Abstract
The diagnosis of syphilis can be complicated when it is based on diverse clinical manifestations, dark-field microscopy, and serology. In the present study, therefore, we examined the additional clinical value of a Treponema pallidum real-time TaqMan PCR for the detection of primary and secondary syphilis. The additional value of the T. pallidum real-time PCR for the diagnosis of primary syphilis was evaluated by the use of three different algorithms: (i) a head-to-head comparison of the dark-field microscopy result and the T. pallidum real-time PCR result, (ii) comparison of the clinical diagnosis made in a sexually transmitted infection clinic (STI) (including by dark-field microscopy) and the T. pallidum real-time PCR result, and (iii) comparison of the clinical diagnosis made in a general practitioner's office (without dark-field microscopy) and the T. pallidum real-time PCR result. A fourth algorithm was used to determine the performance of the T. pallidum real-time PCR regarding the detection of secondary syphilis. From December 2006 to April 2008, 716 patients with suspected cases of primary syphilis and 133 patients with suspected cases of secondary syphilis were included in the study. A kappa value of 0.601 was found for the agreement between dark-field microscopy and the T. pallidum real-time PCR. Good agreement was found between the T. pallidum real-time PCR and both the diagnosis of the general practitioner (kappa = 0.745) and the diagnosis of the STI clinic (kappa = 0.769). The sensitivity with respect to the STI clinic diagnosis was 72.8%, the specificity was 95.5%, the positive predictive value was 89.2%, and the negative predictive value was 95.0%. The T. pallidum real-time PCR is a fast, efficient, and reliable test for the diagnosis of primary syphilis in an STI outpatient clinic and a general practitioner setting, but it has no added diagnostic value for the diagnosis of secondary syphilis.
The etiologic agent of syphilis, Treponema pallidum subsp. pallidum, causes a multistage sexually transmitted infection (STI). During the last decade, there has been an increase in the reported incidence of syphilis in industrialized countries, emphasizing the need for reliable diagnostics for syphilis.
The slow generation time and the inability to survive and multiply outside the mammalian body make T. pallidum unsuitable for in vitro culturing (11). The reliable and fast diagnosis of syphilis and early treatment could improve public health. Until recently, the laboratory diagnosis of syphilis was based on dark-field microscopy and/or syphilis serology. Dark-field microscopy is mainly used for the diagnosis of primary syphilis. For optimal interpretation of the test result, dark-field microscopy requires the laboratory technician performing the microscopy to have a great deal of experience and expertise. In many settings in which patients with (ano)genital ulceration are seen, such as the office of a general practitioner (GP), dark-field microscopy is not available and a definite diagnosis of syphilis depends solely on the clinical picture in combination with the syphilis serology. In the case of primary syphilis, false-negative serological results might specifically occur due to the window period. Consequently, follow-up visits with repeated serological testing are required for a period of 3 months. This might, however, result in a treatment delay, causing disease progression that results in potentially serious health complications and continued transmission. On the other hand, false-positive serological results may occur due to the persistence of antibodies from infections in the past.
A fast and reliable PCR is therefore of great potential value for the diagnosis of primary syphilis, specifically in settings in which it is not possible to perform dark-field microscopy (3). The advantages of a real-time PCR are the ability to detect the pathogen directly, the short turnaround time, and the ease of performance. We previously developed a real-time PCR that targets the polA gene and that is performed with swabs from the (ano)genital ulceration(s) (10).
In the present study, we investigated the clinical value of this T. pallidum real-time PCR with regard to both primary and secondary syphilitic lesions. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the T. pallidum real-time PCR were determined in a clinical setting of a very large, low-threshold outpatient STI clinic in Amsterdam, The Netherlands.
MATERIALS AND METHODS
Study population.
The STI outpatient clinic of the Amsterdam Public Health Service in Amsterdam, The Netherlands, is a low-threshold clinic serving approximately 27,000 clients annually. Clients can visit the STI clinic anonymously, free of charge, and without a referral by a medical doctor. All high-risk clients are routinely screened for Treponema pallidum, Chlamydia trachomatis, and Neisseria gonorrhoeae. Diagnostics for other STIs, such as (ano)genital herpes simplex virus (HSV) infection, are based also on clinical symptoms. A considerable number of clients make multiple visits per year; some of these clients are diagnosed with one or more STIs at a time.
Clients were included consecutively in the study as having suspected cases of primary syphilis if they presented with an (ano)genital ulcer or were included as having suspected cases of secondary syphilis if they presented with a skin manifestation possibly related to secondary syphilis, like macular or papular rashes, condyloma latum lesions, roseolas, mucous patches, or alopecia.
