Abstract
We report the case of a 55-year-old man who exhibited a nodular pneumonia 4 months after an allogeneic hematopoietic stem cell transplantation. Culture of the bronchoalveolar lavage fluid revealed Nocardia pseudobrasiliensis. This recently described carbapenem-resistant species should be included in the differential diagnosis of fungal infection in this setting.
CASE REPORT
We report the case of a 55-year-old man who was admitted to our unit on 7 May 2008 for recent-onset cough and fever. His main medical history was multiple myeloma, diagnosed in February 2001. Treatment of the myeloma included several chemotherapy avenues, including two autologous stem cell transplantations, before he underwent allogeneic hematopoietic stem cell transplantation (HSCT) while in partial remission. Allogeneic HSCT from a matched, unrelated donor was performed at the end of January 2008 following conditioning with intravenous fludarabine (120 mg/m2), oral busulfan (8 mg/kg of body weight), and anti-thymocyte globulin (ATG; 7.5 mg/kg). Prevention of graft-versus-host disease (GVHD) consisted of cyclosporine and mycophenolate mofetil (MMF). Interleukin-2 (IL-2) receptor monoclonal antibody (daclizumab) was added to steroids, tacrolimus, and MMF for steroid-resistant acute GVHD. He received posaconazole (200 mg three times a day) for primary prophylaxis of invasive fungal infection, oral penicillin V (2 millions units/day), and monthly aerosolized pentamidine (300 mg). At the time of admission to our department, the myeloma remained in stable partial response.
Four months after allogeneic HSCT, the patient developed cough, dyspnea, and fever (38°C). On examination, he appeared asthenic; his blood pressure was 135/60 mm Hg, pulse beats were 90 per minute, respiratory rate was 20 per minute, and temperature was 38°C. Physical examination was normal. Laboratory test results were hemoglobin, 9.1 g/dl; polymorphonuclear cells, 1,700/mm3; platelet count, 51,000/mm3; serum creatinine, 142 μmol/liter; C-reactive protein level, 69 mg/liter (normal is below 10 mg/liter); and partial O2 pressure, 74 mm Hg.
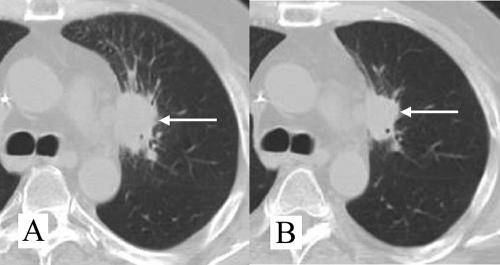
Chest radiography was normal, and a computed tomography (CT) scan of the chest revealed a spiculated nodule of the culmen (Fig. 1A).
FIG. 1.
(A) CT scan of the patient's chest on admission showing a spiculated macronodule (white arrow) of the culmen. (B) CT scan of the patient's chest after 2 weeks of antibiotic treatment: the size of the pulmonary nodule was markedly decreased.
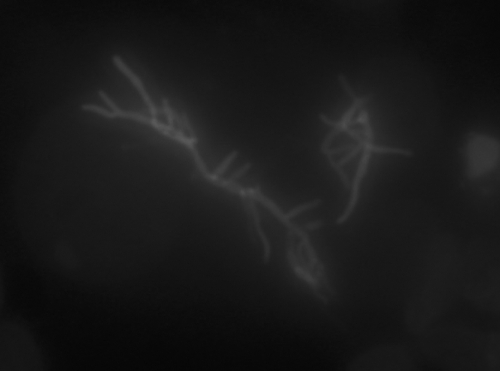
Blood cultures were negative. A serum Aspergillus galactomannan assay was negative. Because of neutropenia occurring a second time, the patient received piperacillin-tazobactam and amikacin. The neutropenia was related to valganciclovir. Bronchoscopy was macroscopically normal, and bronchoalveolar lavage (BAL) was performed; the fluid recovered contained 200 leukocytes per ml (with 64% macrophages and 34% polymorphonuclear neutrophils), with neither Pneumocystis jirovecii nor tumoral cells. Mycologic and viral examinations were negative, but direct microbiological examination of the BAL sample revealed numerous branched Gram-positive filamentous rods that fluoresced upon staining with Uvitex (optical brightener belonging to the stilbene derivatives that fluoresces upon excitation with UV light) (Fig. 2). Bacterial cultures were positive for Nocardia spp. Identification at the species level was based on the sequence of the hsp65 gene, which was amplified and sequenced according to the method of Rodriguez-Nava et al. (18). The 430-bp amplified DNA sequence was 100% homologous with that of the Nocardia pseudobrasiliensis reference strain DSM44290 (GenBank sequence accession number AY756530; NCBI BLAST and BiBi [http://pbil.univ-lyon1.fr/] phylogenetic tools).
