Abstract
Purpose
A significant proportion of adults surviving childhood cancer are smokers. Although these estimated rates of smoking are slightly lower than those in the US population, they remain alarmingly high for this high-risk group. The purpose of this study was to examine the predictive validity of adolescent self-reported smoking intentions for later smoking among childhood cancer survivors.
Patients and Methods
Baseline tobacco intentions were collected from 119 nonsmoking cancer survivors, age 10 to 18 years, who participated in a tobacco-based clinical trial during the late 1990s. Follow-up smoking status was systematically collected annually up to 10 years postintervention (median follow-up, 6.0 years; interquartile range, 3.0 to 6.9 years) as part of clinical survivorship care.
Results
Twenty-seven participants (22.7%) subsequently initiated tobacco use within 5 years of study enrollment. The 5-year cumulative incidence was 29.8% ± 6.0% for those who were susceptible to smoking compared with 12.8% ± 5.4% for those who were committed never smokers (P = .022). Past use (P < .001) and having friends who smoked (P = .038) were also associated (univariate model) with tobacco initiation, and there was a trend for an association for older adolescents (P = .073). Every unit increase on the intentions scale was associated with a 17% increase in the risk for tobacco initiation (P = .002) after adjusting for age group and past tobacco use in a multivariable model.
Conclusion
Because early intentions to smoke are predictive of later tobacco use, survivors as young as 10 years of age who waver in their commitment to remain tobacco abstinent should be targeted for tobacco prevention interventions.
INTRODUCTION
Despite the known health risks associated with tobacco use, smoking remains a significant public health problem, particularly among high-risk populations such as survivors of childhood cancer. Although tobacco use in this population generally occurs at rates equal to or below those of their healthy counterparts, reports indicate current rates of smoking among adult survivors of childhood cancer to be 17%, with 28% reporting past smoking.1 Whereas only 2% to 8% of teens on treatment for cancer report current smoking, 20% report past tobacco use, which is consistent with US population rates.2–6 Among adolescent survivors of childhood cancer, smoking has been reported to be as high as 15% and increases with age.2–6
The implications of tobacco use are significant in that pediatric cancer patients may be at greater risk to develop tobacco-related conditions because of compromised health status resulting from cancer treatment.7 An investigation from the Childhood Cancer Survivor Study estimated that 42% of pediatric cancer survivors will experience serious, disabling, or life-threatening illnesses by 30 years postdiagnosis, including cardiovascular disease, stroke, kidney failure, pulmonary fibrosis, and second malignancies, conditions that can be exacerbated by tobacco use.8 Several antineoplastic therapies commonly used in the treatment of pediatric malignancies have been associated with cardiopulmonary toxicities and organ compromise that can be potentiated by tobacco use. Adults who continue to smoke after diagnosis experience increased rates of tobacco-related complications, diminished efficacy of radiation therapy, reduced survival time, and greater risk of disease reoccurrence/second primary tumor compared with those who stop smoking postdiagnosis.9–11
Based on these health risks, adolescents are strongly encouraged to abstain from tobacco use. Among healthy adolescents, intent to use tobacco is a proximal, reliable outcome measure of future tobacco use among nonsmokers and has subsequently been utilized in smoking prevention efforts. A number of prospective studies have demonstrated that intention to smoke is predictive of adolescents' later smoking outcomes, whether the reports are self-reported or biologically verified.12–14 When compared with healthy peers, nonsmoking adolescents with cancer are one third less likely to report future smoking intentions; however, 48% report some intention to use tobacco in the future.3 Other studies have reported that up to 57% of nonsmoking adolescents with a cancer history intend to smoke in the future15 suggesting that half of adolescent cancer patients waver in their commitment to remain tobacco free. Yet, the ability of smoking intentions to predict future tobacco use has not been evaluated in the context of surviving cancer. Thus, the purpose of this study is to examine the predictive validity of self-reported intentions to smoke among pediatric cancer survivors who were nonsmokers in adolescence/preadolescence.
