Abstract
Purpose
We previously validated disease-free survival (DFS) as a surrogate for overall survival (OS) in fluorouracil-based adjuvant colon cancer clinical trials. New therapies have extended survival after recurrence from 1 to approximately 2 years. We examined the possible impact of this improvement on the DFS/OS association.
Methods
The Adjuvant Colon Cancer Endpoints (ACCENT) data set of 20,898 patients was analyzed. In an exploratory fashion, time from recurrence to death in patients experiencing recurrence was extended using several algorithms, and the association of DFS after 3 years of median follow-up and OS after varying lengths of follow-up (median of 5, 6, and 7 years) was assessed.
Results
Seven thousand four hundred two patients (35%) experienced recurrence. Median time from recurrence to death was 24 months in the hypothetical data sets. When times from recurrence to death were doubled, the association between treatment effects on DFS and 5-year OS was modest (R2 = 0.51 for both 2- and 3-year DFS) but remained strong for DFS and 6-year OS (R2 = 0.67 for both 2- and 3-year DFS) and 7-year OS (R2 = 0.70 for both 2- and 3-year DFS). The reduced DFS/OS association with extended survival after recurrence was greater in stage II than stage III patients. Multiple simulations provided consistent findings.
Conclusion
Extended survival after recurrence reduces the association between treatment effects on 3-year DFS and 5-year OS, particularly in stage II patients; longer follow-up strengthens the association. In modern adjuvant trials, 6 or 7 years may be required to demonstrate OS improvements, further supporting DFS as the preferred primary end point for future adjuvant colon cancer clinical trials.
INTRODUCTION
In the past decade, multiple, incremental advances in the treatment of colon cancer have improved patient outcomes in the advanced-disease setting. Combination chemotherapy with fluorouracil (FU)/leucovorin (LV) plus oxaliplatin,1,2 as well as FU/LV plus irinotecan,3 results in improved progression-free survival and overall survival (OS) when compared with FU/LV alone. At the trial level, exposure to all three cytotoxic agents (FU/LV, oxaliplatin, and irinotecan) at some point in the treatment of advanced disease is associated with prolonged median OS.4,5 Targeted therapies such as bevacizumab, cetuximab, and panitumumab have also extended progression-free survival or OS in the advanced-disease setting.6–10 In addition, the use of postrecurrence metastasectomy has been shown to result in long-term survival in selected patients.11
In the adjuvant setting, the Multicenter International Study of Oxaliplatin/5FU-LV in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial randomly assigned patients to oxaliplatin or not in combination with FU/LV as postoperative therapy for stage II and III colon cancer.12 In this trial, there was a significant increase in disease-free survival (DFS) favoring the oxaliplatin group, which led to the approval of oxaliplatin for adjuvant therapy in stage III colon cancer. Among patients with stage III disease, the beneficial effects observed for DFS at the 3-year time point were maintained at 5 years, but oxaliplatin did not result in a significant benefit in OS until 6 years of follow-up.13 These findings are supported by the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial, where oxaliplatin again provided a highly significant improvement in 3-year DFS but a borderline improvement in 5-year OS.14,15
The use of DFS with 3 years of median follow-up as a primary end point in MOSAIC and NSABP C-07 was supported by analyses from the Adjuvant Colon Cancer Endpoints (ACCENT) database, a pooled database of data on individual patients with colon cancer from multiple phase III clinical trials involving FU-based adjuvant therapy. The initial ACCENT analysis showed a strong correlation between treatment effects on DFS assessed after 3 years of median follow-up and 5-year OS.16 Subsequent analyses also demonstrated that the treatment effect on DFS after 2 years of median follow-up was highly associated with the effect on OS after 5 years of follow-up.17
Improvements in the detection and treatment of recurrent colon cancer, including secondary resections, have translated into a median OS from recurrence to death of approximately 2 years in recent trials.13,15,18-20 In the initial ACCENT report, the median time from recurrence to death was 12 months.16 This observation motivated the current study to determine the impact of longer survival after recurrence on the association between DFS and OS in adjuvant colon cancer therapy trials.
