Abstract
It is clear that sex hormones impact ventilation. While the effects of the menstrual cycle, pregnancy, testosterone, and progesterone on resting ventilation have been well documented, effects of sex hormones on the hypoxic (HVR) and hypercapnic ventilatory responses (HCVR) are inconclusive. In addition, in no study have systemic sex steroid hormone levels been measured. Age and sex differences in long-term facilitation in response to episodic hypoxia were found in anesthetized rats. The purpose of the present study was to assess the effects of sex and age [young, 3–4 mo; middle age, 12–13 mo; and old, >20 mo] on the HVR and the HCVR of awake rats relative to systemic hormone levels. Based on findings from long-term facilitation studies, we hypothesized that the HVR would be influenced by both sex and age. We found no age-related changes in the HVR or HCVR. However, female rats have a greater HVR than male rats at old age, and at middle age female rats have a greater HCVR than male rats. Additionally, we found no correlation between the minute ventilation/oxygen consumption and the progesterone-to-estrogen ratio during hypoxia or hypercapnia. However, changes in ventilatory responses with age were not similar between the sexes. Thus it is critical to take sex, age, estrous cycle stage, and systemic hormone levels into consideration when conducting and reporting studies on respiratory control.
Keywords: ventilation, sex hormones, hypoxia, hypercapnia
sex hormones have a significant impact on ventilation in humans. The effects of the menstrual cycle, pregnancy, testosterone, and progesterone on resting ventilation have been well documented (7–9, 12, 25, 27, 31, 37). Studies of (natural) sex hormone effects on ventilatory responses to hypoxia [hypoxic ventilatory response (HVR)] or hypercapnia [hypercapnic ventilatory response (HCVR)] have had mixed results. For example, in some studies, males had a greater HVR than females (10, 31), while in other studies there were no sex differences (14, 21), and still other studies found females to have a greater HVR than males (15, 30). Similarly, the HCVR was either greater in males than in females (23, 24, 31), or no sex differences were found (1, 21). To add to the complexity, Schlenker and Goldman report that the HVR but not the HCVR changed with age (24).
Likely sources of disagreement from these studies may be the range of ages tested (18–87 yr), variable sex hormone levels, especially in older animals (16, 28), and different levels of hormones associated with stages of the menstrual cycle. While some studies report menstrual or cycle stages (4, 14, 31), others do not (1, 10, 21). In no study has a complete sex hormone level panel (estradiol, progesterone, testosterone) been measured systematically in intact subjects. Unfortunately, because circulating sex hormone levels in human subjects were not reported in previous studies, differences in ventilatory responses might reasonably be attributed to differences in hormone levels in pre-, peri-, and postmenopausal women.
Accordingly, the purpose of the present study was to conduct a comprehensive assessment of the effects of age, sex, and systemic hormone levels on the HVR and the HCVR. Although ventilatory measurement is complicated by potential changes in pulmonary mechanics and/or gas exchange with increasing age (11), unanesthetized studies offers the power to assess age- and sex-dependent effects under more physiological (awake, unrestrained) conditions. Based on sex differences and age-associated changes in long-term facilitation (35, 36), we hypothesized that the HVR would be affected by age and sex. We further hypothesized that the sex hormone ratio would affect the HVR or HCVR.
METHODS
Surgical procedures.
All experimental procedures were approved by the Animal Care and Use Committee of the School of Veterinary Medicine at the University of Wisconsin. All animals were given food and water ad libitum, except during plethysmographic studies. Rats were kept on a 12:12-h light-dark cycle (6 AM to 6 PM). At least 1 wk before ventilation studies, sterile surgery was performed for implantation of chronic indwelling femoral arterial catheters and telemetry transducers (Mini-Mitter, Sunriver, OR) in young (Y, 3–4 mo), middle-aged (MA, 12–13 mo), and old (>20 mo) male and female Fisher 344 rats (NIA). Briefly, anesthesia was initiated and maintained with 3–5% isoflurane via muzzle mask inhalation. A 3-cm lateral incision was made at the junction of the leg and abdomen. The femoral artery was dissected, and the distal end tied off. An arterial catheter (PE-50, 50 cm) was inserted into the artery and advanced (2 cm) into the abdominal aorta. The catheter was secured with suture, tunneled dorsally, and externalized on the midline between the scapulas. To implant the telemetry transducer for body temperature measurements, a ventral midline incision (3 cm) was made into the abdominal cavity, the transmitter inserted, and incision closed. Due to intolerance of old rats to these surgical procedures, arterial catheters were implanted in Y and MA rats only. Ventilation was measured 1 wk after surgery.
Experimental procedures.
For plethysmography studies, rats were continuously monitored (see ventilatory and metabolic measures below). Arterial blood was sampled after 35–40 min of normoxia. Rats were then immediately exposed to hypoxia (12% O2, balance N2) for 10 min, followed by a 10-min exposure to normoxia before a final 10-min exposure to hypercapnia (7% CO2). Arterial blood samples were drawn during the final 4 min of exposure to each condition (Y and MA rats only). Immediately following the plethysmography study, a final blood sample (1 ml) was drawn for measurement of hormone levels (all ages). Because of the lack of an indwelling femoral catheter in the old rats, following the plethysmography study, old rats were anesthetized, and blood samples taken directly from the heart for measurement of sex hormone levels.