FIG. 2.
Direct microbiological examination showed branched filaments upon treatment with fluorescent fungal stain (Uvitex).
Mycologic and viral cultures of the BAL fluid were negative. Brain magnetic resonance imaging (MRI) results were normal.
In vitro susceptibility testing (Table 1) was performed by Etest (AB Biodisk, Solna, Sweden) on Mueller-Hinton plates (4). Although broth microdilution is now recommended by the NCCLS (16), two reports suggest that MIC determination by broth microdilution and Etest give similar results (1, 2), making the Etest suitable and more convenient for routine work. The results were recorded after 48 h (or after 72 h if growth was insufficient after 48 h) and interpreted according to the MIC breakpoints published by the NCCLS (16). Nevertheless, the Etest method was not available for every antibiotic in our laboratory, but only for ciprofloxacin, imipenem, clarithromycin, and amoxicillin-clavulanic acid. Therefore, the agar disk diffusion method on Mueller-Hinton plates in ambient air (26) was used for the other antibiotics, and in the absence of consensual breakpoints, the interpretation of inhibition zones has been made according to the proposed guidelines of the French Society for Microbiology (9). Susceptibility testing revealed resistance to imipenem (Table 1). The addition of EDTA to imipenem on the agar plate restored susceptibility to imipenem, suggesting the presence of a metallo-beta-lactamase (17). Treatment was changed from piperacillin-tazobactam and amikacin to ciprofloxacin and gentamicin. In addition, a progressive tapering off of immunosuppressive drugs was performed, including decreased steroid dosage and discontinuation of MMF. After 15 days of this double antimicrobial therapy, gentamicin was stopped and replaced with sulfamethoxazole-trimethoprim (SXT).
TABLE 1.
Comparative antibiotic susceptibility patterns of Nocardia brasiliensis and Nocardia pseudobrasiliensis, including the case reported here and others from the literaturee
| Drug | MIC breakpoint(s)a | Results for N. pseudobrasiliensis isolate(s) from: |
Result for N. brasiliensis isolates from reference 4d | |||
|---|---|---|---|---|---|---|
| This case report |
Reference |
|||||
| MIC (interpretation) | Inhibition zoneb (interpretation) | 25 (% with susceptibility)c | 15d | |||
| Amikacin | ≤8, ≥16 | ND | 24 (S) | S (100) | S | S |
| Amoxicillin-clavulanic acid | ≤8/4, ≥32/16 | 2 (S) | 12 (R) | R (81) | ND | S |
| Ceftriaxone | ≤8, ≥64 | ND | 20 (S) | S (69) | R | S |
| Ciprofloxacin | ≤1, ≥4 | 0.125 (S) | 33 (S) | S (95) | S | R |
| Clarithromycin | ≤2, ≥8 | 0.125 (S) | ND | S (91) | S | R |
| Imipenem | ≤4, ≥16 | >32 (R) | 8 (R) | R (>90) | R | S |
| Linezolid | ≤8 | ND | 35 (S) | ND | S | S |
| Minocycline | ≤1, ≥8 | ND | 27 (S) | ND | R | S |
| Sulfamethoxazole | ≤32, ≥64 | ND | 6 (S) | S (94) | R | S |
| Tobramycin | ≤4, ≥16 | ND | 32 (S) | ND | ND | ND |
MIC resistance breakpoints are those from the NCCLS (16): the first value indicates the susceptibility breakpoint, and the second value indicates the resistance breakpoint.
Inhibition zones, measured in millimeters, were determined by the Mueller-Hinton agar disk diffusion method.
The percentage of susceptible or resistant strains as determined by the Etest method.
MICs were determined by the Etest method.
