Abstract
Purpose
The sixth edition of the American Joint Committee on Cancer (AJCC) rectal cancer staging subdivided stage II into IIA (T3N0) and IIB (T4N0) and stage III into IIIA (T1-2N1M0), IIIB (T3-4N1M0), and IIIC (anyTN2M0). Subsequent analyses supported revised substaging of stage III as a result of improved survival with T1-2N2 versus T3-4N2 and survival of T4N1 more similar to T3-4N2 than T3N1. The AJCC Hindgut Taskforce sought population-based validation that depth of invasion interacts with nodal status to affect survival.
Methods
Surveillance, Epidemiology, and End Results (SEER) population-based data from January 1992 to December 2004 for 35,829 patients with rectal cancer were compared with rectal pooled analysis data (3,791 patients). T4N0 cancers were stratified by tumors that perforate visceral peritoneum (T4a) versus tumors that invade or are adherent to adjacent organs or structures (T4b). N1 and N2 were stratified by number of positive nodes as follows: N1a/N1b (one v two to three nodes) and N2a/N2b (four to six v ≥ seven nodes). Five-year observed and relative survival rates were obtained for each TN category.
Results
SEER rectal cancer analyses confirm that T1-2N2 cancers have better prognosis than T3-4N2, T4bN1 have similar prognosis to T4N2, T1-2N1 have similar prognosis to T2N0/T3N0, and T1-2N2a have similar prognosis to T2N0/T3N0 (T1N2a) or T4aN0 (T2N2a). Prognosis for T4a lesions is better than T4b by N category. The number of positive nodes affects prognosis.
Conclusion
This SEER population-based rectal cancer analysis validates the rectal pooled analyses and supports the shift of T1-2N2 lesions from IIIC to IIIA or IIIB and T4bN1 from IIIB to IIIC. SEER outcomes support subdividing T4, N1, and N2 and revised substaging of stages II and III. Survival by TN category suggests a complex biologic interaction between depth of invasion and nodal status.
INTRODUCTION
Survival and disease relapse after surgery alone or combined with adjuvant treatment for rectal cancer are a function of both degree of bowel wall penetration of the primary lesion (T classification) and nodal status (N classification), as suggested for 40 to 50 years.1–3 Nodal involvement alone is inadequate as the sole pathologic factor to predict survival and relapse rates.4–13 However, through the fifth edition of the American Joint Committee on Cancer (AJCC) staging manual, marked differences in survival by TN category of disease within stages II and III were not taken into account by appropriate substaging.
In the sixth edition of AJCC staging, stage II was subdivided into IIA (T3N0) and IIB (T4N0), and stage III was subdivided into IIIA (T1-2N1M0), IIIB (T3-4N1M0), and IIIC (anyTN2M0).14 The addition of substaging for stages II and III was based on existing outcomes for IIA versus IIB and for IIIA and IIIB. The placement of all TN2 patients into IIIC was based on data that patients with N2 cancers (four or more positive nodes) had poorer outcomes than patients with N1 cancers (one to three positive nodes).
Subsequent rectal cancer pooled analyses demonstrated the independent prognostic significance of each TN and NT category of resected rectal cancer (N subcategory within T category and T subcategory within N category).15,16 The outcomes in the rectal pooled analyses supported revised substaging of stage III as a result of improved survival for patients with T1-2N2 cancers versus T3-4N2 and survival rates with T4N1 lesions that are more similar to rates seen with T3-4N2 than T3N1.
Before making such changes in the seventh edition of AJCC staging, the AJCC Hindgut Taskforce (HTF) sought validation in a population-based data set that depth of invasion interacts with nodal status to impact survival. Data were obtained for patients with both rectal and colon cancers; the colon cancer data in 109,953 evaluable patients with invasive cancer are reported in a separate article.16a
METHODS
Surveillance, Epidemiology, and End Results (SEER) population-based data were obtained from January 1, 1992 to December 2004 for 55,011 patients with rectal and rectosigmoid cancer (C19.9 and 20.9); 35,829 patients had invasive rectal cancer and evaluable TN category of disease (T1-4N0-2), 17,302 were categorized as NX, and 1,880 were categorized as Tis or T1 polyp. Data of the 35,829 patients with invasive cancers and evaluable TN category of disease were compared with cooperative group rectal pooled analysis data on 2,551 and 3,791 patients (pooled analyses 1 and 2, respectively).15,16 Patients who died within 30 days of surgical resection were not included in the current analysis. The effects of different treatment approaches (surgical technique [total mesorectal resection], adjuvant chemoradiotherapy [pre- or postoperative], adjuvant chemotherapy, or other) were not felt to be pertinent to the current analysis; such data were not analyzed in depth. Of the total group of 55,011 patients, only 4,821 (8.8%) were recorded as having received preoperative irradiation as a component of treatment (T1-4N0-2, 3,353 patients [9.4% of 35,829 patients with evaluable TN category of disease]; NX category, 1,400 patients; Tis/T1 polyp N0-2, 68 patients).
Tumors were stratified by SEER's extent of disease and number of positive nodes coding schemes. T4N0 cancers were stratified by tumors that perforate visceral peritoneum (T4a) versus tumors that invade or are adherent to adjacent organs or structures (T4b). N1 (metastasis in one to three regional nodes) and N2 (metastasis in ≥ four regional nodes) were stratified by number of positive lymph nodes, as follows: N1a (one positive node), N1b (two to three positive nodes), N2a (four to six positive nodes), and N2b (≥ seven positive nodes). T1 to T3 categories were defined as per prior AJCC staging (T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades pericolorectal tissues).
Both observed and relative survival data were obtained for each TN category of disease. Observed survival (≃ overall survival [OS]) is the proportion of cancer patients surviving for a specified time interval after diagnosis. Relative survival (survival corrected by age-related morbidity; ≃ disease-specific survival) is a net survival measure representing cancer survival in the absence of other causes of death.