Arterial blood gases and pH were measured and corrected to body temperature (Radiometer, model 500, Copenhagen, Denmark). Data from the last 20 min of normoxia and the last 4 min (chronic response) of each gas challenge were used for data analysis.
Serum hormone levels.
Blood samples were centrifuged to collect serum, which was then immediately frozen at −80°C. Total testosterone, estradiol, and progesterone levels were analyzed using ELISA (Immuno-Biological Laboratories, Minneapolis, MN; 17β-estradiol, catalog no. RE50421, sensitivity 4.6–3,000 pg/ml; progesterone, catalog no. RE52231, sensitivity 0.05–36 ng/ml; testosterone catalog no. IB79106, sensitivity 0–16 ng/ml).
Ventilatory and metabolic measurements.
Ventilatory and metabolic measurements have been described in detail elsewhere (17). Before each study, the plethysmograph was calibrated by rapid repeated injection of 0.2 ml of air into the chamber. The calibration signal and chamber temperature were used to calculate tidal volume; barometric pressure was measured at the start of each experiment (barometer). Ventilatory and metabolic measurements were made in awake, unrestrained rats during quiet rest in a 2-liter, flow-through plethysmograph that was connected to a compensatory box by a leak with a 10-s half time. Air (100% humidity) was forced through the chamber at a rate of 2 l/min through a high-impedance opening and removed via vacuum through a variable impedance valve. Fluctuations in pressure relative to a 2-liter reference chamber were detected with a pressure transducer (PM15E, Statham Instruments, Hato Rey, PR) and used to calculate respiratory variables. Data were collected on a computer using customized software that provided breath-by-breath analysis of ventilatory variables [frequency, tidal volume, and minute ventilation (V̇e)]. Inflow and outflow gases were monitored by O2 (FCX-MV, Fujikura, Tokyo, Japan) and CO2 (LB-2, Beckman Instruments, Schiller Park, IL) gas analyzers and used to calculate metabolic rates at 10-min intervals.
Data analysis.
For plethysmograph studies, data from the last 4 min of each study (encompassing the times arterial blood samples were taken) were binned into 15-s samples and averaged. Averages were compared using a two-way repeated-measures ANOVA with a Bonferroni t-test for multiple comparisons. For all analyses, P < 0.05 was considered significant. The HVR is defined as the change (Δ) in V̇e-to-oxygen consumption (V̇o2) ratio (ΔV̇e/V̇o2), and the HCVR is defined as ΔV̇e-to-V̇o2-to-Δarterial Pco2 (ΔV̇e/Vo2/ΔPaCO2).
RESULTS
Ventilatory, metabolic, blood gas, and sex hormone data for Y, MA, and old rats in normoxia, hypoxia, and hypercapnia are shown in Table 1. During normoxic baseline conditions, there were significant sex differences and age-related changes in metabolic rates, even when V̇o2 was normalized for body weight. However, the most notable age-related change was an increase in resting PaCO2 in female rats.
Table 1.
Ventilatory, blood gas, and metabolic rate of Y, MA, and old male and female rats during eupnea, hypoxia, and hypercapnia
| Males |
Females | |||||
|---|---|---|---|---|---|---|
| Y | MA | Old | Y | MA | Old | |
| n | 11 | 11 | 6 | 13 | 10 | 5 |
| Weight, g | 283±25.7‡ | 310±15.5‡ | 453±8.4 | 191±5.6*‡ | 189±7.4*‡ | 261±5.8* |
| Normoxia | ||||||
| V̇e, l/min | 253.67±16.35 | 192.49±14.07 | 221.59±37.60 | 147.25±12.26* | 157.35±13.35 | 127.10±20.47 |
| Vt, ml | 1.95±0.14 | 1.86±0.16 | 2.53±0.25 | 1.48±0.08 | 1.57±0.16 | 1.56±0.13 |
