Abstract
Purpose
The optimal treatment for medically inoperable stage I non–small-cell lung cancer (NSCLC) has not been defined.
Patients and Methods
Cancer and Leukemia Group B trial 39904 prospectively assessed accelerated, once-daily, three-dimensional radiotherapy for early-stage NSCLC. The primary objectives were to define the maximally accelerated course of conformal radiotherapy and to describe the short-term and long-term toxicity of therapy. Entry was limited to patients with clinical stage T1N0 or T2N0 NSCLC (< 4 cm) and pulmonary dysfunction. The nominal total radiotherapy dose remained at 70 Gy, while the number of daily fractions in each successive cohort was reduced.
Results
Thirty-nine eligible patients were accrued (eight patients each on cohorts 1 to 4 and seven patients on cohort 5) between January 2001 and July 2005. One grade 3 nonhematologic toxicity was observed in both cohort 3 (dyspnea) and cohort 4 (pain). The major response rate was 77%. After a median follow-up time of 53 months, the actuarial median survival time of all eligible patients was 38.5 months. Local relapse was observed in three patients.
Conclusion
Accelerated conformal radiotherapy was well tolerated in a high-risk population with clinical stage I NSCLC. Outcomes are comparable to prospective reports of alternative therapies, including stereotactic body radiation therapy and limited resection, with less apparent severe toxicity. Further investigation of this approach is warranted.
INTRODUCTION
Five-year survival rates exceeding 50% can generally be expected after standard surgery (eg, lobectomy) for fit patients with stage I non–small-cell lung cancer (NSCLC).1 Unfortunately, a substantial number of patients with early-stage NSCLC have associated cardiopulmonary dysfunction and/or other comorbid medical illness and are not suitable candidates for standard anatomic surgical resection.2 Although myriad retrospective studies have been published, high-risk patients have been relatively neglected with regard to prospective clinical research. Two factors have contributed to a recent increased interest in studying poor-risk early-stage NSCLC. First, several reviews have strongly suggested that even patients with severe underlying cardiopulmonary dysfunction may benefit from definitive therapy because the majority of patients not receiving therapy die from their cancer and not from comorbid disease.3,4 The second factor is the emergence of advanced technologies with the potential to favorably shift the therapeutic index. As a result, innovative prospective studies have recently been launched including trials assessing stereotactic body radiation therapy (SBRT), brachytherapy, and radiofrequency ablation.5–7
Nevertheless, there are relatively few mature prospective multi-institutional trials available to guide treatment decisions. The Cancer and Leukemia Group B (CALGB) previously conducted the first cooperative group study designed expressly for high-risk T1N0 NSCLC, CALGB 9335. This phase II trial evaluated the feasibility of thoracoscopic video-assisted wedge resection and postoperative irradiation but concluded that the approach was not feasible in a multi-institutional setting.8 Moreover, despite pathologic staging, median overall survival time was 32 months, with a 5-year survival rate of approximating 29%, for patients with T1N0 disease. Given these disappointing results, we decided to prospectively study alternative (nonsurgical) treatment options in high-risk patients with stage I NSCLC.
Three-dimensional conformal radiotherapy (3DCRT) offers the ability to deliver therapy with increased precision to a specified target with improved sparing of the surrounding normal tissues. Prospective 3DCRT studies from the University of Michigan and the Radiation Therapy Oncology Group, which were not specific to stage I disease, explored an approach of dose escalation by increasing the number of radiation treatment sessions.9,10 Although high doses ranging from 83.4 to 102.9 Gy were feasible when the volume of irradiated lung was limited, overall treatment time was extended to between 8 and 10 weeks. Using the favorable dose distribution of 3DCRT, we hypothesized that it would be possible to deliver larger single daily fractions resulting in an acceleration of the radiotherapy course. Accelerated radiotherapy may lead to improved tumor control by increasing radiation dose-intensity and allowing less time for tumor repopulation during treatment.11 Moreover, patients with comorbid disease should benefit from the practical advantage of a less demanding travel requirement compared with conventionally fractionated therapy. The mature results of CALGB 39904 are detailed in this report.