RESULTS
Survival Outcomes: SEER Versus Rectal Pooled Analyses
Observed survival outcomes at 5 years in the current SEER rectal cancer analysis were compared with 5-year OS outcomes in the two rectal cancer pooled analyses for patients with invasive rectal cancer and evaluable TN category of disease (SEER database, 35,829 patients; rectal pooled analysis 1, 2,551 patients; rectal pooled analysis 2, 3,791 patients). As shown in Table 1, SEER analyses confirmed the findings of the two pooled analyses with regard to differential prognosis by NT/TN category of disease for patients with stage II and III cancers. For most TN categories, survival rates in the SEER analysis were 7% to 10% lower than in the rectal pooled analyses.
Table 1.
Survival Outcomes at 5 Years by NT Category and Series in Rectal Adjuvant Pooled Analyses and SEER Analysis
| NT Category | Pooled Analysis 1* |
Pooled Analysis 2† |
SEER Analysis‡ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | OS Rate (%) | P | No. of Patients | OS Rate (%) | P | No. of Patients | Observed Survival Rate (%) | SE (%) | |
| N0T1-2 | — | — | — | — | 9,961 | 77.6 | 0.5 | ||
| N0T3 | 668 | 74 | .046 | 1,060 | 75 | .02 | 10,615 | 64.0 | 0.5 |
| N0T4 | 95 | 65 | 111 | 65 | 1,587 | 50.5 | 1.4 | ||
| N1T1-2 | 225 | 81 | < .001 | 355 | 79 | < .001 | 2,008 | 72.1 | 1.2 |
| N1T3 | 544 | 61 | 887 | 60 | 5,787 | 52.4 | 0.8 | ||
| N1T4 | 59 | 33 | 62 | 35 | 903 | 37.4 | 1.8 | ||
| N2T1-2 | 180 | 69 | < .001 | 226 | 67 | < .001 | 508 | 56.1 | 2.6 |
| N2T3 | 663 | 48 | 935 | 44 | 3,755 | 37.5 | 0.9 | ||
| N2T4 | 84 | 38 | 108 | 37 | 705 | 26.4 | 1.9 | ||
| Total | 2,551 | 3,791 | 35,829‡ | ||||||
NOTE. See Table 3 for observed survival data expansion.
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; OS, overall survival.
Modified from Gunderson et al.15
Modified from Gunderson et al.16
Current series, observed survival data (observed survival ≃ OS); lymph node status unknown, n = 17,302; Tis or T1 polyp, n = 1,880.
For patients with stage II cancers, the differential survival outcomes for T3N0 and T4N0 lesions are listed in Table 1. In both pooled analyses, patients with T3N0 lesions had improved 5-year OS relative to patients with T4N0 (P = .046 and P = .02). In the SEER rectal analysis, large differences in 5-year observed survival were found between T3 versus T4N0 cancers, and SEs were small based on large patient numbers (T3N0, 64.0% ± 0.5%, n = 10,615; T4N0, 50.5% ± 1.4%, n = 1,587).
For stage III cancers, patients with T1-2 lesions (confined to rectal wall) had much better prognoses than patients with T3-4 lesions for both N1 and N2 category of disease. These differences were statistically significant in both the SEER and rectal pooled analyses. In addition, patients with T4N1 lesions had prognoses more akin to those of patients with T3N2 or T4N2 cancers in both the SEER and rectal pooled analyses.
The 5-year survival of patients with T1-2 lesions was better than expected for both N1 and N2 category of disease in both the SEER and rectal pooled analyses. For patients with T1-2N1 lesions, 5-year survival was similar to that of patients with T3N0 lesions. For patients with T1-2N2 lesions, 5-year survival was more akin to that of patients with T3-4N0 or T3N1 lesions.
Survival Outcomes by TN Category: SEER Analysis
The large patient numbers in the SEER rectal cancer database allowed the evaluation of both observed and relative survival at 5 years for each TN category of disease, including patients with Tis, T1 polyp, and NX lesions (Tables 2 and 3). Analyses of highest pertinence to this article included the 35,829 patients with invasive T1-4N0-2 cancers.
Table 2.