| f, breaths/min | 134.8±20.0 | 103.2±6.7 | 78.5±8.1 | 101.5±6.9 | 103.3±5.9 | 83.3±7.3 |
| PaCO2, Torr | 35.6±0.6 | 34.7±1.5 | 33.6±3.0† | 39.8±1.8 | ||
| PaO2, Torr | 86.3±2.6 | 89.8±3.9 | 89.4±2.7 | 90.5±4.7 | ||
| V̇o2, ml·min−1·100 g body wt−1 | 1.8±0.1‡ | 1.7±0.1‡ | 1.12±0.1 | 2.3±0.1*‡ | 2.0±0.2*‡ | 1.19±0.2 |
| V̇o2, ml/min | 4.87±0.36 | 5.21±0.30 | 5.08±0.47 | 4.41±0.10‡ | 3.77±0.23* | 3.11±0.39* |
| V̇co2, ml·min−1·100 g body wt−1 | 1.36±0.1 | 1.18±0.1 | 0.9±0.1 | 1.64±0.1*‡ | 1.54±0.1*‡ | 1.05±0.07 |
| V̇co2, ml/min | 3.63±0.21 | 3.53±0.25 | 4.07±0.40 | 3.12±0.13* | 2.87±0.12* | 2.74±0.17 |
| V̇e/V̇o2 | 47.2±4.20 | 34.4±1.26 | 48.4±7.06 | 38.2±5.06 | 44.4±5.14* | 39.2±3.92 |
| V̇e/V̇co2 | 62.15±4.71 | 50.55±1.54 | 62.05±12.0 | 53.36±6.23 | 56.45±5.89 | 42.70±1.74 |
| Hypoxia | ||||||
| V̇e, l/min | 389.04±32.14 | 315.34±24.34‡ | 541.46±64.67 | 246.00±20.94* | 314.59±31.15 | 333.93±30.74 |
| Vt, ml | 2.31±0.17‡ | 2.23±0.18‡ | 3.68±0.38 | 1.66±0.11* | 1.91±0.18 | 2.34±0.22* |
| f, breaths/min | 172.7±16.9 | 143.90±9.48 | 143.33±3.50 | 149.44±8.79 | 168.67±7.14 | 142.79±5.76 |
| PaCO2, Torr | 27.7±1.10 | 29.9±1.22 | 27.0±1.35 | 28.6±1.84 | ||
| PaO2, Torr | 38.5±1.93 | 40.1±1.41 | 40.9±0.96 | 39.7±1.95 | ||
| V̇o2, ml·min−1·100 g body wt−1 | 1.49±0.09†‡ | 1.23±0.06 | 1.02±0.10 | 1.54±0.07 | 1.47±0.16* | 1.29±0.18* |
| V̇o2, ml/min | 4.02±0.23 | 3.77±0.25 | 4.65±0.26 | 2.91±0.14*‡ | 2.80±0.17*‡ | 3.37±0.15* |
| V̇co2, ml·min−1·100 g body wt−1 | 1.37±0.09 | 1.26±0.06 | 1.80±0.19 | 1.53±0.07 | 1.55±0.06 | 1.20±0.12* |
| V̇co2, ml/min | 3.68±0.14‡ | 3.83±0.17‡ | 4.42±0.20 | 2.91±0.13* | 2.92±0.15* | 3.13±0.07 |
| V̇e/V̇o2 | 96.12±7.54 | 79.0±3.33 | 79.12±8.22 | 82.5±8.74 | 105.23±6.50* | 88.18±10.14 |
| V̇e/V̇co2 | 103.75±7.69 | 77.02±3.94 | 81.57±16.86 | 79.11±5.68* | 100.24±6.52* | 95.02±15.65 |
| CO2 | ||||||
| V̇e, l/min | 969.82±40.99‡ | 812.90±55.52‡ | 1362.25±179.80 | 712.96±75.82*† | 567.24±55.00* | 626.86±81.91* |
| Vt, ml | 5.05±0.35‡ | 4.70±0.39‡ | 7.99±0.80 | 3.82±0.28* | 3.39±0.32* | 4.10±0.22* |
| f, breaths/min | 197.0±6.2 | 177.23±6.33 | 166.32±6.12 | 183.04±6.91 | 170.0±7.36 | 150.30±13.09 |
| PaCO2, Torr | 49.1±0.81 | 53.7±2.09 | 53.44±2.85 | 52.82±2.53 | ||
| PaO2, Torr | 135.3±4.57 | 143.7±5.31 | 146.63±3.56 | 138.3±5.96 | ||
| V̇o2, ml·min−1·100 g body wt−1 | 1.85±0.07†‡ | 1.59±0.10 | 1.35±0.12 | 2.16±0.17 | 2.01±0.15* | 1.74±0.18* |
| V̇o2, ml/min | 4.95±0.17 | 4.85±0.36 | 5.97±0.51 | 4.09±0.29*‡ | 3.40±0.36*‡ | 4.54±0.32* |
| V̇e/V̇o2 | 172.2±11.10 | 143.0±9.08 | 160.78±11.48 | 148.45±15.10* | 139.92±11.52 | 123.12±9.93* |
| Testosterone, ng/ml | 1.30±0.11 | 1.44±0.16 | 1.50±0.33 | |||
| Progesterone, ng/ml | 45.86±7.38 | 62.98±17.95 | 68.9±4.28 | |||
| Estradiol, pg/ml | 7.12±1.89 | 12.23±1.48 | 11.19±1.48 | |||
| P/E, ng/pg | 9.381±3.84 | 5.637±1.76 | 6.235±0.55 | |||
Values are means ± SE; n, no. of rats. Y, young; MA, middle aged; V̇e, minute ventilation; Vt, tidal volume; f, breathing frequency; PaCO2, arterial Pco2; PaO2, arterial Po2; V̇o2, minute O2 consumption; V̇co2, minute CO2 production; P/E, ratio of progesterone to estradiol.
P < 0.05 vs. male of same age;
P < 0.05 vs. MA of same sex;
P < 0.05 vs. old of same sex.
HVR.
As previously discussed by Mortola et al. (15), when considering small mammals, changes in metabolic rate have significant impact on ventilation. Thus we evaluated the change in the ventilatory equivalent (ΔV̇e/V̇o2) as the index of the HVR (Fig. 1A).
