Abstract
Purpose
Flavopiridol downmodulates antiapoptotic proteins associated with resistance to fludarabine and rituximab and is effective against p53-mutated chronic lymphocytic leukemia (CLL). We conducted a phase I study of flavopiridol, fludarabine, and rituximab (FFR) in patients with mantle-cell lymphoma (MCL), indolent B-cell non-Hodgkin's lymphomas (B-NHL), and CLL to determine the activity of FFR.
Patients and Methods
Therapy included fludarabine 25 mg/m2 intravenously (IV) days 1 to 5 and rituximab 375 mg/m2 day 1 every 28 days for 6 cycles. We administered flavopiridol 50 mg/m2 by 1-hour IV bolus (IVB) day 1 (n = 15); day 1 to 2 (n = 6); 20 mg/m2 30-minute IVB + 20 mg/m2 4-hour IV infusion (n = 3); or 30 mg/m2 + 30 mg/m2 (n = 14).
Results
Thirty-eight patients (median age, 62 years) with MCL (n = 10); indolent B-NHL including follicular (n = 9), marginal zone (n = 4), lymphoplasmacytic (n = 1), or small lymphocytic lymphoma (n = 3); and CLL (n = 11), were enrolled. Twenty-two patients were previously untreated; 16 had received one to two prior therapies. Two patients in cohort 2 developed grade 3 dose-limiting toxicity (seizures, renal insufficiency). The median number of treatment cycles was 4, with cytopenias (n = 10) and fatigue (n = 3) the most common reasons for early discontinuation. Overall response rate was 82% (complete response, 50%; unconfirmed complete response, 5%; partial response, 26%), including 80% of patients with MCL (median age, 68; seven complete responses, one partial response). Median progression-free survival (PFS) was 25.6 months. Median PFS of patients with nonblastoid variant MCL (n = 8) was 35.9 months.
Conclusion
FFR was active in MCL, indolent B-NHL, and CLL and should be studied for older patients with MCL who are not candidates for aggressive chemotherapy.
INTRODUCTION
Despite advances in the treatment of mantle-cell lymphoma (MCL), indolent B-cell non-Hodgkin's lymphoma (B-NHL), and chronic lymphocytic leukemia (CLL), patients with these diseases invariably relapse and become resistant to therapy.1–3 This conundrum is exemplified by rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), which achieves an 87% complete response (CR) rate, 64% 3-year failure-free survival (FFS), and 82% 3-year overall survival (OS) in previously untreated patients with MCL, but is not curative. Furthermore, in patients older than 65 years of age, rituximab plus hyper-CVAD is more toxic and less effective, with 3-year FFS 50%.4 The majority of patients with MCL are in this older age group, so less toxic yet effective therapies are needed. While fludarabine-based regimens have achieved excellent results in follicular lymphoma (FL) and CLL,5–11 high-risk patients have shortened response duration and inferior survival, largely due to dysfunctional p53.12,13 Thus, identifying novel agents that target p53 and other resistance mechanisms remains a high priority in MCL, FL, and CLL.
Flavopiridol (NSC-649890, alvocidib; sanofi-aventis, Bridgewater, NJ) is an investigational N-methylpiperidinyl, chlorophenyl flavone which inhibits cyclin-dependent kinase (CDK) 1 and CDK214–17 by altering tyrosine phosphorylation.18 Flavopiridol inhibits CDK4-cyclin D1 in vitro and induces apoptosis in leukemia and lymphoma cell lines.16 Furthermore, flavopiridol induces apoptosis by downregulating critical antiapoptotic proteins, such as Mcl-1, which is associated with drug resistance to both fludarabine and rituximab.19 Flavopiridol induces apoptosis via a p53-independent mechanism, suggesting that flavopiridol may be able to eliminate p53-deficient tumor cells resistant to agents such as fludarabine and rituximab.20
We have pursued the clinical development of flavopiridol in lymphomas and CLL. Phase I and II studies administering single-agent flavopiridol using a pharmacokinetically derived schedule to 116 patients with relapsed and refractory CLL, including many with high-risk cytogenetics, achieved response rates of 40% and 53%, respectively.20a,21 The most serious toxicity was tumor lysis syndrome (TLS), which required transient hemodialysis for refractory hyperkalemia in 3% of patients.22 Based on our success with single-agent flavopiridol in CLL, we initiated a phase I study of flavopiridol in combination with fludarabine and rituximab (FFR) in MCL, indolent B-NHL, and CLL with the goal of developing a tolerable, effective regimen.
