Gram-negative pathogens producing metallo-β-lactamases (MβLs) capable of hydrolyzing carbapenems have achieved worldwide spread (1). The most frequent MβLs reported so far belong to the VIM and IMP types. VIM-1 producers constitute the majority of the multiresistant Klebsiella pneumoniae isolates in Greek hospitals (6). MβL-positive isolates among other members of the family Enterobacteriaceae such as Proteus mirabilis have also sporadically occurred (8). Detection of MβLs is mostly based on carbapenem-EDTA synergy tests in various formats. There are, however, sensitivity problems due to low carbapenem MICs, as well as discrepancies between the various methods (1, 3).
P. mirabilis exhibiting resistance to newer β-lactams was recently noticed at Evgenidion General Hospital in Athens. The automated system used in the clinical laboratory (Vitek 2 using the AST-N103 and AST-EXN8 susceptibility cards; bioMérieux) invariably characterized these isolates as cephalosporinase positive. Sixteen P. mirabilis isolates from 2008 were studied. MICs of ceftazidime and cefotaxime were >64 mg/liter. Isolates were also resistant to penicillins, penicillin-clavulanate combinations, and cefoxitin. MIC ranges of cefepime and aztreonam were 8 to 16 and 2 to 4 mg/liter, respectively. Isolates were fully or intermediately susceptible to imipenem (MICs of 2 to 8 mg/liter) and meropenem (MICs of 0.25 to 2 mg/liter), as determined by broth microdilution. PCR assays specific for a variety of bla genes and sequencing of the amplicons showed that all isolates carried blaTEM-1, blaCMY-16, and blaVIM-1. Isoelectric focusing of cell extracts confirmed production of the respective β-lactamases. Comparable imipenem-hydrolyzing activities were also observed by spectrophotometry in crude cell extracts from all isolates, although lower (10 to 15 U) than those usually found in VIM-1-producing K. pneumoniae (40 to 80 U; 1 U was the amount of enzyme hydrolyzing 1 nmol of imipenem/min/mg of protein) (5). All but two isolates appeared MβL negative by the imipenem-EDTA double disk synergy test (DDST) (2). The EDTA-imipenem combined disk test (2) failed to detect any of the isolates. The lack of sensitivity of the EDTA-based tests prompted us to evaluate synergy between dipicolinic acid (DPA) and imipenem. Disks containing 250 μg DPA and imipenem (10 μg) were placed at a distance of 8 mm (edge to edge) as recommended (Dipicolinic Acid Diatabs; Rosco Diagnostika, Taastrup, Denmark). All 16 isolates tested were MβL positive by this method, producing synergy images (Fig. 1). To validate the specificity of the DPA-imipenem synergy method, eight P. mirabilis strains from the collection of the Hellenic Pasteur Institute were used as negative controls. Of these, two strains produced TEM-1 (imipenem MICs were 0.25 and 1 mg/liter), four lacked any acquired β-lactamase (imipenem MIC range of 0.25 to 2 mg/liter), and the remaining two, also lacking detectable β-lactamase activity, were mutants selected in vitro on imipenem (MICs of 4 and 8 mg/liter). All eight control isolates were consistently negative by the DPA-imipenem synergy method. We also tried to apply a combined disk test using imipenem disks (10 μg) each supplemented with 200 μg of DPA (Sigma-Aldrich, St. Louis, MO). Although a slight increase in the imipenem inhibition zone was observed for all but three VIM-1 producers, reproducibility was low and further standardization was not attempted.
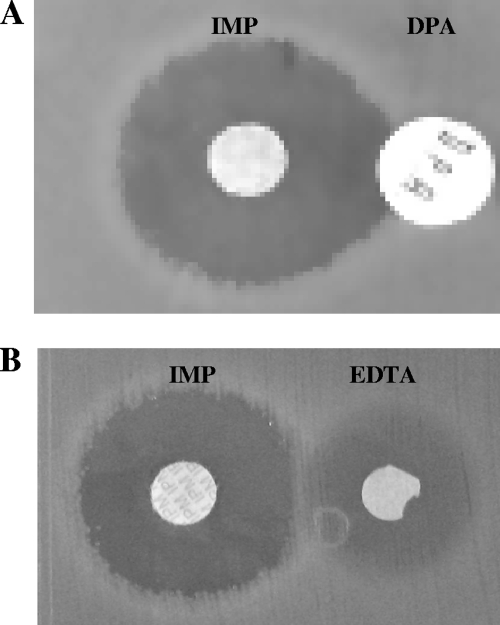
FIG. 1.
