Abstract
Purpose
Vascular endothelial growth factor (VEGF) Trap (aflibercept) is an angiogenesis inhibitor comprising portions of the extracellular domains of human VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin G. This phase I study was designed to evaluate the safety, pharmacokinetics, and pharmacodynamics of VEGF Trap administered intravenously (IV) every 2 weeks.
Patients and Methods
Patients with refractory solid tumors or non-Hodgkin's lymphoma with adequate organ function were eligible. Pharmacokinetic/pharmacodynamic markers included measurement of plasma VEGF bound to VEGF Trap and free VEGF Trap. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was incorporated to measure the biologic effects of the drug on tumor vascularity and permeability.
Results
The study enrolled 47 patients at doses ranging from 0.3 to 7.0 mg/kg IV every 2 weeks. Dose-limiting toxicities were rectal ulceration and proteinuria at the 7.0 mg/kg dose. Other mechanism-specific toxicities included hypertension. On the basis of these observations and on pharmacokinetics, the recommended phase II dose of VEGF Trap as a single agent is 4 mg/kg every 2 weeks. Three RECIST (Response Evaluation Criteria in Solid Tumors) –defined partial responses were observed, one at the 3.0 mg/kg and two at the 7.0 mg/kg dose level. Maximum plasma concentration of free VEGF Trap increased proportionally with dose. Maximal VEGF-bound VEGF Trap complex levels were reached at doses ≥ 2.0 mg/kg. Changes in volume transfer constant measured by DCE-MRI at baseline and at 24 hours after administration indicate a possible dose-related change in this pharmacodynamic marker.
Conclusion
IV VEGF Trap was well tolerated at the dose levels tested. Pharmacodynamic and pharmacokinetic markers were indicative of VEGF blockade.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a cytokine critical to angiogenesis.1,2 The biologic effects of VEGF-A are mediated through the tyrosine kinase receptors VEGF receptor (VEGFR) -1 (ie, flt-1) and VEGFR-2 (ie, KDR, flk-1), which are expressed in normal and tumor vasculature.3–9 Ligand binding to VEGF receptors initiates angiogenesis signaling events, including increased vascular permeability and endothelial cell proliferation and migration.10–12 Studies in animals and human clinical trials have validated anti-VEGF approaches as anticancer strategies.13–15
VEGF Trap (aflibercept; Regeneron Pharmaceuticals, Tarrytown, NY, and sanofi-aventis Oncology, Bridgewater, NJ) is a recombinant protein consisting of domain 2 from VEGFR-1 fused to domain 3 from VEGFR-2, attached to the hinge region of the Fc(a) domain of human immunoglobulin (Ig) G1. VEGF Trap is a circulating antagonist that prevents VEGF receptor binding. In preclinical studies, VEGF Trap compared favorably with other VEGF inhibitors, and it had increased binding affinity (dissociation constant [Kd], 0.5 pM for VEGF-A), a longer circulating half-life, and binding of placental growth factors 1 and 2.16
The objectives of this phase I study were to assess the safety and tolerability of VEGF Trap administered intravenously (IV) every 2 weeks, the maximum-tolerated dose (MTD), preliminary antitumor activity, the pharmacokinetics, and the incidence of VEGF Trap antibody development. The study consisted of two separately approved phases, the dose-escalation phase and the long-term safety phase. Pharmacodynamic assays also were incorporated to define a biologically effective dose of VEGF Trap; these assays included measurement of free and complexed VEGF Trap, which indicated ligand inhibition, and measurement of tumor vascular permeability by using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI).
PATIENTS AND METHODS
Eligibility
The institutional review boards of Vanderbilt University Medical Center and Memorial Sloan-Kettering Cancer Center approved this study for patients with refractory solid tumors or non-Hodgkin's lymphoma. Eligible patients were men or nonpregnant women, age ≥ 18 years, with Eastern Cooperative Oncology Group performance status ≤ 2, with no treatment (including surgery) for 3 weeks before enrollment, with measurable tumors by RECIST (Response Evaluation Criteria in Solid Tumors), and amenable to DCE-MRI scanning. Additional key eligibility criteria included adequate cardiovascular, bone marrow, liver, and renal (ie, serum creatinine ≤ upper limit of normal, urine protein–to-creatinine ratio > 1) functions. Patients with active HIV, hepatitis B or C, primary CNS tumor or metastases, or squamous cell lung carcinoma were excluded. Mechanism-based exclusion criteria were as follows: uncontrolled hypertension ≥ 150/100 mmHg, hypersensitivity to recombinant proteins, or receipt of excluded medications (ie, anticoagulants, antiplatelet drugs, nonsteroidal anti-inflammatory drugs, cellular growth factors, or corticosteroids).
Drug Dosage and Administration
VEGF Trap was supplied (by Regeneron Pharmaceuticals) in sterile, single-use vials (concentration, 25 mg/mL) and was stored under recommended conditions (2°C to 8°C). VEGF Trap was diluted (concentration, 4 mg/mL) immediately before administration and was infused into a peripheral vein or central venous catheter over 1 to 2 hours by using a Deltec CADD Legacy infusion cassette or Deltec IV bag (Deltec, St Paul, MN) and pump every 2 weeks. The selected starting dose of 0.3 mg/kg was 10-fold less than the human equivalent dose, or the dose of the no observed adverse effect level from primate studies. If participants tolerated the first two doses of VEGF Trap on the dose-escalation study, they were eligible to receive treatment on the long-term tolerability study.