PATIENTS AND METHODS
Subjects
Patients were enrolled on this National Cancer Institute (NCI) –sponsored clinical study (NCI 5745) following approval by The Ohio State University institutional review board. All patients provided written informed consent; had MCL, indolent B-NHL, or CLL; were age 18 years or older; had received zero to three prior treatment regimens including no more than six prior cycles of fludarabine; had Eastern Cooperative Oncology Group performance status 0 to 2; and met the following criteria: creatinine ≤ 2.0 mg/dL, bilirubin less than 2 times the upper limit of normal (ULN), transaminases ≤ 2 times ULN, hemoglobin ≥ 9.0 g/dL, absolute neutrophil count (ANC) ≥ 1,500/mm3, and platelet count ≥ 100,000/mm3 unless cytopenias were due to disease. Patients could not have received chemotherapy within 4 weeks of enrollment. Pregnant women were excluded.
Treatment Plan
All patients received fludarabine 25 mg/m2 intravenous (IV) day 1 to 5 and rituximab 375 mg/m2 day 1 every 28 days for up to 6 cycles (Table 1). In the initial planned dose escalation, patients received flavopiridol 50 mg/m2 by 1-hour IV bolus (IVB) day 1 (cohort 1), day 1 to 2 (cohort 2), or day 1 to 3. Dose-limiting toxicity (DLT) was observed at the cohort 2 dose. Twelve additional patients were enrolled at the cohort 1 dose, as specified by the protocol. The study was then amended to incorporate our single-agent hybrid dosing schedule of flavopiridol. In cohorts 3 to 4, patients received fludarabine and rituximab as above, and flavopiridol was initiated during cycle 2 to minimize the risk of TLS. In cohort 3, patients received flavopiridol 20 mg/m2 by 30-minute IVB followed by 20 mg/m2 4-hour IV infusion. In cohort 4, patients received flavopiridol 30 mg/m2 30-minute IVB + 30 mg/m2 4-hour IV infusion; cohort 4 was expanded to gain safety and efficacy data. Patients received prophylactic sulfamethoxazole/trimethoprim double strength twice daily thrice weekly and acyclovir 400 mg three times per day. Growth factor support was given according to American Society of Clinical Oncology guidelines in cohorts 3 to 4.
Table 1.
Treatment Plan (N = 38)
| Cohort | No. | Drug and Dose |
|---|---|---|
| All | Fludarabine 25 mg/m2 days 1-5 | |
| Rituximab 375 mg/m2 day 1 | ||
| Every 28 days for up to 6 cycles | ||
| 1 | 15 | Flavopiridol 50 mg/m2 day 1 |
| 2 | 6 | Flavopiridol 50 mg/m2 days 1 to 2 |
| 3 | 3 | Flavopiridol 20 mg/m2 30-minute intravenous + 20 mg/m2 4-hour intravenous day 1* |
| 4 | 14 | Flavopiridol 30 mg/m2 30-minute intravenous + 30 mg/m2 4-hour intravenous day 1* |
Flavopiridol was given by 30-minute intravenous bolus followed by 4-hour intravenous infusion starting in cycle 2.