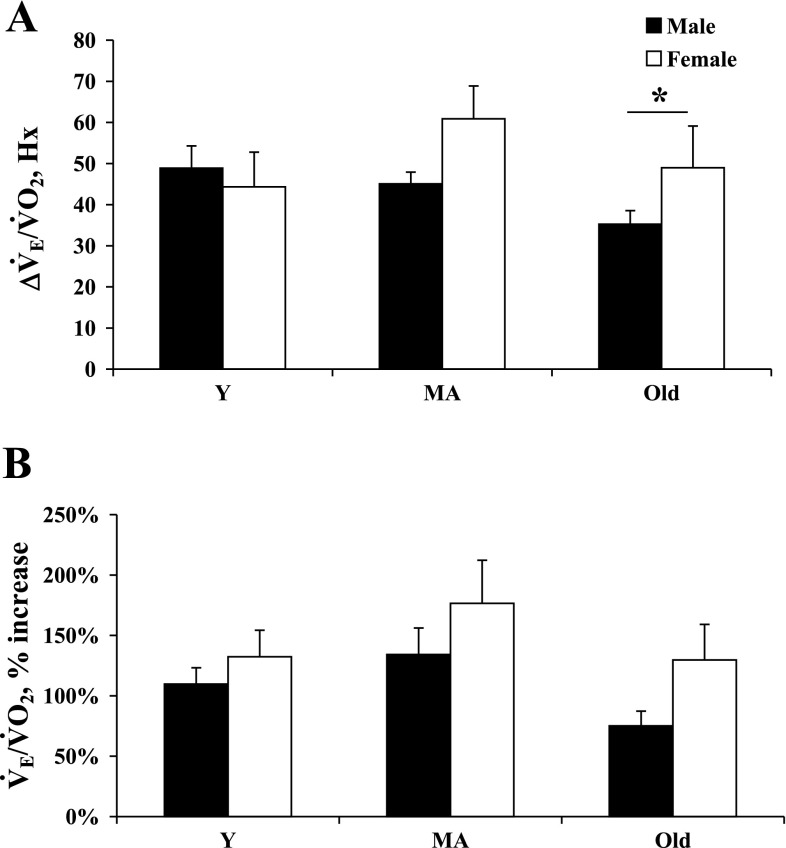
Fig. 1.
Ventilatory response to hypoxia (Hx) reported as the change (Δ) in ventilatory equivalents [Δminute ventilation (V̇e)/O2 consumption (V̇o2), hypoxic ventilatory response (HVR); A], and the percent change in ventilatory equivalent ventilation from control (B) in young (Y), middle-aged (MA), and old male and female rats during eucapnic Hx (inspired O2 fraction = 12%). *P < 0.05.
The most interesting sex-related finding was a greater HVR in old female compared with old male rats (Fig. 1A). Conversely, there were no sex differences in the HVR of Y or MA rats. While MA and old females had a significantly higher (weight-corrected) V̇o2 in hypoxia compared with their male counterparts, there was no difference in the percent change (from control) in V̇o2 in male vs. female rats (MA, 27.6 ± 2.6 vs. 21.2 ± 7.3%; old, 4.7 ± 10.4 vs. 17.6 ± 19%, respectively, Table 1). Finally, while female rats had a significantly higher V̇e/V̇o2 compared with male rats (MA, Table 1), there were no significant sex differences in the percent ΔV̇e/V̇o2 at any age (Fig. 1B).
With regard to age-related changes in hypoxia, we found no significant age-related changes in the HVR per se. We did find that V̇o2 significantly decreased (compared to control) in Y and MA female rats and in MA male rats, but not in Y male rats (P = 0.058) or in old rats of either sex. Of all of the parameters measured, the only age-related effect was a significant percent change of (weight-corrected) V̇o2 in Y and MA vs. old female rats (Y, 33.6 ± 3.6%; MA, 21.2 ± 7.3%; and old, −17.6 ± 18.9%).
HCVR.
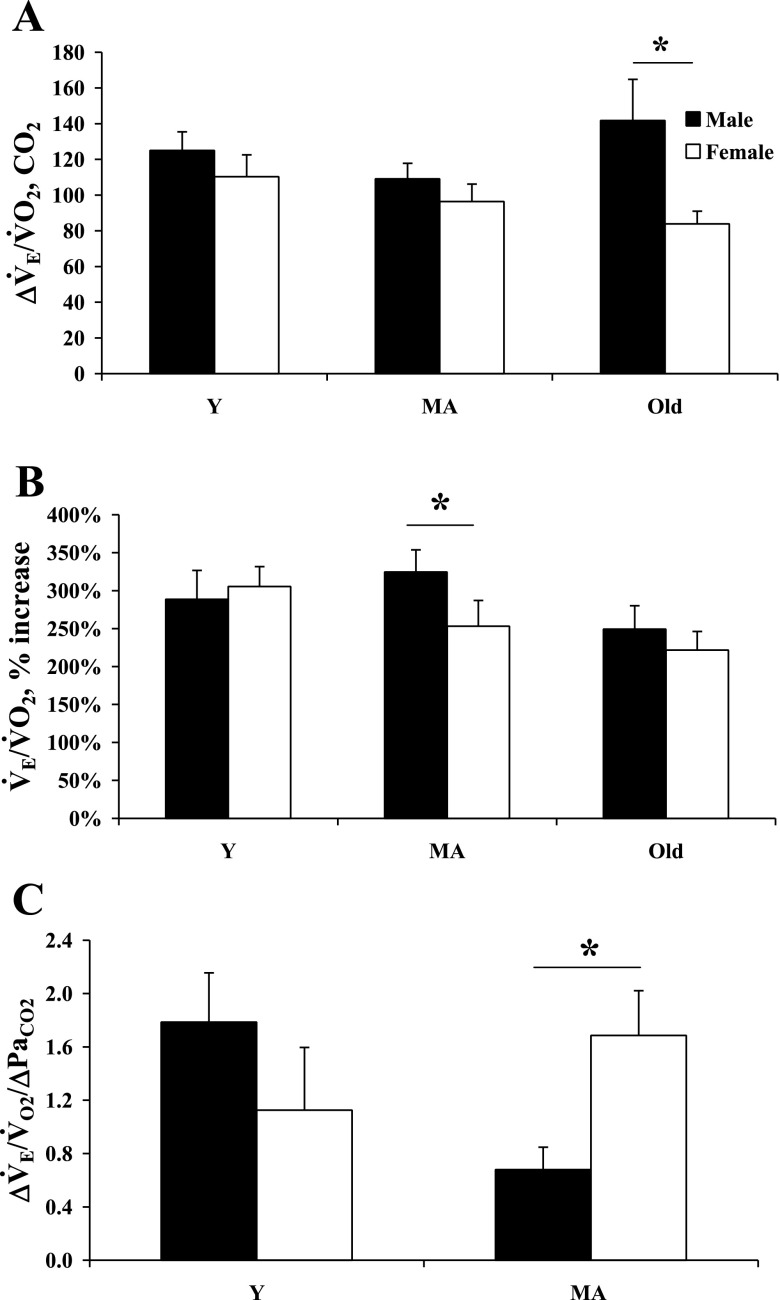