Safety and Efficacy Assessments
After drug administration, patients were assessed on days 2, 3, 4, 8, 11,15 (ie, second dose), and 22 and then every 2 weeks for toxicities, vital signs, hematology, chemistries, prothrombin time/partial thromboplastin time, urinalysis, and spot urine protein–to-creatinine ratio. Toxicities were graded by using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). DLTs were considered during the escalation phase of the study. Hematologic DLTs were febrile neutropenia, grade 4 neutropenia ≥ 7 days, grade 4 thrombocytopenia, and grade 3 thrombocytopenia with hemorrhage. Nonhematologic DLTs were grade 4 nonhematologic toxicities and any grade 3 nonhematologic toxicity except fatigue, anorexia, nausea, or altered alkaline phosphatase. Exceptions were proteinuria, immunologic reactions to VEGF Trap, and hypertension that required medical treatment. Regarding hypertension and proteinuria, DLTs included uncontrolled hypertension (despite medical management) ≥ 150/100 mmHg or ≥ 180/90 mmHg if the patient had a history of hypertension and proteinuria greater than 3.5 g per 24 hours (grade 3) that did not recover to less than 2.0 g per 24 hours within 2 weeks or grade 4 proteinuria. Toxicity of any grade leading to drug discontinuation was a possible DLT. The MTD of VEGF Trap was defined as the highest dose at which two of three to six patients experienced DLT.
Tumor response was assessed by RECIST after every two cycles (ie, four treatments per eight weeks) by using MRI and/or computed tomography (CT).17 Patients could continue to receive VEGF Trap until disease progression, unacceptable toxicities, or consent withdrawal occurred.
Pharmacokinetics and Pharmacodynamics
Blood samples for VEGF Trap pharmacokinetics were obtained as follows: pretreatment, postdose, and 1, 2, 4, 8, 24, 30, 48, 96, 168, and 240 hours after treatment. At the second dose, blood samples for VEGF Trap pharmacokinetics were obtained pre- and postdose and at subsequent patient visits. Four milliliters of whole blood was collected in citrate, theophylline, adenosine, and dipyridamole vacutainer tubes (1 mL citrate buffer, sodium citrate, and 4.2 mg of citric acid) for assessing circulating free and complexed VEGF Trap. Levels of free VEGF Trap were measured by enzyme-linked immunosorbent assay (ELISA) by using VEGF-165 as the capture and an antibody to the IgG2 domain of VEGFR-1 as the report. VEGF-to–VEGF Trap complex was measured by using an antibody to human VEGF as the capture and an antibody to human Fc as the report. Pharmacokinetic parameters, including area under the concentration-time curve (AUC), maximum plasma concentration (Cmax), last measurable (non-zero) plasma concentration (Clast), clearance, steady-state volume of distribution (Vss), and elimination half-life for free VEGF Trap and for complexed VEGF Trap were estimated by using noncompartmental methods (WINNonlin; Scientific Consultant, Apex, NC; version 4.1, PharSight, Raleigh-Durham, NC). The limits of quantification of free and bound VEGF Trap were 31 ng/mL and 44 ng/mL, respectively. The mean recovery of calibration standards and quality controls (QCs) for passing plates ranged from 90% to 108% and 93% to 103%, respectively, for free VEGF Trap and from 89% to 109% and 90% to 98%, respectively, for bound VEGF Trap. For pharmacodynamics, relationships between plasma levels of complexed VEGF Trap and dose were explored.18
Detection of anti-VEGF Trap antibodies was performed with a microtiter plate coated with extracellular receptor domains of VEGF Trap. Anti-VEGF Trap antibodies, if present, were detected with peroxidase-conjugated mouse antihuman IgG Fab2) that was fragment specific.
DCE-MRI
The utility of DCE-MRI for monitoring the effect of VEGF Trap on vascular permeability and on extravascular and extracellular spaces was assessed. MRIs were performed by utilizing a 1.5 Tesla MRI (GE Medical Systems, Milwaukee WI or Philips, Andover, MA) and an eight-channel, phased array coil. Anatomic images, including T1 and T2 weighted images, three-dimensional T1 weighted pre- and postcontrast images, and fast gradient echo (GRE) images were acquired pretreatment and post-treatment at 24 hours and at 8 weeks. A dose (0.1 mmol/kg) of Gd-DTPA (ie, gadolinium diethylenetriamine pentaacetic acid; Magnevist; Berlex, Montvale, NJ) was administered to all patients through a bolus injection at 2 mL/sec, a delay of 6 seconds, and a saline flush of 20 mL with an automatic injector (Medrad; Warrendale, PA).