Rectal SEER Analysis: Relative Survival at 5 Years by NT Category of Disease
| NT Category | No. of Patients | 5-Year Survival Rate (%) | SE |
|---|---|---|---|
| N0 | 23,902 | ||
| Tis | 821 | 95.9 | 1.9 |
| T1 polyp | 918 | 97.3 | 1.7 |
| T1-2 | 9,961 | 93.6 | 0.6 |
| T1 | 3,348 | 96.6 | 0.9 |
| T2 | 6,613 | 92.1 | 0.7 |
| T3 | 10,615 | 78.7 | 0.7 |
| T4 | 1,587 | 61.6 | 1.7 |
| T4a* | 818 | 69.2 | 2.4 |
| T4b* | 769 | 53.6 | 2.5 |
| NX | 17,302 | ||
| Tis | 2,718 | 89.4 | 1.1 |
| T1 polyp | 2,668 | 91.2 | 1.1 |
| T1-2 | 7,585 | 80.9 | 0.8 |
| T1 | 5,688 | 81.5 | 0.9 |
| T2 | 1,897 | 79.0 | 1.6 |
| T3 | 2,834 | 51.8 | 1.3 |
| T4 | 1,497 | 23.8 | 1.4 |
| T4a* | 238 | 42.8 | 4.2 |
| T4b* | 1,259 | 20.2 | 1.4 |
| N1 (1-3 positive nodes) | 8,817 | ||
| Tis | 60 | 86.7 | 6.9 |
| T1 polyp | 59 | 84.8 | 9.2 |
| T1-2 | 2,008 | 85.1 | 1.4 |
| T1 | 444 | 88.1 | 2.8 |
| T2 | 1,564 | 84.3 | 1.6 |
| T3 | 5,787 | 63.1 | 0.9 |
| T4 | 903 | 44.9 | 2.2 |
| T4a* | 480 | 58.7 | 3.1 |
| T4b* | 423 | 28.5 | 2.9 |
| N1a (1 positive node) | 4,419 | ||
| Tis | 34 | 92.7 | 7.2 |
| T1 polyp | 45 | 87.7 | 7.1 |
| T1-2 | 1,197 | 86.5 | 1.8 |
| T1 | 274 | 88.4 | 3.7 |
| T2 | 923 | 86.0 | 2.0 |
| T3 | 2,758 | 66.9 | 1.4 |
| T4 | 419 | 48.6 | 3.3 |
| T4a* | 218 | 65.6 | 4.6 |
| T4b* | 201 | 28.9 | 4.3 |
| N1b (2-3 positive nodes) | 4,338 | ||
| Tis | 26 | 75.4 | 11.5 |
| T1 polyp | 14 | 57.4 | 26.2 |
| T1-2 | 811 | 83.1 | 2.2 |
| T1 | 170 | 85.9 | 4.4 |
| T2 | 641 | 81.8 | 2.5 |
| T3 | 3,029 | 59.7 | 1.3 |
| T4 | 484 | 41.6 | 2.9 |
| T4a* | 262 | 52.6 | 4.1 |
| T4b* | 222 | 27.8 | 4.0 |
| N2 (≥ 4 positive nodes) | 4,990 | ||
| Tis | 15 | 71.7 | 16.1 |
| T1 polyp | 7 | 65.4 | 21.8 |
| T1-2 | 508 | 64.9 | 3.0 |
| T1 | 86 | 77.0 | 6.4 |
| T2 | 422 | 62.4 | 3.3 |
| T3 | 3,755 | 44.1 | 1.1 |
| T4 | 705 | 31.2 | 2.2 |
| T4a* | 397 | 40.6 | 3.2 |
| T4b* | 308 | 18.4 | 3.0 |
| N2a (4-6 positive nodes) | 2,683 | ||
| Tis | 12 | 64.1 | 17.9 |
| T1 polyp | 2 | 100 | 0 |
| T1-2 | 364 | 70.7 | 3.5 |
| T1 | 62 | 82.7 | 7.0 |
| T2 | 302 | 67.7 | 4.0 |
| T3 | 1,964 | 49.9 | 1.5 |
| T4 | 355 | 39.5 | 3.4 |
| T4a* | 199 | 53.1 | 4.8 |
| T4b* | 156 | 22.1 | 4.3 |
| N2b (≥ 7 positive nodes) | 2,285 | ||
| Tis | 3 | — | — |
| T1 polyp | 5 | 54.5 | 25.9 |
| T1-2 | 144 | 49.5 | 5.4 |
| T1 | 24 | 59.3 | 14.6 |
| T2 | 120 | 46.2 | 5.8 |
| T3 | 1,791 | 37.5 | 1.5 |
| T4 | 350 | 22.8 | 2.9 |
| T4a* | 198 | 28.5 | 4.0 |
| T4b* | 152 | 14.1 | 4.0 |
NOTE. Relative survival data (≃ disease-specific survival) were available for 55,011 patients (T1-4N0-2, n = 35,829; NX, n = 17,302; Tis or T1 polyp, n = 1,880).
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
T4a = tumors that penetrate to the surface of visceral peritoneum; T4b = tumors that invade or are adherent to adjacent organs or structures.
Table 3.
Rectal SEER Analysis: Observed Survival at 5 Years by NT Category of Disease
| NT Category | No. of Patients | 5-Year Survival Rate (%) | SE |
|---|---|---|---|
| N0 | 23,902 | ||
| Tis | 821 | 80.8 | 1.6 |
| T1 polyp | 918 | 83.6 | 1.4 |
| T1-2 | 9,961 | 77.6 | 0.5 |
| T1 | 3,348 | 81.4 | 0.8 |
| T2 | 6,613 | 75.7 | 0.6 |
| T3 | 10,615 | 64.0 | 0.5 |
| T4 | 1,587 | 50.5 | 1.4 |
| T4a* | 818 | 55.7 | 1.9 |
| T4b* | 769 | 44.7 | 2.1 |
| NX | 17,302 | ||
| Tis | 2,718 | 74.5 | 1.0 |
| T1 polyp | 2,668 | 76.3 | 1.0 |
| T1-2 | 7,585 | 65.3 | 0.6 |
| T1 | 5,688 | 66.4 | 0.7 |
| T2 | 1,897 | 62.1 | 1.3 |
| T3 | 2,834 | 41.4 | 1.1 |
| T4 | 1,497 | 19.1 | 1.1 |
| T4a* | 238 | 35.1 | 3.4 |
| T4b* | 1,259 | 16.2 | 1.1 |
| N1 (1-3 positive nodes) | 8,817 | ||
| Tis | 60 | 76.5 | 6.1 |
| T1 polyp | 59 | 74.3 | 8.0 |
| T1-2 | 2,008 | 72.1 | 1.2 |
| T1 | 444 | 75.8 | 2.4 |
| T2 | 1,564 | 71.1 | 1.3 |
| T3 | 5,787 | 52.4 | 0.8 |
| T4 | 903 | 37.4 | 1.8 |
| T4a* | 480 | 48.2 | 2.5 |
| T4b* | 423 | 24.3 | 2.5 |
| N1a (1 positive node) | 4,453 | ||
| Tis | 34 | 86.4 | 6.4 |
| T1 polyp | 45 | 82.1 | 6.2 |
| T1-2 | 1,197 | 73.4 | 1.5 |
| T1 | 274 | 75.7 | 3.2 |