METHODS
The ACCENT data set consists of individual patient data from 18 trials that investigated FU-based adjuvant therapy in colon cancer. These trials, conducted from 1978 to 1999, involved 20,898 patients and 43 treatment arms (34 active treatment arms and nine surgery-only arms). Details regarding the individual clinical trials and patient characteristics are listed in Appendix Tables A1 and A2 (online only). Approval for this analysis was granted by the Mayo Clinic Investigational Review Board; individual trials were approved through local mechanisms at the time the trials were conducted.
We created multiple hypothetical data sets based on all patients in ACCENT. In the primary hypothetical data set, for all patients who experienced a recurrence, keeping all other factors identical, each patient's time from recurrence to death was doubled. In patients who experienced recurrence but did not die in the follow-up period, the patient's time from recurrence to censoring was similarly doubled. The survival time remained unchanged for patients without recurrence events. The association between treatment effects on DFS assessed after 2 and 3 years of median follow-up (DFS-2 and DFS-3, respectively) and the hypothetical OS after 5, 6, and 7 years of median follow-up (OS-5, OS-6, and OS-7 respectively) was examined in the simulated data set. Trial-level association (R2) between the two end points was estimated by using a two-stage bivariate copula survival model.21 Briefly, the copula model considers the association between the hazard ratios comparing treatment with control within each trial, based on the complete data available for each patient in each analysis after censoring. The trial-level R2 is based on a 0 to 1 scale; levels closer to 1 indicate a stronger association, thereby indicating a better candidate for a surrogate end point.
The median follow-up time across all trials was greater than 8 years. For all analyses, efforts were made to replicate the actual conduct of a clinical trial, where patients enter over a period of several years and thus at any given calendar date have differing durations of follow-up. For each analysis conducted at a specific time point (eg, 3 years), the outcome data for each patient were censored at the point in time at which the median follow-up in the trial was 3 years. Thus, as the time for the OS analysis changed from 5 to 6 to 7 years, the number of events available for analysis increased. In all calculations of median follow-up, only patients without an event were considered in the median calculation.
Analyses were performed for all patients and also separately for patients with initial stage II and III disease. Additional simulations were performed by extending survival after recurrence differentially for individual patients based on patient-specific characteristics. Survival extensions were modified for the use of adjuvant therapy, time from random assignment to recurrence, and initial stage of colon cancer (I or II v III), factors previously shown to significantly impact the time from recurrence to death.22 For each setting, time from recurrence to death of individual patients was extended by a factor that depended on that patient's individual characteristics. On the basis of previously reported hazard ratios for time from recurrence to death for each characteristic,22 the multiplication factors were normalized such that the weighted average extension in the overall population equaled 2 (ie, a doubling of survival after recurrence).
RESULTS
Among patients in the original ACCENT database, 7,402 (35%) of 20,898 patients experienced recurrence. Eleven percent of patients died without recurrence, and 90% of patients who experienced recurrence subsequently died within the initial trial's follow-up period. The median time from recurrence to death was 12 months in the original data set and 24 months in the hypothetical data sets.
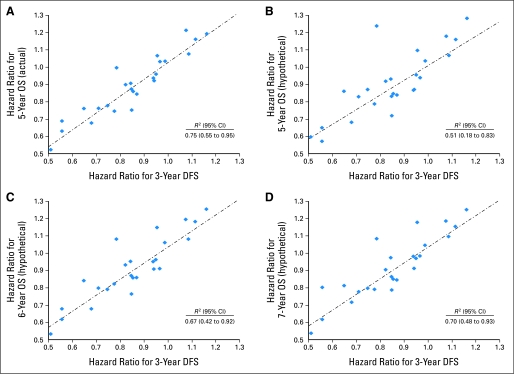
In the original data set, the treatment effect on DFS-3 was highly predictive of the effect on OS-5, with an R2 value of 0.75 (95% CI, 0.55 to 0.95; Fig 1A), as well as for OS-6 and OS-7, with R2 values of 0.78 (95% CI, 0.60 to 0.96) and 0.82 (95% CI, 0.67 to 0.97), respectively (Table 1). The association between treatment effects on DFS-3 and OS-5 deteriorated substantially in the hypothetical data set, with an R2 value of 0.51 (95% CI, 0.18 to 0.83; Fig 1B), but remained strong between DFS-3 and OS-6, although not as strong as in the original data set, with an R2 value of 0.67 (95% CI, 0.42 to 0.92; Fig 1C). When the hypothetical data set follow-up for OS was extended to 7 years, the correlation showed modest continued improvement, with an R2 value of 0.70 (95% CI, 0.48 to 0.93; Fig 1D).