Assessment of Toxicity and Response
National Cancer Institute Common Toxicity Criteria, version 2.0, was used to evaluate toxicity. DLT was defined as any grade 3 to 4 nonhematologic toxicity that did not resolve or decrease to grade 1 to 2 within 2 weeks, or any grade 4 hematologic toxicity that caused more than a 1-week delay in treatment. DLT included toxicity directly attributable to flavopiridol and toxicity of fludarabine or rituximab that was exacerbated by flavopiridol. Patients were assessed for clinical response after 3 and 6 cycles, using International Working Group criteria for lymphomas23 and NCI 96 criteria for CLL.24 Progression-free survival (PFS) was calculated from the date of study entry until time of disease progression or death, whichever came first, censoring patients alive and relapse-free at last follow-up. Patients who withdrew from study to receive alternative therapy before response evaluation (n = 2) or undergo allogeneic stem cell transplantation (SCT) after responding (n = 2) were censored at time of treatment or transplant, respectively.
Statistical Analysis
The primary end point was to define the maximum-tolerated dose (MTD) of FFR. Secondary end points were to determine the overall response (OR) and CR rates to FFR. The study employed a traditional 3 × 3 phase I design without dose de-escalation. The MTD was defined as the dose level below which 2 or more of six patients experienced DLT, as defined above. Patient characteristics, toxicities, and responses were summarized for all enrolled patients. Median PFS was estimated using the Kaplan-Meier method.
RESULTS
Patient Characteristics
Table 2 describes the 38 patients who were enrolled on this study. Median age was 62 years (range, 38 to 81). Twenty-two patients (58%) were male. Patients had MCL (n = 10); indolent B-NHL including FL (n = 9), marginal zone (n = 4), lymphoplasmacytic (n = 1), or small lymphocytic lymphoma (n = 3); or CLL (n = 11). Median β-2-microglobulin was 3.0 mg/dL (range, 1.3 to 6.5). Fifty-eight percent of patients were previously untreated, and 18% and 24% had received one or two prior therapies, respectively. Two patients (5%) had received prior fludarabine. Ten (26%) had received prior rituximab, including single-agent rituximab (n = 6); rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (n = 3); and fludarabine, cyclophosphamide, and rituximab (n = 1). Five patients did not respond to rituximab-based therapy.
Table 2.
Patient Characteristics (N = 38)
| Characteristic | All Patients |
|
|---|---|---|
| No. | % | |
| Median age, years | 62 | |
| Range | 38-81 | |
| Male sex | 22 | 58 |
| Disease | ||
| Mantle cell lymphoma | 10 | 26 |
| Follicular lymphoma | 9 | 24 |
| Marginal zone lymphoma | 4 | 11 |
| Lymphoplasmacytic lymphoma | 1 | 3 |
| Small lymphocytic lymphoma | 3 | 8 |
| Chronic lymphocytic leukemia | 11 | 29 |
| β2-microglobulin, mg/dL | ||
| Median | 3.0 | |
| Range | 1.3-6.5 | |
| No. of prior treatments | ||
| 0 | 22 | 58 |
| 1 | 7 | 18 |
| 2 | 9 | 24 |
| Prior fludarabine | 2 | 5 |
| Prior rituximab | 10 | 26 |
Toxicity and Delivery of Therapy
Three patients were treated in cohort 1; no DLT was observed. Two of six patients in cohort 2 experienced DLT. One patient developed grade 3 confusion and a grand mal seizure 2 days after finishing cycle 2 of FFR. Lumbar puncture and magnetic resonance imaging revealed no evidence of metastases, and she suffered no further confusion or seizures. A second patient developed nausea, vomiting, and anorexia, resulting in grade 3 renal insufficiency which resolved with IV hydration. Both patients were removed from study. One dose of flavopiridol 50 mg/m2 on day 1 of each cycle (cohort 1) was defined as the MTD when FFR employed 1-hour IVB dosing of flavopiridol. In cohorts 3 to 4, which gave flavopiridol by 30-minute IVB followed by 4-hour IV infusion, DLT was not observed, and the MTD was defined as the highest dose level tested (cohort 4).
