Abstract
Purpose
Rituximab has been given after autologous hematopoietic cell transplantation for recurrent or refractory B-cell lymphoma with the goal of eradicating minimal residual disease. Our previous report showed that administration of two courses of rituximab after transplantation is feasible, with encouraging clinical outcomes after a short follow-up. However, neutropenia after the first or second post-transplantation rituximab treatment occurred in 52% of patients. We previously reported that polymorphisms of two immunoglobulin G Fc receptors predict rituximab response, presumably because of their role in antibody-dependent cellular cytotoxicity. In the current report, we determine whether FcγR polymorphisms are correlated with clinical outcomes in 33 patients with B-cell non-Hodgkin's lymphoma who received post-transplantation rituximab.
Patients and Methods
Genomic DNA was used for FcγRIIIa V/F or the FcγRIIa H/R genotyping. The FcγR polymorphisms were then correlated with the incidence of rituximab-induced neutropenia, event-free survival (EFS), and overall survival (OS).
Results
The FcγRIIIa 158 V allele dose was correlated with a higher incidence of rituximab-induced neutropenia. The odds of neutropenia after the first or second post-transplantation rituximab increased three-fold with each V allele (robust z = 2.08, P = .038). The FcγRIIa polymorphism had no impact on rituximab-induced neutropenia. We did not observe a correlation of either FcγRIIIa or FcγRIIa polymorphism with EFS or OS.
Conclusion
The high affinity FcγRIIIa 158 V allele is associated with rituximab-induced neutropenia after autologous transplantation. This is a potential tool to identify a high-risk population for developing neutropenia after antibody therapy.
INTRODUCTION
Autologous hematopoietic cell transplantation (HCT) is the standard of care for patients with recurrent or refractory non-Hodgkin's lymphoma (NHL).1 However, only 40% to 50% patients achieve long-term disease control. One strategy to improve the outcome is to administer rituximab after HCT with the goal of eradicating minimal residual disease.2 Hypothesis is that post-transplantation rituximab will provide better disease control, especially for those who have not received rituximab before transplantation. Our prior report showed that post-transplantation rituximab infusions resulted in promising clinical outcomes.2 We previously reported that two immunoglobulin G (IgG) Fc receptor (FcγR), FcγRIIIa 158 V/V and FcγRIIa 131 H/H genotypes, predict response to single-agent rituximab therapy in patients with follicular lymphoma, probably due to their role in mediating antibody-dependent cellular cytotoxicity (ADCC).3,4 With this background, we correlated FcγR polymorphism with event-free survival (EFS), overall survival (OS), and toxicity in patients receiving post-transplantation rituximab. Although post-transplantation rituximab infusions were relatively safe, grade 3 or 4 neutropenia was observed after rituximab infusion in 52% of patients.2 This incidence was significantly higher than previously reported with single-agent rituximab.5 One possibility is that this is a patient population exposed to high cumulative doses of myelotoxic agents. Indeed, high rates of rituximab-induced neutropenia were also observed in a group of heavily treated patients who underwent allogeneic HCT.6 Although the mechanism of rituximab-induced neutropenia is unknown, several models have been proposed, which include the toxic effect of T-large granular lymphocytes (T-LGLs),7 development of autoantibodies against neutrophils,8,9 and perturbations of stromal-derived factor 1 (SDF-1) and granulopoiesis homeostasis.10 In this study, we hypothesized that FcγR polymorphisms may influence the rate of rituximab-induced neutropenia because high-affinity FcγR genotypes have been associated with superior clinical efficacy of rituximab.3,4,11
PATIENTS AND METHODS
Patient Population
This study included 35 patients with recurrent or refractory B-cell NHL as previously described.2 The median age was 51 years (range, 28 to 70 years). Twenty-five patients had diffuse large B-cell lymphoma (DLBCL), three patients had mantle-cell lymphoma, three patients had transformed large-cell lymphoma, and four patients had other NHL (follicular mixed, n = 2; chronic lymphatic leukemia, n = 1; and diffuse small-cell lymphoma, unspecified, n = 1). Eighteen patients had experienced relapse after initial complete response, 10 patients had disease that was refractory to their first chemotherapy regimen, and seven patients were considered at high risk in first complete response. Thirty-two patients received carmustine, etoposide, and cyclophosphamide, and three patients received total body irradiation in place of carmustine.2 All patients received in vitro purged peripheral-blood progenitor cell product mobilized with a single dose of cyclophosphamide 4 g/m2 and granulocyte colony-stimulating factor (G-CSF). The peripheral-blood progenitor cell product was enriched for CD34+ cells and purged with a panel of anti–B-cell antibodies and rabbit complement.12 At day 42 after HCT, all patients received four weekly infusions of rituximab 375 mg/m2. The first four patients were to receive one course of rituximab, and the remaining patients were scheduled to receive a second course of four weekly rituximab beginning 6 months after HCT. CBCs were monitored weekly during each course of rituximab infusion, 1 week and 3 weeks after the last day of rituximab infusion in each course, and then monthly for 9 months. This study was conducted according to an institutional review board–approved protocol, and informed consent was obtained from all patients.