Scanning was performed under shallow breathing conditions, and images were manually corrected for respiratory motion. Nominal DCE-MRI parameters were as follows: single GRE coronal oblique plane, echo time (TE) of 2 msec, repetition time (TR) of 9 msec, flip-angle of 30 degrees, slice thickness of 7 mm, matrix size of 256 × 128, field of view (FOV) of 360 mm, bandwidth (BW) of 23.8 kHz, number of excitations as 1.0, and number of images as 225. A slice was prescribed through the center of tumor to maximize spatial and temporal resolutions.
The dynamic images were analyzed on the basis of two-compartmental general kinetic models by using a vascular space (VS) and an extracellular extravascular space (EES) for pharmacokinetic characterization of tumors.19,20 An average biexponential vascular input function was used in analysis (Cine Research Software; GE Medical Systems, Milwaukee, WI).21,22 Kinetic parameters, including volume transfer constant from VS to EES (Ktrans, min−1), rate constant from EES to VS (kep, min−1), fractional volume of EES (ve), and AUC of Gd contrast agent greater than 90 and 180 seconds (AUC90 and AUC180, mM) were determined at baseline and at 24 hours by using region of interest (ROI) analysis.20 Ktrans was calculated by using the Gd AUC, model vascular input function, and fractional plasma volume (vp). The change (%) was computed as 100 × (Tx(i) − Tx(0)) ÷ Tx(0), for which Tx(i) and Tx(0) were values at the treatment cycles (i = 1,2,3, and so on) and at baseline, respectively.
Statistical Analysis
Descriptive statistics summarized the safety data and the pharmacokinetics of IV VEGF Trap. Mean differences in Ktrans between baseline and 24 hours were estimated for each VEGF dose by using an analysis of covariance (ANCOVA). The ANCOVA used consists of a linear model of the differences regressed on the natural log of baseline Ktrans and dose, in which dose is represented as a series of indicator variables. ANCOVA results include the estimated mean and 95% CI of Ktrans at each dose. Dose-effect relationships between the 0.3 mg/kg and the other doses were compared by using the linear ANCOVA model. Only scans from baseline and 24 hours were included in the analyses, because too few scans were performed at the 8-week time point to allow for appropriate missing data techniques to be used. However, the percent decrease in the mean Ktrans is reported at both 24 hours and 8 weeks. Similarly, change in AUC is examined by using ANCOVA. Analysis was performed with R software (http://www.r-project.org).
RESULTS
General
Between April of 2004 and March of 2007, a total of 59 patients were enrolled at the two participating institutions, and 47 received IV VEGF Trap at doses ranging from 0.3 to 7.0 mg/kg every 2 weeks. Demographic data for the treated patients are listed in Table 1. Patients were treated at seven dose levels (ie, 0.3, 1.0, 2.0, 3.0, 4.0, 5.0, and 7.0 mg/kg); three, seven, six, seven, seven, four, and 13 patients were treated at each respective dose level. The 7.0 mg/kg dose level was expanded after agreement between the investigators and the sponsor without defining a protocol-specified MTD. The disposition of each patient treated on the study is listed in Appendix Table A1 (online only).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. of Patients by Dose Level (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 47) | 0.3 (n = 3) | 1.0 (n = 7) | 2.0 (n = 6) | 3.0 (n = 7) | 4.0 (n = 7) | 5.0 (n = 4) | 7.0 (n = 13) | |
| Sex | ||||||||
| Male | 15 | 0 | 1 | 3 | 3 | 2 | 2 | 4 |
| Female | 32 | 3 | 6 | 3 | 4 | 5 | 2 | 9 |
| Age, years | ||||||||
| Median | 56 | 60 | 51 | 57.5 | 61 | 48 | 51.5 | 56 |
| Range | 36-78 | 57-65 | 39-58 | 48-73 | 36-74 | 44-68 | 36-78 | 42-72 |
| ECOG PS | ||||||||
| 0 | 19 | 3 | 2 | 2 | 2 | 4 | 1 | 5 |
| 1 | 26 | 0 | 5 | 4 | 5 | 3 | 3 | 6 |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Prior chemotherapy regimens | ||||||||
| Median | 5 | 4 | 5.5 | 5 | 7 | 3 | 4 | 6 |
| Range | 1-13 | 4-5 | 3-11 | 1-13 | 2-11 | 1-7 | 1-9 | 3-10 |
| Tumor site | ||||||||
| Ovarian/fallopian/peritoneal | 14 | 2 | 0 | 3 | 1 | 1 | 2 | 5 |
| Renal | 7 | 0 | 1 | 2 | 0 | 3 | 1 | 0 |
| Colorectal | 7 | 0 | 0 | 0 | 2 | 3 | 0 | 2 |
| Biliary | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Uterine | 4 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Other | 13 | 0 | 2 | 1 | 3 | 0 | 1 | 6 |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety/Toxicity
Encountered toxicities at all dose levels are listed in Table 2. The DLTs for VEGF Trap were proteinuria (n = 1) and rectal ulceration (n = 1) that occurred at the 7 mg/kg dose level. Participants experienced DLTs at lower dose levels, including grade 3 elevation in ALT at the 1.0 mg/kg dose, grade 3 dyspnea and arthalgia at the 2.0 mg/kg dose, and grade 3 hypertension at the 4.0 mg/kg dose. In these instances, dose escalation proceeded after cohort expansion or study amendments to include more intensive blood pressure control before defining a DLT and to allow for higher levels of proteinuria. The most common adverse events were fatigue (63.8%), nausea (36.2%), and vomiting (27.7%; Table 2). These symptoms were generally grade 1 and resolved with drug discontinuation. There were no relationships between doses of VEGF Trap and these common toxicities. Toxicities associated with antiangiogenic therapy and observed with VEGF Trap treatment were dysphonia (46.8%), hypertension (38.3%), and proteinuria (10.6%). The frequency of these toxicities with increasing doses of VEGF Trap were as follows: hypertension of any grade occurred in 0%, 14.3%, 16.7%, 14.3%, 57.1%, 75.0%, and 61.5% of patieints at the increasing dose levels in order, and hypertension of grades 3 to 4 occurred in 0%, 0%, 16.7%, 0%, 42.9%, 75.0%, and 46.2%.