During hypercapnia, female rats had significantly lower V̇e (Y, MA, and old) and V̇e/V̇o2 (Y and old), but higher V̇o2 (MA and old, body weight corrected) compared with age-matched male rats. Old male rats had a significantly greater ΔV̇e/V̇o2 compared with female rats (Fig. 2A); however, the percent increase was not different. While male rats did have a significantly greater percent increase in their V̇e/V̇o2 during hypercapnia compared with female rats (MA, Fig. 2B), when the percent increase of V̇e/V̇o2 is normalized to the change in PaCO2, MA male rats have a lower HCVR than MA female rats (Fig. 2C). Additionally, blood gases during CO2 exposure were similar in all four groups measured (Table 1). It is important to note, however, that potential sex differences in the HCVR in old rats could not be determined due to lack of an arterial blood sample.
Fig. 2.
Ventilatory response to hypercapnia reported as the change in ventilatory equivalents (ΔV̇e/V̇o2; A), the percent change in ventilatory equivalent ventilation from control (B), and the change in ventilatory equivalents normalized to the change in arterial Pco2 (PaCO2) (HCVR; C) in Y, MA, and old male and female rats during hypercapnia (inspired CO2 fraction = 7%). *P < 0.05.
Sex hormones.
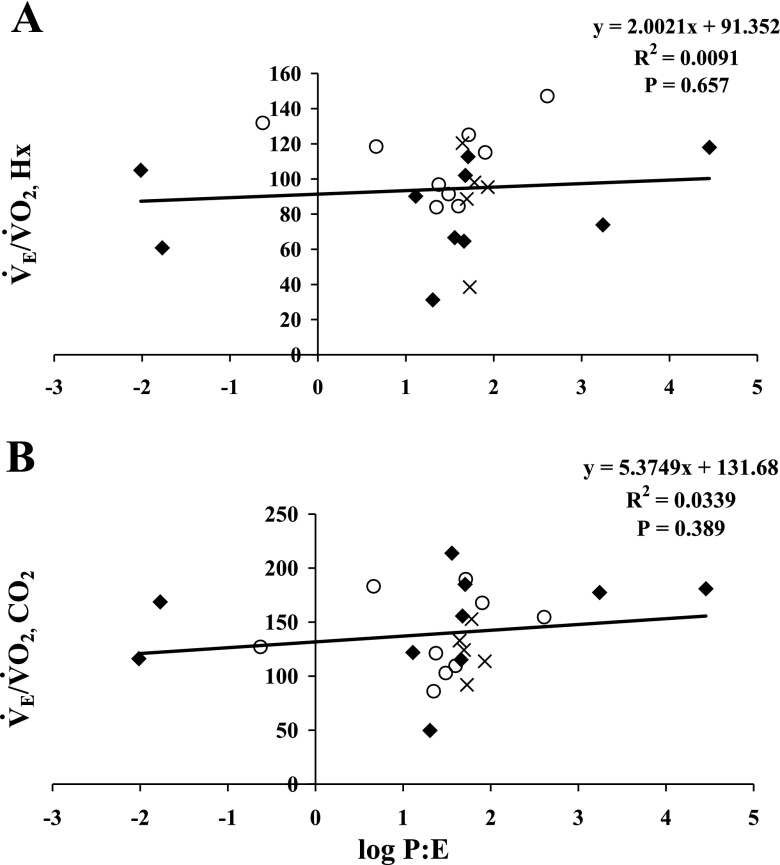
There was no age-associated decline in testosterone levels in male rats (Table 1). Hormone levels in regularly cycling Y and MA female rats varied greatly, while estradiol and progesterone levels in acyclic old female rats were stable (Table 1 and Fig. 3). We found no correlation between the ratio of progesterone to estradiol (P/E) and the HVR or HCVR (Fig. 3, A and B, respectively).
Fig. 3.
The ventilatory equivalents (V̇e/V̇o2) in Hx (A) and in hypercapnia (B) plotted against the log of the ratio of progesterone to estradiol (log P/E) in female rats. ⧫, Y; ○, MA; X, old.