PATIENTS AND METHODS
Patients eligible for study entry were required to have a histologic or cytologic diagnosis of stage IA or IB NSCLC with a solitary parenchymal lung lesion measuring ≤ 4 cm. Study enrollment was restricted to patients at high risk for complications after standard lobectomy, as defined by pulmonary dysfunction (Table 1). Patients who did not meet any of these criteria were eligible if they were deemed to have high-risk features of comorbid medical illness making them unsuitable for surgical resection. Evaluation by a thoracic surgeon (for suitability for lobectomy) was mandated if criteria for pulmonary dysfunction were not met.
Table 1.
CALGB 39904 Eligibility Criteria
| Criterion |
|---|
| FEV1: < 40% predicted |
| DLco: < 50% predicted |
| Pco2: > 45 mmHg |
| VO2max: < 15 mL (kg/min) |
| Oxygen requirement |
Abbreviations: CALGB, Cancer and Leukemia Group B; FEV1, forced expiratory volume in 1 second; DLco, diffusion capacity of carbon monoxide; Pco2, partial pressure of carbon dioxide; VO2max, maximum oxygen consumption.
Initial staging consisted of plain lateral and posteroanterior chest x-ray and a chest computed tomography (CT) scan that included visualization of the liver and adrenal glands. If mediastinal lymph nodes were more than 1.0 cm on prestudy chest CT, a mediastinoscopy (anterior, cervical, or both) was recommended, but the absence of [18F]fluorodeoxyglucose (FDG) uptake in the mediastinum and hilum on positron emission tomography (PET) was acceptable in lieu of mediastinoscopy. FDG-PET scans otherwise were not required. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and weight loss less than 10% in the 6 months before protocol entry and could not have received prior chemotherapy for lung cancer or prior radiotherapy to the chest.
This trial was approved by the institutional review boards of the CALGB institutions that participated in this trial. Patients were required to give informed consent before enrollment onto this trial. Patient registration and data collection were managed by the CALGB Statistical Center. Statistical analyses were performed by CALGB statisticians.
Treatment
Patients were assigned to receive an accelerated course of radiotherapy using once-daily fractionation. Treatment was administered on consecutive weekdays. The daily radiation fraction size was escalated and the number of fractions reduced, while the nominal total radiotherapy dose was maintained at 70 Gy, resulting in progressive acceleration of the radiotherapy course (Table 2). 3DCRT treatment planning was mandated, and each institution was required to submit a 3DCRT benchmark to the Quality Assurance Review Center (QARC, Providence, RI) before entering patients onto the trial. Custom immobilization was required for the treatment planning CT and for daily treatment. X-ray beams with a nominal energy between 4 and 25 MV were used. The radiation-targeted volumes were defined on the treatment planning CT. The gross target volume (gross tumor volume [GTV]) consisted of the primary lung tumor as defined on the lung windows of the planning CT scan. The clinical target volume included the GTV with an expansion of 1 cm in all directions. Elective irradiation of lymph node regions was not used. The planning target volume (PTV) allowed for 0.5 cm around the clinical target volume, with further expansion based on respiratory motion. Four-dimensional CT scans, respiratory gating, and abdominal compression were not routinely available when the trial was designed, and these measures for the assessment and management of tumor motion were not included in this study. Tissue heterogeneity factors were used for lung, soft tissue, and bone. Dose was prescribed to the isocenter, and the 95% isodose line was required to encompass the PTV. Normal tissue constraints were specified for the lung, heart, brachial plexus, spinal cord, and chest wall. No more than 25% of the combined lung volume was to receive more than 18 Gy, and no more than 10% of the combined lung volume was to receive more than 2.2 Gy per fraction. Central review of treatment plans at the Quality Assurance Review Center was mandated before initiating therapy. Final review and summary of submitted data were performed by the study chair.
Table 2.