Hematologic toxicity was observed in most patients, regardless of treatment cohort. Although filgrastim was allowed in cohorts 3 to 4, 87% developed leukopenia (68% grade 3 to 4), and 87% experienced neutropenia (66% grade 3 to 4). Anemia (61% grade 1 to 2, 26% grade 3) and thrombocytopenia (45% grade 1 to 2, 16% grade 3 to 4) were also common, although only one patient required platelet transfusion. Ten (26%) of 38 patients were admitted for IV antibiotic therapy for documented grade 3 infections including central venous catheter infection (n = 1), acute appendicitis (n = 1), urinary tract infection (n = 1), bacterial pneumonia (n = 2), and upper respiratory infection (n = 2). Five additional patients (13%) received outpatient antibiotics for upper respiratory infection (n = 4), cytomegalovirus reactivation (n = 1), and oral candidiasis (n = 1). Five patients (13%) developed febrile neutropenia or fever without documented infection and received empiric antibiotics.
The most common nonhematologic toxicities were fatigue (79% grade 1 to 2; 13% grade 3); nausea (71% grade 1 to 2; 8% grade 3); vomiting (53% grade 1 to 2; 8% grade 3); diarrhea (47% grade 1 to 2; 24% grade 3); and anorexia (61% grade 1 to 2; 3% grade 3). Biochemical toxicities included hypocalcemia (45% grade 1 to 2; 5% grade 3) and TLS (13% grade 3). Table 3 summarizes toxicity data. There were no significant differences in hematologic or nonhematologic toxicities between previously untreated and relapsed patients.
Table 3.
Toxicity (N = 38 patients)
| Toxicity | Toxicity by Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1-2 |
3 |
4 |
All |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic/infections | ||||||||
| Leukopenia | 7 | 18 | 13 | 34 | 13 | 34 | 33 | 87 |
| Neutropenia | 8 | 21 | 9 | 24 | 16 | 42 | 33 | 87 |
| Anemia | 23 | 61 | 10 | 26 | — | — | 33 | 87 |
| Thrombocytopenia | 17 | 45 | 4 | 11 | 2 | 5 | 23 | 61 |
| Documented infections | 5 | 13 | 10 | 26 | — | — | 15 | 39 |
| Febrile neutropenia | — | — | 5 | 13 | — | — | 5 | 13 |
| Nonhematologic | ||||||||
| Fatigue | 30 | 79 | 5 | 13 | — | — | 35 | 92 |
| Nausea | 27 | 71 | 3 | 8 | — | — | 30 | 79 |
| Vomiting | 20 | 53 | 3 | 8 | — | — | 23 | 61 |
| Diarrhea | 18 | 47 | 9 | 24 | — | — | 27 | 71 |
| Anorexia | 23 | 61 | 1 | 3 | — | — | 24 | 63 |
| Hypocalcemia | 17 | 45 | 2 | 5 | — | — | 19 | 50 |
| Tumor lysis syndrome | — | — | 5 | 13 | — | — | 5 | 13 |
The median number of delivered cycles was 4, and 16 (42%) of 38 patients completed all 6 planned cycles of therapy. Hematologic toxicity was common and resulted in early treatment discontinuation in 10 patients (three pancytopenia, five neutropenia, two thrombocytopenia). One previously untreated patient with CLL developed grade 4 idiopathic thrombocytopenia purpura after cycle 2. Filgrastim was not allowed in cohorts 1 to 2, but was allowed per American Society of Clinical Oncology guidelines in cohorts 3 to 4. However, the percentage of patients completing 6 cycles of therapy was similar in both cohorts 1 to 2 and 3 to 4 (43% v 41%). Therapy was discontinued early for fatigue (n = 3), fever (n = 2), and disease progression (n = 2). Significant TLS requiring dialysis was not observed.
Response to Therapy
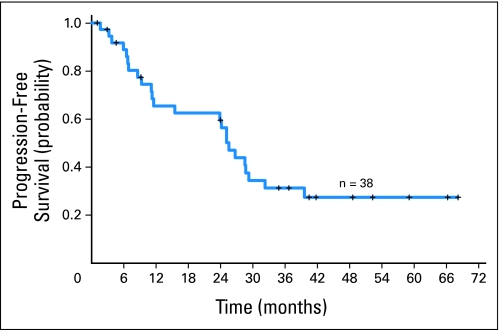
All 38 patients were evaluated for response (Table 4). The OR rate was 82% (95% CI, 69% to 94%), with 50% of patients achieving CR, 5% unconfirmed CR (CRu) and 26% partial response (PR). Median PFS was 25.6 months (Fig 1). Three responders died of unrelated causes (head and neck cancer, cirrhosis, coronary artery disease) but were in remission 28.7, 26.7, and 11.1 months after enrollment, respectively, at the time of death.