| T2 | 923 | 72.7 | 1.7 |
| T3 | 2,758 | 55.4 | 1.1 |
| T4 | 419 | 40.1 | 2.7 |
| T4a* | 218 | 53.2 | 3.7 |
| T4b* | 201 | 24.4 | 3.6 |
| N1b (2-3 positive nodes) | 4,364 | ||
| Tis | 26 | 65.6 | 10.0 |
| T1 polyp | 14 | 50.9 | 23.2 |
| T1-2 | 811 | 70.3 | 1.9 |
| T1 | 170 | 75.9 | 3.8 |
| T2 | 641 | 68.9 | 2.1 |
| T3 | 3,029 | 49.7 | 1.1 |
| T4 | 484 | 35.2 | 2.5 |
| T4a* | 262 | 43.9 | 3.4 |
| T4b* | 222 | 24.0 | 3.4 |
| N2 (≥ 4 positive nodes) | 4,990 | ||
| Tis | 15 | 65.5 | 14.4 |
| T1 polyp | 7 | 64.3 | 21.0 |
| T1-2 | 508 | 56.1 | 2.6 |
| T1 | 86 | 68.6 | 5.7 |
| T2 | 422 | 53.6 | 2.9 |
| T3 | 3,755 | 37.5 | 0.9 |
| T4 | 705 | 26.4 | 1.9 |
| T4a* | 397 | 34.3 | 2.7 |
| T4b* | 308 | 15.6 | 2.5 |
| N2a (4-6 positive nodes) | 2,697 | ||
| Tis | 12 | 58.6 | 16.1 |
| T1 polyp | 2 | 100 | 0 |
| T1-2 | 364 | 61.0 | 3.1 |
| T1 | 62 | 73.8 | 6.2 |
| T2 | 302 | 58.2 | 3.4 |
| T3 | 1,964 | 42.5 | 1.3 |
| T4 | 355 | 32.9 | 2.8 |
| T4a* | 199 | 44.3 | 4.0 |
| T4b* | 156 | 18.5 | 3.6 |
| N2b (≥ 7 positive nodes) | 2,293 | ||
| Tis | 3 | — | — |
| T1 polyp | 5 | 53.3 | 24.8 |
| T1-2 | 144 | 43.4 | 4.7 |
| T1 | 24 | 53.2 | 13.0 |
| T2 | 120 | 41.7 | 5.0 |
| T3 | 1,791 | 32.0 | 1.3 |
| T4 | 350 | 19.6 | 2.5 |
| T4a* | 198 | 24.5 | 3.4 |
| T4b* | 152 | 12.3 | 3.5 |
NOTE. Observed survival data (≃ overall survival) were available for 55,011 patients (T1-4N0-2, n = 35,829; NX, n = 17,302; Tis or T1 polyp, n = 1,880).
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
T4a = tumors that penetrate to the surface of visceral peritoneum; T4b = tumors that invade or are adherent to adjacent organs or structures.
A clear survival difference for patients with stage I versus IIA cancers is seen in the SEER analysis (Tables 1 to 3). For patients with T1-2N0 lesions, 5-year observed survival was 77.6% ± 0.5% v 64.0% ± 0.5% for patients with T3N0 lesions.
Subcategories of patients with T1-2N2 cancers had survival rates similar to those in patients with N0 disease. Patients with T1N2a lesions had similar 5-year observed survival to patients with T2N0 or T3N0 cancers (T2N0, 75.7% ± 0.6%; T1N2a, 73.8% ± 6.2%; T3N0, 64.0% ± 0.5%). Patients with T2N2a lesions had similar 5-year observed survival to patients with T4aN0 cancers (T2N2a, 58.2% ± 3.4%; T4aN0, 55.7% ± 1.9%).
Prognosis for patients with T4a lesions (tumor penetrates to the surface of visceral peritoneum; revised definition, AJCC seventh edition) is better than the prognosis for patients with T4b lesions (tumor directly invades or is adherent to other organs or structures) for each N category. Relative and observed 5-year survival rates for T4aN0 versus T4bN0 lesions were 69.2% ± 2.4% v 53.6% ± 2.5% (relative) and 55.7% ± 1.9% v 44.7% ± 2.1% (observed), respectively. For N1 and N2 categories, the survival differences for T4a versus T4b were even more striking, as shown in Tables 2 and 3 (N1: relative, 58.7% ± 3.1% v 28.5% ± 2.9%; observed, 48.2% ± 2.5% v 24.3% ± 2.5%; N2: relative, 40.6% ± 3.2% v 18.4% ± 3.0%; observed, 34.3% ± 2.7% v 15.6% ± 2.5%, respectively).
The number of positive nodes affects prognosis for most TN categories of disease (Tables 2 and 3). Patients with only one metastatic regional node (N1a) have a 3% to 10% better 5-year relative and observed survival than patients with two to three positive nodes (N1b) for most TN categories of disease. Patients with four to six involved nodes (N2a) have a 5% to 20% better 5-year survival than patients with ≥ seven positive nodes (N2b) by TN category (Tables 2 and 3).
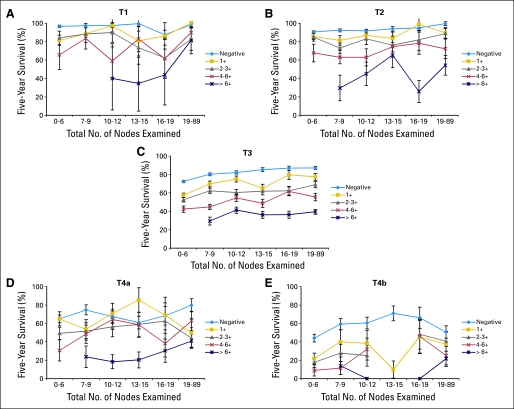
The relative survival impact of both the number of positive nodes and number of nodes evaluated by the pathologist is presented in Figure 1. Relative survival improves for some TN categories as the number of nodes examined increases, most obvious in the larger T3 category of disease (Fig 1C). Prognosis as a function of percentage of nodes involved was not evaluated in the current analysis.
Fig 1.