Under this treatment, the disease progression was initially favorable: the patient became apyretic, and the size of the pulmonary nodule decreased markedly (Fig. 1B). The SXT treatment was complicated by neutropenia. However, the patient's clinical status worsened and a cerebral lesion appeared despite the withdrawal of immunosuppressive drugs. This lesion was compatible with disseminated nocardiosis according to four points: pulmonary nocardiosis was microbiologically proven, this patient presented a predisposing setting, cerebral MRI findings were compatible with this diagnosis (nodular lesion with ventriculitis), and finally, there was no evidence of a differential diagnosis. A complete microbiological workup returned negative results for blood cultures, bronchoalveolar lavage fluid culture (bacteriologic, mycologic, and viral examinations), cerebrospinal fluid culture (bacteriologic, mycologic, and viral examinations), urine sample culture, and serum Aspergillus galactomannan assay. Histological examination of a lung biopsy specimen showed inflammation with histiocytes and polymorphonuclear neutrophils. Grocott methenamine silver staining showed numerous branched filamentous rods. The patient died 5 months later, and this fatal progression is considered a therapeutic failure.
The genus Nocardia currently contains more than 80 species that have been characterized by phenotypic and molecular methods (8, 10). Disseminated nocardiosis is a well-known opportunistic infection occurring in conjunction with solid organ transplantation (5), AIDS (13), chronic granulomatous disease (CGD) (7), and prolonged steroid therapy but rarely after allogeneic HSCT (4, 24).
Nocardia pseudobrasiliensis is a newly described species that was formerly considered to belong to the N. brasiliensis species. Phenotypically, this new taxon is similar to N. brasiliensis but hydrolyzes adenine, has nitrate reductase activity less frequently, and displays two specific and early mycolic acid ester peaks in high-performance liquid chromatography (19). Genotypically, this new taxon was confirmed to indeed constitute a new bacterial species on the basis of 16S rRNA gene sequence analysis and DNA-DNA hybridization (19). Furthermore N. pseudobrasiliensis exhibits a specific restriction pattern in PCR-restriction fragment length polymorphism analysis of the 65-kDa heat shock protein gene (19, 25).
Wallace et al. (25), in a study of 43 isolates of this new species, described different anatomical sites of infection but reported a strong association with invasive disease, including involvement of the lungs in 30/43 (70%) cases, of the brain and/or meninges in 10/43 (23%), of skin and/or soft tissue in 13/43 (30%), of joints in 3/43 (7%), and disseminated disease in 16/43 (37%). Most of these patients presented with underlying immunosuppression, with 92% (after excluding HIV infection and alcoholism) under corticosteroid therapy.
Ruimy et al. analyzed this taxon by sequencing the gene encoding the small ribosomal subunit (16S RNA) and with DNA-DNA hybridization. They confirmed that this new taxon is indeed a new species and proposed the name N. pseudobrasiliensis (19). Three additional cases have since been reported, including invasive infections; these include ventriculitis in an immunocompetent child (15), pneumonia in a 71-year-old man with a recent history of esophageal and gastric cancer (12), and disseminated infection in a patient with AIDS (3). In contrast, N. brasiliensis is mostly isolated from posttrauma cutaneous infection (21) and is responsible for the majority (approximately 80%) of primary cutaneous nocardiosis (4, 14, 20).
The antimicrobial susceptibilities of Nocardia spp. are heterogenous, and six antimicrobial susceptibility patterns have been proposed to classify the most frequently isolated Nocardia spp. strains. Importantly, N. pseudobrasiliensis does not fit this classification and its antimicrobial susceptibilities differ from those of N. brasiliensis. In Nocardia spp., several mechanisms may lead to imipenem resistance, including decreased permeability, efflux pump overexpression, and high-level beta-lactamase production (11, 23). In N. brasiliensis, mutations in the beta-lactamase gene can lead to acquired resistance to clavulanic acid, sulbactam, tazobactam, cloxacillin, and imipenem (22). As for N. pseudobrasiliensis, patterns of susceptibility to imipenem may vary between strains, which can be either resistant or susceptible, but no data are available concerning the precise mechanisms by which resistance occurs (11). In our patient, the observation that EDTA restored susceptibility to imipenem suggests the presence of a metallo-beta-lactamase (17).