Diagnostic tests. (i) Dark-field microscopy.
Dark-field microscopy was performed for all clients who presented with an (ano)genital ulcer. A microscope equipped with a reflecting dark-field condenser and a ×40 objective was used. Depending on the quality of the ulcer sample collected (no epithelial cells, small amount of erythrocytes), a minimum of two slides and a maximum of four slides were prepared for examination. A trained dermatologist or a trained resident in dermatology examined the dark-field slides, and the result for those found to be positive for T. pallidum were confirmed by another examiner.
(ii) Syphilis serology.
The sera of all clients were tested at the Public Health Laboratory of the Amsterdam Health Service. The syphilis serology included a quantitative T. pallidum particle agglutination (TPPA) test (Serodia-TP.PA; Fujirebio Europe BV) as a primary screening test and both a quantitative rapid plasma reagin (RPR) flocculation test (RPR-Nosticon II; bioMérieux, Boxtel, The Netherlands) and a fluorescent treponemal antibody (FTA) test (Trepo-Spot IF; bioMérieux) as confirmation tests. The FTA and RPR tests were performed with TPPA test-positive sera. To rule out false-positive results, TPPA test titers of <1:1,000 were also confirmed by a supplementary line immunoassay (INNO-LIA syphilis score). All diagnostic tests for treponemal and nontreponemal syphilis were performed according to the manufacturer's instructions.
(iii) T. pallidum real-time PCR.
For testing by the T. pallidum real-time PCR, two dry swab specimens were obtained from the (ano)genital ulcer or skin scraping. Within 24 h, these swabs were transported to the Public Health Laboratory at room temperature. All ulcerations were also examined for HSV infections by real-time PCR (see below). The swabs were combined and eluted in a 600-μl phosphate-buffered salt solution. Part of the T. pallidum eluate (100 μl) was lysed by adding 600 μl of a 5 M guanidine thiocyanate buffer (L6; bioMérieux) containing 0.04 mg/ml glycogen (Roche, Almere, The Netherlands) and was incubated at 65°C for 30 min. T. pallidum chromosomal DNA was precipitated by adding 700 μl isopropanol (−20°C; Merck, Darmstadt, Germany), and the pellet was subsequently washed twice with 500 μl 70% ethanol. The precipitated total DNA was dissolved in 50 μl 10 mM Tris-HCl (pH 8.0) and directly amplified (10).
A real-time PCR assay targeting the polA gene of T. pallidum was performed (10). The forward primer sequence was 5′-GGTAGAAGGGAGGGCTAGTA, and the reverse primer sequence was 5′-CTAAGATCTCTATTTTCTATAGGTATGG. The TaqMan probe sequence was 5′-FAM-ACACAGCACTCGTCTTCAACTCC-BHQ1 (where FAM is 6-carboxyfluorescein and BHQ1 is Black Hole Quencher). After a denaturation cycle of 5 min at 93°C, the amplification profile consisted of 50 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C. The cutoff positive cycle threshold (CT) was <36. CT values of 36 to 40 were considered to be in the gray zone. These samples were retested and were considered positive if the CT value was <40. The amplification was performed in a Rotor-Gene3000 apparatus (Corbett, Westburg, The Netherlands).
The T. pallidum real-time PCR was shown to be specific, as no cross-reactivity with nontreponemal pathogens occurred (10). DNA samples from T. pertenue and T. endemicum, however, could successfully be amplified by the T. pallidum real-time PCR.
HSV-1 and HSV-2 real-time PCR.
The two eluted dry swab specimens of an (ano)genital ulcer or skin scraping obtained for the T. pallidum real-time PCR were also used for an HSV real-time PCR. HSV DNA was released by heat extraction: a 30-μl swab eluate was incubated at 95°C for 15 min.
A real-time PCR assay targeting the gG gene of HSV-1 and the gD gene of HSV-2 was performed (20). For HSV-1, the forward primer sequence was 5′-GGTTCCGACGCCTCAACATAC and the reverse primer sequence was 5′-GGTGTGGATGACGGTGCTG. The TaqMan probe sequence was 5′-HEX-CCGCTGTTCTCGTTCCTCACTGCCTC-TAMRA (where HEX is 4,4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). For HSV-2, the forward primer sequence was 5′-CGCCAAATACGCCTTAGCA, the reverse primer sequence was 5′-GAAGTTCTTCCCGCGAAAT, and the TaqMan probe sequence was 5′-FAM-CTCGCTTAAGATGGCCGATCCCAATC-TAMRA (20). After a denaturation cycle of 2 min at 95°C, the amplification profile consisted of 45 cycles of 15 s at 95°C and 60 s at 45°C. The cutoff positive CT value was <36. The amplification was performed in a Rotor-Gene3000 apparatus (Corbett).