Analysis of FcγR Polymorphism
Genomic DNA was prepared from peripheral-blood mononuclear cells using a Qiagen DNA extraction kit (Qiagen, Valencia, CA). Genotyping of FcγR polymorphisms was performed using TaqMan technology on an ABI Prism 7900HT Sequence Detector System (Applied Biosystems, Foster City, CA) as previously described.13,14 In brief, FcγRIIIa, FcγRIIa-specific primer pairs flanking the polymorphic sites were used for amplification of genomic DNA in the presence of probes specific to different alleles. Probes specific to FcγRIIIa 158 V and FcγRIIa 131 H alleles were labeled with VIC, and probes specific to FcγRIIIa 158 F and FcγRIIa 131 R alleles were labeled with FAM. Each sample was analyzed in duplicate. The final determination of FcγR genotypes was performed using Allelic Discrimination protocol in SDS software provided by Applied Biosystems.
Statistical Analysis
The EFS was defined as the time period between the day of transplantation and the time of disease relapse/progression or death from any cause. The OS was defined as the time period from the day of transplantation to death from any cause. The differences in EFS and OS were determined using log-rank statistic (PRISM for Macintosh, GraphPad Software, San Diego, CA). They were considered statistically significant for P < .05 between different genotype groups. We analyzed the effects of FcγR genotype on rituximab-induced neutropenia in three ways, to account for the correlated incidence data and imbalance in numbers receiving rituximab over time. We first used the Generalized Estimating Equation method (GEE package for R, version 2.5.0, http://cran.r-project.org/web/packages/gee/index.html) with binomial family and logistic links to estimate the increase in odds of neutropenia associated with each extra V allele and report the robust z statistic that accounts for the within-subject clustering on all 33 patients. Because GEE makes strong assumptions about the reasons some patients do not receive a second course, we used two additional simpler methods to reduce the data to a single summary per patient before correlating with allelic dose. In the first analysis, we scored every patient (N = 33) by number of neutropenic episodes/number of courses of rituximab in the following order: 0/2 < 0/1 < 1/2 < 1/1 < 2/2. In the second analysis, we looked only at patients who received two courses of treatment (n = 24) and counted the number of neutropenic episodes (0, 1, 2). These “scores” were correlated with number of alleles (Spearman and Kendall coefficients). The results of three methods were in agreement.
