Abstract
Purpose
Dasatinib is effective therapy for chronic myeloid leukemia (CML) after imatinib failure. In this study, we investigate the efficacy of dasatinib as initial therapy for patients with CML in early chronic phase.
Patients and Methods
Patients with newly diagnosed CML in early chronic phase were randomly assigned to receive dasatinib 100 mg once daily or 50 mg twice daily as initial therapy.
Results
Among 50 patients observed for at least 3 months, 49 patients (98%) achieved a complete cytogenetic response (CCyR), and 41 patients (82%) achieved a major molecular response (MMR). Responses occurred rapidly, with 94% of patients achieving CCyR by 6 months. There was no difference in response rate by treatment arm. The projected event-free survival rate at 24 months is 88%, and all patients are alive after a median follow-up time of 24 months. Grade ≥ 3 neutropenia and thrombocytopenia occurred in 21% and 10% of patients, respectively. Nonhematologic toxicity was usually grade 1 to 2. There was no significant difference in toxicity between the two arms, and the actual median dose at 12 months was 100 mg (range, 20 to 100 mg).
Conclusion
Dasatinib is an effective agent for the initial management of CML in early chronic phase, producing high rates of CCyR and MMR.
INTRODUCTION
Imatinib is the standard front-line therapy for Philadelphia chromosome (Ph) –positive chronic myeloid leukemia (CML). In early chronic-phase CML, a complete cytogenetic response (CCyR) is achieved in 82% of patients, with a 7-year projected event-free survival (EFS) rate of 81%.1 Most patients who achieve CCyR maintain this response, but eventually, some patients require alternative therapies because of loss of response or progression to accelerated phase (AP) or blastic phase (BP). The projected 7-year survival rate of 86% with imatinib1 compares favorably to the historical median survival time of 4 to 5 years in the preimatinib era.2,3 Despite these excellent results, a significant proportion of patients do not achieve optimal outcomes on imatinib, including patients who do not achieve CCyR (18% in the International Randomized Study of Interferon Versus STI571 [IRIS] trial), patients who experience relapse after CCyR (approximately 10%), and patients who discontinue therapy for safety reasons or intolerance (approximately 4% to 8%). Thus, it is desirable to improve the long-term outcome in CML by improving initial therapy.
High-dose imatinib has resulted in earlier cytogenetic responses, but the impact on long-term outcome remains to be established.4,5 Using second-generation tyrosine kinase inhibitors (dasatinib, nilotinib, and bosutinib)6 as initial therapy is another approach to improve long-term outcome. Potential advantages of these agents include their increased kinase inhibitory potency against mutated or unmutated BCR-ABL and their favorable toxicity profile.7 The clinical benefit of these agents in CML patients after imatinib failure has already been established.8–10
Dasatinib, a potent inhibitor of ABL, is approximately 300 times more potent in vitro than imatinib. It is active against most BCR-ABL mutants with the exception of T315I.11 Dasatinib also inhibits SRC family of kinase (SFK), c-kit, and platelet-derived growth factor receptor.12 After imatinib failure, dasatinib therapy induces CCyR in approximately 50% of patients, with a 2-year progression-free survival rate of 81%.9 We hypothesized that dasatinib could improve the outcome in CML when used as initial therapy.
PATIENTS AND METHODS
From November 2005 to March 2009, patients with chronic-phase CML referred to our institution were offered front-line dasatinib therapy. To be eligible, patients should have been diagnosed within 6 months from the start of study treatment. Patients should have received no prior CML therapy other than hydroxyurea or a maximum of 1 month of therapy with standard-dose imatinib. Other eligibility criteria included age ≥ 18 years, performance status of 0 to 2, and adequate organ functions. Patients with clonal evolution were eligible provided they met all other criteria for chronic phase (blasts < 15%, blasts plus promyelocytes < 30%, basophils < 20%, and platelets > 100 × 109/L unless related to therapy). Patients with cardiac conditions including uncontrolled angina, congenitally prolonged QTc or pretreatment QTc more than 450 milliseconds, uncontrolled hypertension, or history of clinically significant ventricular arrhythmias were excluded. The study was approved by the institutional review board; all patients signed an institutional review board–approved informed consent.