Table 2.
Common and Mechanism-Related Toxicities of Intravenously Administered VEGF Trap by Dose and Grade
| Toxicity by Grade | No. of Patients by Dose Level (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | 0.3 (n = 3) | 1.0 (n = 7) | 2.0 (n = 6) | 3.0 (n = 7) | 4.0 (n = 7) | 5.0 (n = 4) | 7.0 (n = 13) | |
| Most common | ||||||||
| Fatigue | ||||||||
| 1 | 10 | 1 | 2 | 0 | 1 | 1 | 1 | 4 |
| 2 | 16 | 2 | 1 | 1 | 3 | 3 | 1 | 5 |
| 3 | 4 | 0 | 2 | 0 | 1 | 1 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | ||||||||
| 1 | 13 | 1 | 3 | 0 | 2 | 3 | 1 | 3 |
| 2 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 1 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | ||||||||
| 1 | 4 | 1 | 0 | 1 | 2 | 0 | 0 | 0 |
| 2 | 11 | 0 | 3 | 0 | 2 | 3 | 0 | 3 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | ||||||||
| 1 | 4 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| 2 | 5 | 1 | 1 | 0 | 2 | 1 | 0 | 0 |
| 3 | 2 | 0 | 1 | 1* | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | ||||||||
| 1 | 11 | 0 | 1 | 0 | 5 | 2 | 0 | 3 |
| 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anorexia | ||||||||
| 1 | 4 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 2 | 4 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| 3 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | ||||||||
| 1 | 7 | 2 | 0 | 0 | 2 | 1 | 0 | 2 |
| 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 3 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | ||||||||
| 1 | 9 | 1 | 2 | 0 | 2 | 2 | 0 | 2 |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | ||||||||
| 1 | 7 | 0 | 1 | 0 | 1 | 1 | 1 | 3 |
| 2 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| 3 | 2 | 0 | 1* | 0 | 0 | 0 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Back pain | ||||||||
| 1 | 4 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| 2 | 6 | 0 | 0 | 0 | 3 | 0 | 2 | 1 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | ||||||||
| 1 | 5 | 0 | 1 | 0 | 1 | 1 | 0 | 2 |
| 2 | 3 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mechanism-related | ||||||||
| Dysphonia | ||||||||
| 1 | 18 | 1 | 1 | 1 | 4 | 2 | 2 | 7 |
| 2 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 3 | 1 | 0 | 1* | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | ||||||||
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2 | 4 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| 3 | 13 | 0 | 0 | 1 | 0 | 3* | 3 | 6 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | ||||||||
| 1 | 13 | 0 | 1 | 1 | 1 | 3 | 2 | 5 |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteinuria | ||||||||
| 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 3 | 0 | 0 | 0 | 0 | 2* | 1 | 0 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1* |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epistaxis | ||||||||
| 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombosis | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amenorrhea | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI perforation | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviation: VEGF, vascular endothelial growth factor.
Dose-limiting toxicity.
The median times to the onset of hypertension and proteinuria were 3.5 days (range, 1 to 21 days) and 15 days (range,14 to 16 days), respectively. These events were reversible or manageable on drug discontinuation or initiation of appropriate supportive measures. No patient experienced drug-related grades 4 or 5 adverse events.
On the basis of the clinical observations and given the increase in frequency and severity of adverse events (particularly hypertension) at VEGF Trap doses of 4.0 mg/kg and greater, the recommended phase II dose of VEGF Trap as a single agent is 4 mg/kg.
Immunogenicity
No patient developed detectable anti-VEGF Trap antibodies. One patient in the 3.0 mg/kg cohort experienced grade 2 hypersensitivity after developing skin rash and flushing (both grade 2) that were temporally related to VEGF Trap administration.