RESULTS
FcγRIIIa and FcγRIIa Polymorphisms and Survival
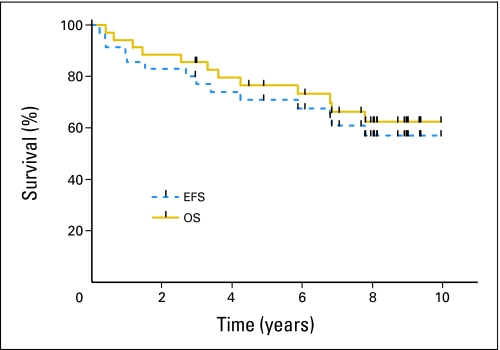
Thirty-five patients were treated in the study as planned. With current median follow-up of 7.7 years, the estimated 5-year EFS was 71% (95% CI, 55% to 86%), and the 5-year OS was 77% (95% CI, 62% to 91%) for all patients on an intent-to-treat basis (Fig 1). Twelve patients died: eight patients of progressive disease, one patient in remission from pneumonitis, one patient of secondary malignancy, one patient of suicide, and one patient of amyotrophic lateral sclerosis. Eleven patients experienced disease progression or relapse after transplantation (DLBCL, n = 5; mantle cell, n = 3; transformed NHL, n = 3).
Fig 1.
Kaplan-Meier estimates of event-free survival (EFS) and overall survival (OS).
We previously showed that clinical efficacy of single-agent rituximab was associated with high-affinity FcγRIIIa and FcγRIIa genotypes in patients with follicular lymphoma. The goal of this clinical study was to improve clinical outcome by eliminating minimal residual disease with post-transplantation rituximab. We first determined whether FcγRIIIa 158 V/F or FcγRIIa 131 H/R polymorphisms influenced EFS or OS. Genomic DNA was available in 33 patients for FcγR genotyping. For the FcγRIIIa, four patients (12%) were homozygous valine/valine (158 V/V), 14 patients (42%) were heterozygous valine/phenylalanine (158 V/F), and 15 patients (46%) were homozygous phenylalanine/phenylalanine (158 F/F). For the FcγRIIa, eight patients (24%) were homozygous histidine/histidine (131 H/H), 16 patients (49%) were heterozygous histidine/arginine (131 H/R), and nine patients (27%) were homozygous arginine/arginine (131 R/R). Five-year EFS by genotype were as follows: FcγRIIIa 158 V/V, 75% (95% CI, 50% to 100%); V/F, 70% (95% CI, 45% to 94%); F/F, 73% (95% CI, 51% to 96%); FcγRIIa 131 H/H, 75% (95% CI, 50% to 100%); H/R, 74% (95% CI, 53% to 96%); and R/R, 67% (95% CI, 36% to 97%). Five-year OS was not different for FcγRIIIa 158 V/V at 75% (95% CI, 50% to 100%), V/F at 77% (95% CI, 54% to 99%), F/F at 80% (95% CI, 60% to 100%) nor FcγRIIa 131 H/H at 75% (95% CI, 50% to 100%), H/R at 87% (95% CI, 64% to 100%), and R/R at 67% (95% CI, 37% to 97%).
FcγRIIIa Genotypes and Neutropenia
Among the 33 patients studied, a total of 57 courses (four weekly infusions) of rituximab were administered. Four patients received a single course by study design and five patients received a single course because of disease progression (n = 2), pneumonitis (n = 1), disseminated zoster (n = 1), and excess rituximab infusion reaction (n = 1). No difference in the actual frequency of blood count monitoring was observed according to FcγR genotype (data not shown). Seventeen (52%) of the 33 patients developed grade 3 or 4 neutropenia (absolute neutrophil count < 1,000/μL) in one or both post-transplantation rituximab courses. As shown in Table 1 for all 33 patients, rituximab-induced neutropenia occurred in 37% of treatment courses. The median time to develop neutropenia was 40 days (range, 7 to 117 days) after the last rituximab infusion in each course. For patients who developed neutropenia, the median time to neutropenia was 25 days (range, 7 to 70 days) in V/V, 51 days (range, 27 to 102 days) in V/F, and 44 days (range, 24 to 117 days) in F/F patients after first rituximab course. The median time to neutropenia was 18 days (range, 15 to 21 days) in V/V, 57 days (range, 27 to 102 days) in V/F, and 23 days in one F/F patient after second rituximab course. Using Kaplan-Meier estimation on all 33 patients, there was a trend for rituximab-induced neutropenia to occur earlier after the first rituximab course with each dose of V allele (hazard ratio for each dose of V allele = 1.92, P = .092, in the order of V/V, V/F, F/F), but the time to neutropenia was not statistically different between the three genotypes. All neutropenia episodes either resolved spontaneously or responded to 2 to 4 days of G-CSF. We then determined whether FcγR polymorphisms influenced the rates of rituximab-induced neutropenia. Taking account of the varying number of courses of rituximab administered, we found that each additional V allele was associated with a three-fold increase in the odds of neutropenia with each rituximab course using the generalized estimating equation method (robust z = 2.08, two-sided P = .038). For confirmation, in a nonparametric analysis, we also scored each patient according to the number of occurrences of neutropenia over the number of courses of rituximab, with the order 0/2 < 0/1 < 1/2 < 1/1 < 2/2. The number of V alleles is significantly associated with this score (Spearman's correlation coefficient = 0.39, P = .025).