Patients were randomly assigned to receive dasatinib 100 mg once daily or 50 mg twice daily. Treatment was continued until disease progression or unacceptable toxicity. Patients with grade 3 to 4 nonhematologic toxicity had treatment transiently interrupted, and dasatinib was restarted (after resolution to grade ≤ 1) at one dose level reduction to 80 mg once daily or 40 mg twice daily. For hematologic toxicity, treatment was interrupted for grade 4 neutropenia (neutrophils < 0.5 × 109/L) or thrombocytopenia (platelets < 40 × 109/L). Treatment was restarted at the same dose if recovery to above these levels occurred within 2 weeks. Treatment was restarted with a reduction of one dose level if recovery time was more than 2 weeks.
Patients had a CBC and blood chemistry every 1 to 2 weeks for the first 3 months and then every 6 weeks. Bone marrow aspiration with cytogenetic analysis and peripheral-blood quantitative reverse transcription polymerase chain reaction for BCR-ABL fusion transcripts were performed at baseline, every 3 months for 1 year, and every 6 months thereafter. Response criteria were as previously defined.13 Cytogenetic response was based on G-banding with at least 20 metaphases counted and categorized as complete (0% Ph-positive metaphases), partial (1% to 35% Ph-positive metaphases), and minor (36% to 95% Ph-positive metaphases). A major molecular response (MMR) was defined as a BCR-ABL/ABL transcript ratio of less than 0.1% (international scale), and complete molecular response was defined as undetectable BCR-ABL transcripts with a detection sensitivity of ≥ one in 105 cells.14 Patients with no CCyR by 6 months after treatment had sequencing of the ABL kinase domain (codons 220 to 500) after standard two-step quantitative reverse transcription polymerase chain reaction amplification of the BCR-ABL fusion transcript.
Levels of activated (phosphorylated) ABL kinase and a BCR-ABL target (CrkL) were measured in pretreatment and post-treatment (1, 3, 6, 12, and 18 months) protein lysates isolated from peripheral-blood leukocytes. Multiprobe Western blot analysis was performed using phospho (p) -ABL (Tyr245) and p-CrkL (Tyr207) antisera (Cell Signaling Technology, Danvers, MA), with normalization to levels of β-actin protein (AC-15; Sigma-Aldrich, St Louis, MO). Levels of p-LYN detected by anti-Tyr507 antisera (Cell Signaling Technology) in unsorted blood leukocytes or magnetic bead antibody-purified T cells and myeloid cells were used as a control for phosphoprotein preservation in all samples (EasySep kits; StemCell Technologies, Vancouver, British Columbia, Canada).
Statistical Analysis
The primary objective was to improve the MMR rate at 12 months from the expected 40% based on historical experience with standard-dose imatinib.15 Two end points were monitored, the MMR probability at 12 months and the toxicity rate. The goal was to determine whether dasatinib would improve the MMR rate while maintaining an acceptable toxicity rate. A maximum of 50 patients could be treated per arm, with early stopping rules for response and toxicity. The stopping rules for response were to be applied 12 months after the first patient was accrued to the study (and every 3 months thereafter), whereas the toxicity rules were applied 3 months after the first patient was accrued (and every 3 months thereafter). The distributions of time-to-event end points (overall survival and EFS) were estimated using the Kaplan-Meier method. Survival was measured from the time treatment was started to the date of death from any cause or date of last follow-up. EFS was measured from the start of treatment to the date of any of the following events while on therapy: death from any cause, loss of complete hematologic response (CHR), loss of complete cytogenetic response, discontinuation of therapy for toxicity or lack of efficacy, or progression to AP or BP. Transformation-free survival was measured from the start of therapy to the date of transformation to AP or BP while on therapy or to the date of last follow-up.
RESULTS
A total of 62 patients were enrolled (31 in each arm). The median time from diagnosis to treatment was 0.9 months (range, 0 to 6 months), and 19 patients (31%) had received imatinib before the start of dasatinib for a median of 17 days (range, 3 to 30 days). Patient characteristics were similar in the once-daily and twice-daily schedules except for WBC (Table 1). The median follow-up time for all patients is 24 months (range, 1 to 39 months).
Table 1.