DISCUSSION
Following a history of inconsistent results, no single study can be expected to completely clarify the question of whether there are age-related effects or sex differences in ventilation. However, this study does attempt to provide a more comprehensive and focused look at ventilatory changes that occur with aging in both male and female rats. The major findings of this study are as follows: 1) resting PaCO2 increases with age (Y to MA) in female but not in male rats; 2) the HVR and HCVR are conserved with age in both sexes; 3) there are no sex differences in the HVR in Y and MA rats; 4) the HVR is greater in old female rats than old male rats; and 5) the HCVR is greater in MA female than in MA male rats.
Normoxia.
The most intriguing result from the normoxic data are the age-related decrease in PaCO2 in female rats (Y vs. MA) and the greater V̇o2 in female compared with male rats (Y and MA). The change in PaCO2 with age in female rats is difficult to explain, given that hormone levels in Y and MA female rats are similar, and that resting metabolic rates (V̇o2 and CO2 production) are not different in Y vs. MA female rats. Although not reported in a study by Crapo et al. (5), an age-related increase in resting PaCO2 in MA compared with Y women was observed in a study by Loeppky et al. (Ref. 14; M. Jensen, personal communication). While we found no sex differences in resting blood gases in this study, despite sex differences in resting metabolic rates (Table 1), several investigators report sex differences in resting PaCO2. In those studies, both human and feline males had a higher resting PaCO2 than their respective female counterparts [1, 14, 30 (cat), 31 (human)]. Since a disconnect between resting blood-gas levels and metabolic rates cannot be explained by differences in pH (data not shown), it may be unique to this species or rat strain.
The lack of age-related change in V̇o2 in male rats could simply be attributed to a lack of age-related decline in testosterone levels. Whereas the overall female hormone levels do not appear to be different, the lack of fluctuation of progesterone and estrogen levels in acyclic old female rats may contribute to the age-related decrease in resting V̇o2 (Y and MA vs. old) in female rats.
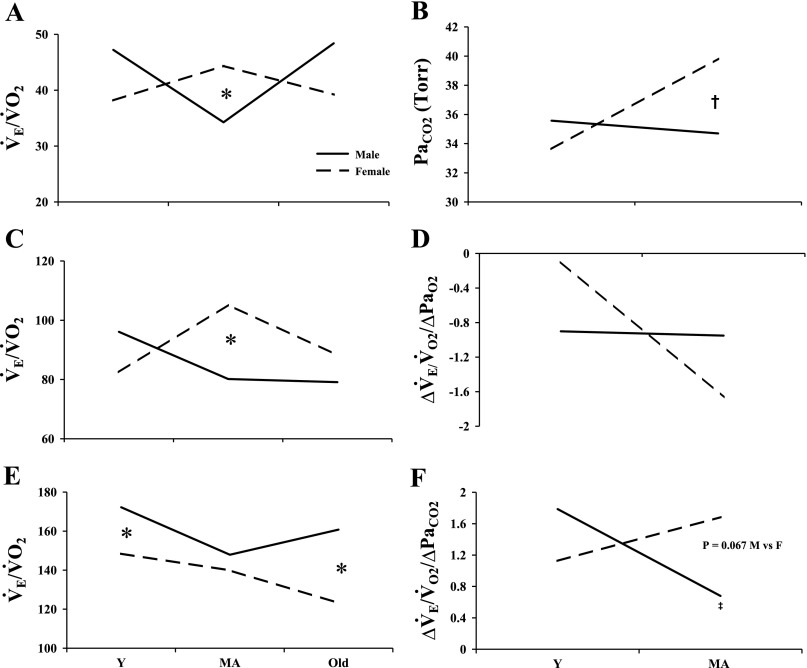
Finally, there are some subtle age-related ventilatory changes in male and female rats. Most interesting is that the pattern of ventilatory age-related changes is not similar between the sexes. In ventilation for example, V̇e in male rats is lowest at middle age, whereas, in female rats, V̇e peaks at middle age (Table 1). When metabolic changes are also considered, the character of the age-related changes is altered such that, at middle age, males have a nadir, while females reach a peak. This sex difference at MA is significant (V̇e/V̇o2, Table 1, Fig. 4). To our knowledge, we are the first to demonstrate such consistent ventilatory and metabolic changes over a wide range of ages in both sexes.
Fig. 4.
General pattern of age-related changes in ventilation in Y, MA, and old male (solid lines) and female rats (dashed lines) during normoxic (A and B), hypoxic (C and D), and hypercapnic (E and F) breathing. *P < 0.05, male vs. female; ‡P < 0.05, Y vs. MA male; †P < 0.05 Y vs. MA female. PaO2, arterial Po2.
Hypoxia.
The published data on sex differences in the HVR are conflicting. While some report a greater HVR in females [1 (human), 15 (rat), 30 (cat)], some report a greater HVR in (human) males (10, 31), and yet others report no sex differences in the HVR (humans; Refs. 14, 21). A factor contributing to disparities in these findings is the wide range of ages of the subjects.
The only sex difference we found within the HVR occurred in old rats. Thus our data agree with those who report no sex difference in the HVR in MA subjects (14, 21, 24), but contrasts with findings by others who found sex differences in the HVR (1, 15, 30–32). The assorted findings cannot be explained by differences in how HVR was reported or defined [V̇e/V̇o2 vs. A (slope of the hyperbolic curve) vs. Pa − Pa vs. A/(V̇i·kg)], where Pa is alveolar pressure, Pa is arterial pressure, and V̇i is inspiratory flow, since reporting methods in some studies were used in both categories of findings (sex differences vs. no sex differences). It is important to note that, in the study by Tatsumi et al. in cats, the HVR is reported as A/(V̇i·kg) (30). In that same study, however, they report no significant difference in the slope of the hyperbolic curve of the HVR (A). In recent studies by Davis et al. (6) and Penatti et al. (19), not normalizing to body weight is suggested to be a more appropriate means of reporting ventilatory responses. Thus the result of no sex difference in A in the study by Tatsumi et al. (30) may be the more accurate finding, and we might argue that Tatsumi et al. actually found no sex differences in the HVR of adult cats.