CALGB 39904 Fraction Size Escalation Scheme
| Cohort No. | Total Dose (Gy) | Fraction Size (Gy) | No. of Fractions | No. of Weeks |
|---|---|---|---|---|
| 1 | 70 | 2.41 | 29 | 5.8 |
| 2 | 70 | 2.69 | 26 | 5.2 |
| 3 | 70 | 3.04 | 23 | 4.6 |
| 4 | 70 | 3.5 | 20 | 4 |
| 5 | 70 | 4.11 | 17 | 3.4 |
Abbreviation: CALGB, Cancer and Leukemia Group B.
Response and Toxicity Evaluation
Patients were assessed weekly during therapy including a progress note, physical examination, and radiation toxicity assessment. Pulmonary function tests and a posteroanterior and lateral chest x-ray were obtained at completion of therapy. Patients were assessed 3 weeks, 6 weeks, and 3 months after the completion of therapy, then at least every 3 months for 2 years, and then every 6 months for 3 years. A chest CT was obtained at 6 weeks, 3 months, and 6 months after the completion of therapy and then every 6 months for 5 years or as clinically indicated by patient symptoms. Pulmonary function testing was performed at 6 weeks, 3 months, 6 months, and 12 months after the completion of therapy and then yearly for 4 years.
Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria (version 2.0). Response to therapy was assessed at the completion of therapy and then during follow-up. Standard Response Evaluation Criteria in Solid Tumors (RECIST) were used for response evaluation.12
Statistical Considerations
The primary objective of CALGB 39904 was to determine the maximally accelerated course of 3DCRT. The decision to escalate treatment was dependent on both the outcome in previous cohorts and that observed to date in the current cohort. After completion of a cohort's accrual, escalation to the subsequent cohort was indicated if both of the following criteria were satisfied. The first criterion required that, within each of the previous cohorts, ≤ two patients developed ≥ grade 3 treatment-related toxicity and ≤ one patient developed ≥ grade 4 treatment-related toxicity. Second, in the current cohort, either of the following had to be satisfied: at least five patients in the current cohort were observed for more than 3 months and no ≥ grade 3 toxicity was observed; or all patients (minimum of seven patients) were observed for at least 3 months and ≤ two patients developed ≥ grade 3 treatment-related toxicity and ≤ one patient developed ≥ grade 4 treatment-related toxicity.
Overall survival was defined as the time between protocol registration and death. Failure-free survival was defined as the time between protocol registration and initial failure (disease progression, relapse, or death). Overall survival and failure-free survival curves were calculated using the Kaplan-Meier life-table method.13
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 14 (35%) of the 40 patients in this study.
RESULTS
CALGB 39904 was activated on December 15, 2000 and closed on July 29, 2005. Forty patients were accrued, with eight patients on each cohort. One patient on cohort 5 declined protocol treatment, leaving 39 eligible patients. Fifty-three percent of patients were female. Sixty-seven percent of patients had an ECOG performance status of 1, and 28% had an ECOG performance status of 2. The median age was 75 years (range, 48 to 87 years), and the majority of patients (67%) had T1N0M0 disease. Eleven (28%) of 39 patients required supplemental oxygen, between 2 and 4 L nasal canula, before the start of therapy. Pulmonary function at the time of study entry was available for 38 of 39 eligible patients (Table 3). Data regarding the use of FDG-PET imaging as part of the staging evaluation was not captured.
Table 3.
Pretreatment Pulmonary Function (n = 38)
| Measure | Median | Nonparametric 95% CI |
|---|---|---|
| FEV1, % (prebronchodilator predicted) | 40.50 | 34 to 59 |
| FEV1, L (prebronchodilator observed) | 0.96 | 0.83 to 1.31 |
| DLco, % predicted | 40.50 | 32 to 50 |
| DLco, mL/min/mmHg | 7.9 | 6.0 to 10.2 |
Abbreviations: FEV1, forced expiratory volume in 1 second; DLco, diffusion capacity of carbon monoxide.
Radiotherapy treatment plans varied between three and seven conformal fields, and the majority of patients (83%) were treated with 6-MV photons. The PTV encompassed the GTV with a median expansion of 1.5 cm, and an additional margin of 7 to 10 mm to the beam edge was typical. All plans used coplanar beam arrangements.