Demographic and Clinical Characteristics of the Study Group at the Start of Dasatinib Therapy
| Characteristic | Total (N = 62) |
Twice-Daily Treatment (n = 31) |
Once-Daily Treatment (n = 31) |
P | |||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | ||
| Age, years | 47 | 18-76 | 46 | 18-70 | 47 | 22-76 | .72 |
| Splenomegaly | .79 | ||||||
| No. of patients | 21 | 10 | 11 | ||||
| % | 34 | 32 | 35 | ||||
| Hemoglobin, g/dL | 11.9 | 6.7-14.9 | 11.7 | 6.7-14.8 | 12.0 | 8.4-14.9 | .15 |
| WBC, × 109/L | 22.2 | 0.8-193 | 13.5 | 0.8-112.1 | 37.3 | 4.4-193 | .01 |
| Platelets, × 109/L | 315 | 94-1,906 | 278 | 131-1,769 | 384 | 94-1,906 | .36 |
| Peripheral blood blasts, % | 0 | 0-5 | 0 | 0-3 | 0 | 0-5 | .33 |
| Peripheral blood basophils, % | 3 | 0-17 | 4 | 0-18 | 3 | 0-17 | .27 |
| Bone marrow blasts, % | 2 | 0-8 | 2 | 0-6 | 2 | 0-8 | .86 |
| Bone marrow basophils, % | 3 | 0-12 | 3 | 0-12 | 2 | 0-6 | .11 |
| Sokal risk group | .75 | ||||||
| Low | |||||||
| No. of patients | 50 | 26 | 24 | ||||
| % | 81 | 84 | 77 | ||||
| Intermediate | |||||||
| No. of patients | 8 | 3 | 5 | ||||
| % | 13 | 10 | 16 | ||||
| High | |||||||
| No. of patients | 4 | 2 | 2 | ||||
| % | 6 | 6 | 6 | ||||
| Ph-positive metaphases at start of therapy, % | 100 | 10-100 | 100 | 10-100 | 100 | 81-100 | .37 |
| Clonal evolution | .97 | ||||||
| No. of patients | 4 | 2 | 2 | ||||
| % | 7 | 6 | 6 | ||||
Efficacy
One patient (once-daily schedule) discontinued therapy after three doses for personal reasons, and 11 patients have been observed for less than 3 months (too early to be evaluated for response). Among the 45 patients who started therapy not in CHR, 45 (100%) achieved CHR, with a median time to CHR of 4 weeks (range, 0.3 to 24 weeks). Fifty patients have been observed for ≥ 3 months and are thus evaluable for cytogenetic and molecular response. Among these patients, 49 (98%) achieved a CCyR at any time. The patient who never achieved CCyR had grade 3 thrombocytopenia after 13 weeks of therapy requiring a prolonged treatment interruption (63 days). The best response for this patient was a transient minor cytogenetic response (70% Ph positive); no mutation was detected at diagnosis or at 6 and 12 months after treatment. An MMR was achieved in 41 (82%) of 50 evaluable patients, and complete molecular response was achieved in five patients (10%). The median time to CCyR was 3 months (range, 3 to 9 months), and the median time to MMR was 6 months (range, 3 to 18 months).
Table 2 lists the rates of cytogenetic response over time. For this analysis, all 50 evaluable patients are kept in the denominator up to the time of their last follow-up, with patients who discontinued therapy included up to the follow-up time that would correspond to the time of this report had they stayed on the study. Cytogenetic responses occurred early, with 41 patients (82%) achieving CCyR by 3 months and 46 patients (94%) achieving CCyR by 6 months (92% in the once-daily arm and 96% in the twice-daily arm). There was a rapid reduction in transcript levels, with a median BCR-ABL/ABL transcript ratio of 0.042 at 6 months and a steady but slower decline thereafter (Table 2). The MMR rate was 71% at 12 months and 79% at 18 months.
Table 2.