Pharmacokinetics/Pharmacodynamics
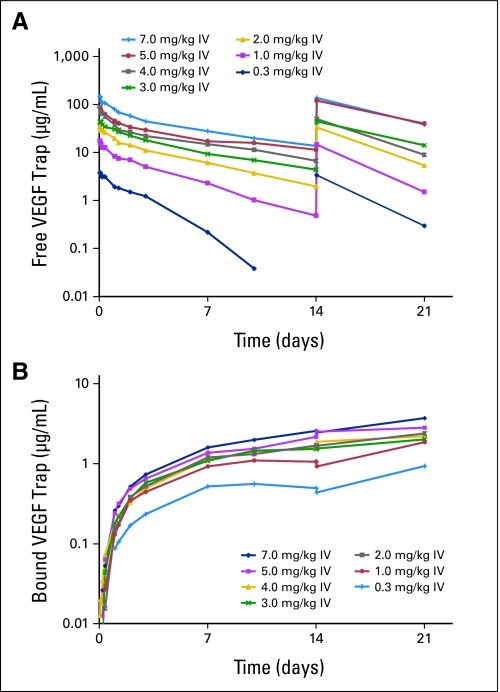
The mean Cmax of free VEGF Trap ranged from 4 to 159 μg/mL and increased with dose (Table 3). Free VEGF Trap exposure increased more than dose proportionally in the 0.3 to 2.0 mg/kg dose range. The apparent half-life increased with doses from 1.7 days at 0.3 mg/kg to 5.1 days at 7.0 mg/kg. Mean clearance values of free VEGF Trap decreased from 1.94 L/d estimated at 0.3 mg/kg to 1.13 L/d at 2.0 mg/kg and then were stable between 2.0 and 7.0 mg/kg (Fig 1A). The dose-dependent clearance indicates binding saturation of endogenously produced VEGF. The pharmacokinetics of free VEGF Trap were linear in the 2.0 to 7.0 mg/kg dose range. There was no accumulation of free VEGF Trap between treatment cycles.
Table 3.
Mean Pharmacokinetics of Free VEGF Trap After Intravenous Administration of Aflibercept
| Dose Level (mg/kg) | Pharmacokinetic Parameters |
|||||
|---|---|---|---|---|---|---|
| Cmax (μg/mL) | Clast (μg/mL) | AUC (day × μg/mL) | T1/2λz (day) | Vss (L) | Cl (L/d) | |
| 0.3 (n = 3) | ||||||
| Coefficient of variation | 4.00 | 0.147 | 9.34 | 1.70 | 4.51 | 1.95 |
| % | 9 | 38 | 15 | 21 | 29 | 42 |
| 1.0 (n = 7) | ||||||
| Coefficient of variation | 17.9 | 0.659 | 50.9 | 2.58 | 5.88 | 1.87 |
| % | 31 | 116 | 54 | 50 | 22 | 51 |
| 2.0 (n = 6) | ||||||
| Coefficient of variation | 34.5 | 2.36 | 125 | 3.76 | 5.58 | 1.13 |
| % | 11 | 71 | 35 | 42 | 21 | 31 |
| 3.0 (n = 7) | ||||||
| Coefficient of variation | 48.7 | 4.06 | 226 | 6.18 | 7.74 | 1.14 |
| % | 30 | 63 | 34 | 38 | 33 | 48 |
| 4.0 (n = 7) | ||||||
| Coefficient of variation | 97.4 | 11.0 | 293 | 5.51 | 7.88 | 1.10 |
| % | 43 | 51 | 15 | 18 | 38 | 38 |
| 5.0 (n = 4) | ||||||
| Coefficient of variation | 86.8 | 9.63 | 428 | 7.43 | 9.89 | 1.27 |
| % | 34 | 28 | 64 | 38 | 31 | 65 |
| 7.0 (n = 12) | ||||||
| Coefficient of variation | 159 | 14.4 | 605 | 5.14 | 6.12 | 0.915 |
| % | 21 | 55 | 46 | 37 | 29 | 39 |
Abbreviations: VEGF, vascular endothelial growth factor; Cmax, maximum observed plasma concentration; Clast, last measurable (non-zero) plasma concentration; AUC, area under the curve extrapolated to infinity; tλz, apparent terminal half-life; Vss, volume of distribution at steady-state; Cl, total body clearance.
Fig 1.
Mean plasma concentration versus time profiles for (A) free and (B) bound vascular endothelial growth factor (VEGF) Trap (log scale). (A) After the first two doses (on days 0 and 14) of intravenous (IV) VEGF Trap, there is a dose-dependent increase in free VEGF Trap maximum plasma concentration, and detectable levels are present at doses of 2.0 mg/kg and greater. (B) At dose levels of 2.0 mg/kg and greater, bound VEGF Trap levels appear to saturate and have no appreciable increase with dose escalation.
Maximal VEGF-bound VEGF Trap complex levels were reached at doses ≥ 2.0 mg/kg (Fig 1B), which indicates complete ligand blockade. Bound VEGF Trap concentrations increased after cycle 1 and up to the last sampling time, which indicates that steady-state was not reached at 3 weeks after the first dose (Table 4). Free VEGF Trap levels remained greater than bound VEGF Trap levels throughout the dosing intervals at dose levels of 2.0 mg/kg and greater. Detectable free VEGF Trap at the end of the dosing period (ie, minimum plasma concentration or Ctrough) was interpreted as an indication that all available VEGF was bound.
Table 4.