Diagnostic criteria.
To evaluate the clinical value of the T. pallidum real-time PCR for the diagnosis of primary syphilis, we used three different diagnostic algorithms. The first was a head-to-head comparison of the results of dark-field microscopy and those of the T. pallidum real-time PCR (irrespective of previous syphilis episodes or syphilis serology test results), since both are direct treponemal tests.
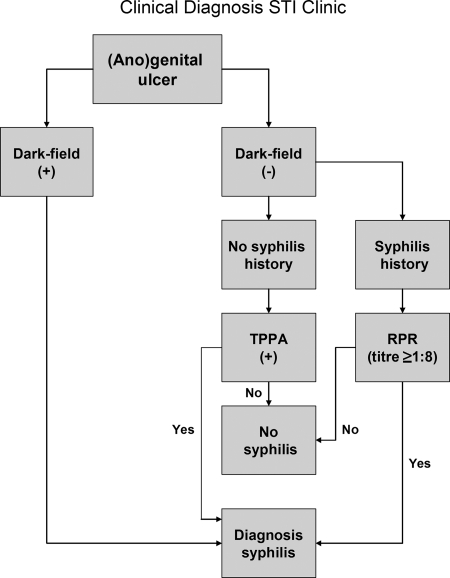
The second algorithm, which we refer to as an STI clinic-based clinical diagnosis, is based on an algorithm conceivable for use in an equipped STI outpatient clinic and includes comparison of the results of dark-field microscopy of the ulcer sample, previous syphilis episodes, and the syphilis serology results with the results of the T. pallidum real-time PCR (Fig. 1).
FIG. 1.
Representation of the procedure used to diagnose primary syphilis in the STI outpatient setting.
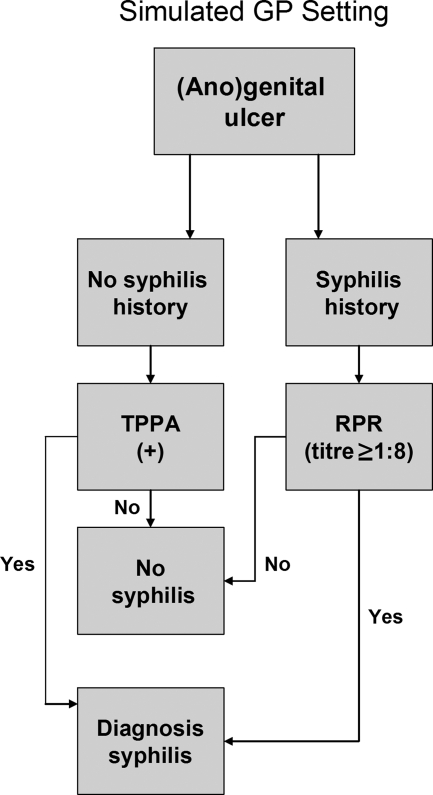
Since the T. pallidum real-time PCR also has great potential for GP, the third algorithm, which we refer to as the clinical diagnosis in a GP setting, simulates a GP setting without the availability of dark-field microscopy and is based only on comparison of the presence of an (ano)genital ulcer, previous syphilis episodes, and syphilis serology results with the results of the T. pallidum real-time PCR (Fig. 2).
FIG. 2.
Representation of the procedure used to diagnose primary syphilis in a simulated GP setting.
To evaluate the clinical value of the T. pallidum real-time PCR with respect to the diagnosis of secondary syphilis, the following diagnostic criteria were used: a skin manifestation possibly related to secondary syphilis, as stated in the inclusion criteria above under “Study population,” and a reactive quantitative RPR test (titer ≥ 1:8).
Statistical analysis.
Evaluation of the clinical accuracy of the T. pallidum real-time PCR was performed by determining the sensitivity, specificity, PPV, NPV, and kappa value (a measurement of agreement). The Wilcoxon signed-rank test was used to describe differences in age between T. pallidum real-time PCR-positive and -negative cases. The Pearson χ2 test was used to examine the differences in the proportion of cases with an HSV-1 and/or HSV-2 infection between groups. Analyses were performed by using SPSS (version 15.0) software (SPSS Inc., Chicago, IL).