Fig 1.
Scatter plots of hazard ratios by trial (disease-free survival [DFS] v overall survival [OS]). (A) Three-year DFS versus 5-year OS (actual data). (B) Three-year DFS versus 5-year OS (hypothetical data, doubled time from recurrence to death). (C) Three-year DFS versus 6-year OS (hypothetical data, doubled time from recurrence to death). (D) Three-year DFS versus 7-year OS (hypothetical data, doubled time from recurrence to death).
Table 1.
Copula R2 Values and Corresponding 95% CIs
| Factor | 5-Year Median OS |
6-Year Median OS |
7-Year Median OS |
|||
|---|---|---|---|---|---|---|
| R2 | 95% CI | R2 | 95% CI | R2 | 95% CI | |
| Association with 3-year DFS (median) | ||||||
| Actual data | 0.75 | 0.55 to 0.95 | 0.78 | 0.60 to 0.96 | 0.82 | 0.67 to 0.97 |
| Double TRD | 0.51 | 0.18 to 0.83 | 0.67 | 0.42 to 0.92 | 0.70 | 0.48 to 0.93 |
| Recurrence dependent | 0.54 | 0.23 to 0.85 | 0.64 | 0.37 to 0.91 | 0.76 | 0.56 to 0.95 |
| Stage dependent | 0.44 | 0.10 to 0.78 | 0.61 | 0.32 to 0.89 | 0.68 | 0.43 to 0.92 |
| Treatment dependent (surgery only v chemotherapy) | 0.29 | 0.0 to 0.64 | 0.41 | 0.06 to 0.76 | 0.49 | 0.16 to 0.82 |
| Association with 2-year DFS (median) | ||||||
| Actual data | 0.73 | 0.51 to 0.94 | 0.77 | 0.58 to 0.96 | 0.80 | 0.63 to 0.96 |
| Double TRD | 0.51 | 0.18 to 0.83 | 0.67 | 0.41 to 0.92 | 0.70 | 0.46 to 0.93 |
Abbreviations: OS, overall survival; DFS, disease-free survival; TRD, time from recurrence to death.
Similar results were obtained for the relationship between treatment effect on DFS-2 and OS (Table 3); R2 values in the original data set for the association between DFS-2 and OS-5, OS-6, and OS-7 were 0.73, 0.77, and 0.80, respectively; R2 values on the hypothetical data set were 0.51, 0.67, and 0.70, respectively.
When analyzed by stage, extending survival after recurrence reduced the association between treatment effects on DFS-3 and OS-5 in both stage II and stage III patients. In the original data set in stage II patients, the R2 value for association between treatment effects on DFS-3 and OS-5 was 0.57 (95% CI, 0.27 to 0.87), which fell to 0.38 (95% CI, 0.02 to 0.73) when survival after recurrence was doubled. Extending OS follow-up to 6 or 7 years resulted in limited improvement, with an R2 of 0.53 for OS-6 and 0.49 for OS-7 in stage II patients. In stage III patients, extending survival after recurrence reduced the R2 values for the association between treatment effects on DFS-3 and OS-5 from 0.84 (95% CI, 0.70 to 0.97) in the original data set to 0.55 (95% CI, 0.25 to 0.86); extending OS median follow-up to 6 or 7 years increased the R2 values to 0.69 and 0.72, respectively.
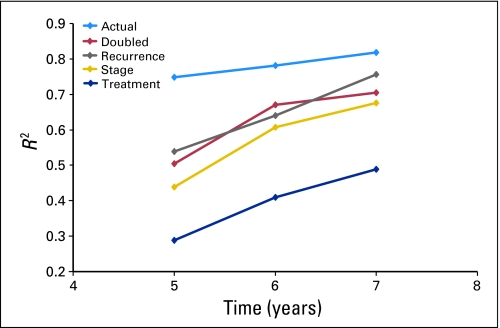
The analysis was repeated on additional simulated data sets based on differential extension of survival based on patient characteristics (Table 2); results are shown in Figure 2. Consistent findings were observed in sensitivity analyses where the association between treatment effects on DFS-3 and OS-5 suffered when survival after recurrence was extended. The association improved between treatment effects on DFS-3 and OS-6, with further improvement between DFS-3 and OS-7. Among the three sensitivity analyses, the most substantial deterioration of the association between DFS and OS was observed when patient survival after recurrence was differentially extended based on initial adjuvant therapy versus control.