Cytogenetic and Molecular Response to Dasatinib Over Time
| Time on Therapy (months) | Cytogenetic |
Molecular |
||||
|---|---|---|---|---|---|---|
| No. of Patients at Risk | Major Response (%) | Complete Response (%) | No. of Patients at Risk | Major Response (%) | Complete Response (%) | |
| 3 | 50 | 96 | 82 | 50 | 24 | 0 |
| 6 | 49 | 98 | 94 | 49 | 63 | 0 |
| 12 | 42 | 98 | 98 | 42 | 71 | 7 |
| 18 | 35 | 91 | 89 | 34 | 79 | 6 |
| 24 | 31 | 87 | 84 | 31 | 87 | 6 |
| 30 | 23 | 83 | 83 | 21 | 81 | 0 |
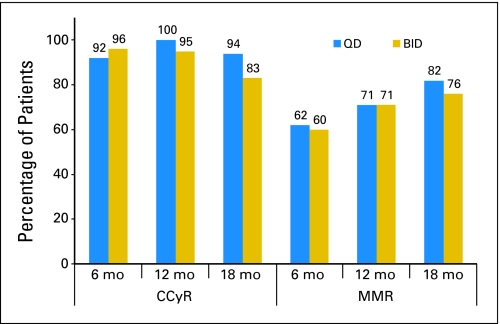
The overall rate of CCyR was 100% for the once-daily arm and 96% for the twice-daily arm (P = 1.00); the corresponding MMR rates were 79% and 85% (P = .72), respectively. There was no significance difference in response by treatment arm at different times. At 12 months, CCyR had been achieved in 100% of patients in the once-daily arm and 95% of patients in the twice-daily arm (P = 1.00), and MMR had been achieved in 71% and 71% of patients (P = 1.00), respectively. At 18 months, CCyR had been achieved in 94% of patients in the once-daily arm and 83% of patients in the twice-daily arm (P = .6), and MMR had been achieved in 82% and 76% of patients (P = 1.00), respectively (Fig 1).
Fig 1.
Cytogenetic and molecular response by treatment arm. QD, once daily; BID, twice daily; CCyR, complete cytogenetic response; MMR, major molecular response; mo, months.
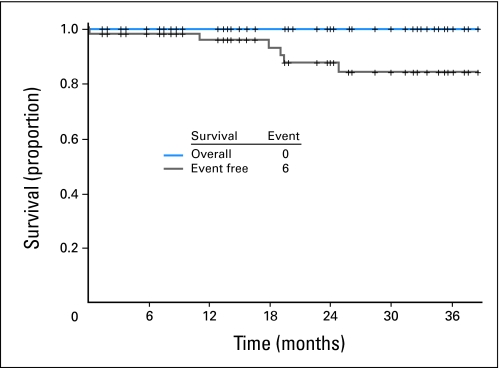
Responses have been durable, with 45 (94%) of 48 patients who achieved CCyR maintaining this response (one patient has not yet had follow-up cytogenetic analysis). Three patients lost CCyR; one lost CCyR to 25% Ph-positive metaphases as a result of prolonged treatment interruption (87 days) because of pleural effusion; another patient lost CCyR to 75% Ph-positive metaphases as a result of noncompliance/financial problems and recently resumed therapy; and the third patient lost CCyR to 70% Ph-positive metaphases because of noncompliance and was lost to follow-up. Among 39 patients who achieved MMR and who had subsequent molecular assessments, five lost MMR; one of these patients also lost CCyR as a result of prolonged dose interruption for pleural effusion; the other four patients maintained CCyR. The projected EFS rate for all 62 patients is 88% at 24 months (90% when excluding the two patients who experienced relapse because of noncompliance; Fig 2). All patients are alive, and no patient has transformed to AP or BP.
Fig 2.
Overall and event-free survival for all patients treated with dasatinib as initial therapy for chronic myeloid leukemia in chronic phase.
As demonstrated by Western blot phosphoprotein analysis on peripheral-blood leukocytes, most patients had residual detectable activated ABL kinase or CrkL target phosphorylation in the 1-month post-treatment samples, but 29 (81%) of 36 and 34 (90%) of 38 patients had complete absence of p-CrkL by 3 and 6 months after treatment, respectively (Table 3). All seven patients with residual p-CrkL at 6 months had not achieved MMR; two patients had not achieved CCyR and had residual p-CrkL also at 6 months. Only two patients had residual p-CrkL at 12 months, and one patient acquired p-CrkL expression in an 18-month sample.
Table 3.