RESULTS
During the period from December 2006 to April 2008, a total of 716 suspected cases of primary syphilis and another 133 suspected cases of secondary syphilis were consecutively included. For primary and secondary syphilis, we included 40% and 87% men who have sex with men (MSM), respectively; 30% and 9% men who have sex with women, respectively; and 30% and 4% of women who have sex with men, respectively (Table 1). We identified 93 T. pallidum real-time PCR-positive cases of primary syphilis, and the median age was 42 years (interquartile ratio [IQR], 36 to 47 years) (Table 1). Infections with herpes simplex virus type 1 (17%) or type 2 (28%) and infections with Chlamydia trachomatis (7%) and Neisseria gonorrhoeae (4%) occurred frequently, reflecting the high risk for STIs in this population. The T. pallidum real-time PCR-positive cases were 91% MSM, 7% heterosexual men, and 2% women. Patients with a positive T. pallidum real-time PCR result were significantly older (P < 0.001) than those who tested T. pallidum real-time PCR negative.
TABLE 1.
Characteristics of suspected cases of primary and secondary syphilis and T. pallidum real-time PCR results
| Characteristic | Ulcus episodes (primary syphilis) |
Skin manifestations (secondary syphilis) |
||
|---|---|---|---|---|
| All cases | T. pallidum PCR-positive cases | All cases | T. pallidum PCR-positive cases | |
| Total no. (%) of cases | 716 (100) | 93 (100) | 133 (100) | 34 (100) |
| Age (yr) | ||||
| Median | 32 | 42 | 37 | 39 |
| Interquartile range | 25-41 | 36-47 | 32-45 | 32-47 |
| Sexual orientation (no. [%] of cases) | ||||
| Men who have sex with men | 289 (40) | 85 (91) | 116 (87) | 34 (100) |
| Men who have sex with women | 212 (30) | 6 (7) | 12 (9) | 0 (0) |
| Women who have sex with men | 215 (30) | 2 (2) | 5 (4) | 0 (0) |
| Infections (no. [%] of cases) | ||||
| HSV-1a | 122 (17) | 1 (1) | 3 (2) | 1 (3) |
| HSV-2 | 197 (28) | 0 (0) | 0 (0) | 0 (0) |
| Neisseria gonorrhoeae | 28 (4) | 4 (4) | 13 (10) | 5 (15) |
| Chlamydia trachomatis | 53 (7) | 8 (9) | 15 (11) | 8 (24) |
One patient was infected with both HSV-1 and HSV-2.
For secondary syphilis, 34 cases were found to be positive by the T. pallidum real-time PCR. All of the cases were MSM, and their median age was 39 years (IQR, 32 to 47 years). The characteristics of all patients are summarized in Table 1.
Evaluation of T. pallidum real-time PCR for primary syphilis.
In the comparison of dark-field microscopy and the T. pallidum real-time PCR results, 47 cases tested positive for primary syphilis by both dark-field microscopy and the T. pallidum real-time PCR (Table 2). We found 53 discrepant results between dark-field microscopy and the T. pallidum real-time PCR; 7 cases tested dark-field microscopy positive and T. pallidum real-time PCR negative, while 46 cases tested dark-field microscopy negative and T. pallidum real-time PCR positive. The kappa value was 0.601, indicating fair agreement in the head-to-head comparison between dark-field microscopy and the T. pallidum real-time PCR.
TABLE 2.
Diagnosis of primary syphilis obtained by dark-field microscopy versus that obtained by the T. pallidum real-time PCRa
| T. pallidum real-time PCR result | No. of cases with the following dark-field microscopy result: |
|
|---|---|---|
| + | − | |
| + | 47 | 46 |
| − | 7 | 616 |
The data are for a total of 716 cases. The sensitivity of the T. pallidum real-time PCR was 87.0%, and its specificity was 93.1%. The kappa value was 0.601, which indicates fair agreement between the results of the two tests.
On the basis of the diagnosis made by use of the clinical criteria for primary syphilis used in an STI clinic setting, we found the results for 83 cases to be concordantly positive with those of the T. pallidum real-time PCR, while the results for 41 cases were found to be discordant (Table 3). Of these, 31 cases were positive on the basis of the clinical criteria but T. pallidum real-time PCR negative, while 10 cases were negative on the basis of the clinical criteria but T. pallidum real-time PCR positive. The kappa value was 0.769, indicating good agreement between the diagnosis made by use of the STI clinic algorithm and the result of the T. pallidum real-time PCR. The positive and negative predictive values of 89.2% and 95%, respectively, indicate the clinical usability of the T. pallidum real-time PCR for the diagnosis of primary syphilis.