Table 1.
Neutropenia and FcγR Polymorphisms in 33 Patients Receiving One or Two Courses of Rituximab
| Parameter | FcγR IIIa Polymorphism |
FcγR IIa Polymorphism |
All Patients (N = 33) | ||||
|---|---|---|---|---|---|---|---|
| V/V (n = 4) | V/F (n = 14) | F/F (n = 15) | H/H (n = 8) | H/R (n = 16) | R/R (n = 9) | ||
| Patients developing neutropenia* | |||||||
| No. | 3 | 8 | 6 | 3 | 9 | 5 | 17 |
| % | 75 | 57 | 40 | 38 | 56 | 56 | 52 |
| V/V (n = 7) | V/F (n = 23) | F/F (n = 27) | H/H (n = 15) | H/R (n = 27) | R/R (n = 15) | All Courses (N = 57) | |
| Courses with neutropenia | |||||||
| No. | 5 | 10 | 6 | 5 | 11 | 5 | 21 |
| % | 71 | 43 | 22 | 33 | 41 | 33 | 37 |
Grade 3 or 4 (absolute neutrophil count < 1,000/μL).
As described in Table 2, we separately analyzed the 24 patients who received two courses of rituximab in order to increase the power of detecting a difference. In this analysis, the number of episodes of rituximab-induced neutropenia was correlated with the number of FcγRIIIa 158 V alleles (Spearman rank correlation = 0.49, P = .014), with the highest rates of rituximab-induced neutropenia found in patients with V/V genotype. The number of FcγRIIIa 158 V alleles was also correlated with grade 4 rituximab-induced neutropenia. Grade 4 neutropenia was experienced in three (75%) of four V/V patients, affecting 43% of their rituximab courses, and in six (42%) of 14 V/F patients, affecting 30% of their rituximab courses. In contrast, just two (13%) of 15 F/F patients experienced grade 4 neutropenia, affecting only 7% of their rituximab courses. The FcγRIIa 131 H/R polymorphism was not associated with rituximab-induced neutropenia.
Table 2.
Neutropenia and FcγR Polymorphisms in 24 Patients Receiving Two Courses of Rituximab
| Neutropenia | FcγR IIIa Polymorphism |
FcγR IIa Polymorphism |
||||
|---|---|---|---|---|---|---|
| V/V (n = 3) | V/F (n = 9) | F/F (n = 12) | H/H (n = 7) | H/R (n = 11) | R/R (n = 6) | |
| No neutropenia* | 1 | 3 | 9 | 4 | 5 | 4 |
| Neutropenia in one course | 0 | 4 | 3 | 1 | 4 | 2 |
| Neutropenia in both courses | 2 | 2 | 0 | 2 | 2 | 0 |
| Mean | 1.33 | 0.89 | 0.25 | 0.77 | 0.72 | 0.33 |
| Spearman rank correlation | ||||||
| r | 0.495 | 0.140 | ||||
| P | .014 | .520 | ||||
Grade 3 or 4 (absolute neutrophil count < 1,000/μL).