While some report age-related changes in the HVR in both rat and human studies (13, 20, 24), we found no age-related change in either sex; thus our data are in agreement with findings of Rubin et al. (22). However, while we found no significant age-related changes in the HVR per se, in hypoxia, we did find consistent age-related changes in the ventilatory response to hypoxia that were unique to each sex. For example, frequency and V̇e/V̇o2 in male rats peaked in Y male rats, while in female rats they peaked at MA. Why these age-related changes occur is not clear, since testosterone levels in male rats and estrogen and progesterone levels in female rats were not different between Y and MA animals (Table 1 and Fig. 3). One possible explanation could be a sex difference in serotonin receptor density. Recently, Seebart et al. (26) demonstrated significant differences in 5-HT2A receptor immunoreactivity within a respiratory-related nucleus (hypoglossal) in male and female rats. They also found a significant age-related increase in 5-HT2A receptors in females but not in males. These sex-specific, age-associated changes in receptor density could contribute to the sex difference in HVR in old rats.
Hypercapnia.
The published data on sex-related differences in the HCVR are also conflicting. Some report no sex difference in the HCVR (1, 21, 24) (21.6–36 mmHg CO2), while others report males to have a greater HCVR than females (18, 23, 31). On the other hand, we report here that MA female rats have a significantly greater HCVR than male rats. One possible explanation for this could be the metrics by which HCVR is being reported [slope/CO2 production (1), slope of CO2 (21, 31), ΔV̇e/inspired Pco2 (24), ΔV̇e/Δexpired Pco2 (Ref. 23; in hyperoxic hypercapnia) vs. ΔV̇e/V̇o2/ΔPaCO2 (present study)].
Age-related HCVR decreases have been reported in humans, but these differences were found between MA and old subjects (4, 13, 20, 22). Additionally, in some studies, both men and women were included (4, 20). In contrast, Schlenker and Goldman (24) reported an age-related decrease in CO2 responsiveness (in male rats), but this difference was only apparent at low levels of CO2 (0–3%). At higher levels of CO2 (3–5%), closer to levels used in our study (7% CO2), no age-related differences were reported.
Similar to the results reported here under normoxic and hypoxic conditions, in hypercapnia, we found consistent age-related changes in the ventilatory response that were unique to each sex (Fig. 4). Moreover, the age-related and sex-specific responses to hypercapnia were different from the age-related response patterns we found in hypoxia. For example, in hypoxia, during the ventilatory response at MA, male rats reached a nadir, while female rats reached a peak. However, in hypercapnia, ventilatory peaks occurred in the Y age group in both male and female rats. Additionally, the ventilatory response to CO2 (both V̇e and V̇e/V̇o2) rebounded in old male rats, such that old male rats had a significantly higher ventilatory response to CO2 than did old female rats.
Hormones.
Because of the close proximity of surgery to plethysmography studies (1 wk), the time needed to establish a clear estrous cycle (2–3 wk), and the possibility of surgery interfering with or interrupting the estrous cycle, we did not take daily vaginal smears to determine estrous cycle stage, but rather relied on blood hormone (estrogen and progesterone) measurements. Based on hormone profiles, there were no significant correlations between P/E and the HVR or HCVR. While others have shown decreased testosterone levels with age (16, 33), we were unable to detect an age-related decrease in testosterone levels in these male rats. However, testosterone levels reported here (1.30 ± 0.11 ng/ml, Y; 1.44 ± 0.16 ng/ml, MA; and 1.50 ng/ml, old) are similar to those reported by Zabka et al. (33), in MA Fisher 344 male rats. The lack of an age-related decrease in testosterone level in the male rats in the present study may be due to strain or colony differences. Nevertheless, the stable systemic testosterone levels may help explain the lack of age-related effects on the HVR and HCVR in male rats.
In Y and MA female rats, progesterone and estradiol levels fluctuate during the estrous cycle (4–5 days). In contrast to Zabka et al. (34), who reported a negative correlation between hormone levels (P/E) and hypoglossal and phrenic long-term facilitation in response to episodic hypoxia in anesthetized female rats, we found no correlation between the P/E and the HVR or the HCVR in female rats. However, the estrous cycle has been shown to influence eupneic breathing and ventilatory responses (14, 29, 31, 35); thus we cannot rule out the possibility that, during wakefulness, estrous cycle stage may have a stronger correlation with ventilatory responses than systemic hormone levels.
Furthermore, measurements of circulating sex hormone levels are, at best, a physiological snapshot taken many hours after those hormones have exerted their effect in the tissue of interest. Additionally, the absolute levels of progesterone and estradiol in respiratory motor nuclei at the time of the experiment may differ considerably from serum levels.
Conclusion.