Interaction among tumor and node classifications and total nodes examined on 5-year survival in rectal cancer Surveillance, Epidemiology, and End Results (SEER) analysis. (A-E) Relative survival for pT1-4 by N1a (one positive node), N1b (two to three positive nodes), N2a (four to six positive nodes), and N2b (≥ seven positive nodes) on 35,829 patients (SEER analysis). The effect of the total number of nodes examined is categorized along the abscissa. Relative survival improves for some TN categories as number of lymph nodes examined increases, which is most obvious with (C) T3 category. Reprinted with permission.17
DISCUSSION
Survival and disease relapse after surgery alone1–9,13 or combined with adjuvant treatment10–12,14-16,18–44 for rectal cancer patients are a function of both degree of bowel wall penetration of the primary lesion and nodal status. However, nodal involvement alone does not determine survival and relapse rates. Invasion through the bowel wall and number of involved lymph nodes are independent high-risk factors for both relapse and survival.
For patients with a single high-risk factor of either direct tumor extension beyond the wall, nodes negative (T3N0), or positive nodes but primary tumor confined to the wall (T1-2N1-2), local relapse rates published in older surgical series have ranged from 20% to 40%.2–7 For patients with both positive nodes and extension beyond the wall (T3-4N1-2), the risk of pelvic relapse was nearly additive (40% to 65% in clinical series and 70% in a reoperative series).2–7
The rate of systemic metastases is significantly higher for patients with both high-risk pathologic factors (extension beyond rectal wall and positive nodes; T3-4N1-2), as opposed to patients with only a single risk factor (T3-4N0, T1-2N1). In published data from adjuvant rectal cancer patients irradiated at either Massachusetts General Hospital10,11 or Mayo Clinic,12 the incidence of subsequent systemic relapse was approximately 20% for patients with T3-4N0 and T1-2N+ lesions versus 40% to 60% for patients with T3-4N+ lesions.
Single-institution analyses from Massachusetts General Hospital10 and Mayo Clinic12 had previously suggested that patients with T1-2N1-2 lesions who were treated with postoperative irradiation alone or combined with chemotherapy had outcomes similar to T3N0 and T4N0 patients, but patient numbers in each stage subset were small. In the rectal pooled analyses with larger numbers of patients, the 5-year OS rate observed for the T1-2N1 patients was similar to that for T3N0 patients, and the 5-year survival for T1-2N2 patients was similar to that for patients with T4N0 or T3N1 lesions (Table 1).15,16 Results by N2 category were rarely available before the first pooled analysis,15 and results by T subcategory for patients with N2 disease (ie, T1-2N2, T3N2, T4N2) were nonexistent.
Data from the rectal cancer pooled analyses (Table 1)15,16 strongly supported substaging of TNM stages II and III, as accomplished in the sixth edition (2002) of TNM staging.14,18 As shown in Table 1, for TNM stage III patients, three separate prognostic subgroups of lesions exist (intermediate risk, T1-2N1; moderately high risk, T1-2N2 and T3N1; and high risk, T3N2 and T4N1-2). To combine or merge all of these patients into TNM stage III (Dukes C) does not provide full prognostic information for patients or physicians. However, patients with T1-2N1-2 disease had a more favorable prognosis than previously thought, and patients with T4N1 lesions (stage IIIB, AJCC sixth edition, along with T3N1 lesions) had prognoses more akin to those of patients with T3-4N2 lesions (stage IIIC, sixth edition).
For patients with N2 disease, data from the rectal pooled analyses demonstrated that N2 disease does not by itself confer poor prognosis.15,16 Substaging by T category influenced both 5-year OS (N2T1-2, 67%; N2T3, 44%; and N2T4, 37%; P < .001; Table 1) and 5-year disease-free survival (N2T1-2, 58%; N2T3, 36%; and N2T4, 30%; P < .001). Placement of all N2 patients in AJCC IIIC substage in the sixth edition16a did not reflect the markedly different prognosis of N2 patients observed in the rectal pooled analyses.
As shown in Table 1, data in the current large SEER population-based rectal cancer analysis validates the rectal pooled analyses with regard to the more favorable prognosis of patients with T1-2N1-2 lesions (stage IIIC, AJCC sixth edition) and less favorable prognosis of patients with T4N1 cancers (stage IIIB, sixth edition). Both SEER and rectal pooled analyses data support the shift of T1-2N2 lesions from stage IIIC to an earlier stage of disease (IIIA/IIIB) and T4N1 lesions from stage IIIB to IIIC (Tables 4 and 5).
Table 4.