DISCUSSION
In the pivotal trial using single-agent rituximab therapy, 4% of patients developed neutropenia after rituximab infusion, and only one patient had grade 3 or 4 neutropenia.5 However, recent reports have suggested the incidence of neutropenia after rituximab, especially the late-onset neutropenia, is probably higher.15 An increasing number of cases of rituximab-induced neutropenia has been reported in patients who received single-agent rituximab after multiple salvage therapies, including autologous transplantation.16,17 More commonly, late-onset neutropenia was observed in patients receiving rituximab-chemotherapy combinations.16,18,19 In these cases, neutropenia occurred between 4 and 17 weeks from last infusion after initial recovery of neutrophil counts from chemotherapy. One Japanese study reported a 25% incidence of late-onset neutropenia in patients who were treated with rituximab-chemotherapy.19 In another study, 12% of patients with DLBCL who received maintenance single-agent rituximab after induction rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone or cyclophosphamide, doxorubicin, vincristine, and prednisone experienced grade 3 or 4 neutropenia compared with 4% of observation patients.20 Similar to our observation, late-onset neutropenia was found in higher frequency in post-transplantation patients when rituximab was given either as in vivo purging before transplantation 16,21,22 or as consolidation immediately after transplantation.21,23,24 The collective impression is that rituximab-induced neutropenia occurs more frequently in patients who had more intensive prior myelotoxic therapies. However, rituximab-induced neutropenia has been reported in patients with autoimmune disease without intensive myelotoxic therapy.25,26
The mechanism of rituximab-induced neutropenia is unclear. Because CD20 is not expressed by neutrophils or their precursors, direct rituximab-mediated killing is unlikely. Because neutropenia may occur a few months after infusion, production of autoantibodies against neutrophils during recovery of a new immune repertoire has been postulated.8,9 However, this would not explain the quick neutrophils recovery either without intervention or with a short course of G-CSF. Another study suggested that an excess of T-LGL in the marrow may lead to apoptosis of mature neutrophils as a result of the secretion of large amounts of Fas and Fas ligand.7 However, excess of T-LGL is commonly observed in patients undergoing nonmyeloablative allogeneic transplantation who have normal neutrophils counts. A recent study suggested that perturbations of SDF-1 and granulopoiesis homeostasis during B-cell recovery are responsible for rituximab-induced neutropenia.10 However, in our study, neutropenia occurred within 4 months after rituximab infusion, which is too early for B-cell recovery. Instead, we found that the high affinity FcγRIIIa 158 V allele was associated with a higher incidence of rituximab-induced neutropenia. One question is whether pharmacokinetics of rituximab may differ in patients with high affinity FcγRIII 158 V allele. This is still unknown because no study has reported the effect of FcγR polymorphisms on rituximab concentration. On the other hand, pharmacokinetics of rituximab is related to tumor burden at the time of infusion demonstrated both in humans5 or in an animal model.27 In our study, this is not a major factor because all patients had minimal residual disease at the time of rituximab infusion after high-dose therapy. Although this retrospective report should be considered hypothesis-generating rather than establishing a mechanism of rituximab-induced neutropenia, there are important implications from our observations.
First, it is probable that patients with high-affinity FcγRs mediate ADCC on both normal and malignant B lymphocytes more vigorously. During this process, influx of granzyme and lysozyme released by effector cells may kill neutrophils via a bystander effect. This model can explain the self-limiting nature of rituximab-induced neutropenia, because once the target B cells are eliminated, the ADCC will not continue. Neutrophils themselves can mediate ADCC via an inducible FcγRI (CD64).28,29 In this case, neutrophils may be self-lysed after ADCC. However, whether this occurs in rituximab-treated patients and its relationship to FcγRIIIa polymorphism is unknown.