Treatment was well tolerated without grade 4 or higher treatment-related toxicity. One patient in cohort 2 experienced grade 3 hematologic toxicity (lymphopenia), and one nonhematologic toxicity was observed in both cohort 3 (dyspnea) and cohort 4 (pain). There were no treatment interruptions secondary to toxicity. There have not been any additional reports of treatment-related chest wall pain, and severe (≥ grade 3) late toxicity has not been reported. A significant trend for changes in pulmonary function was not observed.
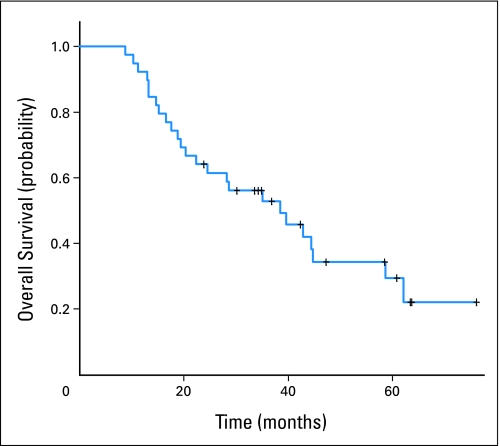
The major response rate was 77% (31% complete response, 46% partial response), and 23% of patients had stable disease. Response rates did not differ among cohorts. After a median follow-up time of 53 months (range, 35 to 61 months), the actuarial median overall survival and progression-free survival times of all eligible patients were 38.5 months (95% CI, 22.4 to 58.7 months; Fig 1) and 28.6 months (95% CI, 17.6 to 44.5 months), respectively.
Fig 1.
Overall survival for all patients on Cancer and Leukemia Group B trial 39904.
Patterns of Relapse
Overall, disease relapse was observed in 10 patients. Distant relapse was the most common site of recurrence, including bone metastases (n = 3), brain metastases (n = 2), and contralateral lung metastases (n = 2). The time to the appearance of distant metastases ranged from 3 to 30 months (median, 13 months), and 3-year freedom from distant metastases was estimated at 78% (95% CI, 59% to 89%). Distant relapse was observed in four of 11 patients with T2N0 disease.
Three patients experienced relapse in the treated region in the primary lung (two patients with T1N0 disease and one patient with T2N0 disease). All local relapses were observed between 13 and 19 months after therapy. One isolated mediastinal lymph node relapse was reported.
DISCUSSION
Our results suggest that accelerated radiotherapy is feasible for high-risk, early-stage NSCLC when three-dimensional conformal techniques are used. Although a maximum accelerated regimen was not defined, the time to complete a definitive course of radiotherapy was safely cut in half, compared with conventionally fractionated treatment (eg, 2-Gy daily fractions to 70 Gy), as the dose per fraction was more than doubled.