Table 2.
Factors Included in Sensitivity Analyses
| Factor | Survival After Recurrence Multiplication Factor | Resultant Median Survival After Recurrence in Subgroup (months) |
|---|---|---|
| Time from adjuvant chemotherapy to recurrence, years | ||
| < 1 | 1.54 | 14 |
| 1-3 | 2.05 | 26 |
| > 3 | 2.59 | 41 |
| Initial therapy | ||
| Surgery alone | 2.34 | 33 |
| Chemotherapy | 1.94 | 22 |
| Initial stage | ||
| I or II | 2.61 | 42 |
| III | 1.84 | 21 |
Fig 2.
R2 values for actual data and hypothetical survival extensions based on patient-specific characteristics.
DISCUSSION
This analysis of simulated data based on the ACCENT database suggests that an extension of patient survival after recurrence will reduce the association between the detection of a treatment effect on DFS assessed after a relatively short duration of follow-up (in this case, a median of 2 or 3 years) and the observed effect on OS assessed at a later but relatively short time point (in this case, 5 years). The association between treatment effects on DFS and OS improved if OS was evaluated at a longer follow-up time. Our results imply that extended survival after disease recurrence will require modern adjuvant colon cancer clinical trials to observe patients for more than 5 years to verify therapeutic benefits of adjuvant therapy on OS. These results do not directly inform as to whether 2-year versus 3-year DFS is the optimal end point for adjuvant trials; to obtain adequate power to use a 2-year DFS end point, a greater number of patients may be required. The resolution of this issue awaits future analyses on modern adjuvant therapy trials.
The decreased association between DFS-3 and OS-5 as a result of increasing survival after recurrence has a clear intuitive rationale. When patients live longer after recurrence, more patients who experience recurrence within the first 3 years from chemotherapy will be alive at the 5-year time point. In the original ACCENT data set, median survival after recurrence was 12 months; thus, most patients who experienced recurrence within the first 3 years had died by 5 years. With a median survival time after recurrence of 24 months, this proportion will decrease substantially. However, even with extended survival after recurrence, most patients with recurrent disease will have died by 6 or 7 years after adjuvant therapy; thus, the association between DFS-3 and OS-6 or OS-7 intuitively should be enhanced. Furthermore, deaths from other causes become increasingly likely as patients become more removed from their primary colon cancer; thus, it is unlikely that the association between DFS-3 and even OS-7 could be as strong as between DFS-3 and OS-5 when survival after recurrence is extended, which was also demonstrated in our hypothetical data sets. Our analyses on the earlier DFS end point, after 2 years of median follow-up, were entirely consistent with the DFS-3 results. The results were also consistent separately in stage II and III patients. Thus, these analyses support the previous ACCENT findings that the association between DFS and OS in trials of stage II patients only is insufficient to declare DFS to be a valid surrogate end point in clinical trials with only a stage II population.17
In two of the three sensitivity analyses performed, the findings were consistent with those from the primary hypothetical data set. However, in the sensitivity analysis where patient survival after recurrence was differentially extended by whether the patient received or did not receive initial adjuvant therapy, the association between treatment effects on DFS and OS at all time points was substantially diminished. This is expected because, in this analysis, patients who experience recurrence after initial surgery without adjuvant therapy have a much longer time to death after recurrence than patients treated initially with adjuvant therapy, thus influencing the association between DFS and OS differentially between the two primary study arms. Data from the MOSAIC study suggest that although there is some differential survival after recurrence between the two treatment arms in that trial (21-month median survival for patients treated with infusional FU, LV, and oxaliplatin; 24-month median survival for patients treated with bolus plus continuous-infusion FU/LV13), the impact is much smaller than we used in our modeling (median survival of 33 months after recurrence in control patients v 22 months in treated patients). It must also be recognized that the modeling approach used here extended the postrecurrence survival of all patients modestly and no patients to a large extreme (such as postrecurrent cure), when in fact, postrecurrence resections now provide a small subset of patients (< 20%) a greatly extended survival after re-resection.