Percent Kinase Target Inhibition of Dasatinib Over Time
| Time on Dasatinib (months) | No. of Evaluable Patients | Detectable p-CrkL-Tyr207 |
Detectable pABL-Tyr245 |
||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| 1 | 38 | 36 | 94.7 | 30 | 78.9 |
| 3 | 36 | 7 | 19.4 | 6 | 16.7 |
| 6 | 38 | 4 | 10.5 | 3 | 7.9 |
| 12 | 32 | 2 | 6.2 | 1 | 3.1 |
| 18 | 23 | 3 | 13.0 | 2 | 8.7 |
Toxicity
Treatment was well tolerated overall. The most common nonhematologic adverse events, regardless of causality, were muscle and joint aches, fatigue, dermatologic complaints such as rash and acne, headaches, and diarrhea (Table 4). Grade ≥ 3 nonhematologic toxicity was uncommon and included fatigue (6%), joint and muscle pain (6%), peripheral neuropathy (5%), dyspnea (5%), and memory impairment (5%). Pleural effusion was uncommon, occurring in 13% of patients and with only one (2%) being grade 3 (once-daily schedule; no grade 4 pleural effusions). Myelosuppression occurred frequently but was usually transient and manageable with temporary treatment interruptions and occasional dose reductions, although one patient discontinued therapy because of prolonged thrombocytopenia. Grade ≥ 3 neutropenia, thrombocytopenia, and anemia occurred in 13 patients (21%), six patients (10%), and four patients (6%), respectively. There were two instances of grade 4 neutropenia and three instances of grade 4 thrombocytopenia, all on the twice-daily schedule. There was no significant difference in the overall rate of adverse events by schedule. The rate of pleural effusion was 3% with the once-daily schedule and 10% with the twice-daily schedule (P = .26). One patient had a grade 1 pericardial effusion. The rates of grade 3 to 4 neutropenia for the once-daily and twice-daily schedules were 13% and 8% (P = .53), respectively, and the rates of grade 3 to 4 thrombocytopenia were 3% and 6%, respectively (P = .67). Myelosuppression usually occurred early; the median time to the first event of grade 3 to 4 myelosuppression was 28 days (range, 8 to 1,035 days). Four patients had neutropenic fever. Bleeding occurred in six patients (three nose bleeds, one conjunctival bleed, one rectal bleed, and one lower GI bleed).
Table 4.
Adverse Events on Dasatinib, Regardless of Causality, Present in at Least 5% of Patients or With at Least One Grade ≥ 3 Event
| Adverse Event | All Patients (N = 62) |
Once-Daily Schedule |
Twice-Daily Schedule |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade 3 or 4 |
Any Grade |
Grade 3 or 4 |
Any Grade |
Grade 3 or 4 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Nonhematologic | ||||||||||||
| Pain (joint, muscle) | 46 | 74 | 4 | 6 | 24 | 39 | 2 | 3 | 22 | 35 | 2 | 3 |
| Fatigue | 45 | 73 | 4 | 6 | 22 | 35 | 4 | 6 | 23 | 37 | 0 | 0 |
| Dyspnea | 28 | 45 | 3 | 5 | 12 | 19 | 2 | 3 | 16 | 26 | 1 | 2 |
| Neuropathy | 19 | 31 | 3 | 5 | 10 | 16 | 1 | 2 | 9 | 15 | 2 | 3 |
| Memory impairment | 12 | 19 | 3 | 5 | 9 | 15 | 2 | 3 | 3 | 5 | 1 | 2 |
| Headache | 35 | 56 | 2 | 3 | 19 | 31 | 2 | 3 | 16 | 26 | 0 | 0 |