Rectal SEER Analysis: 5-Year Relative and Observed Survival by TN Category of Disease in Patients With Invasive Cancer and Evaluable TN Category
| TN Category | No. Patients | SEER: 5-Year Relative Survival Rate (%) | SE (%) | TNM Stage (sixth edition) | Proposed TNM Stage (seventh edition) | SEER: Observed Survival Rate (%) | SE (%) |
|---|---|---|---|---|---|---|---|
| T1N0 | 3,348 | 96.6 | 0.9 | I | I | 81.4 | 0.8 |
| T2N0 | 6,613 | 92.1 | 0.7 | I | I | 75.7 | 0.6 |
| T3N0 | 10,615 | 78.7 | 0.7 | IIA | IIA | 64.0 | 0.5 |
| T4aN0 | 818 | 69.2 | 2.4 | IIB | IIB | 55.7 | 1.9 |
| T4bN0 | 769 | 53.6 | 2.5 | IIB | IIC* | 44.7 | 2.1 |
| T1-2N1 | 2,008 | 85.1 | 1.4 | IIIA | IIIA | 72.1 | 1.2 |
| T1N2a | 62 | 82.7 | 7.0 | IIIC | IIIA* | 73.8 | 6.2 |
| T2N2a† | 302 | 67.7 | 4.0 | IIIC | IIIB* | 58.2 | 3.4 |
| T3N1a | 2,758 | 66.9 | 1.4 | IIIB | IIIB | 55.4 | 1.1 |
| T4aN1a | 218 | 65.6 | 4.6 | IIIB | IIIB | 53.2 | 3.7 |
| T3N1b | 3,029 | 59.7 | 1.3 | IIIB | IIIB | 49.7 | 1.1 |
| T1N2b | 24 | 59.3 | 14.6 | IIIC | IIIB* | 53.2 | 13.0 |
| T4aN2a‡ | 199 | 53.1 | 4.8 | IIIC | IIIC | 44.3 | 4.0 |
| T4aN1b | 262 | 52.6 | 4.1 | IIIB | IIIB | 43.9 | 3.4 |
| T3N2a | 1,964 | 49.9 | 1.5 | IIIC | IIIB* | 42.5 | 1.3 |
| T2N2b | 120 | 46.2 | 5.8 | IIIC | IIIB* | 41.7 | 5.0 |
| T3N2b | 1,791 | 37.5 | 1.5 | IIIC | IIIC | 32.0 | 1.3 |
| T4aN2b | 198 | 28.5 | 4.0 | IIIC | IIIC | 24.5 | 3.4 |
| T4bN1 | 423 | 28.5 | 2.9 | IIIB | IIIC* | 24.3 | 2.5 |
| T4bN2a | 156 | 22.1 | 4.3 | IIIB | IIIC | 18.5 | 3.6 |
| T4bN2b | 152 | 14.1 | 4.0 | IIIC | IIIC | 12.3 | 3.5 |
NOTE. Survival outcomes of 35,829 patients with invasive T1-4N0-2 rectal cancer are shown.
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Changes in substaging of stages II and III are based on expanded outcomes in SEER rectal/colon analyses.
T2N2a rectal lesions did worse than colon T2N2a lesions; both categories are placed in stage IIIB.
T4aN2a rectal lesions did better than colon T4aN2a lesions; both categories are placed in stage IIIC.
Table 5.
Rectal Cancer: Proposed Changes in Substaging of AJCC Stages II and III Based on Rectal Pooled and SEER Analyses
| TN Category | Rectal Pooled Analysis 1 |
Rectal Pooled Analysis 2 |
AJCC TNM Stage (sixth edition) | Proposed TNM Stage (seventh edition) | SEER Rectal Cancer |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | 5-Year Overall Survival Rate (%) | No. of Patients | 5-Year Overall Survival Rate (%) | No. of Patients | 5-Year Observed Survival Rate (%) | SE (%) | |||
| T1-2N0 | — | — | — | — | I | I | 9,961 | 77.6 | 0.5 |
| T1N0 | — | — | — | — | I | I | 3,348 | 81.4 | 0.8 |
| T2N0 | — | — | — | — | I | I | 6,613 | 75.7 | 0.6 |
| T3N0 | 668 | 74 | 1,060 | 75 | IIA | IIA | 10,615 | 64.0 | 0.5 |
| T4N0* | 95 | 65 | 111 | 65 | IIB | T4a, IIB | 818 | 55.7 | 1.9 |
| T4b, IIC* | 769 | 44.7 | 2.1 | ||||||
| T1-2N1 | 225 | 81 | 355 | 79 | IIIA | IIIA | 2,008 | 72.1 | 1.2 |
| T1-2N2* | 180 | 69 | 226 | 67 | IIIC | IIIA/IIIB*† | 508 | 56.1 | 2.6 |
| T3N1 | 544 | 61 | 887 | 60 | IIIB | IIIB | 5,787 | 52.4 | 0.8 |
| T4N1* | 59 | 33 | 62 | 35 | IIIB | T4a, IIIB | 480 | 48.2 | 2.5 |
| T4b, IIIC* | 423 | 24.3 | 2.5 | ||||||
| T3N2* | 663 | 48 | 935 | 44 | IIIC | T3N2a, IIIB* | 1,964 | 42.5 | 1.3 |
| T3N2b, IIIC | 1,791 | 32.0 | 1.3 | ||||||
| T4N2 | 84 | 38 | 108 | 37 | IIIC | T4a, IIIC | 397 | 34.3 | 2.7 |
| T4b, IIIC | 308 | 15.6 | 2.5 | ||||||
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results.
Proposed changes in substaging.
IIIA = T1N2a; IIIB = T2N2a, T1-2N2b.
Expanded SEER rectal cancer outcomes data (Tables 2 to 5) also support subdividing T4, N1, and N2 categories of disease. Patients with T4a lesions (penetrates to the surface of visceral peritoneum [revised definition, AJCC seventh edition]) have a better prognosis than patients with T4b lesions (directly invades or is adherent to other organs or structures) for each N category of disease (N0, N1, and N2). For patients with N0T4a versus N0T4b lesions, there is an approximately 10% improvement in absolute 5-year relative survival and OS, and for patients with N1T4a versus N1T4b and N2T4a versus N2T4b disease, there is a nearly 20% improvement in 5-year survival. Patients with one positive node (N1a) have a better prognosis than patients with two to three positive nodes (N1b), and patients with four to five positive nodes (N2a) have a better prognosis than patients with ≥ seven positive nodes (N2b) by T category.
Previous analyses with much smaller data sets had suggested that patients with perforated T4 lesions may have a worse prognosis than patients with invasion of or adherence to other organs or structures.45,46 However, as shown in the current SEER analysis with large data sets for each TN category of disease, patients with T4 lesions that penetrate to the surface of visceral peritoneum (T4a in AJCC seventh edition) have a more favorable prognosis than patients with invasion of or adherence to other organs or structures (T4b in AJCC seventh edition).
Data in the current SEER analyses combined with rectal pooled analyses data support revised substaging of stages II and III (Tables 4 and 5). The AJCC seventh edition HTF recommended the following changes (Table 5): subdivide IIB into IIB (T4aN0) and IIC (T4bN0); shift more favorable TN2 categories to either IIIA (T1N2a) or IIIB (T2N2a, T1-2N2b, T3N2a); and shift less favorable T4N1 lesions from IIIB to IIIC (T4bN1).
