molecular epidemiology of PPE in Utah in the years following licensure of the PCV-7 vaccine, and (iii) determine, by examining the MLST of PPE isolates, whether serotype replacement or serotype switch was occurring.
MATERIALS AND METHODS
Human subject protection.
The Institutional Review Boards of the University of Utah and Intermountain Healthcare approved this study. A waiver of consent was granted.
Setting and identification of children with clinical empyema.
We determined the incidence of pediatric empyema for the state of Utah by using clinical data obtained from Intermountain Healthcare (Intermountain) and hospital discharge data obtained from Utah's Indicator-Based Information System (IBIS) for Public Health (http://ibis.health.utah.gov/home). Intermountain owns and operates Primary Children's Medical Center (PCMC) in Salt Lake City, UT. PCMC is the only tertiary care children's hospital in Utah and provides care for over 80% of hospitalized children in the state. All Intermountain facilities share a computerized record system, and data are stored in the enterprise data warehouse (EDW). The EDW was queried for cases of empyema or pneumonia in children living in Utah and aged younger than 18 years that were recorded during 2001 to 2007. International Classification of Disease, 9th Revision (ICD-9), codes 510.0 to 510.9 for empyema and 481 to 482.9 for bacterial pneumonia were used to identify cases. The same codes (481 to 482.9) were used to identify cases of presumed bacterial pneumonia resulting in hospital discharge, using the IBIS database. Population-based incidence was then calculated using denominator data that included all children younger than 18 years in Utah as determined by the U.S. Census and a correction factor for the proportion of children cared for in Intermountain facilities.
Identification of culture-confirmed pneumococcal empyema.
The PCMC clinical microbiology laboratory has archived all pneumococcal isolates from children with IPD since 1996. For this study, we recovered all pneumococcal isolates from children with PPE cared for at PCMC from January 2001 to February 2007. Isolates were from blood or pleural fluid.
Serotype and MLST analysis of pneumococcal isolates.
Pneumococcal isolates were serotyped (by E.O.M.) at the time of initial identification, and the serotyping was repeated for this analysis by the capsular swelling method (Statens Seruminstitut and Daco). All pneumococcal isolates underwent MLST analysis (by K.G.H.). Following MLST analysis, an allelic profile query was performed using MLST.net (Imperial College London and Wellcome Trust) (1). Investigators performing the serotype and MLST analyses were blinded as to the year the isolate was recovered and all clinical information.
Nucleotide sequence accession numbers.
The allelic sequence and clinical information for all pneumococcal isolates described in this study were deposited into MLST.net (Imperial College London and Wellcome Trust) on 1 September 2009.
RESULTS
ICD-9 coded cases of pediatric pneumonia and empyema.
During the study period, cases of hospitalized pneumonia in Intermountain facilities represented between 80% and 96% of all pediatric hospital discharges for pneumonia in the IBIS system (mean, 90%). Pediatric pneumonia hospitalization within Intermountain decreased by 1.4% per year (P = 0.23). Conversely, cases of pediatric empyema increased by 5.4% per year (P = 0.015). The rate of empyema in the Utah pediatric population increased from 8.5/100,000 children in 2001 to 12.5/100,000 children in 2007 (P = 0.006) (Table 1). As a consequence of the decreasing rates of hospitalized pneumonia and the increasing rates of empyema, the proportion of hospitalized cases of pediatric pneumonia complicated by empyema, in Utah, increased from 24.8% in 2001 to 35.5% in 2007 (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.1 to 2.5; P = 0.007).
TABLE 1.