(A) Positive synergy test using disks containing DPA (250 μg) and imipenem (IMP, 10 μg) for a P. mirabilis isolate producing VIM-1. (B) The same isolate appearing MβL negative by DDST using a disk containing EDTA (975 μg). Tests were performed with Mueller-Hinton agar and an inoculum of 5 × 105 cells/ml.
Imipenem-DPA synergy has been effectively used for MβL-producing Pseudomonas spp. and Acinetobacter spp (4, 7). To our knowledge, this is the first successful application of this method for P. mirabilis. Yet, it must be pointed out that the isolates examined were clonally related, as indicated by PFGE using NotI for generating macrorestriction fragments (data not shown). Thus, the DPA-based method requires further validation using epidemiologically distinct MβL-positive strains.
Both EDTA and DPA are chelators with high inhibitory activity against MβLs. Therefore, we cannot provide a plausible explanation for the markedly higher sensitivity of the DPA-imipenem DDST. Differences in the interaction of these agents with P. mirabilis cells, other than MβL inhibition, could be hypothesized. Irrespective of the underlying mechanism, it seems that the use of DPA can facilitate detection of VIM-positive P. mirabilis isolates that appear falsely negative by the EDTA-based tests. Also, these data indicate an unnoticed spread of MβL-producing P. mirabilis that warrants investigation.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Cornaglia, G., M. Akova, G. Amicosante, R. Cantón, R. Cauda, J. D. Docquier, M. Edelstein, J.-M. Frère, M. Fuzi, M. Galleni, H. Giamarellou, M. Gniadkowski, R. Koncan, B. Libisch, F. Luzzaro, V. Miriagou, F. Navarro, P. Nordmann, L. Pagani, L. Peixe, L. Poirel, M. Souli, E. Tacconelli, A. Vatopoulos, and G. M. Rossolini. 2007. Metallo-β-lactamases as emerging resistance determinants in gram-negative pathogens: open issues. Int. J. Antimicrob. Agents 29:380-388. [DOI] [PubMed] [Google Scholar]
- 2.Franklin, C., L. Liolios, and A. Y. Peleg. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giakkoupi, P., L. S. Tzouvelekis, G. L. Daikos, V. Miriagou, G. Petrikkos, N. J. Legakis, and A. C. Vatopoulos. 2005. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J. Clin. Microbiol. 43:494-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura, S., Y. Ishii, and K. Yamaguchi. 2005. Evaluation of dipicolinic acid for detection of IMP- or VIM-type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 53:241-244. [DOI] [PubMed] [Google Scholar]
- 5.Loli, A., L. S. Tzouvelekis, E. Tzelepi, A. Carattoli, A. C. Vatopoulos, P. T. Tassios, and V. Miriagou. 2006. Sources of diversity of carbapenem resistance levels in Klebsiella pneumoniae carrying blaVIM-1. J. Antimicrob. Chemother. 58:669-672. [DOI] [PubMed] [Google Scholar]
- 6.Psichogiou, M., P. T. Tassios, A. Avlamis, I. Stefanou, C. Kosmidis, E. Platsouka, O. Paniara, A. Xanthaki, M. Toutouza, G. L. Daikos, and L. S. Tzouvelekis. 2008. Ongoing epidemic of blaVIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J. Antimicrob. Chemother. 61:59-63. [DOI] [PubMed] [Google Scholar]
- 7.Shin, K. S., B. R. Son, S. B. Hong, and J. Kim. 2008. Dipicolinic acid-based disk methods for detection of metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. Diagn. Microbiol. Infect. Dis. 62:102-105. [DOI] [PubMed] [Google Scholar]
- 8.Vourli, S., H. Tsorlini, H. Katsifa, M. Polemis, L. S. Tzouvelekis, A. Kontodimou, and A. C. Vatopoulos. 2006. Emergence of Proteus mirabilis carrying the blaVIM-1 metallo-β-lactamase gene. Clin. Microbiol. Infect. 12:691-694. [DOI] [PubMed] [Google Scholar]