Mean Pharmacokinetics of VEGF Bound to VEGF Trap After Intravenous Administration of Aflibercept, Expressed As Equivalent Free VEGF Trap
| Dose Level(mg/kg) | Pharmacokinetic Parameter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tmax (day) |
Cmax (μg/mL) |
Tlast (day) |
Clast (μg/mL) |
AUClast (day × μg/mL) |
||||||
| Median | Range | Coefficient of Variation | % | Median | Range | Coefficient of Variation | % | Coefficient of Variation | % | |
| 0.3 (n = 3) | 9.96 | 7.03-9.98 | 0.575 | 5 | 13.9 | 13.8-14 | 0.494 | 16 | 5.53 | 5 |
| 1.0 (n = 7) | 9.98 | 7-14 | 1.22 | 29 | 14 | 9.86-14 | 1.12 | 38 | 9.98 | 30 |
| 2.0 (n = 6) | 13.5 | 9.01-13.9 | 1.58 | 32 | 13.9 | 9.01-13.9 | 1.57 | 32 | 12.7 | 41 |
| 3.0 (n = 7) | 13.9 | 7.08-23 | 1.72 | 23 | 14 | 13.9-23 | 1.55 | 34 | 16.1 | 38 |
| 4.0 (n = 7) | 9.99 | 3.04-14 | 1.34 | 45 | 10 | 3.04-14 | 1.27 | 49 | 10.8 | 38 |
| 5.0 (n = 4) | 10.5 | 6.98-21.8 | 1.94 | 25 | 11.9 | 7.04-21.8 | 1.94 | 25 | 12.9 | 18 |
| 7.0 (n = 13) | 14 | 7.02-27.9 | 2.37 | 37 | 14 | 7.03-27.9 | 2.35 | 38 | 20.9 | 26 |
Abbreviations: VEGF, vascular endothelial growth factor; Tmax, time of maximum plasma concentration; Cmax, maximum observed plasma concentration; Tlast, time of last measurable plasma concentration; Clast, last measurable (non-zero) plasma concentration; AUClast, area under the curve from the time of dosing to the last measurable concentration.
Functional Imaging
Forty (85%) of 47 patients had DCE-MRI scans at baseline and at 24 hours; 22 (47%) of 47 had scans at 8 weeks that were available for analysis of Ktrans and AUC.
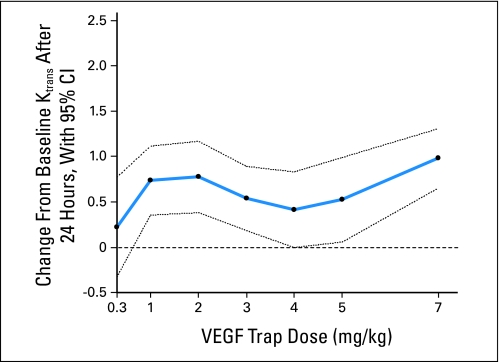
Figure 2 illustrates the change in Ktrans by VEGF Trap dose. All doses greater than 0.3 mg/kg, except 4 mg/kg, are associated with a statistically significant decrease in Ktrans after analysis was adjusted for the baseline Ktrans value. For any fixed baseline Ktrans, the average decreases in Ktrans were 0.22, 0.74, 0.78, 0.54, 0.42, 0.52, and 0.98 for doses of 0.3, 1, 2, 3, 4,5, and 7 mg/kg, respectively. Only the 7.0 mg/kg dose had significantly different Ktrans lowering efficacy compared with the 0.3 mg/kg dose (0.98 [95% CI, 0.65 to 1.31] v 0.22 [95%CI, −0.33 to 0.77]; P = .0276). Appendix Table A2 (online only) shows the percent decrease in the means of Ktrans from baseline to both 24 hours and 8 weeks by dose. Individual tumor types did not differ in terms of Ktrans and Ktrans changes with therapy.
Fig 2.
Mean change in volume transfer constant (Ktrans) between baseline and 24 hours for each vascular endothelial growth factor (VEGF) Trap dose estimated from a linear analysis of covariance model. The average dose effects (points) and 95% CI (dotted lines) are shown. The decrease in Ktrans after 24 hours was significant at all doses except 0.3 and 4.0 mg/kg. Baseline and 24-hour Ktrans measurements were significantly different between the 0.3 and 7.0 mg/kg doses (0.98 v 0.22; P = .0276).
A statistically significant increase in AUC90 between baseline and 24 hours was detected in both the 0.3 mg/kg and 7.0 mg/kg dose levels (P < .05 for both); however, the dose effect was not different between 0.3 mg/kg and 7.0 mg/kg (0.06 v 0.04 increase; P = .31). Dose effects were not detected when AUC180 was analyzed.