Table 4.
Response to Therapy (N = 38)
| Group | No. | OR |
CR* |
PR |
Median PFS (months) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| All patients | 38 | 31 | 82 | 21 | 55 | 10 | 26 | 25.6 |
| MCL | 10 | 8 | 80 | 7 | 70 | 1 | 10 | 21.9 |
| Nonblastoid | 8 | 6 | 75 | 5 | 63 | 1 | 13 | 35.9 |
| FL | 9 | 9 | 100 | 7 | 78 | 2 | 22 | 25.6 |
| MZL/LPL | 5 | 3 | 60 | 0 | 0 | 3 | 60 | 29.3 |
| CLL/SLL | 14 | 11 | 79 | 7 | 50 | 4 | 29 | 24.1 |
| Untreated | 22 | 18 | 82 | 12 | 55 | 6 | 27 | 25.1 |
| Relapsed | 16 | 13 | 81 | 9 | 56 | 4 | 25 | 25.6 |
| 1-hour dosing | 21 | 18 | 86 | 14 | 67 | 4 | 19 | 25.1 |
| Hybrid dosing | 17 | 13 | 76 | 7 | 41 | 6 | 35 | 25.6 |
Abbreviations: OR, overall response; CR, complete response; PR, partial response; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; LPL, lymphoplasmacytic lymphoma; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; PFS, progression-free survival of responding patients.
Includes two patients with follicular lymphoma who had unconfirmed complete remissions.
Fig 1.
Kaplan-Meier estimate of progression-free survival.
Ten MCL patients (median age, 68; range, 58 to 81), including six previously untreated and four relapsed patients who had each received two prior therapies, received a median of 3.5 cycles. Eight patients responded (seven CR, one PR). Median PFS was 21.9 months, ranging from 1.1 months when a patient withdrew to receive another regimen to at least 68.2 months. Two patients with blastoid variant MCL responded but relapsed within 1 year of study entry. Median PFS of the eight patients with nonblastoid MCL was 35.9 months.
Nine FL patients (median age, 59; range, 38 to 67), of whom seven had received one to two prior therapies, received a median of 6 cycles. All nine patients responded (five CR, two CRu, two PR); median PFS was 25.6 months. One responder developed large cell transformation at 3.8 months, and two responders died in remission of unrelated causes (cirrhosis, head and neck cancer) at 26.7 and 28.7 months. Two CR/CRu patients remain in remission at 41.8 and 52.3 months. Four patients with marginal zone lymphoma and one patient with lymphoplasmacytic lymphoma (median age, 54; range, 39-62), of whom two had received one to two prior therapies, were enrolled. Three patients achieved a PR, and median PFS was 29.3 months.
Fourteen patients with CLL/SLL (median age, 57; range, 43 to 72), of whom 11 were previously untreated, received a median of 3 cycles. Eleven patients responded (seven CRs, four PRs). Median PFS was 24.1 months, ranging from 2.9 months when a patient withdrew to receive another regimen to at least 66.2 months. Two of three patients with del(17p13) achieved CR; one went off study to undergo allogeneic SCT at 9.2 months, and the other progressed at 28.5 months. Two of three patients with del(11q22) responded (one CR, two PR) with progression at 25.1 and 8.6 months.
No differences in treatment delivery, response rates, or PFS between previously untreated and relapsed patients were observed. Of 22 previously untreated patients, 18 (82%) responded (12 CR, six PR), and 13 (81%) of 16 relapsed patients responded (nine CR, four PR). The hybrid 30-minute IVB + 4-hour IV infusion schedule did not significantly affect response rates or PFS (Table 4).