PATIENTS AND METHODS
Participants
Baseline data were collected from 162 adolescent survivors of childhood cancer who participated in either pilot or primary phases of a randomized clinical trial examining the efficacy of a health counseling intervention in reducing intentions of future smoking. Eligible participants included youths with a history of cancer, 10 to 18 years of age at baseline, cognitively intact, and currently disease-free. Survivors of brain tumors were ineligible (Tyc et al4 for study details). Eleven participants were identified as tobacco users at baseline, and an additional 32 patients without standardized follow-up tobacco use assessment were subsequently excluded, leaving 119 participants in this analysis of baseline nonsmokers.
Design and Procedures
This study uses a prospective, nonexperimental design to determine the predictive validity of adolescent smoking intentions as they relate to future smoking. Baseline data included demographic (age, sex, race, and socioeconomic status [SES]), medical (diagnosis, type of treatment, relapse status), and psychosocial variables (intervention condition, parent smoking, peer smoking status, tobacco use, and smoking intentions; see Tyc et al4 for a description of these variables). SES was measured using Hollingshead Four Factor Index of Social Status,16 with high SES defined as a score of 1 or 2, middle SES as a score of 3, and low SES as a score of 4 or 5. Parent and peer smoking were not collected among the 53 patients who participated in the pilot phase of the clinical trial.
The smoking intentions scale consists of six questions that measure future intention to use tobacco as rated on a 5-point Likert scale ranging from “very unlikely” to “very likely.” Participants were asked to respond to questions such as “How likely is it that you will use tobacco in the next year?” and “How likely is it that you will be tempted to use tobacco in the future?” Intention scores range from 6 to 30, with 6 representing no intentions of future tobacco use and 30 representing strong intentions. Participants who endorsed all six items as “very unlikely” (score = 6) were considered “committed never smokers” and those who endorsed as least one item as “somewhat unlikely” to “very likely” (score > 6) were identified as being “susceptible to smoking.”17 Cronbach's alpha of .88 has been computed for this scale, which is indicative of strong internal reliability.4 Smoking intentions in this study were examined as a binary variable (given the skewed distribution with 52 of 119 participants being committed never smokers) and as a continuous variable.
Participants' tobacco use status was coded on the basis of their response to a smoking item on a clinic-based health behavior questionnaire that is completed at each survivorship visit. Specifically, participants were asked: “Do you now smoke cigarettes every day, some days, or not at all?” Those who endorsed smoking “every day” or “some days” (either on questionnaire or interview) were coded as current smokers. An identical question querying smokeless tobacco use was also included on the questionnaire. Tobacco use that began before survivorship clinic eligibility was captured by the endorsement of current smoking (eg, within the last 30 days) on the clinical trial follow-up questionnaires or within the patient's active treatment section.
Patients at our institution who are at least 5 years from diagnosis and 2 years disease-free typically transfer from treatment clinics to the After Completion of Therapy (ACT) Clinic (ie, survivorship clinic) where they are seen annually for either 10 years postdiagnosis or until they reach 18 years of age, whichever is later. Tobacco use data are systematically collected by medical staff during ACT Clinic appointments via standardized health behavior questionnaires and follow-up interview, and these data are entered into an ACT clinical database. For this study, the earliest date of tobacco use was reported, along with the most recent contact as a nonsmoker and date of death. Standardized tobacco use data were available for all 119 of the study participants. Follow-up smoking status was systematically collected annually up to 10 years postintervention (median follow-up, 6.0 years; interquartile range, 3.0 to 6.9 years) as part of clinical survivorship care. There was one eligible participant for whom only a baseline assessment of tobacco use was collected as part of the original clinical trial. As a result, this participant's follow-up time was censored at 0 days resulting in a follow-up range of 0 to 10.9 years.