Survival outcomes by TN/NT category in the rectal pooled analyses15,16 and the current SEER rectal cancer analysis suggest a complex biologic interaction between depth of invasion and nodal status. As shown in Tables 1 to 5, some TN categories of patients with positive nodes (T1-2N1 and T1-2N2) have a similar or better prognosis than patients with negative nodes with regard to both relapse (rectal pooled) and survival (SEER, rectal pooled). Patients with T1-2N1 lesions have better 5-year OS (rectal pooled), relative survival, and observed survival (SEER) than patients with T3N0 or T4N0 cancers (Tables 1 to 5), with outcomes more akin to patients with T2N0 lesions (Tables 4 to 5). Accordingly, the indications for adjuvant chemoradiotherapy or chemotherapy in patients with T1-2N1 disease should continue to be evaluated. Patients with N2a category (four to six involved nodes) but limited invasion have 5-year survival outcomes similar to those of patients with T2N0 and T3N0 cancers (T1N2a) or T4aN0 cancers (T2N2a) in the current analysis.
Survival outcomes by TN category of disease in the SEER rectal cancer analysis are more similar to SEER colon cancer outcomes than expected.16a Because of the similarities, the AJCC seventh edition HTF recommended continuance of a common staging system for patients with rectal and colon cancers. These similarities may be the result of common tumor biology, the impact of adjuvant chemoradiotherapy (preoperative or postoperative), and/or adjuvant chemotherapy or other factors.
This revision of TNM classification for rectal cancer demonstrates the critical role of formulating postulates and then assessing them in data sets that are larger than single-institution series. As the AJCC proceeds to the next edition, it will be important to collect data on other points of consideration that include, but are not limited to, the number of peritumoral deposits, the number of positive and total nodes examined, and the magnitude of the circumferential radial margin. Only through prospective data collection will usable data exist that can guide decisions relative to the next edition of the staging manual, when hopefully several molecular markers can be incorporated as an adjunct or modifier to the TNM categories of disease.
Acknowledgment
We acknowledge Lynn A.G. Ries, MS Health Statistician, Cancer Statistics Branch, Cancer Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, for her invaluable assistance in helping navigate the SEER*Stat to retrieve the data used in the current Surveillance, Epidemiology, and End Results (SEER) rectal cancer analysis.
Appendix
American Joint Committee on Cancer (AJCC) Hindgut Taskforce, 7th Edition: John Milburn Jessup, MD, FACS, Chair; Leonard L. Gunderson, MD, MS, FASTRO, Vice-Chair; Jaffer Ajani, MD; Robert W Beart, Jr, MD, FACS; Al B. Benson III, MD; James Brierly, MD, FRCR, FRCPC (International Union Against Cancer [UICC] Representative); John M. Carethers, MD; Paul Catalano, SCD (Statistical Task Force Liaison); George J. Chang, MD; Carolyn A. Compton, MD, PhD (Editorial Board Representative); Julio Garcia-Aguilar, MD, PhD, FACS; Richard M. Goldberg, MD (Cooperative Group Representative; Cancer and Leukemia Group B); Frederick L. Greene, MD, FACS (Editorial Board Representative); Daniel G. Haller, MD, FACP; Stanley R. Hamilton, MD; Donald E. Hanson, MD; Vencine Kelly, CTR; Bruce D. Minsky, MD; Heidi Nelson, MD; Stephen Rubesin, MD; Daniel J. Sargent, PhD (Statistical Task Force Liaison); Leslie H. Sobin, MD (UICC Representative); Mary Kay Washington, MD (College of American Pathologists Representative); Martin Weiser, MD (AJCC grant recipient); and Mark Lane Welton, MD, FACS, FASCRS.
Footnotes
See accompanying article on page 264
Written on behalf of the American Joint Committee on Cancer Hindgut Taskforce.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Leonard L. Gunderson, John Milburn Jessup, Daniel J. Sargent, Frederick L. Greene
Administrative support: Leonard L. Gunderson, John Milburn Jessup
Provision of study materials or patients: John Milburn Jessup
Collection and assembly of data: Leonard L. Gunderson, John Milburn Jessup
Data analysis and interpretation: Leonard L. Gunderson, John Milburn Jessup, Daniel J. Sargent, Frederick L. Greene, Andrew Stewart
Manuscript writing: Leonard L. Gunderson, John Milburn Jessup, Daniel J. Sargent, Frederick L. Greene, Andrew Stewart
Final approval of manuscript: Leonard L. Gunderson, John Milburn Jessup, Daniel J. Sargent, Frederick L. Greene, Andrew Stewart
REFERENCES
- 1.Dukes CE. Cancer of the rectum: An analysis of 1000 cases. J Pathol Bacteriol. 1940;50:527–539. [Google Scholar]
- 2.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copeland EM, Miller LD, Jones RS. Prognostic factors in carcinoma of the colon and rectum. Am J Surg. 1968;116:875–881. doi: 10.1016/0002-9610(68)90458-3. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson LL, Sosin H. Adenocarcinoma of the rectum: Areas of failure found at reoperation (second or symptomatic look) following curative surgery for adenocarcinoma of the rectum—Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278–1292. doi: 10.1002/1097-0142(197410)34:4<1278::aid-cncr2820340440>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SB. The significance of symptomatic local tumor failure following abdominoperineal resection. Int J Radiat Oncol Biol Phys. 1978;4:801–807. doi: 10.1016/0360-3016(78)90039-1. [DOI] [PubMed] [Google Scholar]
- 6.Rich T, Gunderson LL, Galdabini J, et al. Clinical and pathologic factors influencing local failure after curative resection of carcinoma of the rectum and rectosigmoid. Cancer. 1983;52:1317–1329. [Google Scholar]