TABLE 3.
Clinical diagnosis of primary syphilis made in an STI outpatient clinic versus that made by the T. pallidum real-time PCRa
| T. pallidum real-time PCR result | No. of cases with the following clinical diagnosis at STI clinicb: |
|
|---|---|---|
| + | − | |
| + | 83 | 10 |
| − | 31 | 592 |
The data are for a total of 716 cases. The sensitivity of the T. pallidum real-time PCR was 72.8%, the specificity was 95.5%, the PPV was 89.2%, and the NPV was 95.0%. The kappa value was 0.769, which indicates good agreement between the results of the two tests.
The diagnostic criteria for primary syphilis in the STI outpatient clinic setting were dark-field microscopy positive, syphilis serology, and patient history.
On the basis of the diagnosis made by use of the clinical criteria for primary syphilis used in a GP setting, we found the findings for 76 cases to be concordantly positive with those of the T. pallidum real-time PCR (Table 4). Of the 43 discrepant results, 26 cases were positive on the basis of the clinical diagnosis in the GP setting and T. pallidum real-time PCR negative, while 17 cases were negative on the basis of the clinical diagnosis in the simulated GP setting and T. pallidum real-time PCR positive. The kappa value between the results found by use of the criteria in a simulated GP setting and those of T. pallidum real-time PCR was 0.745, which is good agreement.
TABLE 4.
Clinical diagnosis of primary syphilis made by using syphilis serology and patient history as a model for a general practitioner setting versus the result of the T. pallidum real-time PCRa
| T. pallidum real-time PCR result | No. of samples with the following result for clinical diagnosis in GP settingb: |
|
|---|---|---|
| + | − | |
| + | 76 | 17 |
| − | 26 | 597 |
The data are for a total of 716 cases. The sensitivity of the T. pallidum real-time PCR was 75%, and its specificity was 97%. The kappa value was 0.745, which indicates good agreement between the results of the two tests.
Primary syphilis was diagnosed either in patients with a positive TPPA result (irrespective of the RPR test result) without a history of syphilis or in patients with an RPR titer of ≥1:8 and a history of syphilis (Fig. 2).
Genital and anogenital ulcers can be caused by infection with T. pallidum, but in most cases they are caused by an HSV infection; therefore, all swab samples were also examined for both HSV-1 and HSV-2 by a herpes simplex virus type-specific PCR. Among the 716 cases, 122 (17%) were HSV-1 positive and 197 (28%) were HSV-2 positive (Table 1). In one case, an infection with both HSV-1 and HSV-2 was found. We subtabulated the results of both dark-field microscopy and the diagnosis made in the STI clinic setting in relation to the results for HSV and those of the T. pallidum real-time PCR (Table 5). Among the cases confirmed to be positive by both dark-field microscopy and the T. pallidum real-time PCR, only one case was found to be positive by the HSV-1 PCR. Among the dark-field microscopy-negative and the T. pallidum real-time PCR-negative cases, we found 314/616 (51%) HSV-1 and -2 infections (Table 5). Of importance, no HSV-1 and -2 infections were found in the dark-field microscopy-negative and T. pallidum real-time PCR-positive group (0/46). Similarly, when the STI clinic algorithm was used, only 1 (of 83; 1%) HSV-positive case was noted in the T. pallidum real-time PCR-positive group, whereas 306 (of 592; 52%) HSV-1 and HSV-2 infections were found in the STI clinic algorithm-negative and the T. pallidum real-time PCR-negative group. Notably, again, not a single HSV infection (0/10) was found in the STI clinic algorithm-negative and the T. pallidum real-time PCR-positive group. Therefore, for both the dark-field microscopy and the STI clinic algorithms, we found HSV infections significantly more often (P < 0.001) among the T. pallidum real-time PCR-negative cases than among the T. pallidum real-time PCR-positive cases. This shows that most of the time ulcers either are of herpetic origin or are caused by a syphilitic infection and not by double infections with herpes simplex virus and Treponema pallidum.
TABLE 5.