Antitumor Activity
There were three objective tumor responses to VEGF Trap. One patient with malignant thymoma treated with the 3.0 mg/kg dose had a confirmed RECIST-defined partial response of 6 months. This patient's prior therapies were octreotide, cyclophosphamide/adriamycin/cisplatin, capecitabine, fluorouracil, thalidomide, gefitinib, and LY573636 (an apoptosis inducer). A patient with ovarian cancer treated with the 4.0 mg/kg dose had an unconfirmed RECIST-defined partial response of 2 months associated with a 67% decrease in CA-125 and resolution of ascites. This patient's prior therapies were carboplatin with paclitaxel and carboplatin with gemcitabine. Two patients with ovarian cancer treated with the 7.0 mg/kg dose had confirmed partial responses. Two patients with renal cell cancer treated with the 1.0 and 2.0 mg/kg doses had prolonged (ie, > 1year) stable disease. One of these patients had not received prior therapy and is still receiving VEGF Trap on protocol.
Of note, the three patients with objective tumor responses did not have Ktrans parameters statistically differing from the other study participants (median percent decrease of Ktrans for entire study group, 40% v 60% reduction for responders).
DISCUSSION
VEGF inhibition is a validated anticancer strategy, and several agents designed to exploit the VEGF and angiogenesis pathways have entered clinical testing. VEGF Trap is a fusion protein that specifically targets VEGFR-1 and VEGFR-2, as it contains portions of the extracellular domains of both of these receptors. By design, VEGF Trap binds to and is expected to inactivate intravascular and extravascular VEGF. A similar approach has proven successful in the treatment of rheumatoid arthritis, for which the extracellular domain of the tumor necrosis factor receptor was fused to the Fc portion of human IgG (ie, etanercept; Enbrel; Amgen, Thousand Oaks, CA). In this phase I study, IV VEGF Trap was safely administered every 2 weeks.
Forty-seven patients with refractory solid tumors were treated on this study at doses of 0.3 to 7.0 mg/kg. Treatment-related toxicities were consistent with prior studies of anti-VEGF agents; included proteinuria, hypertension, fatigue, and hoarseness; and were reversible on drug discontinuation.14 The antitumor activity of VEGF trap was demonstrated with three RECIST-defined partial responses in patients with thymic and ovarian cancers, and no patients developed antibodies to VEGF Trap.
An additional objective of this study was to define a biologically active dose by using scientifically rational biomarkers. Preclinical studies indicated that the biologic effects of VEGF Trap correlated with free VEGF Trap levels in excess of bound VEGF Trap.18 Therefore, a study objective was to determine whether maximal levels of bound VEGF Trap complex and free VEGF Trap levels in excess of complexed VEGF Trap were obtainable. At doses of 2.0 mg/kg and greater, complexed VEGF Trap levels did not additionally increase, which indicated maximal ligand blockade; and free VEGF Trap levels remained in excess of bound VEGF Trap levels. On the basis of these results, VEGF Trap was administered at a biologically active dose.
DCE-MRI scans were performed to assess the biologic effects of VEGF Trap on tumor perfusion and tumor vascularity.23,24 On the basis of the baseline levels of Ktrans, VEGF Trap decreased the Ktrans at 24 hours, and the highest dose tested (7.0 mg/kg) was the most effective, with an average decrease of 0.98 in Ktrans.
Ktrans is a mixed measure of perfusion, vascularity, and permeability; thus, several factors—including tumor type, tumoral mature-to-immature blood vessel ratio, blood volume, and the analysis model—could influence Ktrans measurements. In the study population, there were no indications that any particular tumor type was overrepresented, either among the tumors that demonstrated higher baseline Ktrans values or among those with more dramatic decreases in the Ktrans measurements.
In this study, we observed that VEGF Trap could be safely administered at doses that result in antitumor activity and modulation of biomarkers indicative of target inhibition and biologic response. On the basis of the toxicity results, the antitumor results, drug exposure, and the biomarkers, a dose of 4 mg/kg administered IV every 2 weeks meets the biologic hypothesis of achieving adequate drug concentrations and free and bound drug levels. On the basis of VEGF Trap tolerability and demonstrated antitumor activity, several single-agent and combination studies are planned and ongoing.
Acknowledgment
We thank Jesse M. Cedarbaum (Regeneron Pharmaceuticals, Tarrytown, NY) and Darrel P. Cohen (sanofi-aventis Oncology, Bridgewater, NJ) for contributions to the completion of this study.
Appendix
Table A1.
Patient Disposition
| Disposition | Patients by Dose Level (mg/kg IV) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 |
1.0 |
2.0 |
3.0 |
4.0 |
5.0 |
7.0 |
Total |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Patients enrolled with informed consent | 59 | 100 | ||||||||||||||
| Patients treated | 3 | 100 | 8 | 100 | 6 | 100 | 7 | 100 | 7 | 100 | 4 | 100 | 12 | 100 | 47 | 79.7 |
| Reason for study discontinuation | ||||||||||||||||
| Adverse event | 0 | 1 | 12.5 | 1 | 16.7 | 2 | 28.6 | 4 | 57.1 | 2 | 50.0 | 3 | 25.0 | 13 | 27.7 | |
| Progressive disease | 3 | 100 | 6 | 75.0 | 4 | 66.7 | 5 | 71.4 | 3 | 42.9 | 2 | 50.0 | 6 | 50.0 | 29 | 61.7 |
| Patient did not wish to continue | 0 | 0 | 1 | 16.7 | 0 | 0 | 0 | 1 | 8.3 | 2 | 4.3 | |||||
| No longer required study treatment | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Protocol violation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Lost to follow-up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Ongoing | 0 | 1 | 12.5 | 0 | 0 | 0 | 0 | 0 | 1 | 2.1 | ||||||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8.3 | 1 | 2.1 | ||||||
NOTE. For those patients receiving different study treatment doses between the dose-escalation phase and the long-term safety phase of the study, the assigned dose in long-term treatment phase is reported in the table.