From this study, it appears that both the HVR and HCVR are well conserved with age. Sex differences in the HVR were not apparent until old age (female > male), while sex differences in the HCVR were seen at middle age (female > male). It is also important to note that breathing during rest, as well as during hypoxic or hypercapnic challenge, changes with age, that age-related changes are not necessarily gradual, and that age-related changes are different in males and females (see Fig. 4). The lack of correlation between the P/E and the HVR or the HCVR in female rats may indicate that estrous cycle status and not systemic hormone levels may have a stronger correlation with ventilatory drives. Thus we recommend that, when conducting studies on female subjects, it is important to stage the estrous cycle (daily vaginal smear for 2–3 wk) to determine whether rats are cycling normally. Knowing the estrous cycle stage might provide additional insight into the physiological effects observed. Additionally, it is also important to measure systemic sex hormone levels to determine that they are within normal range, as well as to help identify the transition between estrus and diestrus.
REFERENCES
- 1.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol 60: 1894–1899, 1986. [DOI] [PubMed] [Google Scholar]
- 2.Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience 130: 725–734, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Behan M, Zabka A, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131: 65–77, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Brischetto MJ, Millman RP, Peterson DD, Silage DA, Pack AI. Effect of aging on ventilatory response to exercise and CO2. J Appl Physiol 56: 1143–1150, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med 160: 1525–1531, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097–1103, 2006. [DOI] [PubMed] [Google Scholar]
- 7.England SJ, Farhi LE. Fluctuations in alveolar CO2 and in base excess during the menstrual cycle. Respir Physiol 26: 157–161, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Goodland RL, Pommerenke WT. Cyclin fluctuation of the alveolar carbon dioxide tension during the normal menstrual cycle. Fertil Steril 3: 394–398, 1952. [DOI] [PubMed] [Google Scholar]
- 9.Hasselbach KA Ein Beitrag zur Respirationsphysiologie der Gravidität. Skand Arch Physiol 27: 1–12, 1912. [Google Scholar]
- 10.Hirshman CA, McCullough RE, Weil JV. Normal values for hypoxic and hypercapnic ventilaroty drives in man. J Appl Physiol 38: 1095–1098, 1975. [DOI] [PubMed] [Google Scholar]
- 11.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 13: 197–205, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S, Guerra LF. Effects of domperidone and medroxyprogesterone acetate on ventilation in man. Respir Physiol 81: 359–370, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest 52: 1812–1819, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeppky JA, Scotto P, Charlton GC, Gates L, Icenogle M, Roach RC. Ventilation is greater in women than men, but the increase during acute altitude hypoxia is the same. Respir Physiol 125: 225–237, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respir Physiol 106: 21–34, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Neill JD (Editor). Knobil and Neill's Physiology of Reproduction (3rd Ed.). Amsterdam: Elsevier, 2006, p. 1027.
- 17.Olson EB, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol 64: 666–671, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Patrick JM, Howard A. The influence of age, sex, body size and lung size on the control and pattern of breathing during CO2 inhalation in Caucasians. Respir Physiol 16: 337–350, 1972. [DOI] [PubMed] [Google Scholar]
- 19.Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol 101: 1177–1188, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis 124: 387–391, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Rebuck AS, Kangalee M, Pengelly LD, Campbell EJ. Correlation of ventilatory responses to hypoxia and hypercapnia. J Appl Physiol 35: 173–177, 1973. [DOI] [PubMed] [Google Scholar]
- 22.Rubin S, Tack M, Cherniack NS. Effect of aging on respiratory responses to CO2 and inspiratory resistive loads. J Gerontol 37: 306–312, 1982. [DOI] [PubMed] [Google Scholar]
- 23.Saunders NA, Heilpem S, Rebuck AS. Relation between personality and ventilatory response to carbon dioxide in normal subjects: a role in asthma? Br Med J 18: 719–21, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlenker EH, Goldman M. Ventilatory responses of aged male and female rats to hypercapnia and to hypoxia. Gerontology 31: 301–308, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Schoene RB, Robertson HT, Pierson DJ, Peterson AP. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J Appl Physiol 50: 1300–1305, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Seebart B, Stoffel RT, Behan M. Age-related changes in the serotonin 2A receptor in the hypoglossal nucleus of male and female rats. Respir Physiol Neurobiol 158: 14–21, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skatrud JB, Dempsey JA, Kaiser DG. Ventilatory response to medroxyprogesterone acetate in normal subjects: time course and mechanism. J Appl Physiol 44: 393–344, 1978. [DOI] [PubMed] [Google Scholar]
- 28.Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav 26: 110–135, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Takano N Changes of ventilation and ventilatory response to hypoxia during the menstrual cycle. Pflügers Arch 402: 312–316, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Influences of gender and sex hormones on hypoxic ventilatory response in cats. J Appl Physiol 71: 1746–1751, 1991. [DOI] [PubMed] [Google Scholar]
- 31.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol 54: 874–879, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Wood SC The effect of gender on ventilatory drive, body temperature, and hypoxia tolerance of rats. In: Hypoxia: Women at Altitude, edited by Houston C and Coates G. Burlington, VT: Queen City Printers, 1998, chapt. 14.
- 33.Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol 563: 557–568, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabka AG, Mitchell GS, Olson EB Jr, Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol 95: 2614–2623, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol 91: 2831–2838, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol 531: 509–514, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwillich CW, Natalino MR, Sutton FD, Weil JV. Effects of progesterone on chemosensitivity in normal men. J Lab Clin Med 92: 262–269, 1978. [PubMed] [Google Scholar]