- 7.Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer. 1976;37:2861–2865. doi: 10.1002/1097-0142(197606)37:6<2861::aid-cncr2820370643>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Chan KW, Boey J, Wong SKC. A method of reporting radial invasion and surgical clearance of rectal carcinoma. Histopathology. 1983;9:1319–1327. doi: 10.1111/j.1365-2559.1985.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 9.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 10.Hoskins RB, Gunderson LL, Dosoretz D, et al. Adjuvant postoperative radiotherapy in carcinoma of the rectum and rectosigmoid. Cancer. 1985;55:61–71. doi: 10.1002/1097-0142(19850101)55:1<61::aid-cncr2820550111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Tepper JE, Cohen AM, Wood WC, et al. Postoperative radiation therapy of rectal cancer. Int J Radiat Oncol Biol Phys. 1987;13:5–10. doi: 10.1016/0360-3016(87)90252-5. [DOI] [PubMed] [Google Scholar]
- 12.Schild SE, Martenson JA, Gunderson LL, et al. Postoperative adjuvant therapy of rectal cancer. Int J Radiat Oncol Biol Phys. 1989;17:55–62. doi: 10.1016/0360-3016(89)90370-2. [DOI] [PubMed] [Google Scholar]
- 13.Adam IJ, Mohamdee MO, Martin JG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 14.Jessup JM, Gunderson LL, Greene FL, et al. Colon and rectum. In: Greene FL, Page AL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer; 2002. pp. 113–124. [Google Scholar]
- 15.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer: A pooled analysis. Int J Radiat Oncol Biol Phys. 2002;54:386–396. doi: 10.1016/s0360-3016(02)02945-0. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 16a.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. ed 7. New York, NY: 2009. Colon and rectal; pp. 143–164. [Google Scholar]
- 18.Greene FL, Stewart A, Norton HJ. New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: An analysis. J Clin Oncol. 2004;22:1778–1784. doi: 10.1200/JCO.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy or rectal cancer: Results from NSABP R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 20.JAMA; National Institutes of Health Consensus Conference: Adjuvant therapy for patients with colon and rectal cancer.; 1990. pp. 1444–1450. [PubMed] [Google Scholar]
- 21.Krook J, Moertel C, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 22.O'Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 23.Tepper JE, O'Connell MJ, Petroni G, et al. Adjuvant postoperative 5-FU-modulated chemotherapy combined with pelvic radiation therapy for rectal cancer: Initial results of Intergroup 0114. J Clin Oncol. 1997;15:2030–2039. doi: 10.1200/JCO.1997.15.5.2030. [DOI] [PubMed] [Google Scholar]
- 24.Gunderson LL. Indications for and results of combined modality treatment of colorectal cancer. Acta Oncol. 1999;38:7–21. doi: 10.1080/028418699431753. [DOI] [PubMed] [Google Scholar]
- 25.Coia LR, Gunderson LL, Haller D, et al. Outcomes of patients receiving radiation for carcinoma of the rectum: Results of the 1988-1989 patterns of care study. Cancer. 1999;86:1952–1958. doi: 10.1002/(sici)1097-0142(19991115)86:10<1952::aid-cncr11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Willett CG, Badizadegan K, Ancukiewicz M, et al. Prognostic factors in stage T3N0 rectal cancer: Do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum. 1999;42:167–173. doi: 10.1007/BF02237122. [DOI] [PubMed] [Google Scholar]
- 27.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 28.Tepper JE, O'Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 29.Tepper JE, O'Connell MJ, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: Analysis of stage, sex and local control—Final report of Intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 30.Smalley SR, Benedetti J, Williamson SK, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006;24:3542–3547. doi: 10.1200/JCO.2005.04.9544. [DOI] [PubMed] [Google Scholar]
- 31.Rich TA, Skibber JM, Ajani JA, et al. Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys. 1995;32:1025–1029. doi: 10.1016/0360-3016(95)00020-y. [DOI] [PubMed] [Google Scholar]
- 32.Mohiuddin M, Hayne M, Regine WF, et al. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075–1080. doi: 10.1016/s0360-3016(00)00732-x. [DOI] [PubMed] [Google Scholar]
- 33.Grann A, Feng C, Wong D, et al. Preoperative combined modality therapy for clinically resectable UT3 rectal cancer. Int J Radiat Oncol Biol Phys. 2001;49:987–995. doi: 10.1016/s0360-3016(00)01529-7. [DOI] [PubMed] [Google Scholar]
- 34.Valentini V, Coco C, Cellini N, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: Acute toxicity, tumor response and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys. 2001;51:371–383. doi: 10.1016/s0360-3016(01)01618-2. [DOI] [PubMed] [Google Scholar]
- 35.Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 36.Nagtegaal ID, Marijnen CAM, Kranenbarg EKM, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: Not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–357. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Marijnen CAM, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: Report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1311–1320. doi: 10.1016/s0360-3016(02)04291-8. [DOI] [PubMed] [Google Scholar]
- 38.Crane CH, Skibber JM, Feig BW, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517–524. doi: 10.1002/cncr.11075. [DOI] [PubMed] [Google Scholar]
- 39.Gérard JP, Chapet O, Nemoz C, et al. Preoperative concurrent chemotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: The Lyon R0-04 phase II trial. J Clin Oncol. 2003;21:1119–1124. doi: 10.1200/JCO.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 40.Sauer R, Becker H, Hohenberger W, et al. For the German Rectal Cancer Group: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 41.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 42.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 43.Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group trial 0012. J Clin Oncol. 2006;24:650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 44.Rodel C, Valentini V, Minsky B. Rectal cancer. In: Gunderson LL, Tepper JE, editors. Clinical Radiation Oncology. ed 2. Philadelphia, PA: Churchill Livingstone/Elsevier; 2007. pp. 1113–1143. [Google Scholar]
- 45.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 46.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]