Distribution of HSV-1 and/or HSV-2 infections within the analysis of the results of dark-field microscopy versus those of the T. pallidum real-time PCR and the analysis of the clinical diagnosis by the STI clinic versus the results of the T. pallidum real-time PCRa
| T. pallidum real-time PCR result | No. of samples with positive HSV result/total no. of samples in the group (%) |
|||
|---|---|---|---|---|
| Dark-field microscopy |
Clinical diagnosis of primary syphilis in STI clinic setting |
|||
| Positive | Negative | Positive | Negative | |
| + | 1/47 (2) | 0/46 (0) | 1/83 (1) | 0/10 (0) |
| − | 3/7 (43) | 314/616 (51) | 11/31 (36) | 306/592 (52) |
The data are for a total of 716 cases.
Evaluation of T. pallidum real-time PCR for diagnosis of secondary syphilis.
To evaluate the performance of the T. pallidum real-time PCR for the diagnosis of secondary syphilis, 133 cases were included in the study. A total of 33 cases were positive both for a diagnosis of secondary syphilis and by the T. pallidum real-time PCR. Of the 45 cases with discordant results, 44 were T. pallidum real-time PCR negative and secondary syphilis positive, while only 1 case was possibly missed by use of a diagnosis of secondary syphilis. The kappa value was 0.372, indicating that there was only slight agreement between a diagnosis of secondary syphilis and the T. pallidum real-time PCR result (Table 6).
TABLE 6.
Diagnosis of secondary syphilis made on the basis of suspected skin or mucosal findings plus an RPR titer of ≥1:8 versus the result of the T. pallidum real-time PCRa
| T. pallidum real-time PCR result | No. of samples with the following result for secondary syphilis: |
|
|---|---|---|
| + | − | |
| + | 33 | 1 |
| − | 44 | 55 |
The data are for a total of 133 cases. The sensitivity of the T. pallidum real-time PCR was 43.0%, and the specificity was 98.0%. The kappa value was 0.372, which indicates slight agreement between the results of the two tests.
DISCUSSION
Syphilis is also known as “the great imitator,” and the diagnosis of syphilis is complicated since its clinical manifestations are diverse or may be totally absent (6, 7). Although genital ulcerations can be caused by Treponema pallidum infection, in The Netherlands they are mainly caused by herpes simplex virus types 2 and 1 (1, 3, 14, 15, 18). A proper diagnosis of syphilis therefore requires the interpretation of both the clinical picture with reference to the laboratory results (the findings of syphilis serology and dark-field microscopy) and the patient's history (previous episodes of syphilis and previous syphilis serology) (17).
The T. pallidum real-time PCR is easy to perform in most present-day microbiological laboratory settings (16). Two dry sterile swab specimens are used for ulcer sampling and can be sent, at room temperature, to the laboratory for testing, making this a fast and efficient process. Moreover, when the T. pallidum real-time PCR is available, there is no further need to consider the syphilis serological window period, which can be up to 3 months (8). Our analysis shows that, in addition to practical advantages, there is a good agreement between a clinical diagnosis of primary syphilis in the STI outpatient setting and a clinical diagnosis of primary syphilis in the simulated general practitioner setting (without the availability of dark-field microscopy).
Both T. pallidum real-time PCR and dark-field microscopy identify the spirochete directly without the use of an indirect parameter, such as antibody production (syphilis serology), or possibly biased information, such as the patient's history. Therefore, we first compared the T. pallidum real-time PCR and dark-field microscopy results and found only fair agreement of the results of the two tests for patients with suspected primary syphilis. If the bacterial load is low or if the viability of the treponemes is reduced, such as may occur in older lesions, the sensitivity of dark-field microscopy may be severely reduced to less than 80%. In combination with the usually high sensitivity of the PCR, this lack of agreement can be envisaged (12, 13, 16). The second and third diagnostic algorithms were better approaches for the clinical diagnosis of primary syphilis. Comparison of the result of the T. pallidum real-time PCR to the diagnoses made by use of both of these diagnostic algorithms for primary syphilis showed good agreement, as discussed by Byrt (5). Looking in more detail at the general practitioner setting, we found that 7 of 17 discrepant cases were positive by the T. pallidum real-time PCR and by dark-field microscopy, indicating that the T. pallidum real-time PCR result was truly positive. In these seven cases, an early primary T. pallidum infection within the window of the serological response may have occurred. In the general practitioner setting, performance of the T. pallidum real-time PCR, in addition to syphilis serology, may thus prevent a false-negative diagnosis of syphilis.