Incidence of pediatric empyema and pneumonia cases in Utah based on data from Intermountain Healthcare facilities
| Yr | Total no. of children with pediatric empyema cared for in Intermountain facilities | No. of pediatric empyema cases/100,000 children in Utaha | Total no. of children with pediatric pneumonia cared for in Intermountain facilities | No. of pediatric pneumonia cases/100,000 children in Utah |
|---|---|---|---|---|
| 2001 | 62 | 8.5 | 250 | 34.2 |
| 2002 | 64 | 8.6 | 267 | 36.0 |
| 2003 | 75 | 9.9 | 271 | 35.9 |
| 2004 | 74 | 9.6 | 264 | 34.3 |
| 2005 | 88 | 11.1 | 253 | 32.0 |
| 2006 | 75 | 9.3 | 241 | 29.8 |
| 2007 | 104 | 12.5 | 293 | 35.1 |
Population-based incidence of empyema for children younger than 18 years (pediatric) was adjusted for the proportion of children (90%) in Utah who receive care at an Intermountain facility.
Culture confirmed pneumococcal empyema at PCMC.
We identified 66 archived pneumococcal isolates from children diagnosed with PPE. Of these, 51 were viable and represent the study population for molecular analysis. The mean age of children with empyema increased from 45 months during the study period from 2001 through 2004 to 61 months during the period from 2005 through 2007 (P < 0.001).
Serotype analysis of culture-confirmed PPE at PCMC.
From 2001 to 2007, 98% of PPE was due to pneumococcal serotypes that are not represented in the PCV-7 vaccine (Table 2). This represented a significant increase from the pre-PCV-7 years, when 62% of empyema was due to nonvaccine serotypes, as shown in Table 2 (P < 0.001) (13). Four serotypes were responsible for 90% of PPE: serotypes 1 (33%), 3 (27%), 19A (26%), and 7F (4%).
TABLE 2.
Serotypes of pediatric empyema isolates pre- and post-PCV-7
| Serotype | No. (%) of isolates |
|---|---|
| Pre-PCV-7 (1993-1999) | |
| Vaccine serotypes | 10 (38) |
| 14 | 4 (15) |
| 9V | 4 (15) |
| 19F | 1 (4) |
| 18C | 1 (4) |
| Nonvaccine serotypes | 16 (62) |
| 1 | 13 (50) |
| Others | 3 (12) |
| Post-PCV-7 (2001-2007) | |
| Vaccine serotypes | 1 (2) |
| 9V | 1 |
| Nonvaccine serotypes | 50 (98) |
| 1 | 17 (33) |
| 3 | 14 (27) |
| 7F | 2 (4) |
| 17 | 1 (2) |
| 19A | 13 (26) |
| 22F | 1 (2) |
| 38 | 1 (2) |
| Nontypeable | 1 (2) |
MLST analysis.
All viable isolates successfully underwent MLST analysis (Table 3). All ST correlated with serotypes, indicating that the ST could predict the serotype. These MLST findings indicate that for the most common serotypes resulting in empyema, 1, 3, 7F, and 19A, serotype replacement was occurring, rather than serotype switch.
TABLE 3.
MLSTs of each of the four serotypes that were most often identified from children with PPE
| Serotype (no. of isolates) | MLST (% of total for serotype)a |
|---|---|
| 1 (17) | 227 (12) |
| 304 (12) | |
| 306 (35) | |
| 2126 (SLV 227) (6) | |
| 4288 (24) | |
| 4289 (6) | |
| 4290 (SLV 306) (12) | |
| 3 (14) | 180 (86) |
| 1468, 2570 (SLV 180) (14) | |
| 19A (13) | 199 (77) |
| 667 (SLV 199) (8) | |
| 4303 (SLV 199) (8) | |
| 2348 (8) | |
| 7F (2) | 191 (50) |
| 4292 (SLV 191) (50) |
SLV indicates that the ST varies at a single locus.