Whereas 77 of the study participants had a timely annual tobacco use assessment after the study intervention, 42 had gaps of more than 2 years between completion of the clinical trial and entry into our survivorship clinic. Medical record review revealed tobacco use status for 27 of these participants during this gap period and found that the tobacco status results were consistent with those included in the ACT database, except for one patient who was found to have initiated tobacco use 2 years earlier, based on the medical record review; the analysis data set was updated to reflect the earlier date from the medical record review. Informed consent was obtained from all study participants before their enrollment on the clinical trial, and both the original clinical trial and this follow-up study were approved by our institutional review board.
Statistical Approach
The association between patient characteristics with intentions group (committed never smokers v susceptible to smoking) was investigated using χ2 and exact χ2 tests. To estimate the probability of first tobacco initiation at 5 years, the cumulative incidence (± standard error) was calculated for study participants who reported being nonsmokers at baseline.18 Time to first documented tobacco use was calculated from the original clinical trial enrollment (when baseline intention was assessed) to the earliest date tobacco use was reported. Patients who were still alive without experiencing an event were censored on their last follow-up date. Death was treated as a competing event for patients who died before initiating tobacco use. The cumulative incidences of tobacco initiation for different patient characteristics were compared with Gray's test, which allows for comparisons of cause-specific failure distributions when competing risks are present.19 Patient characteristics that were significant at the 0.10 level were included in a multivariable model for proportional hazards with competing risks.20
RESULTS
Forty-five percent of participants were between the ages of 10 and 13 years at baseline, while 55% were between the ages of 14 and 18 (median age, 14.5 years; range, 10.2 to 18.9 years) with a median time since diagnosis of 4.4 years (range, 0.3 to 15.7 years). Regarding the race distribution, 82% of patients were white and 18% were African American. The sample was composed of high SES families (50%), whereas 26% of patients came from middle SES and 24% came from low SES families. The majority of patients had leukemia (61%), and the remaining had a solid tumor diagnosis (39%). Almost half the sample (44%) reported no intentions to use tobacco (score = 6); 32% scored 7 to 10, 19% scored 11 to 15, and the remaining 5% scored 16 to 19. The median (interquartile range) intentions score for those susceptible to smoking was 10.0 (range, 8.0 to 12.0). With the exception of past tobacco use (P = .031), participant characteristics were not found to differ significantly between those with and without smoking intentions at baseline (Table 1).
Table 1.
Comparison of Tobacco Use Intentions at Study Baseline by Patient Characteristics (N = 119)
| Characteristic | All Participants(N = 119) |
Baseline Intentions Group |
P‡ | ||||