To our knowledge, this is the first reported case of Nocardia pseudobrasiliensis in an allogeneic HSCT recipient. van Burik et al. (24) retrospectively reported 25 cases of nocardiosis among 6,759 HSCT recipients that included 4,570 allogeneic HSCT recipients. All cases occurred in allogeneic HSCT recipients, with an incidence of 0.54% in this subpopulation. Infections were attributed to Nocardia asteroides in 90% of the cases. However, the infections reported in the study were probably caused by other species. According to current taxonomy, there are very few, if any, clinical isolates with genetic similarity to the type strain of N. asteroides. Pulmonary involvement (nodule and/or infiltrate) was noticed in 56% of the patients, 44% exhibited skin lesions, and 8% had brain abscesses. Those patients exhibited a high prevalence of GVHD (13/25 [52%]). The median day on which nocardiosis was diagnosed after HSCT was day 210.
Another retrospective study (6) reported 10 cases (corresponding to 1.7% of the allogeneic HSCT recipients) and found 40% coinfection with Aspergillus fumigatus. The high mortality rate (70%) was attributed to aspergillosis and GVHD (4/5 patients diagnosed with nocardiosis exhibited GVHD). In that study, cases occurred at a median of 6.2 months after HSCT (ranging from 2 to 18 months) and the Nocardia species found were N. asteroides (6/10), N. nova (3/10), and N. otitidiscaviarum (1/10). In these two studies, the beneficial effect of SXT (used as Pneumocystis jirovecii prophylaxis) remained uncertain, as nocardiosis occurred in 40% (10/25) of patients receiving this prophylaxis (24).
Nocardia spp. infections should be included in the differential diagnosis of invasive fungal infections in allografted patients, and carbapenem-resistant species should be considered when initiating antimicrobial therapy in this setting.
Acknowledgments
We thank Blandine Rammaert, Jean-Paul Viard, Sylvain Poiree, and Louis Puybasset for their clinical assistance in the care of this patient.
The authors declare no conflict of interest.
Footnotes
Published ahead of print on 25 November 2009.
REFERENCES
- 1.Ambaye, A., P. C. Kohner, P. C. Wollan, K. L. Roberts, G. D. Roberts, and F. R. Cockerill III. 1997. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J. Clin. Microbiol. 35:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biehle, J. R., S. J. Cavalieri, M. A. Saubolle, and L. J. Getsinger. 1994. Comparative evaluation of the E test for susceptibility testing of Nocardia species. Diagn. Microbiol. Infect. Dis. 19:101-110. [DOI] [PubMed] [Google Scholar]
- 3.Brown, B. A., J. O. Lopes, R. W. Wilson, J. M. Costa, A. C. de Vargas, S. H. Alves, C. Klock, G. O. Onyi, and R. J. Wallace, Jr. 1999. Disseminated Nocardia pseudobrasiliensis infection in a patient with AIDS in Brazil. Clin. Infect. Dis. 28:144-145. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Elliott, B. A., J. M. Brown, P. S. Conville, and R. J. Wallace, Jr. 2006. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 19:259-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman, S. W., and J. P. Wilson. 1990. Nocardiosis in transplant recipients. Semin. Respir. Infect. 5:74-79. [PubMed] [Google Scholar]
- 6.Choucino, C., S. A. Goodman, J. P. Greer, R. S. Stein, S. N. Wolff, and J. S. Dummer. 1996. Nocardial infections in bone marrow transplant recipients. Clin. Infect. Dis. 23:1012-1019. [DOI] [PubMed] [Google Scholar]
- 7.Dorman, S. E., S. V. Guide, P. S. Conville, E. S. DeCarlo, H. L. Malech, J. I. Gallin, F. G. Witebsky, and S. M. Holland. 2002. Nocardia infection in chronic granulomatous disease. Clin. Infect. Dis. 35:390-394. [DOI] [PubMed] [Google Scholar]
- 8.Euzeby, J. P. 1997. List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet. Int. J. Syst. Bacteriol. 47:590-592. [DOI] [PubMed] [Google Scholar]
- 9.French Society for Microbiology. 2009. Proposed guidelines of the French Society for Microbiology. Comite de l'Antibiogramme de la Societe Francaise De Microbiologie. http://www.sfm.asso.fr/publi/general.php?pa=1.