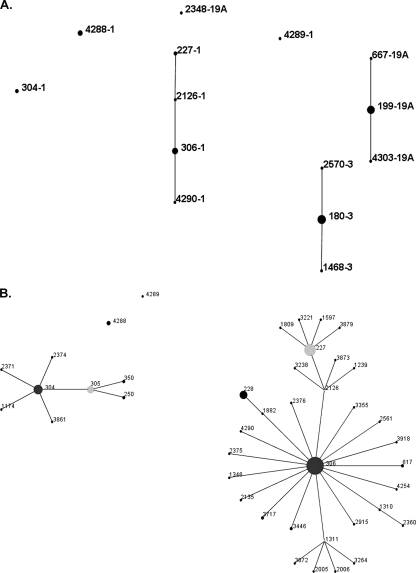
For empyema due to serotype 1, the only ST demonstrated prior to 2001 was 227 (13). In 2003, other ST were demonstrated, including ST 304, 306, and 2126, single-locus variants (SLV) of 227 and 306, and new ST, including 4288 and 4289. Figure 1B demonstrates the relatedness of the serotype 1 ST.
FIG. 1.
(A) eBURST “population snapshot” showing the clusters and unlinked ST of the Streptococcus pneumoniae isolates of serotypes 1, 3, and 19A included in this study. The numbers indicate the ST-serotype designations. (B) eBURST diagram depicting the serotype 1 isolates in this study and related ST available in the MLST database (http://www.mlst.net) hosted at Imperial College London. Circle sizes demonstrate the relative prevalence of each ST in the database. Lines indicate one allele difference between connected circles. A predicted founder ST is in the center of a group and SLV radiate from the founder.
Serotype 3 emerged in 2001 as an important cause of PPE, and MLST indicated that all isolates were ST 180 or SLV. Serotype 19A was first detected in Utah empyema isolates in 2002. Of the 19A isolates, 12/13 (92%) were ST 199 or an SLV of 199, including ST 2704 (n = 1) and ST 667 (n = 1). One isolate of 19A was ST 2348. ST 7F was first detected in Utah children with empyema in 2004. Both isolates were ST 191 or an SLV of 191.
DISCUSSION
This study demonstrates the dramatic increase in pediatric empyema cases in Utah in the years following the licensure of PCV-7. By 2007, more than one-third of pediatric hospitalizations for pneumonia were complicated by empyema. In Utah, PPE is now the most common form of IPD in children. The majority of PPE is due to serotypes 1, 3, 7F, and 19A, and many cases occur in children older than the current recommended age for immunization (4). The molecular analysis of empyema isolates demonstrated that PPE in the postvaccine period in Utah was the result of replacement disease by nonvaccine serotypes.
We have documented high rates of empyema in the Utah pediatric population since 1993 (13). Serotype 1 has consistently been associated with empyema and complicated pneumonia worldwide (24). Serotype 1 was an important cause of IPD in Utah children prior to the introduction of PCV-7. While we continued to see a significant proportion of IPD caused by serotype 1, we also noted increases in serotypes 3, 7F, and 19A in the post-PCV-7 years. Serotypes 7F and 3 have been reported to cause complicated pneumonia and empyema in Spain (37). Furthermore, serotype 7F has been associated with severe or fatal pneumococcal infection in children (43). Serotype 19A has emerged as the most important cause of IPD in the United States in the postvaccine years (40). Infection with 19A has been associated with meningitis (35) in 20 U.S. hospitals, mastoiditis (38) in Houston, TX, and pneumonia and empyema in the Alaskan native population (45).
Our serotype and MLST analysis revealed that replacement ST were responsible for increases in empyema in the postvaccine period. For the serotypes 3, 7F, and 19A, MLST analysis revealed that the dominant ST for each of these three serotypes was the same as that commonly isolated in the United States prior to the introduction of PCV-7 (5). Molecular analysis of serotype 1 isolates demonstrated a decrease in the predominance of ST 227 and expansion of ST 304 and 306, members of the same lineage.