Several factors must be considered in applying these results, based on simulated data sets, to current and future trials. First, there are several mechanisms by which the duration from detected recurrence to death may be extended. Improved imaging modalities may detect recurrent disease earlier, introducing lead-time bias and resulting in an apparent increase in duration from recurrence to death. This fact by itself may alter the time points where the association between DFS and OS may be strongest, but it will not change the overall relationship. However, if earlier detection results in interventions that are potentially curative, such as secondary resection, this association between DFS and OS will likely change as a meaningful proportion of patients who experience recurrence become subsequent long-term survivors. Improvements in therapy after recurrence that extend life without cure for most patients, such as currently provided by chemotherapy and biologic therapy, will also impact the time points of maximal association between treatment effects on DFS and OS but not the fundamental relationship. In general, the paradigm of a steady, modest extended survival after recurrences induced by the new therapies is a simple, but in our opinion reasonable, reflection of results of recent large phase III colon cancer trials. In the last decade, the median survival in patients with advanced disease has increased from approximately 12 months with FU alone to approximately 15 months with the addition of irinotecan,3 to 19 to 20 months with the addition of oxaliplatin to FU,2 to approximately 24 months with biologic agents such as bevacizumab6 and cetuximab.9 The paradigm of gradual improvements in outcomes in patients with advanced disease also has been established in breast and lung cancer.23 If new agents induce dramatic changes in postrecurrence survival, the methods presented here would be inadequate; however, such occurrences have historically been rare. With regard to whether these results are translatable to other disease settings, we stress that end point evaluation must be disease and treatment specific. However, the research methodology used in the current study can be easily applied to other settings.
Critical to the implementation of this analysis to future trials is the need to consider whether new therapeutic interventions will alter the time course of disease recurrence. In the MOSAIC trial, the DFS curves have remained separated out to 6 years of follow-up and have now translated into long-term OS benefit in stage III patients.13 Adjuvant treatments that truly prevent recurrence do not change the relationship between DFS and OS but do affect the time points of correlation. Our results are not highly sensitive to the underlying event times; rather, they are sensitive to the ratio of the typical time from study entry to the full end point (in this case, OS, which is expected to be relatively long) to the typical time to the surrogate end point (in this case, relatively short, with median time to recurrence among patients who experienced recurrence of 18 months). Thus, if future adjuvant therapies truly prevent recurrence, as has been demonstrated for FU-based therapies,24 the strong predictive relationship between DFS and OS should also persist, albeit possibly at different time points. However, it is uncertain how targeted therapies (bevacizumab, cetuximab, and panitumumab) may potentially change the time patterns of recurrence when used in the adjuvant setting. If targeted therapies change the nature of the underlying disease (ie, resulting in less aggressive behavior and thus a later recurrence), then the association between treatment effects on DFS assessed at an early time point and OS at a later time point will likely be altered, and DFS at an early time point such as 3 years may not be a reliable end point to predict OS.
An example of how tumor biology affects this association was shown in a previous ACCENT analysis17 that demonstrated a lower concordance of DFS-3 and OS-5 in patients with stage II disease versus stage III disease. This may have been, in part, a result of a lower proportion of recurrences occurring in the first 3 years for the stage II patients, suggesting less aggressive tumor biology. Subsequent analyses showed that survival after recurrence was significantly longer in patients with initial stage II versus stage III disease, further supporting the theory of differences in intrinsic tumor biology between node-negative and node-positive disease.22 If prolongation of DFS is accepted as a clinical benefit end point on its own, independent of its association with OS, then extending DFS remains an appropriate primary end point regardless of whether the agent truly prevents or delays recurrence.
Continued improvements in survival after recurrence are anticipated with earlier detection of recurrent disease, improved treatment regimens on recurrence, and secondary resection for cure. The potential need for prolonged follow-up further emphasizes the importance of early end points to ensure efficient and timely evaluation of potentially life-extending adjuvant therapies. DFS may be a more accurate reflection of the true patient-relevant benefit of adjuvant treatment if the association between DFS and OS is altered by factors subsequent to recurrence. The extended follow-up required to observe OS improvements further supports using DFS as a full clinical end point, not only as a surrogate end point for survival.