Second, the FcγRIIIa polymorphism may affect the “depth” of B lymphocyte depletion. In patients with the high-affinity V/V genotype, the B-lymphocyte depletion may be more complete, which in turn results in steering the bone marrow into lymphopoiesis and away from granulopoiesis. Studies have demonstrated that reciprocal dynamics of the marrow lymphocyte and neutrophil populations were consistent with a cellular competition within developmental niche, which creates a balance between granulopoiesis and lymphopoiesis.30 This notion is supported by a report that showed an unusually high serum B-cell activating factor (BAFF) level at the time of rituximab-induced neutropenia, indicating severe B-lymphocyte depletion, because BAFF level is correlated inversely with B lymphocyte mass.31 To support this hypothesis, one study has shown that FcγRIIIa 158 V allele was associated with higher degree of B-lymphocyte depletion in patients with lupus who received low-dose rituximab.32 However, whether FcγRIIIa 158 V allele is associated with prolonged B-lymphocyte depletion in patients with NHL is unknown. We did not collect information on B-lymphocyte counts routinely beyond the first 6 months after transplantation. Therefore, we were unable to address this question in the current study. Future studies to monitor B-lymphocyte recovery, serum BAFF level, and SDF-1 status during rituximab-induced neutropenia will help to elucidate its mechanism.
Third, by identifying the association between FcγRIIIa polymorphisms and rituximab-induced neutropenia, we now have a tool to predict who might have an increased risk of developing neutropenia. This becomes clinically important because the use of rituximab-chemotherapy combinations and the use of maintenance rituximab has been expanded.20,33,34 This tool could identify patients who need closer monitoring after rituximab-based therapies. However, confirmation of our observation in a larger study is needed before wider application of this tool should be adopted. One such analysis is underway in a large low-grade population of patients with NHL who received maintenance rituximab after induction cyclophosphamide, vincristine, prednisone under Eastern Cooperative Oncology Group 1496 protocol (S.J. Horning, personal communication, August 2009). In this analysis, FcγR polymorphism will be correlated with rituximab-induced neutropenia and other pertinent clinical outcomes. Recently, predictive value of the FcγRIIIa polymorphism was also observed after treatment with other therapeutic antibodies, including anti–HER-2/neu antibody and cetuximab.35–37 Whether the high-affinity FcγRIIIa genotype is also associated with a higher rate of antibody-induced neutropenia in these patients is of great interest.
Finally, a significant fraction of second-generation antitumor antibodies carry a re-engineered Fc portion to enhance their affinity to activating FcγRs.38,39 It will be important to monitor the incidence of neutropenia after administration of these second-generation antibodies.
Although a higher response rate after single-agent rituximab therapy has been observed in patients with follicular lymphoma with high-affinity FcγRIIIa or FcγRIIa genotypes, we found no such association between FcγR polymorphism and EFS or OS in this patient population. There are several explanations for this observation. First, whether FcγR polymorphisms influence the clinical outcome in rituximab-treated patients with histology other than follicular lymphoma is still unclear.40,41 Because the majority (71%) of the patients in this study had DLBCL, this is an important question. One study has linked FcγRIIIa polymorphism to the clinical outcomes of patients with DLBCL who were treated with a rituximab-chemotherapy combination.42 In contrast, another showed no such association.43 It is also possible that the correlation between FcγR polymorphisms and clinical response only apply to patients who receive single-agent rituximab, but not in combination with chemotherapy. One such example was the inability to link FcγR polymorphisms to clinical outcome even in patients with follicular lymphoma who received a sequential cyclophosphamide, doxorubicin, vincristine, prednisone–rituximab combination.44 Second, the small number of FcγRIIIa V/V homozygotes (n = 4) in our study limits the ability to detect a difference in the EFS and OS, even if one exists. As shown in Results, the 95% CIs for the EFS and OS in different genotypes were quite large, which made it extremely difficult to detect even a moderate difference in EFS or OS between different groups. Third, rituximab was used in the minimal disease setting after autologous transplantation. To more definitely address the impact of FcγR polymorphisms on the clinical efficacy in patients receiving post-transplantation rituximab, a similar analysis from larger clinical studies, such as the ongoing Collaborative Trial in Relapsed Aggressive Lymphoma study,45 is required.