|---|---|---|---|---|---|---|---|
| Committed Never Smokers*(n = 52) |
Susceptible to Smoking†(n = 67) |
||||||
| No. | % | No. | Row % | No. | Row % | ||
| Age at baseline, years | |||||||
| 10-13 | 54 | 45.4 | 25 | 46.3 | 29 | 53.7 | .602 |
| 14-18 | 65 | 54.6 | 27 | 41.5 | 38 | 58.5 | |
| Sex | |||||||
| Male | 61 | 51.3 | 26 | 42.6 | 35 | 57.4 | .809 |
| Female | 58 | 48.7 | 26 | 44.8 | 32 | 55.2 | |
| Race | |||||||
| White | 98 | 82.4 | 46 | 46.9 | 52 | 53.1 | .124 |
| Black | 21 | 17.6 | 6 | 28.6 | 15 | 71.4 | |
| SES | |||||||
| Low | 29 | 24.4 | 12 | 41.4 | 17 | 58.6 | .063 |
| Middle | 31 | 26.0 | 19 | 61.3 | 12 | 38.7 | |
| High | 59 | 49.6 | 21 | 35.6 | 38 | 64.4 | |
| Diagnosis | |||||||
| Leukemia | 73 | 61.3 | 31 | 42.5 | 42 | 57.5 | .733 |
| Solid tumor | 46 | 38.7 | 21 | 45.7 | 25 | 54.3 | |
| Intervention arm | |||||||
| Standard | 37 | 31.1 | 14 | 37.8 | 23 | 62.2 | .820 |
| Intervention | 43 | 36.1 | 18 | 41.9 | 25 | 58.1 | |
| Pilot | 39 | 32.8 | 20 | 51.3 | 19 | 48.7 | |
| Past tobacco use | |||||||
| Established/experimenter | 16 | 13.4 | 3 | 18.8 | 13 | 81.2 | .031 |
| Never | 103 | 86.6 | 49 | 47.6 | 54 | 52.4 | |
| Peer tobacco use§ | |||||||
| Tobacco user | 32 | 40.0 | 11 | 34.4 | 21 | 65.6 | .472 |
| Non-tobacco user | 39 | 48.7 | 17 | 43.6 | 22 | 56.4 | |
| Don't know | 9 | 11.3 | 4 | 44.4 | 5 | 55.6 | |
| Parent tobacco use§ | |||||||
| Tobacco user | 31 | 38.8 | 11 | 35.5 | 20 | 64.5 | .483 |
| Non-tobacco user | 45 | 56.2 | 20 | 44.4 | 25 | 55.6 | |
| Don't know | 4 | 5.0 | 1 | 25.0 | 3 | 75.0 | |
Abbreviation: SES, socioeconomic status.
Those with smoking intentions scores equal to 6 at study baseline.
Those with smoking intentions scores above 6 at study baseline.
P values are from a test of the association between patient characteristic and intentions group. χ2 or exact χ2 tests were used. For testing, “Don't know” (peer and parent use) and “pilot” (intervention group) categories were ignored.
Data provided only on the non-pilot cohort of 80 nonsmokers.
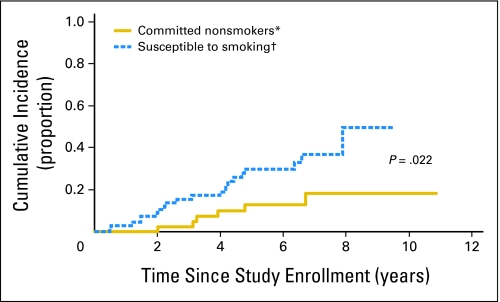
Of the 119 participants, 27 (22.7%) subsequently initiated tobacco use, 89 (74.8%) never initiated tobacco use by last study follow-up, and three (2.5%) died without initiating tobacco use. Of the 27 tobacco users, 20 (74.1%) reported smoking cigarettes, four (14.8%) reported smokeless tobacco use, and three (11.1%) reported both cigarette and smokeless tobacco use. Univariate analyses were conducted to determine the cumulative incidence of reported tobacco use according to patient characteristics (Table 2). Results indicated that binary baseline smoking intentions (committed never smokers v susceptible to smoking) were associated with risk of tobacco initiation, with the 5-year cumulative incidence being 29.8% ± 6.0% versus 12.8% ± 5.4% for those with and without intentions to use tobacco (P = .022; Fig 1). Past tobacco use (59.7% ± 14.7% v 17.4% ± 4.1%; P < .001) and peer tobacco use at baseline (35.2% ± 10.8% v 11.9% ± 5.7%; P = .038) were associated with later tobacco initiation. There was also a trend for older patients (age 14 to 18 years) to be more likely to initiate tobacco use (33.7% ± 7.1% v 13.2% ± 4.7%; P = .073) compared with younger patients.
Table 2.