CALGB 39904 is the first cooperative group study designed to specifically study primary radiotherapy for patients with clinical stage I NSCLC. This trial adds to CALGB 9335, the group's initial experience studying patients with early-stage lung cancer and cardiopulmonary dysfunction, in demonstrating that it is possible to prospectively study high-risk stage I NSCLC. The mature results of this trial demonstrate encouraging outcomes for a population with defined cardiopulmonary dysfunction or other severe medical comorbidity. The median survival of 38.5 months compares favorably with reports of prospective trials assessing alternative treatment approaches in poor-risk patients. For example, treatment with thoracoscopic wedge resection and postoperative radiotherapy resulted in a median survival of 32 months on CALGB 9335, which is the only other mature cooperative group trial for high-risk early NSCLC at this time. Updated outcomes from the widely referenced prospective phase II SBRT study from Indiana University were recently reported, with a median survival of 32.4 months overall and 38.7 months for T1N0 lesions.14
This experience also suggests that accelerated conformal radiotherapy may be better tolerated than alternative treatment approaches without sacrificing efficacy. Local in-field tumor recurrence was observed in only 7% of patients after 53 months of median follow-up, which compares well with the results of SBRT, including the Indiana University report of 88.1% 3-year local tumor control. Importantly, few severe treatment-related toxic events were observed. Moreover, treatment was not limited to peripheral lesions (six patients had central tumors), which may be a condition for safe delivery of aggressive SBRT regimens. For example, excessive treatment-related toxicity was observed on the prospective Indiana University trial in patients with central lesions, including four deaths that were potentially attributable to SBRT.15 In that study, the 2-year freedom from severe toxicity rate was 83% for patients with peripheral tumors compared with 54% for patients with central tumors. Because of these findings, the subsequent Radiation Therapy Oncology Group prospective SBRT study restricted enrollment to patients with peripheral tumors.5
This trial has obvious limitations inherent to many pilot studies. Although entry criteria were well defined for high-risk characteristics, patient selection bias is still a concern given the small size of this study. For example, just more than half of patients were female, which may have favorably influenced outcomes. However, adverse factors included advanced patient age (median age, 75 years) and the frequency of severe pulmonary dysfunction (> 25% of patients required supplemental oxygen at the time of study enrollment). Although local tumor control was excellent, this assessment was based on findings from serial anatomic imaging (eg, chest CT), as is the case for most contemporaneous studies. FDG-PET imaging was not generally available when this trial was initiated and therefore was not mandated. The routine use of functional imaging (in conjunction with anatomic imaging) may provide a more accurate assessment of local tumor control compared with anatomic imaging alone, and some recently activated trials will include routine FDG-PET imaging to assess tumor response.
One of the primary end points of the trial, to define the maximal accelerated course of therapy, was not realized. The study was designed with five cohorts with the goal of reducing the treatment time by at least 50% compared with conventional (eg, 2 Gy per fraction) therapy administered to the same nominal total dose of 70 Gy. The intent was to reduce the number of treatments required to complete a definitive course of therapy, not to develop an SBRT-type of treatment regimen. Preserving some degree of daily fractionation may be advantageous given the potential radiobiologic advantages of fractionated therapy.16 At the same time, the accelerated approach potentially increases efficacy while allowing treatment to be completed in a reduced number of patient visits. A dose response was not observed for more accelerated treatment cohorts, although the small number of patients in each cohort limits this analysis. Whether there is a differential therapeutic index between an approach of accelerated 3DCRT and SBRT is an open question that could only be addressed in a prospective randomized trial, although additional data will be available as prospective SBRT studies mature. In addition, a phase II trial of accelerated 3DCRT (60 Gy in 4-Gy fractions) for early-stage peripheral NSCLC was completed by the National Cancer Institute of Canada Clinical Trials Group, and results should be available in the near future.17
A consistent observation from recent studies of intensive therapy for early-stage, high-risk NSCLC, including CALGB 39904, is the inverse relationship between local and distant tumor control.18–20 As local relapse decreases and patients live longer, the risk of developing distant metastases seems to increase. In the current study, seven patients were found to have distant relapse, including four of 11 patients with clinical T2N0 NSCLC. Whether adjuvant systemic therapy may be of benefit for this population has not been well studied. CALGB 9633 tested adjuvant chemotherapy for resected stage IB NSCLC in good-risk patients who were suitable for lobectomy. Adjuvant chemotherapy did not result in improved overall survival for the entire study population, but subset analysis suggested a survival benefit with the addition of chemotherapy for tumors more than 4 cm.21 CALGB is currently planning a phase II study of accelerated conformal radiotherapy and systemic chemotherapy, and other trials are in development that will assess chemotherapeutic or molecular targeted agents in conjunction with SBRT. The tolerability and efficacy of combined-modality therapy in the high-risk population remains to be defined.
Appendix
The following institutions participated in this study: Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Southeast Cancer Control Consortium Inc. Community Clinical Oncology Program, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA–Barbara A. Parker, MD, supported by CA11789; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, MD, supported by CA77440.
Footnotes
Written on behalf of the Cancer and Leukemia Group B.