Appendix
The Adjuvant Colon Cancer Endpoints (ACCENT) Collaborative Group consists of D.J. Sargent, E. Green, A. Grothey, S.R. Alberts, B. Bot, M. Campbell, Q. Shi (Mayo Clinic, Rochester, MN); G. Yothers, M.J. O'Connell, N. Wolmark (National Surgical Adjuvant Breast and Bowel Project [NSABP] Biostatistical and Operations Centers, Pittsburgh, PA); A. de Gramont (Hopital Saint Antoine, Paris, France); R. Gray, D. Kerr (QUASAR Collaborative Group, Birmingham and Oxford, United Kingdom); D.G. Haller (Abramson Cancer Center at the University of Pennsylvania, Philadelphia, PA); J. Benedetti (Southwest Oncology Group Statistical Center, Seattle, WA); M. Buyse (International Drug Development Institute, Louvain-la-Neuve, Belgium); R. Labianca (Ospedali Riuniti, Bergamo, Italy); J.F. Seitz (University of the Mediterranean, Marseilles, France); C.J. O'Callaghan (National Cancer Institute of Canada Clinical Trials Group, Queens University, Kingston, Ontario, Canada); G. Francini (University of Siena, Siena, Italy); P.J. Catalano (Eastern Cooperative Oncology Group Statistical Center, Boston, MA); C.D. Blanke (British Columbia Cancer Agency, Vancouver, British Columbia, Canada); T. Andre (Groupe Hospitalier Piti e-Salpetriere, Paris, France); R.M. Goldberg, H. Sanoff (University of North Carolina, Chapel Hill, NC); A. Benson (Northwestern University, Chicago, IL).
Table A1.
Trials Included in the ACCENT Data Set
| Trial | Accrual Period | Treatment Arm(s) | No. of Patients |
|---|---|---|---|
| NSABP C-01 | 1977-1983 | Control v MOF | 724 |
| NCCTG 784852 | 1978-1984 | Control v FU/LEV | 247 |
| FFCD | 1982-1990 | Control v FU/LV | 239 |
| NSABP C-02 | 1984-1988 | Control v PVI FU | 896 |
| INT 0035 | 1984-1987 | Control v FU/LEV | 926 |
| Siena | 1985-1990 | Control v FU/LV | 256 |
| NCIC | 1987-1992 | Control v FU/LV | 359 |
| NSABP C-03 | 1987-1989 | MOF v FU/LV | 1,042 |
| NCCTG 874651 | 1988-1989 | Control v FU/LV | 408 |
| GIVIO | 1989-1992 | Control v FU/LV | 867 |
| NCCTG 894651 | 1989-1991 | FU/LV ± LEV for 6 or 12 months | 915 |
| NSABP C-04 | 1989-1990 | FU/LEV v FU/LV v FU/LV/LEV | 2,083 |
| INT 0089 | 1990-1992 | FU/LEV v FU/LV (HD or LD) v FU/LV/LEV | 3,561 |
| NSABP C-05 | 1991-1994 | FU/LV v FU/LV + IFN | 2,136 |
| NCCTG 914653 | 1993-1998 | FU/LV + HD or standard LEV | 878 |
| SWOG 9415 | 1994-1999 | Bolus v infusional FU/LEV/LV | 939 |
| QUASAR | 1994-1997 | FU/LV (HD or LD) ± LEV | 3,517 |
| GERCOR | 1996-1999 | Bolus v infusional FU/LV | 905 |
| Total | 20,898 |
Abbreviations: ACCENT, Adjuvant Colon Cancer Endpoints; NSABP, National Surgical Adjuvant Breast and Bowel Project; MOF, semustine, vincristine, and fluorouracil; NCCTG, North Central Cancer Treatment Group; FU, fluorouracil; LEV, levamisole; FFCD, Fédération Francophone de Cancérologie Digestive; LV, leucovorin; PVI, protracted venous infusion; INT, Intergroup; NCIC, National Cancer Institute of Canada; GIVIO, Gruppo Interdisciplinare Valutazione Interventi Oncologia; HD, high dose; LD, low dose; IFN, interferon alfa-2a; SWOG, Southwest Oncology Group; QUASAR, Quick and Simple and Reliable; GERCOR, Groupe Cooperateur Multidisciplinaire en Oncologie.