Abbreviation: IV, intravenous.
Table A2.
Percent Decrease in the Mean Ktrans From Baseline to 24 Hours and 8 Weeks
| Aflibercept Dose Level, mg/kg | Time Point |
|||
|---|---|---|---|---|
| 24 Hours |
8 Weeks |
|||
| % Decrease | No. of Patients | % Decrease | No. of Patients | |
| 0.3 | −32.4* | 3 | 64.9 | 1 |
| 1.0 | 56.8 | 6 | 96.9 | 1 |
| 2.0 | 39.8 | 6 | 50.7 | 5 |
| 3.0 | 39.6 | 7 | 75.4 | 4 |
| 4.0 | 39.6 | 6 | 19.6 | 2 |
| 5.0 | 50.5 | 4 | 26.5 | 3 |
| 7.0 | 43.9 | 8 | 45.6 | 4 |
NOTE. The very small sample size demands caution in interpretation.
Abbreviation: Ktrans, volume transfer constant.
The negative decrease indicates the change was an increase.
Footnotes
Supported by sanofi-aventis and by National Cancer Institute Grant No. 5K23CA098011 (A.C.L.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00082823.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Paul Chevalier, sanofi-aventis (C); Lars Sternas, sanofi-aventis (C); Giliane Buzenet, sanofi-aventis (C); Isabelle Dancy, sanofi-aventis (C) Consultant or Advisory Role: Mace L. Rothenberg, sanofi-aventis (C) Stock Ownership: Paul Chevalier, sanofi-aventis; Lars Sternas, sanofi-aventis; Giliane Buzenet, sanofi-aventis; Isabelle Dancy, sanofi-aventis Honoraria: Jeffrey A. Sosman, Roche Research Funding: A. Craig Lockhart, sanofi-aventis; Mace L. Rothenberg, sanofi-aventis; Jakob Dupont, sanofi-aventis; Jeffrey A. Sosman, Bristol-Myers Squibb, Pfizer; David R. Spriggs, sanofi-aventis, Regeneron; William P. Tew, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jakob Dupont, Paul Chevalier, Lars Sternas, Lawrence H. Schwartz, David H. Gultekin, Jason A. Koutcher
Financial support: Lars Sternas, Giliane Buzenet, Isabelle Dancy
Administrative support: Wendy Cooper, Giliane Buzenet, Ric Andal, Isabelle Dancy
Provision of study materials or patients: A. Craig Lockhart, Mace L. Rothenberg, Jakob Dupont, Wendy Cooper, Jeffrey A. Sosman, David H. Gultekin, David R. Spriggs, William P. Tew
Collection and assembly of data: A. Craig Lockhart, Jakob Dupont, Wendy Cooper, Lawrence H. Schwartz, David H. Gultekin, Jason A. Koutcher, Edwin F. Donnelly, Ric Andal, William P. Tew
Data analysis and interpretation: A. Craig Lockhart, Mace L. Rothenberg, Jakob Dupont, Paul Chevalier, Lars Sternas, Elizabeth Koehler, Lawrence H. Schwartz, David H. Gultekin, Jason A. Koutcher, Edwin F. Donnelly, David R. Spriggs, William P. Tew
Manuscript writing: A. Craig Lockhart, Mace L. Rothenberg, Paul Chevalier, Elizabeth Koehler, David H. Gultekin, David R. Spriggs, William P. Tew
Final approval of manuscript: A. Craig Lockhart, Mace L. Rothenberg, Lars Sternas, William P. Tew
REFERENCES
- 1.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 2.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 3.Berkman RA, Merrill MJ, Reinhold WC, et al. Expression of the vascular permeability factor/ vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993;91:153–159. doi: 10.1172/JCI116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LF, Berse B, Jackman RW, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 6.Ferrara N. VEGF: An update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 7.Guidi AJ, Abu-Jawdeh G, Berse B, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J Natl Cancer Inst. 1995;87:1237–1245. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- 8.Guidi AJ, Abu-Jawdeh G, Tognazzi K, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in endometrial carcinoma. Cancer. 1996;78:454–460. doi: 10.1002/(SICI)1097-0142(19960801)78:3<454::AID-CNCR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 10.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 12.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 16.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Rudge JS, Holash J, Hylton D, et al. Inaugural article: VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104:18363–18370. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kety SS. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3:1–41. [PubMed] [Google Scholar]
- 20.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. I. Fundamental concepts. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 22.Weinmann HJ, Laniado M, Mutzel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR. 1984;16:167–172. [PubMed] [Google Scholar]
- 23.Knopp MV, Giesel FL, Marcos H, et al. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging. 2001;12:301–308. doi: 10.1097/00002142-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: Results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]