Prior to the introduction of PCV-7, all of serotype 1 isolates obtained from children in Utah were ST 227 (13). Following licensure of the PCV-7 vaccine in the United States, we noted a persistence of serotype 1 as a cause of empyema; however, by 2003, multiple ST, including 304, 306, 2126, and SLV of these, were demonstrated. Brueggemann and Spratt demonstrated in 2003 that ST 227, 304, and 306 are all related and members of a major lineage with a geographic distribution that includes Europe and North America (9). ST 2126 is also closely related to ST 227, differing at only the spi locus. Clonal expansion of specific ST of serotype 1 have been reported previously, including an epidemic of meningitis in Ghana and Burkina Faso associated with ST 217 (29, 48) and the carriage of ST 306 in healthy children following immunization with PCV-7 (36).
The analysis of serotypes 3, 7F, and 19A revealed a limited number of ST. All serotype 3 isolates were ST 180, and both serotype 7F isolates were either ST 191 or an SLV of 191. In the United States, ST 180 and ST 191 represent the most common ST seen in serotypes 3 and 7F, respectively, both before and after the introduction of PCV-7 (5). Similarly, 12/13 (92%) of 19A isolates were ST 199 or SLVs of 199, the most common 19A ST in the United States, especially among children (5, 33, 40). We were not able to demonstrate serotype switching in the 19A isolates from Utah children that resulted in empyema. Our findings were similar to those seen in Alaska, where the most common ST seen was ST 199 and serotype replacement that occurred following immunization with PCV-7 involved ST that existed in control communities (31).
The pneumococcal strain distributions associated with pediatric empyema in Utah in the PCV-7 period is reflective of the complex ecology and epidemiology of pneumococci. In our analysis of pediatric empyema, we note an overall increase in empyema cases and a similar disease phenotype for infections that resulted from multiple ST of serotype 1 and from serotypes 3, 7F, and 19A. Serotype 1 ST 227 was a predominant cause of IPD, along with vaccine serotypes, prior to the introduction of PCV-7. With the selective pressure of the vaccine, PCV-7 serotypes have decreased dramatically in the United States (47) and, in Utah, account for <5% of IPD in 2008 and 2009 (C. L. Byington, unpublished data). The decrease in vaccine serotypes likely created ecological niches that allowed for replacement by other pneumococcal serotypes.
The reasons for the increased rate of empyema in our population are unknown. S. pneumoniae strains differ in their abilities to invade different tissue sites, certain pneumococcal serotypes have a propensity to cause invasive disease and others are more often associated with colonization (6, 8). It is also possible that the pneumococcal isolates in our study population contain virulence genes that confer an increased ability to invade the pleural space. Recent studies have demonstrated that strains of identical serotypes and ST can differ in the presence of important virulence determining loci (42, 44). Ehrlich and others have observed that S. pneumoniae possesses a supragenome, comprised of all genes available for a species (20, 21). Individual pneumococcal isolates contain core genes present in all members of the species, distributed genes shared in subsets of strains, and unique genes found only in a single strain (25). Genetic material available in the supragenome may include unknown genes that confer the ability to invade the pleural space and thus have resulted in the significant increase in pediatric empyema that we have observed. We are currently testing this hypothesis through comparative genomic analysis of empyema isolates of multiple serotypes and ST.
Our study has limitations. First, not all empyema isolates were viable. It is possible that some serotypes or ST may be less viable and are underrepresented. In addition, all of our pneumococcal isolates come from children in Utah, a single geographic location. Thus, our findings may not be representative, especially if pneumococcal diversity is related to geographic location. However, studies by Hiller et al. failed to demonstrate a correlation between genetic diversity and geographic distance (25). Our own findings related to empyema caused by serotype 1 and the emergence of serotypes 3, 7F, and 19A are similar to reports from other parts of North America and Europe (28, 37, 45).
In spite of the limitations, we believe our findings have several important implications. First, serotype replacement occurred rapidly following the introduction of the PCV-7 vaccine and is continuing to occur. The introduction of broader pneumococcal vaccines, such as PCV-13, may provide protection for the dominant serotypes present at the time of vaccine licensure, but new pathogenic strains may continue to emerge. The ecology of the organism changes under vaccine selective pressure, and we must be alert to changes in IPD clinical presentations and susceptible populations. Finally, in the monitoring of emerging pathogens, it will become increasingly important to identify genes from the supragenome that facilitate survival in different environments and develop surveillance mechanisms for these (20).