Cumulative Incidence of Reported Tobacco Use According to Patient Characteristics (N = 119)
| Characteristic | No. of Patients | Estimate + SE (%) at Year 5 | P* |
|---|---|---|---|
| Age, years | |||
| 10-13 | 54 | 13.2 ± 4.7 | .073 |
| 14-18 | 65 | 33.7 ± 7.1 | |
| Sex | |||
| Male | 61 | 23.9 ± 5.9 | .831 |
| Female | 58 | 22.0 ± 6.3 | |
| Race | |||
| White | 98 | 26.6 ± 4.9 | .131 |
| Black | 21 | 5.6 ± 5.6 | |
| SES | |||
| Low | 29 | 27.5 ± 9.9 | .478 |
| Middle | 31 | 14.5 ± 6.9 | |
| High | 59 | 25.6 ± 6.3 | |
| Diagnosis | |||
| Leukemia | 73 | 23.0 ± 5.5 | .336 |
| Solid tumor | 46 | 22.4 ± 6.7 | |
| Past tobacco use | |||
| Established/experimenter | 16 | 59.7 ± 14.7 | < .001 |
| Never | 103 | 17.4 ± 4.1 | |
| Intentions category | |||
| Committed never smokers† | 52 | 12.8 ± 5.4 | .022 |
| Susceptible to smoking‡ | 67 | 29.8 ± 6.0 | |
| Intervention arm§ | |||
| Standard control | 37 | 24.5 ± 8.5 | .756 |
| Intervention | 43 | 22.8 ± 7.3 | |
| Peer tobacco use§ | |||
| Tobacco user | 32 | 35.2 ± 10.8 | .038¶ |
| Non-tobacco user | 39 | 11.9 ± 5.7 | |
| Don't know | 9 | 40.7 ± 20.4 | |
| Parent tobacco use§ | |||
| Tobacco user | 31 | 31.3 ± 9.6 | .286¶ |
| Non-tobacco user | 45 | 19.4 ± 6.8 | |
| Don't know | 4 | 0 |
Abbreviations: SE, standard error; SES, socioeconomic status.
Gray's test.
Those with smoking intentions scores equal to 6 at study baseline.
Those with smoking intentions scores above 6 at study baseline.
Data provided only on the non-pilot cohort of 80 nonsmokers.
Excluding those who responded “Don't know.”
Fig 1.
Cumulative incidence of first reported tobacco use. (*) Those with smoking intentions scores = 6 at study baseline. (†) Those with smoking intentions scores > 6 at study baseline.
The effect of baseline intentions on later tobacco initiation was examined in a multivariable proportional hazards model controlling for past tobacco use and age. Peer use, which was significant (univariate model), was not included in the multivariable model since it was not assessed in the pilot phase of the tobacco trial. We found that binary intentions (P = .18) were not significant in the multivariable model, but continuous intentions were. Every unit increase in intentions conferred a 17% (95% CI, 6% to 30%) increase in the risk for tobacco initiation (P = .002). Reported past tobacco use for baseline nonsmokers was associated with a 3.60-fold increase in the risk of future tobacco use (95% CI, 1.50-fold to 8.62-fold; P = .004). The univariate trend for age was no longer apparent in the multivariable model when age was characterized either as a group or continuous variable.
DISCUSSION
The results of our study found that young nonsmoking survivors who waver in their commitment to remain tobacco free were at significantly greater risk to initiate tobacco use compared with patients without intentions. These findings are reason for concern in that intention of future smoking is the strongest modifiable predictor of later smoking and has been found to be more influential than either parent or best friend smoking among adolescents.21 The results of this study are also congruent with prior research examining intention to smoke as a predictor for future tobacco use among healthy preadolescents and adolescents, and with validated models of health behavior.22–24
In addition to smoking intentions, similarities in findings between our sample and those published on healthy adolescents have also emerged specific to smoking prevalence and risk factors; 23% of our sample eventually engaged in tobacco use, and factors such as older age, tobacco use history, and peer use differentiated those who had/had not used tobacco. While Tyc et al3 reported parallel risk factor findings among adolescents being treated for cancer, to the best of our knowledge, we are the first to identify these findings in adolescents surviving childhood cancer. Despite these similarities, the role that cancer and its treatment have on the progression of smoking remains undetermined and represents an important focus for future research. It is important to note that although survivors of childhood cancer are not immune to environmental factors that promote smoking intentions and onset (eg, media and marketing messages relating to tobacco),25–26 many of these factors are modifiable and responsive to intervention.