- 10.Kaewkla, O., and C. M. Franco. 14 August 2009. Nocardia callistridis sp. nov., an endophytic actinobacterium isolated from a surface-sterilized root of an Australian native pine tree. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijs.0.016337-0. [DOI] [PubMed]
- 11.Kageyama, A., Y. Hoshino, M. Watanabe, K. Yazawa, and Y. Mikami. 2004. Clinical isolates of Nocardia brasiliensis from Japan exhibit variable susceptibility to the antibiotic imipenem. Mycopathologia 158:275-278. [DOI] [PubMed] [Google Scholar]
- 12.Kageyama, A., H. Sato, M. Nagata, K. Yazawa, M. Katsu, Y. Mikami, K. Kamei, and K. Nishimura. 2002. First human case of nocardiosis caused by Nocardia pseudobrasiliensis in Japan. Mycopathologia 156:187-192. [DOI] [PubMed] [Google Scholar]
- 13.Long, P. F. 1994. A retrospective study of Nocardia infections associated with the acquired immune deficiency syndrome (AIDS). Infection 22:362-364. [DOI] [PubMed] [Google Scholar]
- 14.Maraki, S., S. Chochlidakis, E. Nioti, and Y. Tselentis. 2004. Primary lymphocutaneous nocardiosis in an immunocompetent patient. Ann. Clin. Microbiol. Antimicrob. 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongkolrattanothai, K., S. Ramakrishnan, M. Zagardo, and B. Gray. 2008. Ventriculitis and choroid plexitis caused by multidrug-resistant Nocardia pseudobrasiliensis. Pediatr. Infect. Dis. J. 27:666-668. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS/CLSI. 2003. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes; approved standard. NCCLS document M24-A (ISBN 1-56238-500-3). NCCLS, Wayne, PA. [PubMed]
- 17.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Nava, V., A. Couble, G. Devulder, J. P. Flandrois, P. Boiron, and F. Laurent. 2006. Use of PCR-restriction enzyme pattern analysis and sequencing database for hsp65 gene-based identification of Nocardia species. J. Clin. Microbiol. 44:536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruimy, R., P. Riegel, A. Carlotti, P. Boiron, G. Bernardin, H. Monteil, R. J. Wallace, Jr., and R. Christen. 1996. Nocardia pseudobrasiliensis sp. nov., a new species of Nocardia which groups bacterial strains previously identified as Nocardia brasiliensis and associated with invasive diseases. Int. J. Syst. Bacteriol. 46:259-264. [DOI] [PubMed] [Google Scholar]
- 20.Satterwhite, T. K., and R. J. Wallace, Jr. 1979. Primary cutaneous nocardiosis. JAMA 242:333-336. [PubMed] [Google Scholar]
- 21.Smego, R. A., Jr., and H. A. Gallis. 1984. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev. Infect. Dis. 6:164-180. [DOI] [PubMed] [Google Scholar]
- 22.Steingrube, V. A., R. J. Wallace, Jr., B. A. Brown, Y. Pang, B. Zeluff, L. C. Steele, and Y. Zhang. 1991. Acquired resistance of Nocardia brasiliensis to clavulanic acid related to a change in beta-lactamase following therapy with amoxicillin-clavulanic acid. Antimicrob. Agents Chemother. 35:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steingrube, V. A., R. J. Wallace, Jr., B. A. Brown, Y. Zhang, L. C. Steele, G. Young, and D. R. Nash. 1993. Partial characterization of Nocardia farcinica beta-lactamases. Antimicrob. Agents Chemother. 37:1850-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Burik, J. A., R. C. Hackman, S. Q. Nadeem, J. W. Hiemenz, M. H. White, M. E. Flowers, and R. A. Bowden. 1997. Nocardiosis after bone marrow transplantation: a retrospective study. Clin. Infect. Dis. 24:1154-1160. [DOI] [PubMed] [Google Scholar]
- 25.Wallace, R. J., Jr., B. A. Brown, Z. Blacklock, R. Ulrich, K. Jost, J. M. Brown, M. M. McNeil, G. Onyi, V. A. Steingrube, and J. Gibson. 1995. New Nocardia taxon among isolates of Nocardia brasiliensis associated with invasive disease. J. Clin. Microbiol. 33:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace, R. J., Jr., and L. C. Steele. 1988. Susceptibility testing of Nocardia species for the clinical laboratory. Diagn. Microbiol. Infect. Dis. 9:155-166. [DOI] [PubMed] [Google Scholar]