Acknowledgments
The University of Utah received a grant from Wyeth to perform the MLST analysis described here. C.L.B. is supported by Public Health Services research grant UL1-RR025764 from the National Center for Research Resources, NIH/NIAID U01 AI074419 and U01-A1061611, CDC P01HK000030, and NIH/Eunice Kennedy Shriver NICHD K24-HD047249. M.P. is supported by NIH/NIAID R01AI068043. A.J.B. is supported by the NIH/NIAID K-23 award.
Footnotes
Published ahead of print on 16 December 2009.
REFERENCES
- 1.Aanensen, D. M., and B. G. Spratt. 2005. The multilocus sequence typing network: mlst.net. Nucleic Acids Res. 33:W728-W733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advisory Committee on Immunization Practices. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 49:1-35. [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. Committee on Infectious Diseases. 2000. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362-366. [DOI] [PubMed] [Google Scholar]
- 4.Ampofo, K., X. Sheng, K. Korgenski, E. O. Mason, J. Daly, A. T. Pavia, and C. L. Byington. 2009. The changing age and serotype distribution of invasive pneumococcal diseases in the PCV-7 era; implications for new pneumococcal vaccines, abstr. 4315.5. Abstr. 2009 PAS Annu. Meet. Pediatric Academic Societies, Baltimore, MD.
- 5.Beall, B., M. C. McEllistrem, R. E. Gertz, Jr., S. Wedel, D. J. Boxrud, A. L. Gonzalez, M. J. Medina, R. Pai, T. A. Thompson, L. H. Harrison, L. McGee, and C. G. Whitney. 2006. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J. Clin. Microbiol. 44:999-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann, A. B., R. Pai, D. W. Crook, and B. Beall. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 9.Brueggemann, A. B., and B. G. Spratt. 2003. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J. Clin. Microbiol. 41:4966-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckingham, S. C., M. D. King, and M. L. Miller. 2003. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr. Infect. Dis. J. 22:499-504. [DOI] [PubMed] [Google Scholar]
- 11.Byington, C. L., K. Korgenski, J. Daly, K. Ampofo, A. Pavia, and E. O. Mason. 2006. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr. Infect. Dis. J. 25:250-254. [DOI] [PubMed] [Google Scholar]
- 12.Byington, C. L., M. H. Samore, G. J. Stoddard, S. Barlow, J. Daly, K. Korgenski, S. Firth, D. Glover, J. Jensen, E. O. Mason, C. K. Shutt, and A. T. Pavia. 2005. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin. Infect. Dis. 41:21-29. [DOI] [PubMed] [Google Scholar]
- 13.Byington, C. L., L. Y. Spencer, T. A. Johnson, A. T. Pavia, D. Allen, E. O. Mason, S. Kaplan, K. C. Carroll, J. A. Daly, J. C. Christenson, and M. H. Samore. 2002. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin. Infect. Dis. 34:434-440. [DOI] [PubMed] [Google Scholar]
- 14.Calbo, E., A. Diaz, E. Canadell, J. Fabrega, S. Uriz, M. Xercavins, M. A. Morera, E. Cuchi, M. Rodriguez-Carballeira, and J. Garau. 2006. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin. Microbiol. Infect. 12:867-872. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2007. Estimated vaccination coverage with individual vaccines and selected vaccination series among children 19-35 months of age by state and local area. U.S. National Immunization Survey, Q3/2006-Q2/2007. http://www.cdc.gov/vaccines/stats-surv/nis/data/tables_0607.htm.
- 16.Centers for Disease Control and Prevention. 2004. Estimated vaccination coverage with individual vaccines and selected vaccination series among children 19-35 months of age by state. U.S. National Immunization Survey 2002-2003. http://www.cdc.gov/vaccines/stats-surv/nis/data/tables_0203.htm.