Table A2.
Patient Demographics and Clinical Characteristics (N = 20,898)
| Demographic or Clinical Characteristic | Patients (%) |
|---|---|
| Age, years | |
| < 50 | 16 |
| 50-59 | 26 |
| 60-69 | 39 |
| ≥ 70 | 19 |
| Sex | |
| Male | 55 |
| Female | 45 |
| Stage | |
| I | 1 |
| II | 33 |
| III | 66 |
| Treatment | |
| Surgery alone | 12 |
| Surgery plus chemotherapy | 88 |
Footnotes
Written on behalf of the Adjuvant Colon Cancer Endpoints (ACCENT) Group; see Appendix for full group membership.
Supported by Grants No. CA25224, CA12027, CA69974, CA37372, and CA69651 from the National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard M. Goldberg, sanofi-aventis (C), Pfizer (C); Charles D. Blanke, Pfizer (C); Thierry Andre, Roche (C) Stock Ownership: None Honoraria: Thierry Andre, Baxter Research Funding: Richard M. Goldberg, sanofi-aventis, Pfizer; Steven R. Alberts, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Aimery de Gramont, Marc Buyse, Axel Grothey, Daniel Sargent
Financial support: Steven R. Alberts, Daniel Sargent
Administrative support: Aimery de Gramont, Steven R. Alberts, Daniel Sargent
Provision of study materials or patients: Aimery de Gramont, Michael J. O'Connell, Jacqueline Benedetti, Chris O'Callaghan, Greg Yothers, Thierry Andre, Norman Wolmark, Daniel Sargent
Collection and assembly of data: Greg Yothers, Daniel Sargent
Data analysis and interpretation: Aimery de Gramont, Qian Shi, Michael J. O'Connell, Marc Buyse, Jacqueline Benedetti, Brian Bot, Greg Yothers, Qiqi Deng, Thierry Andre, Axel Grothey, Daniel Sargent
Manuscript writing: Aimery de Gramont, Joleen Hubbard, Qian Shi, Michael J. O'Connell, Marc Buyse, Jacqueline Benedetti, Richard M. Goldberg, Charles D. Blanke, Al Benson, Thierry Andre, Axel Grothey, Daniel Sargent
Final approval of manuscript: Aimery de Gramont, Joleen Hubbard, Qian Shi, Michael J. O'Connell, Marc Buyse, Jacqueline Benedetti, Brian Bot, Chris O'Callaghan, Greg Yothers, Richard M. Goldberg, Charles D. Blanke, Al Benson, Qiqi Deng, Steven R. Alberts, Thierry Andre, Norman Wolmark, Axel Grothey, Daniel Sargent
REFERENCES
- 1.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American Intergroup Trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer: Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-liine therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 8.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 11.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 12.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 13.Andre T, Boni C, Navarro M. Improved overall survival with oxaliplatin, fluorouracil and leucovorin as adjuvant treatment in stage II/III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 14.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2008;26(suppl 15S) doi: 10.1200/JCO.2006.08.2974. abstr LBA4005. [DOI] [PubMed] [Google Scholar]
- 15.Wolmark N, Wieand S, Kuebler PJ, et al. A phase III trial comparing FULV to FULV + oxaliplatin in stage II or III carcinoma of the colon: Survival results of NSABP Protocol C-07. J Clin Oncol. 2005;23:246s. abstr 3500. [Google Scholar]
- 16.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 17.Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: Observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 19.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 20.André T, Quinaux E, Louvet C, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: Final results of GERCOR C96.1. J Clin Oncol. 2007;25:3732–3738. doi: 10.1200/JCO.2007.12.2234. [DOI] [PubMed] [Google Scholar]
- 21.Burzykowski T, Molenbergs G, Buyse M, et al. Validation of surrogate endpoints in multiple randomized clinical trials with failure time end points. Appl Stat. 2001;50:405–422. [Google Scholar]
- 22.O'Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: Findings from the ACCENT data set. J Clin Oncol. 2008;26:2336–2341. doi: 10.1200/JCO.2007.15.8261. [DOI] [PubMed] [Google Scholar]
- 23.Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 24.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]