| Cardiac | 13 | 21 | 2 | 3 | 3 | 5 | 2 | 3 | 10 | 16 | 0 | 0 |
| Skin toxicity | 36 | 58 | 1 | 2 | 20 | 32 | 0 | 0 | 16 | 26 | 1 | 2 |
| Diarrhea | 33 | 53 | 1 | 2 | 15 | 24 | 1 | 2 | 18 | 29 | 0 | 0 |
| Ocular/vision | 20 | 32 | 1 | 2 | 13 | 21 | 1 | 2 | 7 | 11 | 0 | 0 |
| Hyperglycemia | 15 | 24 | 1 | 2 | 9 | 15 | 1 | 2 | 6 | 10 | 0 | 0 |
| Mood alteration | 15 | 24 | 1 | 2 | 11 | 18 | 0 | 0 | 5 | 8 | 1 | 2 |
| Pleural effusion | 8 | 13 | 1 | 2 | 2 | 3 | 1 | 2 | 6 | 10 | 0 | 0 |
| Pruritus | 5 | 8 | 1 | 2 | 3 | 5 | 0 | 0 | 2 | 3 | 1 | 2 |
| Renal, genitourinary | 5 | 8 | 1 | 2 | 3 | 5 | 1 | 2 | 2 | 3 | 0 | 0 |
| Weight gain | 4 | 6 | 1 | 2 | 1 | 2 | 0 | 0 | 3 | 5 | 1 | 2 |
| Hypophosphatemia | 4 | 6 | 1 | 2 | 2 | 3 | 0 | 0 | 2 | 3 | 1 | 2 |
| Hyponatremia | 3 | 5 | 1 | 2 | 1 | 2 | 0 | 0 | 2 | 3 | 1 | 2 |
| Nausea | 28 | 45 | 0 | 0 | 12 | 19 | 0 | 0 | 16 | 26 | 0 | 0 |
| GI | 23 | 37 | 0 | 0 | 10 | 16 | 0 | 0 | 13 | 21 | 0 | 0 |
| Dizziness | 22 | 35 | 0 | 0 | 11 | 18 | 0 | 0 | 11 | 18 | 0 | 0 |
| Edema | 20 | 32 | 0 | 0 | 11 | 18 | 0 | 0 | 9 | 15 | 0 | 0 |
| Cough | 16 | 26 | 0 | 0 | 8 | 13 | 0 | 0 | 8 | 13 | 0 | 0 |
| Vomiting | 13 | 21 | 0 | 0 | 7 | 11 | 0 | 0 | 6 | 10 | 0 | 0 |
| Insomnia | 13 | 21 | 0 | 0 | 9 | 15 | 0 | 0 | 4 | 6 | 0 | 0 |
| Elevated ALT | 10 | 16 | 0 | 0 | 4 | 6 | 0 | 0 | 6 | 10 | 0 | 0 |
| Elevated AST | 9 | 15 | 0 | 0 | 6 | 10 | 0 | 0 | 3 | 5 | 0 | 0 |
| Alopecia | 9 | 15 | 0 | 0 | 3 | 5 | 0 | 0 | 6 | 10 | 0 | 0 |
| Allergy | 8 | 13 | 0 | 0 | 6 | 10 | 0 | 0 | 2 | 3 | 0 | 0 |
| Chills/rigors | 7 | 11 | 0 | 0 | 3 | 5 | 0 | 0 | 4 | 6 | 0 | 0 |
| Sweating | 7 | 11 | 0 | 0 | 4 | 6 | 0 | 0 | 3 | 5 | 0 | 0 |
| Hemorrhage | 6 | 10 | 0 | 0 | 2 | 3 | 0 | 0 | 4 | 6 | 0 | 0 |
| Hematologic | ||||||||||||
| Neutropenia | 39 | 63 | 13 | 21 | 19 | 31 | 8 | 13 | 20 | 32 | 5 | 8 |
| Anemia | 50 | 81 | 4 | 6 | 24 | 39 | 1 | 2 | 26 | 42 | 3 | 5 |
| Thrombocytopenia | 43 | 69 | 6 | 10 | 20 | 32 | 2 | 3 | 23 | 37 | 4 | 6 |
Throughout the study period, 30 (48%) of 62 patients have required 87 transient treatment interruptions, with 21 patients requiring more than one interruption (median, two interruptions; range, one to seven interruptions). The most common causes for these 87 treatment interruptions were pleural effusion (n = 20 treatment interruptions), dyspnea (n = 16), and headache (n = 12). Dose reductions were required in 22 patients (35%). The actual median daily dose at 12 months was 100 mg (range, 20 to 100 mg). Five patients discontinued therapy; three patients discontinued because of intolerance, including two patients with pleural effusion (one patient on each schedule) and one patient with prolonged myelosuppression (twice-daily schedule); the other two patients discontinued as a result of patient's choice (n = 1) and noncompliance (n = 1). Treatment interruptions occurred in 18 patients (58%) in the once-daily arm and 12 patients (39%) in the twice-daily arm, and dose reductions occurred in 10 patients (33%) and 12 patients (39%), respectively.