Footnotes
Supported by National Institutes of Health/National Cancer Institute K08 Grant No. CA111827 (W.K.W.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Sandra J. Horning, Genentech (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: Sandra J. Horning, Roche, Genentech Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Wen-Kai Weng, Robert S. Negrin, Sandra J. Horning
Collection and assembly of data: Wen-Kai Weng
Data analysis and interpretation: Wen-Kai Weng, Philip Lavori, Sandra J. Horning
Manuscript writing: Wen-Kai Weng, Philip Lavori
Final approval of manuscript: Wen-Kai Weng, Robert S. Negrin, Philip Lavori, Sandra J. Horning
REFERENCES
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz SM, Negrin RS, Blume KG, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–783. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 3.Weng W-K, Levy R. Two immunoglobulin G Fc receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Weng W-K, Levy R. Genetic polymorphism of the inhibitory IgG Fc receptor FcγRIIb is not associated with clinical outcome of rituximab treated follicular lymphoma patients. Leuk Lymphoma. 2009;50:723–727. doi: 10.1080/10428190902829441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 6.Shimoni A, Hardan I, Avigdor A, et al. Rituximab reduced relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin's lymphoma. Br J Haematol. 2003;122:457–464. doi: 10.1046/j.1365-2141.2003.04446.x. [DOI] [PubMed] [Google Scholar]
- 7.Papadaki T, Stamatopoulos K, Stavroyianni N, et al. Evidence for T-large granular lymphocyte-mediated neutropenia in Rituximab-treated lymphoma patients: Report of two cases. Leuk Res. 2002;26:597–600. doi: 10.1016/s0145-2126(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 8.Chaiwatanatorn K, Lee N, Grigg A, et al. Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol. 2003;121:913–918. doi: 10.1046/j.1365-2141.2003.04385.x. [DOI] [PubMed] [Google Scholar]
- 9.Voog E, Morschhauser F, Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348:2691–2694. doi: 10.1056/NEJM200306263482620. [DOI] [PubMed] [Google Scholar]
- 10.Dunleavy K, Hakim F, Kim HK, et al. B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood. 2005;106:795–802. doi: 10.1182/blood-2004-08-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγR IIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 12.Negrin RS, Kusnierz-Glaz CR, Still BJ, et al. Transplantation of enriched and purged peripheral blood progenitor cells from a single apheresis product in patients with non-Hodgkin's lymphoma. Blood. 1995;85:3334–3341. [PubMed] [Google Scholar]
- 13.Weng W-K, Czerwinski D, Levy R. Humoral immune response and immunoglobulin G Fc receptor genotype are associated with better clinical outcome following idiotype vaccination in follicular lymphoma patients regardless of their response to induction chemotherapy. Blood. 2007;109:951–953. doi: 10.1182/blood-2006-03-013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng W-K, Levy R. Immunoglobulin G Fc receptor polymorphisms do not correlate with response to chemotherapy or clinical course in patients with follicular lymphoma. Leuk Lymphoma. 2009;50:1494–1500. doi: 10.1080/10428190903128660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin P. Late-onset neutropenia following rituximab. Leuk Lymphoma. 2006;47:965–966. doi: 10.1080/10428190600649349. [DOI] [PubMed] [Google Scholar]
- 16.Cattaneo C, Spedini P, Casair S, et al. Delayed-onset peripheral blood cytopenia after rituximab: Frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47:1013–1017. doi: 10.1080/10428190500473113. [DOI] [PubMed] [Google Scholar]
- 17.Kaya H, Keung Y-K, Case D, et al. Efficacy and safety of monoclonal anti-CD20 antibody (rituximab) for the treatment of patients with recurrent low-grade non-Hodgkin's lymphoma after high dose chemotherapy and autologous hematopoietic cell transplant. Biol Blood Marrow Transplant. 2002;8:544–549. doi: 10.1053/bbmt.2002.v8.pm12434949. [DOI] [PubMed] [Google Scholar]