The implications of this study are significant. Preadolescent and adolescent patients' intentions to use tobacco reliably predict later smoking initiation; these patients are thus prime targets for preventive counseling among health care providers. Oncologists and other health care providers should begin assessing their patients' intentions of tobacco use early during the elementary years (via clinical interview or reliable screening tools like the one used in this study), because students who initiate smoking before participation in middle-school prevention programs are unaffected by such programs.27–30 In the latest update of the Clinical Practice Guidelines, Fiore et al31 specifically identify and consider children/adolescents and medically ill populations in their discussion of smoking prevention and cessation. While recommendations developed by the Committee on Substance Abuse32 urge clinicians to anticipate and target those children/adolescents who are at risk for becoming later smokers, these recommendations direct clinicians to ask about household smoking status and to advise all family members to become/remain “tobacco free.” Screening and counseling can be provided prophylactically for nonsmokers and as a means of cessation for active smokers. Collectively, these guidelines ensure that preventive advice is provided to youths and their parents, and that those who are vulnerable to smoking onset are identified before the initiation of tobacco use. Health care providers should be particularly sensitive to smoking among pediatric cancer survivors and assess risk of initiating tobacco use using known predictors such as intentions to smoke and exposure to tobacco-using peers.
The provision of tobacco counseling among adolescent survivors of pediatric cancer has been shown to be potentially effective in the short term. Cox et al33 reported that behavioral risk counseling was marginally effective at maintaining smoking abstinence at one year post intervention, while Hollen et al34 found that an educational/social support intervention improved smoking outcomes at 6 months follow-up. In the clinical trial that provided the baseline data for this study, Tyc et al4 found that patients randomly assigned to a health counseling intervention experienced greater changes in tobacco knowledge, tobacco-related perceived vulnerability, and intentions to smoke (at 1 year, but not at 6 months) compared with those in the control condition. Determining long-term efficacy of the original intervention on realized tobacco use was not a goal of this study, because a brief, single-session intervention similar to what should be delivered by health care providers in the clinical setting may not be useful in affecting tobacco outcomes.4 We do note that this intervention targeting intentions did not affect later smoking outcomes (as can be seen in Table 2), but collectively, these studies suggest that educational and behavioral risk counseling interventions may be beneficial in reducing short-term smoking risk among adolescents with a cancer history. Although effective smoking cessation interventions are available for adult survivors of childhood cancer,35 more studies are needed to improve the effectiveness of interventions and establish whether pediatric interventions are effective long-term in preventing smoking onset and progression during the high-risk adolescent and young adult years. Concurrently, the applicability of smoking prevention interventions developed for healthy adolescents should be tested in the oncology context.
Despite the contributions of this study, there are several limitations that should be taken into consideration. First, patient smoking status was established via patient self-report but not validated through biologic markers. Reports of tobacco use were also collected within the medical setting resulting in a potential social desirability bias, which may have affected the validity of reported intentions to use tobacco and tobacco initiation among patients. While attempts were made to include the most robust factors on smoking initiation, our study results may be limited by the exclusion of influential variables such as cancer-related worry, pain, and fatigue.33 Our sample was predominantly white and almost 50% were of high SES; however, we did not detect significant race and SES differences in contrast to the findings of others.1,36 Finally, we chose to exclude patients who did not have a structured tobacco assessment. As a result, potential for selection bias exists. Interestingly, in an analysis that did not exclude the 32 subjects with tobacco use status determined from medical records only (N = 151, total smokers = 37, total deaths = 10), the findings were consistent. The 5-year cumulative incidence in this case was 28.8% ± 5.4% for those susceptible to smoking compared with 14.0% ± 5.0% for committed never smokers (P = .024). After adjusting for age group and past tobacco use in a multivariable model, every unit increase on the intentions scale was associated with a 13% increase in the risk for tobacco initiation (95% CI, 4% to 24%; P = .005).