DISCUSSION
The FFR regimen was effective in MCL, indolent B-NHL, and CLL. More than 80% of patients responded to therapy, and 55% attained CR/CRu. Responses were durable; estimated median PFS was 25.6 months across all diseases. Due to concern about potential hematologic toxicity, enrollment was limited to patients with no more than three prior treatments and normal hematologic parameters unless due to bone marrow infiltration. Sixteen of 38 patients had received prior treatment. Only two patients had received previous fludarabine-based therapy. A CLL patient with del(11q22) and a complex karyotype who failed both fludarabine and FC achieved a PR (PFS 8.6 months), and a lymphoplasmacytic lymphoma patient refractory to fludarabine, cyclophosphamide, and rituximab failed to respond. Thus, our study population was essentially fludarabine naïve, which obviously influences interpretation of our findings.
While interpretation of these results is limited by the small size, phase I design, and mixture of different histologies of this trial, review of the findings in individual disease types provides insight regarding whether further study of this regimen is indicated. Although single-agent flavopiridol showed minor clinical activity when given by 1-hour IVB to patients with MCL (11% PR, 71% stable disease),25 FFR appeared promising in elderly patients with MCL (median, 68 years). All 10 patients had low-risk MCL International prognostic index (MIPI) scores (median, 3.25; range, 2.59 to 4.59).26 Ki-67 information was available on only three patients (median 35% positivity; range, 20% to 45%). Four patients with MCL had each received two prior systemic regimens (CHOP 2, hyper-CVAD 2, etoposide, methylprednisolone, cytarabine and platinum 1, rituximab 2, high-dose therapy 1). In comparison, rituximab, fludarabine, cyclophosphamide, and mitoxantrone achieved an OR rate of 73% (CR, 23%), with a median response duration of 12 months, in relapsed and refractory MCL.27 The absence of patients with intermediate-risk or high-risk MIPI scores indicates that a larger phase II study is required to determine the true clinical efficacy of FFR in older patients with MCL, particularly intermediate-risk and high-risk patients.26 Despite these limitations, our findings suggest that FFR or a similar flavopiridol-based combination regimen may be a potential therapeutic option for older MCL patients who are not candidates for more aggressive therapies.
While single-agent flavopiridol is highly active in patients with refractory, genetically high-risk CLL (Lin TS et al, submitted),21,22 our results did not provide compelling evidence that FFR is more active than other fludarabine-based regimens already in clinical use in CLL. While FFR achieved respectable OR, CR, and median PFS in patients with CLL/SLL, these results are not necessarily better than what would be expected with other fludarabine-based regimens.8–11 Nonetheless, FFR demonstrated activity in patients with high-risk cytogenetic features who typically fare poorly with conventional fludarabine-based regimens. Two of three patients with del(17p13), which corresponds to loss of the p53 tumor suppressor gene and confers a very poor outcome in CLL,12,13 responded with one undergoing stem cell transplantation at 9.2 months and the other progressing at 28.5 months. Furthermore, the duration of PFS may have been compromised by a median of only 3 cycles of therapy due to cytopenias. Given the activity of single-agent flavopiridol in high-risk genomic CLL, the cytopenias observed with FFR, and fludarabine's T-cell immunosuppressive toxicity, we are piloting a nonfludarabine-based regimen of cyclophosphamide, flavopiridol, and rituximab in high-risk CLL, using the hybrid dosing schedule of 30-minute IVB followed by 4-hour IV infusion crucial for single-agent clinical activity in CLL.21–23
Interestingly, the hybrid dosing schedule did not improve results with FFR compared to 1-hour IVB dosing, although the number of patients was small. We elected not to pursue further dose escalation of flavopiridol due to the severe TLS observed in the single-agent CLL study and our paramount concern that patient safety be first priority.23 Our findings indicate that drugs which are active but achieve limited clinical responses as a single agent may still improve the activity of fludarabine or other standard therapies when given in combination. Furthermore, our results demonstrate that different dosing schedules may need to be explored if an investigational agent is studied in different cancers or as part of combination therapy rather than as a single agent.