- 17.Chonmaitree, T., and K. R. Powell. 1983. Parapneumonic pleural effusion and empyema in children. Review of a 19-year experience, 1962-1980. Clin. Pediatr. (Phila.) 22:414-419. [DOI] [PubMed] [Google Scholar]
- 18.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 19.Eastham, K. M., R. Freeman, A. M. Kearns, G. Eltringham, J. Clark, J. Leeming, and D. A. Spencer. 2004. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax 59:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrlich, G. D., N. L. Hiller, and F. Z. Hu. 2008. What makes pathogens pathogenic. Genome Biol. 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich, G. D., F. Z. Hu, K. Shen, P. Stoodley, and J. C. Post. 2005. Bacterial plurality as a general mechanism driving persistence in chronic infections. Clin. Orthop. Relat. Res. 2005(437):20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144(Pt 11):3049-3060. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari, C. A., G. M. Pirez, A. A. Martinez, R. G. Algorta, V. F. Chamorro, B. M. Guala, C. Zabala Ch, L. G. Giachetto, and L. A. Montano. 2007. Etiology of community acquired pneumonia in inpatients children. Uruguay 1998-2004. Rev. Chilena Infectol. 24:40-47. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 25.Hiller, N. L., B. Janto, J. S. Hogg, R. Boissy, S. Yu, E. Powell, R. Keefe, N. E. Ehrlich, K. Shen, J. Hayes, K. Barbadora, W. Klimke, D. Dernovoy, T. Tatusova, J. Parkhill, S. D. Bentley, J. C. Post, G. D. Ehrlich, and F. Z. Hu. 2007. Comparative genomic analyses of seventeen Streptococcus pneumoniae strains: insights into the pneumococcal supragenome. J. Bacteriol. 189:8186-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh, Y. C., C. H. Hsiao, P. N. Tsao, J. Y. Wang, P. R. Hsueh, B. L. Chiang, W. S. Lee, and L. M. Huang. 2006. Necrotizing pneumococcal pneumonia in children: the role of pulmonary gangrene. Pediatr. Pulmonol. 41:623-629. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan, S. L., E. O. Mason, Jr., E. R. Wald, G. E. Schutze, J. S. Bradley, T. Q. Tan, J. A. Hoffman, L. B. Givner, R. Yogev, and W. J. Barson. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113:443-449. [DOI] [PubMed] [Google Scholar]
- 28.Langley, J., J. Kellner, and J. Robinson. 2008. PICNIC (Pediatric Investigators Collaborative Study of Infections in Canada) study of empyema due to community-acquired pneumonia in Canadian children: etiology, clinical manifestations and management variation, abstr. 4465.5. Abstr. 2008 PAS ASPR Joint Meet. Pediatric Academic Societies and Asian Society for Pediatric Research, Honolulu, HI.
- 29.Leimkugel, J., A. Adams Forgor, S. Gagneux, V. Pfluger, C. Flierl, E. Awine, M. Naegeli, J. P. Dangy, T. Smith, A. Hodgson, and G. Pluschke. 2005. An outbreak of serotype 1 Streptococcus pneumoniae meningitis in northern Ghana with features that are characteristic of Neisseria meningitidis meningitis epidemics. J. Infect. Dis. 192:192-199. [DOI] [PubMed] [Google Scholar]
- 30.Li, S. 2008. Empyema hospitalizations increasing in the US despite decreasing invasive pneumococcal disease post-introduction of the pneumococcal conjugate vaccine, abstr. 4055.4. Abstr. 2008 PAS ASPR Joint Meet. Pediatric Academic Societies and Asian Society for Pediatric Research, Honolulu, HI.