In summary, this study found that tobacco intentions are a valid predictor of future tobacco use among survivors of pediatric cancer. Consequently, tobacco intentions should be used to screen vulnerable youth, so that tobacco prevention interventions can be delivered to these patients before tobacco initiation. Given that survivors of pediatric cancer are at high risk for tobacco-related complications, and that 17% of adult survivors of childhood cancer report current smoking, prevention of tobacco onset in this group is essential. By averting tobacco use in this group, we will be improving not only quality of life but also long-term survivorship.
Footnotes
Supported by the American Lebanese Syrian Associated Charities and the National Cancer Institute, Cancer Center Support (CORE) Grants No. CA21765 and CA23099.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: James L. Klosky, Vida L. Tyc
Administrative support: James L. Klosky, Shelly Lensing
Provision of study materials or patients: James L. Klosky, Vida L. Tyc, Melissa M. Hudson
Collection and assembly of data: James L. Klosky, Vida L. Tyc, Joanna Buscemi
Data analysis and interpretation: James L. Klosky, Vida L. Tyc, Ashley Hum, Shelly Lensing, Joanna Buscemi, Danette M. Garces-Webb, Melissa M. Hudson
Manuscript writing: James L. Klosky, Vida L. Tyc, Ashley Hum, Shelly Lensing, Joanna Buscemi, Danette M. Garces-Webb, Melissa M. Hudson
Final approval of manuscript: James L. Klosky, Vida L. Tyc, Ashley Hum, Shelly Lensing, Joanna Buscemi, Danette M. Garces-Webb, Melissa M. Hudson
REFERENCES
- 1.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2002;20:1608–1616. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 2.Mulhern RK, Tyc VL, Phipps S, et al. Health-related behaviors of survivors of childhood cancer. Med Pediatr Oncol. 1995;25:159–165. doi: 10.1002/mpo.2950250302. [DOI] [PubMed] [Google Scholar]
- 3.Tyc VL, Lensing S, Klosky J, et al. A comparison of tobacco-related risk factors between adolescents with and without cancer. J Pediatr Psychol. 2005;30:359–370. doi: 10.1093/jpepsy/jsi030. [DOI] [PubMed] [Google Scholar]
- 4.Tyc VL, Rai SN, Lensing S, et al. Intervention to reduce intentions to use tobacco among pediatric cancer survivors. J Clin Oncol. 2003;21:1366–1372. doi: 10.1200/JCO.2003.11.148. [DOI] [PubMed] [Google Scholar]
- 5.Tyc VL, Hadley W, Crockett G. Brief report: Predictors of intentions to use tobacco among adolescent survivors of cancer. J Pediatr Psychol. 2001;26:117–121. doi: 10.1093/jpepsy/26.2.117. [DOI] [PubMed] [Google Scholar]
- 6.Tyc VL, Hadley W, Crockett G. Prediction of health behaviors in pediatric cancer survivors. Med Pediatr Oncol. 2001b;37:42–46. doi: 10.1002/mpo.1161. [DOI] [PubMed] [Google Scholar]
- 7.Hollen PJ, Hobbie WL. Decision making and risk behaviors of cancer-surviving adolescents and their peers. J Pediatr Oncol Nurs. 1996;13:121–133. doi: 10.1177/104345429601300304. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 9.Cinciripini PM, Gritz ER, Tsoh JY, et al. Smoking cessation and cancer prevention. In: Holland JC, editor. Psycho-Oncology. New York, NY: Oxford University Press; 1998. pp. 27–44. [Google Scholar]
- 10.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 11.Des Rochers C, Dische S, Saunders MI. The problem of cigarette smoking in radiotherapy for cancer in the head and neck. Clin Oncol (R Coll Radiol) 1992;4:214–216. doi: 10.1016/s0936-6555(05)81053-2. [DOI] [PubMed] [Google Scholar]