DISCUSSION
Improving the long-term outcome of patients with CML remains an important objective. Despite the excellent results achieved with imatinib, at least 25% to 30% of patients do not achieve an optimal outcome.1 In a recently reported single-institution analysis on 204 consecutive patients treated with standard-dose imatinib, the 5-year probability of EFS was 81%. However, considering intolerance to imatinib or lack of achievement of the desirable end points (eg, major cytogenetic response) as events, the 5-year EFS was 63%.16 To improve these results, we investigated dasatinib as initial therapy for CML. Dasatinib is more potent in vitro than imatinib,11 is effective in at least 50% of patients in whom imatinib has failed,9 and may be less prone to development of mutations than imatinib.17
In this study, dasatinib induced a high rate of CCyR (98%), with most responses occurring early. For example, by the sixth month of therapy, more than 90% of patients had already achieved a CCyR. These results compare favorably with the historical experience at our institution in similar patients treated with standard- or high-dose imatinib. Responses with dasatinib occurred considerably faster and ultimately at a higher rate compared with responses reported with standard-dose imatinib (45% at 6 months) and with high-dose imatinib (57% at 6 months).18 Still, considering that ultimately, the CCyR rate with standard-dose imatinib reaches 82%, the main advantage achieved with front-line dasatinib therapy might be the faster achievement of response. It is unclear what the long-term implications of these earlier responses may be. A subanalysis of the IRIS study suggested that the time of achievement of CCyR does not impact the probability of EFS.19 For this particular subanalysis, only patients who achieved a CCyR were considered. Because achieving CCyR is known to favorably affect long-term outcome, such analysis is affected by a selection bias of patients who are already known to have a favorable outcome. However, it is important to consider that a patient who has not achieved CCyR at any given time may either improve and eventually achieve CCyR or may not improve and eventually experience progression. A recent report has suggested that as more time evolves without achieving CCyR, the probability of eventually achieving CCyR decreases and the risk of progression increases.20 MMR was also achieved earlier, with 71% of patients achieving MMR at 12 months with dasatinib, compared with the reported rates of 46% with standard-dose imatinib and 54% with high-dose imatinib.18
These extrapolations should be considered with caution because the comparisons refer to historical controls. The inclusion criteria for all of these studies (standard- and high-dose imatinib and dasatinib) were identical, and there were no obvious differences in the patient characteristics. Still, the long-term impact of dasatinib or other second-generation tyrosine kinase inhibitors as initial therapies for CML will require prospective randomized trials. The results reported here are comparable to those achieved with nilotinib in a parallel study reported separately conducted in parallel to this study, with patients allocated to each study in alternating sequence.
Overall, treatment with dasatinib is well tolerated. The toxicity profile compares favorably with what has been reported with imatinib. For example, the rates of grade 3 or 4 anemia, neutropenia, and thrombocytopenia (6%, 21%, and 10%, respectively) are similar to what has been reported with standard-dose imatinib (4%, 25%, and 17%, respectively)21 and high-dose imatinib (5% to 10%, 14% to 36%, and 18% to 25%, respectively).5,22 Nonhematologic adverse events are generally mild and manageable. The most common nonhematologic adverse events (musculoskeletal pain and fatigue, 6% each) occurred at a similar frequency to what has been reported with high-dose imatinib (musculoskeletal pain, 3% to 7%; fatigue, 1% to 6%), but they are probably more frequent compared with standard-dose imatinib (fatigue, 1%; pain, 3%). One adverse event not previously noted is peripheral neuropathy manifested as numbness and tingling in hands (most frequently) or feet. These were usually grade 1 to 2, but three patients had grade 3 peripheral neuropathy requiring treatment interruptions and dose reductions, after which symptoms improved.
There was no significant difference in toxicity between the two schedules, except for a trend for less pleural effusions with the once-daily schedule. Results from a randomized trial suggested a decreased rate of adverse events, particularly myelosuppression and pleural effusion, with 100 mg once daily compared with 50 mg twice daily or higher doses.23 The overall rate of adverse events is lower in the front-line setting than after imatinib failure.9 This is not unexpected and is similar to what was reported with imatinib front-line therapy or after interferon failure.24,25 This lower rate of events would make it difficult to demonstrate a significant difference with the once-daily versus twice-daily schedules, particularly in a relatively small sample size. Because of the mature results of the previously mentioned large-scale randomized trial and the trends in favor of the once-daily schedule in this trial, we have discontinued random assignment to the twice-daily schedule. The study continues with the once-daily schedule only.