On the basis of experience, a considerable number of visitors to the STI outpatient clinic did not return after the initial visit for their test results and could not be traced because they were tested anonymously. Therefore, as standard practice, all patients with a suspected syphilitic lesion and a positive dark-field microscopy result received presumptive treatment, the consequence being that seroconversion to syphilis antibodies or a rise in titer at the end of the window period cannot be registered, even though this would prove with more certainty that the individual had primary syphilis.
Since no laboratory test is perfect, there are several explanations for the false-negative T. pallidum PCR results that we found in our comparisons. There is always the possibility of sampling errors, but dark-field microscopy may also give false-positive results, for example, because commensal spirochetes in a perianal ulceration are falsely classified as Treponema pallidum. False-positive serological results are also known to occur, even if the patient's history is known in some detail. In our study, additional information on the patient's history and follow-up data indicated that 21 of 26 discordant cases, in which the individual was positive by use of the algorithm for the GP setting and T. pallidum real-time PCR negative, had a suspected case of secondary or latent syphilis or had a serology result that corresponded with previous syphilis treatment. Of these 21 cases, 12 cases had a herpes simplex virus infection and 2 cases had lymphogranuloma venereum, which were the more probable causes of ulceration. In four other cases, the serology and history data were inconclusive and in only one case the dark-field microscopy result was positive, supporting the notion that a case was missed by the T. pallidum real-time PCR.
Using the dark-field microscopy and clinical diagnosis algorithms, we observed a significantly higher number of HSV-1 and -2 infections in the T. pallidum real-time PCR-negative group (>50%) than in the T. pallidum real-time PCR-positive group (<1%) (Table 5). Thus, for the cases in which the T. pallidum real-time PCR result was negative, it is reasonable to assume that the (ano)genital ulceration was caused by HSV-1 and/or HSV-2 (3, 9). In Amsterdam, it is standard practice to perform both a T. pallidum and a herpes simplex virus type-specific PCR with the same swab samples from ulcers. In the present study, 44% of the cases suspected of having primary syphilis included were found to have an HSV-1 or an HSV-2 infection.
For a considerable number of individuals with genital ulcers, no causative agent could be isolated, and in our study this was the case for 40% of the individuals, which is highly comparable to the findings of other studies (3, 19).
Secondary syphilis is often missed because of the wide diversity of clinical presentations (2). In contrast, laboratory confirmation of secondary syphilis is not complicated, since it is almost always accompanied by a highly reactive anticardiolipin antibody titer. Consequently, we evaluated our T. pallidum real-time PCR with samples from patients with skin or mucosal lesions suspected of being secondary syphilis. A diagnosis of secondary syphilis was confirmed by an RPR test titer of ≥1:8. Although we found that the T. pallidum real-time PCR had a high specificity (98.0%) for the detection of secondary syphilis, the sensitivity was disappointingly low (43.0%). This might be explained by the low bacterial load present in most secondary syphilitic lesions and/or by the method used to obtain material for PCR (skin scrapings of abraded lesions). In contrast to our data, a study in which directly frozen skin biopsy specimens and a PCR that used the tp47 gene as a target were used to diagnose secondary syphilis reported a sensitivity of 75% (4). On the basis of the low level of agreement, we conclude that the T. pallidum real-time PCR has no added value for the clinical diagnosis of secondary syphilis cases.
In summary, the T. pallidum real-time PCR is a fast and reliable test for the detection of primary syphilis in the STI outpatient clinic setting, as well as in the general practitioner setting. Its added value for the diagnosis of secondary syphilis seems to be limited, however. The earlier diagnosis of primary syphilis enabled by a fast PCR result will lead to timely treatment and the prevention of disease progression, as well as a reduction in the length of exposure of the patient's sexual partners.
Acknowledgments
We thank the public health nurses from the STI clinic (Cluster of Infectious Diseases, Amsterdam Health Service) for collecting samples and the laboratory technicians of the Public Health Laboratory (Cluster of Infectious Diseases, Amsterdam Health Service) for analyzing them. We also thank T. Heijman for assistance with data interpretation and I. Evertse for editing the final manuscript.
We hereby state that we do not have a commercial or other association that might pose a conflict of interest regarding the study presented in this paper.