Nonhematologic toxicity was generally well tolerated. In particular, no severe TLS was observed, in contrast to the need for transient hemodialysis for severe hyperkalemia in a small subset of relapsed CLL patients receiving flavopiridol monotherapy. DLT was observed in cohort 2. It is unclear which of the individual drugs contributed to the confusion and seizures observed in the first patient with DLT, and she experienced no further episodes after treatment discontinuation. The second DLT might have been avoided had the patient sought medical attention earlier and received antiemetic medications and IV hydration before elevation of the serum creatinine. However, further escalation of the flavopiridol dose is likely not possible, since FFR was associated with high rates of hematologic toxicity, most notably prolonged neutropenia, which limited treatment delivery. This observation is not surprising, as 77% of relapsed CLL patients who received single-agent flavopiridol experienced transient, reversible grade 3 to 4 neutropenia.23 It is interesting to speculate whether a higher CR rate or longer PFS would result if more aggressive growth factor support were used to combat myelosuppression. However, the significant incidence of hematologic toxicity of FFR even in previously untreated patients highlights the challenge of combining flavopiridol with myelosuppressive agents, particularly in relapsed patients with limited marrow reserve.
Despite the high incidence of grade 3 to 4 neutropenia, infectious toxicity was acceptable. While 10 patients were hospitalized for IV antibiotic therapy for documented infections, there were no grade 4 to 5 infections and patients were able to resume therapy after appropriate treatment of their infections. Opportunistic infections were not observed, similar to our experience with single-agent flavopiridol in refractory CLL.21–23 In contrast to fludarabine, flavopiridol does not cause significant T-cell lymphopenia and therefore is not associated with an increased risk of opportunistic infections.23
In summary, our findings demonstrate that the FFR regimen is generally well tolerated but causes significant hematologic toxicity which limits therapy in a subset of patients. FFR was active against indolent B-NHL and CLL but appears most promising in older patients with MCL, in whom FFR attained promising results with acceptable toxicity. Our results indicate that a larger phase II study of FFR in older patients with previously untreated or relapsed MCL is needed to define this regimen's activity across MIPI risk groups.27 Furthermore, our findings in MCL suggest that FFR may be active in a particular histology even if flavopiridol demonstrates limited clinical activity as monotherapy for that particular lymphoma.26 Finally, the promising findings of this study suggest that other combination regimens using flavopiridol may constitute a potential treatment strategy for patients with relapsed B-NHL and CLL.
Acknowledgment
We thank the patients who participated in this trial and the nurses and nurse practitioners who cared for them in the in-patient Hematology unit, Clinical Treatment Unit, Chemotherapy Unit and Immediate Care Center.
Footnotes
Supported by National Cancer Institute Grants No. U01-CA76576 (M.R.G.) and K23 CA102276-01A1 (T.S.L.), Leukemia and Lymphoma Society Specialized Center of Research (J.C.B.).
Presented in poster format at the 46th Annual Meeting of the American Society of Hematology, December 4-7, 2004, San Diego, CA; the 47th Annual Meeting of the American Society of Hematology December 10-13, 2005, Atlanta, GA; the 42nd Annual Meeting of the American Society of Clinical Oncology June 2-6, 2006, Atlanta, GA; and the 50th Annual Meeting of the American Society of Hematology December 6-8, 2008, San Francisco, CA.