We conclude that dasatinib is effective as initial therapy for CML in chronic phase, with a high rate of response and rapid achievement of CCyR in nearly all patients by 6 months from the start of therapy. These results are achieved with a favorable toxicity profile. The possible superiority of this approach compared with standard therapy will need to be confirmed in randomized studies to evaluate a possible impact on EFS and, ultimately, overall survival. Results from such a study are expected shortly.
Acknowledgment
We thank Neelima Reddy, MS, and Jie Qiang Guo, MD, for assistance with phosphoprotein analysis.
Footnotes
Supported in part by National Cancer Institute Grant No. CA49639.
Presented in part at the 50th Annual Meeting of the American Society of Hematology, December 6-9, 2008, San Francisco, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00254423.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan O'Brien, Genta (C), sanofi-aventis (C), Celgene (C), Genmab (C), GlaxoSmithKline (C), Gemin X (U), Biogen Idec (U), Eli Lilly (U); Farhad Ravandi, Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Elias Jabbour, Novartis, Bristol-Myers Squibb; Farhad Ravandi, Bristol-Myers Squibb; Gautam Borthakur, Bristol-Myers Squibb Research Funding: Jorge E. Cortes, Bristol-Myers Squibb, Novartis, Wyeth; Susan O'Brien, Genentech, Berlex Laboratories, Biogen Idec, Eli Lilly, Novartis, Bristol-Myers Squibb, Gemin X, Genta, Hana BioSciences; Farhad Ravandi, Bristol-Myers Squibb; Hagop Kantarjian, Bristol-Myers Squibb, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jorge E. Cortes, Dan Jones, Hagop Kantarjian
Administrative support: Jorge E. Cortes, Hagop Kantarjian
Provision of study materials or patients: Jorge E. Cortes, Susan O'Brien, Elias Jabbour, Farhad Ravandi, Charles Koller, Gautam Borthakur, Hagop Kantarjian
Collection and assembly of data: Jorge E. Cortes, Dan Jones, Gautam Borthakur, Brenda Walker, Weiqiang Zhao, Hagop Kantarjian
Data analysis and interpretation: Jorge E. Cortes, Dan Jones, Gautam Borthakur, Weiqiang Zhao, Jianqin Shan, Hagop Kantarjian
Manuscript writing: Jorge E. Cortes, Dan Jones, Hagop Kantarjian
Final approval of manuscript: Jorge E. Cortes, Dan Jones, Susan O'Brien, Elias Jabbour, Hagop Kantarjian
REFERENCES
- 1.O'Brien SG, Guilhot F, Goldman J, et al. International Randomized Study of Interferon Versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM) Blood. 2008;112:186. abstr. [Google Scholar]
- 2.Kantarjian HM, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 3.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: Historical comparison between two phase 3 trials. Blood. 2006;108:1478–1484. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 4.Baccarani M, Martinelli G, Rosti G, et al. Imatinib and pegylated human recombinant interferon-alpha2b in early chronic-phase chronic myeloid leukemia. Blood. 2004;104:4245–4251. doi: 10.1182/blood-2004-03-0826. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, Kantarjian H. Beyond dose escalation: Clinical options for relapse or resistance in chronic myelogenous leukemia. J Natl Compr Canc Netw. 2008;6(suppl 2):S22–S30. [PubMed] [Google Scholar]
- 7.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 9.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 10.Cortes J, Kantarjian H, Kim D-W, et al. Efficacy and safety of bosutinib (SKI-606) in patients with chronic phase (CP) Ph+ chronic myelogenous leukemia (CML) with resistance or intolerance to imatinib. Blood. 2008;112:1098. doi: 10.1182/blood-2011-05-355594. abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 12.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 13.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 16.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: Incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 17.Bradeen HA, Eide CA, O'Hare T, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: High efficacy of drug combinations. Blood. 2006;108:2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes J, Baccarani M, Guilhot F, et al. A phase III, randomized, open-label study of 400 mg versus 800 mg of imatinib mesylate (IM) in patients (pts) with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase (CML-CP) using molecular endpoints: 1-year results of TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) study. Blood. 2008;112:335. doi: 10.1200/JCO.2009.25.3724. abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilhot F, Larson R, O'Brien S. Time to complete cytogenetic response (CCyR) does not affect long-term outcomes for patients on imatinib therapy. Blood. 2007;110:27. abstr. [Google Scholar]
- 20.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113:6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 22.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 25.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]