This study was supported by the RIVM Centre for Infectious Diseases.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Alfa, M. 2005. The laboratory diagnosis of Haemophilus ducreyi. Can. J. Infect. Dis. Med. Microbiol. 16:31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughn, R. E., and D. M. Musher. 2005. Secondary syphilitic lesions. Clin. Microbiol. Rev. 18:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruisten, S. M., I. Cairo, H. Fennema, A. Pijl, M. Buimer, P. G. Peerbooms, E. Van Dyck, A. Meijer, J. M. Ossewaarde, and G. J. van Doornum. 2001. Diagnosing genital ulcer disease in a clinic for sexually transmitted diseases in Amsterdam, The Netherlands. J. Clin. Microbiol. 39:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buffet, M., P. A. Grange, P. Gerhardt, A. Carlotti, V. Calvez, A. Bianchi, and N. Dupin. 2007. Diagnosing Treponema pallidum in secondary syphilis by PCR and immunohistochemistry. J. Invest. Dermatol. 127:2345-2350. [DOI] [PubMed] [Google Scholar]
- 5.Byrt, T. 1996. How good is that agreement? Epidemiology 7:561. [DOI] [PubMed] [Google Scholar]
- 6.DiCarlo, R. P., and D. H. Martin. 1997. The clinical diagnosis of genital ulcer disease in men. Clin. Infect. Dis. 25:292-298. [DOI] [PubMed] [Google Scholar]
- 7.Dourmishev, L. A., and A. L. Dourmishev. 2005. Syphilis: uncommon presentations in adults. Clin. Dermatol. 23:555-564. [DOI] [PubMed] [Google Scholar]
- 8.French, P. 2007. Syphilis. BMJ 334:143-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jethwa, H. S., J. L. Schmitz, G. Dallabetta, F. Behets, I. Hoffman, H. Hamilton, G. Lule, M. Cohen, and J. D. Folds. 1995. Comparison of molecular and microscopic techniques for detection of Treponema pallidum in genital ulcers. J. Clin. Microbiol. 33:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koek, A. G., S. M. Bruisten, M. Dierdorp, A. P. van Dam, and K. Templeton. 2006. Specific and sensitive diagnosis of syphilis using a real-time PCR for Treponema pallidum. Clin. Microbiol. Infect. 12:1233-1236. [DOI] [PubMed] [Google Scholar]
- 11.LaFond, R. E., and S. A. Lukehart. 2006. Biological basis for syphilis. Clin. Microbiol. Rev. 19:29-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen, S. A., B. M. Steiner, and A. H. Rudolph. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie, D. E., F. Azzato, T. Karapanagiotidis, J. Leydon, and J. Fyfe. 2007. Development of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay's performance by comparison with serological testing. J. Clin. Microbiol. 45:93-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay, I. M., G. Harnett, N. Jeoffreys, I. Bastian, K. S. Sriprakash, D. Siebert, and T. P. Sloots. 2006. Detection and discrimination of herpes simplex viruses, Haemophilus ducreyi, Treponema pallidum, and Calymmatobacterium (Klebsiella) granulomatis from genital ulcers. Clin. Infect. Dis. 42:1431-1438. [DOI] [PubMed] [Google Scholar]
- 15.Morre, S. A., J. Spaargaren, J. S. Fennema, and H. J. de Vries. 2005. Molecular diagnosis of lymphogranuloma venereum: PCR-based restriction fragment length polymorphism and real-time PCR. J. Clin. Microbiol. 43:5412-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orle, K. A., C. A. Gates, D. H. Martin, B. A. Body, and J. B. Weiss. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J. Clin. Microbiol. 34:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnam, S. 2005. The laboratory diagnosis of syphilis. Can. J. Infect. Dis. Med. Microbiol. 16:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi, G., E. Allason-Jones, J. Richens, N. T. Annan, D. Hawkins, A. Ekbote, S. Alexander, and J. White. 2009. Lymphogranuloma venereum presenting as genital ulceration and inguinal syndrome in men who have sex with men in London, United Kingdom. Sex Transm. Infect. 85:165-170. [DOI] [PubMed] [Google Scholar]
- 19.Suntoke, T. R., A. Hardick, A. A. Tobian, B. Mpoza, O. Laeyendecker, D. Serwadda, P. Opendi, C. A. Gaydos, R. H. Gray, M. J. Wawer, T. C. Quinn, and S. J. Reynolds. 2009. Evaluation of multiplex real-time PCR for detection of Haemophilus ducreyi, Treponema pallidum, herpes simplex virus type 1 and 2 in the diagnosis of genital ulcer disease in the Rakai District, Uganda. Sex Transm. Infect. 85:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Doornum, G. J., J. Guldemeester, A. D. Osterhaus, and H. G. Niesters. 2003. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J. Clin. Microbiol. 41:576-580. [DOI] [PMC free article] [PubMed] [Google Scholar]