- 31.Lipsitch, M., K. O'Neill, D. Cordy, B. Bugalter, K. Trzcinski, C. M. Thompson, R. Goldstein, S. Pelton, H. Huot, V. Bouchet, R. Reid, M. Santosham, and K. L. O'Brien. 2007. Strain characteristics of Streptococcus pneumoniae carriage and invasive disease isolates during a cluster-randomized clinical trial of the 7-valent pneumococcal conjugate vaccine. J. Infect. Dis. 196:1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore, M. R., R. E. Gertz, Jr., R. L. Woodbury, G. A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L. H. Harrison, J. L. Hadler, N. M. Bennett, A. R. Thomas, L. McGee, T. Pilishvili, A. B. Brueggemann, C. G. Whitney, J. H. Jorgensen, and B. Beall. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016-1027. [DOI] [PubMed] [Google Scholar]
- 34.Muρoz-Almagro, C., I. Jordan, A. Gene, C. Latorre, J. J. Garcia-Garcia, and R. Pallares. 2008. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin. Infect. Dis. 46:174-182. [DOI] [PubMed] [Google Scholar]
- 35.Nigrovic, L. E., N. Kuppermann, and R. Malley. 2008. Children with bacterial meningitis presenting to the emergency department during the pneumococcal conjugate vaccine era. Acad. Emerg. Med. 15:522-528. [DOI] [PubMed] [Google Scholar]
- 36.Nunes, S., R. Sa-Leao, L. C. Pereira, and H. Lencastre. 2008. Emergence of a serotype 1 Streptococcus pneumoniae lineage colonising healthy children in Portugal in the seven-valent conjugate vaccination era. Clin. Microbiol. Infect. 14:82-84. [DOI] [PubMed] [Google Scholar]
- 37.Obando, I., C. Munoz-Almagro, L. A. Arroyo, D. Tarrago, D. Sanchez-Tatay, D. Moreno-Perez, S. S. Dhillon, C. Esteva, S. Hernandez-Bou, J. J. Garcia-Garcia, W. P. Hausdorff, and A. B. Brueggemann. 2008. Pediatric parapneumonic empyema, Spain. Emerg. Infect. Dis. 14:1390-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ongkasuwan, J., T. A. Valdez, K. G. Hulten, E. O. Mason, Jr., and S. L. Kaplan. 2008. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics 122:34-39. [DOI] [PubMed] [Google Scholar]
- 39.Pai, R., R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Clonal association between Streptococcus pneumoniae serotype 23A, circulating within the United States, and an internationally dispersed clone of serotype 23F. J. Clin. Microbiol. 43:5440-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988-1995. [DOI] [PubMed] [Google Scholar]
- 41.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26:468-472. [DOI] [PubMed] [Google Scholar]
- 42.Pettigrew, M. M., K. P. Fennie, M. P. York, J. Daniels, and F. Ghaffar. 2006. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect. Immun. 74:3360-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rückinger, S., R. von Kries, A. Siedler, and M. van der Linden. 2009. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr. Infect. Dis. J. 28:118-122. [DOI] [PubMed] [Google Scholar]
- 44.Silva, N. A., J. McCluskey, J. M. Jefferies, J. Hinds, A. Smith, S. C. Clarke, T. J. Mitchell, and G. K. Paterson. 2006. Genomic diversity between strains of the same serotype and multilocus sequence type among pneumococcal clinical isolates. Infect. Immun. 74:3513-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784-1792. [DOI] [PubMed] [Google Scholar]
- 46.Tan, T. Q., E. O. Mason, Jr., E. R. Wald, W. J. Barson, G. E. Schutze, J. S. Bradley, L. B. Givner, R. Yogev, K. S. Kim, and S. L. Kaplan. 2002. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 110:1-6. [DOI] [PubMed] [Google Scholar]
- 47.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 48.Yaro, S., M. Lourd, B. Naccro, B. M. Njanpop-Lafourcade, A. Hien, M. S. Ouedraogo, Y. Traore, L. M. Schouls, I. Parent du Chatelet, and B. D. Gessner. 2006. The epidemiology of Haemophilus influenzae type b meningitis in Burkina Faso. Pediatr. Infect. Dis. J. 25:415-419. [DOI] [PubMed] [Google Scholar]