A patent was filed on this method of administering flavopiridol, but it was rejected. Inventors on this patent included J.C.B, M.R.G., and T.S.L.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00058227.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Thomas S. Lin, sanofi-aventis (C); Pierluigi Porcu, Therakos (C); Michael R. Grever, sanofi-aventis (C) Stock Ownership: None Honoraria: Thomas S. Lin, sanofi-aventis Research Funding: Thomas S. Lin, sanofi-aventis; Pierluigi Porcu, Millennium; John C. Byrd, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Thomas S. Lin, Larry J. Schaaf, Michael R. Grever, John C. Byrd
Financial support: Thomas S. Lin, Michael R. Grever, John C. Byrd
Administrative support: Thomas S. Lin, Diane Beth Fischer, Sarah M. Mitchell, Larry J. Schaaf, Michael R. Grever, John C. Byrd
Provision of study materials or patients: Thomas S. Lin, Kristie A. Blum, Pierluigi Porcu, Eric H. Kraut, Robert A. Baiocchi, Mollie E. Moran, John C. Byrd
Collection and assembly of data: Thomas S. Lin, Kristie A. Blum, Diane Beth Fischer, Sarah M. Mitchell, Pierluigi Porcu, Eric H. Kraut, Robert A. Baiocchi, Mollie E. Moran, John C. Byrd
Data analysis and interpretation: Thomas S. Lin, Diane Beth Fischer, Sarah M. Mitchell, Amy S. Ruppert, Amy J. Johnson, Larry J. Schaaf, John C. Byrd
Manuscript writing: Thomas S. Lin, Amy S. Ruppert, John C. Byrd
Final approval of manuscript: Thomas S. Lin, Kristie A. Blum, Diane Beth Fischer, Sarah M. Mitchell, Amy S. Ruppert, Pierluigi Porcu, Eric H. Kraut, Robert A. Baiocchi, Mollie E. Moran, Amy J. Johnson, Larry J. Schaaf, Michael R. Grever, John C. Byrd
REFERENCES
- 1.Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2003;362:139–146. doi: 10.1016/S0140-6736(03)13868-8. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Zucca E, Cavalli F. Mantle cell lymphoma. Curr Opin Hematol. 2004;11:411–418. doi: 10.1097/01.moh.0000138682.13354.da. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi MK, Marcus RE. Follicular lymphoma: Time for a re-think? Blood Rev. 2005;19:165–178. doi: 10.1016/j.blre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin P, Hagemeister FB, Romaguera JE, et al. Fludarabine, mitoxantrone, and dexamethasone: An effective new regimen for indolent lymphoma. J Clin Oncol. 1996;14:1262–1268. doi: 10.1200/JCO.1996.14.4.1262. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin P, Hagemeister FB, Rodriguez MA, et al. Safety of fludarabine, mitoxantrone, and dexamethasone combined with rituximab in the treatment of stage IV indolent lymphoma. Semin Oncol. 2000;27:37–41. [PubMed] [Google Scholar]
- 7.Forstpointer R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 8.Bosch F, Ferrer A, Lopez-Guillermo A, et al. Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukaemia. Br J Haematol. 2002;119:976–984. doi: 10.1046/j.1365-2141.2002.03959.x. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 10.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 11.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Dohner H, Fischer K, Bentz M, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85:1580–1589. [PubMed] [Google Scholar]
- 13.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 14.Carlson BA, Dubay MM, Sausville EA, et al. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–2978. [PubMed] [Google Scholar]
- 15.Kaur G, Stetler-Stevenson M, Sebers S, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84:1736–1740. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 16.Sausville EA, Johnson J, Alley M, et al. Inhibition of CDKs as a therapeutic modality. Ann N Y Acad Sci. 2000;910:207–222. doi: 10.1111/j.1749-6632.2000.tb06710.x. [DOI] [PubMed] [Google Scholar]
- 17.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92:376–387. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
- 18.Worland PJ, Kaur G, Stetler-Stevenson M, et al. Alteration of the phosphorylation state of p34cdc2 kinase by the flavone L86-8275 in breast carcinoma cells: Correlation with decreased H1 kinase activity. Biochem Pharmacol. 1993;46:1831–1840. doi: 10.1016/0006-2952(93)90590-s. [DOI] [PubMed] [Google Scholar]
- 19.Kitada S, Zapata JM, Andreeff M, et al. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–397. [PubMed] [Google Scholar]
- 20.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 20a.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia: High response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6015–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase I study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Grever MR, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 25.Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle cell lymphoma: Results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–1745. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphomas. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 27.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108:4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]