- 12.Choi WS, Gilpin EA, Farkas AJ, et al. Determining the probability of future smoking among adolescents. Addiction. 2001;96:313–323. doi: 10.1046/j.1360-0443.2001.96231315.x. [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt L, Woodruff SI, Elder JP. A longitudinal analysis of adolescent smoking and its correlates. J Sch Health. 1994;64:67–72. doi: 10.1111/j.1746-1561.1994.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoving C, Reubsaet A, de Vries H. Predictors of smoking stage transitions for adolescent boys and girls. Prev Med. 2007;44:485–489. doi: 10.1016/j.ypmed.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Tyc VL, Lensing S, Rai SN, et al. Predicting perceived vulnerability to tobacco-related health risks and future intentions to use tobacco among pediatric cancer survivors. Patient Educ Couns. 2006;62:198–204. doi: 10.1016/j.pec.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 17.Pierce JP, Distefan JM, Kaplan RM, et al. The role of curiosity in smoking initiation. Addict Behav. 2005;30:685–696. doi: 10.1016/j.addbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. ed 2. New York, NY: John Wiley & Sons; 2002. pp. 247–254. [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Pierce JP, Choi WS, Gilpin EA, et al. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- 22.Ajzen I, Fishbein M. Understanding Attitudes and Predicting Social Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- 23.Weinstein ND. Why it won't happen to me: Perceptions of risk factors and susceptibility. Health Psychol. 1984;3:431–457. doi: 10.1037//0278-6133.3.5.431. [DOI] [PubMed] [Google Scholar]
- 24.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 25.Wellman RJ, Sugarman DB, DiFranza JR, et al. The extent to which tobacco marketing and tobacco use in films contribute to children's use of tobacco. A meta-analysis. Arch Pediatr Adolesc Med. 2006;160:1285–1296. doi: 10.1001/archpedi.160.12.1285. [DOI] [PubMed] [Google Scholar]
- 26.Wakefield M, Flay B, Nichter M, et al. Role of the media in influencing trajectories of youth smoking. Addiction. 2003;98(suppl 1):79–103. doi: 10.1046/j.1360-0443.98.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellickson PL, Bell RM, McGuigan KA. Prevention of adolescent drug use: Long-term results of a junior high program. Am J Public Health. 1993;83:856–861. doi: 10.2105/ajph.83.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flay BR, Koepke D, Thomson SJ, et al. Six-year follow-up of the first Waterloo school smoking prevention trial. Am J Public Health. 1989;79:1371–1376. doi: 10.2105/ajph.79.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray DM, Pirie P, Luepker RV, et al. Five- and six-year follow-up results from four seventh-grade smoking prevention strategies. J Behav Med. 1989;12:207–218. doi: 10.1007/BF00846551. [DOI] [PubMed] [Google Scholar]
- 30.Vartiainen E, Fallonen U, McAlister AL, et al. Eight-year follow-up results of an adolescent smoking prevention program: The North Karelia Youth Project. Am J Public Health. 1990;80:78–79. doi: 10.2105/ajph.80.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. May, [Google Scholar]
- 32.Committee on Substance Abuse. American Academy of Pediatrics: Tobacco's toll—Implications for the pediatrician. Pediatrics. 2001;107:794–798. [PubMed] [Google Scholar]
- 33.Cox CL, McLaughlin RA, Rai SN, et al. Adolescent survivors: A secondary analysis of a clinical trial targeting behavior change. Pediatr Blood Cancer. 2005;45:144–154. doi: 10.1002/pbc.20389. [DOI] [PubMed] [Google Scholar]
- 34.Hollen PJ, Hobbie WL, Finley SM. Testing the effects of a decision-making and risk-reduction program for cancer-surviving adolescents. Oncol Nurs Forum. 1999;26:1475–1486. [PubMed] [Google Scholar]
- 35.Emmons KM, Puleo E, Mertens A, et al. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol. 2008;27:52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]