The research for Cancer and Leukemia Group B (CALGB) 39904 was supported, in part, by Grant No. CA31946 from the National Cancer Institute to the CALGB (Richard L. Schilsky, MD, Chairman) and Grant No. CA33601 to the CALGB Statistical Center (Stephen George, PhD). This study was also supported by National Cancer Institute Grants No. CA21060, CA11789, CA03927, CA45808, CA41287, and CA03927.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00009789.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: A. William Blackstock, Eli Lilly (U), sanofi-aventis (U) Stock Ownership: None Honoraria: None Research Funding: A. William Blackstock, Eli Lilly, AstraZeneca, sanofi-aventis, OSI Pharmaceuticals, Merck, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey A. Bogart, Robert Lenox, Andrew T. Turrisi III, Mark R. Green
Administrative support: Lydia Hodgson, Xiaofei Wang
Provision of study materials or patients: Jeffrey A. Bogart, Stephen L. Seagren, A. William Blackstock, John Reilly, Ajeet Gajra
Collection and assembly of data: Lydia Hodgson, Xiaofei Wang
Data analysis and interpretation: Lydia Hodgson, Xiaofei Wang
Manuscript writing: Jeffrey A. Bogart, Everett E. Vokes
Final approval of manuscript: Jeffrey A. Bogart, Lydia Hodgson, Stephen L. Seagren, A. William Blackstock, Xiaofei Wang, Robert Lenox, Andrew T. Turrisi III, John Reilly, Ajeet Gajra, Everett E. Vokes, Mark R. Green
REFERENCES
- 1.Mountain CF. A new international staging system for lung cancer. Chest. 1986;89(suppl 4):225S–233S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 3.Kyasa MJ, Jazieh AR. Characteristics and outcomes of patients with unresected early-stage non-small cell lung cancer. South Med J. 2002;95:1149–1152. [PubMed] [Google Scholar]
- 4.McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: Poor outcome. Chest. 2002;121:1155–1158. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman RD, Paulus R, Galvin J, et al. Toxicity analysis of RTOG 0236 using stereotactic body radiation therapy to treat medically inoperable early stage lung cancer patients. Int J Radiat Oncol Biol Phys. 2007;69(suppl 3):S86. [Google Scholar]
- 6.National Cancer Institute. Phase III randomized study of sublobar resection with versus without intraoperative brachytherapy in high-risk patients with stage I non-small cell lung cancer. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=422346&version=HealthProfessional&protocolsearchid=5691197.
- 7.National Cancer Institute. Phase II pilot study of radiofrequency ablation in high-risk patients with stage IA non-small cell lung cancer. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=426417&version=HealthProfessional&protocolsearchid=5691200.
- 8.Shennib H, Bogart J, Herndon JE, et al. Video-assisted wedge resection and local radiotherapy for peripheral lung cancer in high-risk patients: The Cancer and Leukemia Group B (CALGB) 9335, a phase II, multi-institutional cooperative group study. J Thorac Cardiovasc Surg. 2005;129:813–818. doi: 10.1016/j.jtcvs.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Hayman JA, Ten Haken RK, et al. Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1-3N0 non-small-cell lung cancer: Is low incidence of regional failure due to incidental nodal irradiation? Int J Radiat Oncol Biol Phys. 2006;64:120–126. doi: 10.1016/j.ijrobp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG9311: A phase I-II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 11.Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys. 2000;46:516–517. doi: 10.1016/s0360-3016(99)00364-8. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck S, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys Nov. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Xing L. Optimization of radiotherapy dose-time fractionation with consideration of tumor specific biology. Med Phys. 2005;32:3666–3677. doi: 10.1118/1.2126167. [DOI] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov. Radiation therapy in treating patients with stage I or stage II non-small cell lung cancer that cannot be removed by surgery. http://clinicaltrials.gov/ct2/show/record/NCT00346320.
- 18.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Hof H, Muenter M, Oetzel D, et al. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC) Cancer. 2007;110:148–155. doi: 10.1002/cncr.22763. [DOI] [PubMed] [Google Scholar]
- 21.Strauss GM, Herndon JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non–small-cell lung cancer: CALGB 9633 with Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;31:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]