- 18.Fukuno K, Tsurumi H, Ando N, et al. Late-onset neutropenia in patients treated with rituximab for non-Hodgkin's lymphoma. Int J Hematol. 2006;84:242–247. doi: 10.1532/IJH97.05105. [DOI] [PubMed] [Google Scholar]
- 19.Nitta E, Izutsu K, Sato T, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: A single-institution study. Ann Oncol. 2007;18:364–369. doi: 10.1093/annonc/mdl393. [DOI] [PubMed] [Google Scholar]
- 20.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 21.Flinn IW, O'Donnell PV, Goodrich A, et al. Immunotherapy with rituximab during peripheral blood stem cell transplantation for non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2000;6:628–632. doi: 10.1016/s1083-8791(00)70028-0. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux B, Tartas S, Traulle C, et al. Rituximab-related late-onset neutropenia after autologous stem cell transplantation for aggressive non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33:921–923. doi: 10.1038/sj.bmt.1704467. [DOI] [PubMed] [Google Scholar]
- 23.Cairoli R, Grillo G, Tedeschi A, et al. High incidence of neutropenia in patients treated with rituximab after autologous stem cell transplantation. Haematologica. 2004;89:361–363. [PubMed] [Google Scholar]
- 24.Joyce RM, Regan M, Ottaway J, et al. A phase I-II study for rituximab, ifosfamide, mitoxantrone and etoposide (R-IME) for B cell non-Hodgkin's lymphoma prior to and after high-dose chemotherapy and autologous stem cell transplantation (HDC-ASCT) Ann Oncol. 2003;14(suppl 1):i21–i27. doi: 10.1093/annonc/mdg705. [DOI] [PubMed] [Google Scholar]
- 25.Marotte H, Paintaud G, Watier H, et al. Rituximab-related late-onset neutropenia in a patient with severe rheumatoid arthritis. Ann Rheum Dis. 2008;67:893–894. doi: 10.1136/ard.2007.081166. [DOI] [PubMed] [Google Scholar]
- 26.Rios-Fernández R, Gutierrez-Salmeron MT, Callejas-Rubio J-L, et al. Late-onset neutropenia following rituximab in patients with autoimmune disease. Br J Dermatol. 2007;157:1271–1273. doi: 10.1111/j.1365-2133.2007.08189.x. [DOI] [PubMed] [Google Scholar]
- 27.Daydé D, Ternant D, Ohresser M, et al. Tumor burden influences exposure and response to rituximab: Pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113:3765–3772. doi: 10.1182/blood-2008-08-175125. [DOI] [PubMed] [Google Scholar]
- 28.Valerius T, Repp R, de Wit TPM, et al. Involvement of the high-affinity receptor for IgG (FcγRI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood. 1993;82:931–939. [PubMed] [Google Scholar]
- 29.Perussia B, Kobayashi M, Rossi ME, et al. Immune interferon enhances functional properties of human granulocytes: Role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138:765–774. [PubMed] [Google Scholar]
- 30.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrier B, Ittah M, Tourneur L, et al. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica. 2007;92:e20–e23. doi: 10.3324/haematol.11031. [DOI] [PubMed] [Google Scholar]
- 32.Anolik JH, Campbell D, Felgar RE, et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 33.Aksoy S, Dizdar O, Hayran M, et al. Infectious complications of rituximab in patients with lymphoma during maintenance therapy: A systematic review and meta-analysis. Leuk Lymphoma. 2009;50:357–365. doi: 10.1080/10428190902730219. [DOI] [PubMed] [Google Scholar]
- 34.van Oers MHJ, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 35.Miescher S, Spycher MO, Amstutz H, et al. A single recombinant anti-RhD IgG prevents RhD immunization: Association of RhD-positive red blood cell clearance rate with polymorphism in the FcγRIIA and FcγRIIIA genes. Blood. 2004;103:4028–4035. doi: 10.1182/blood-2003-11-3929. [DOI] [PubMed] [Google Scholar]
- 36.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor-expression metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 38.Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc Optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 39.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farag SS, Flinn IW, Modali R, et al. FcγRIIIa and FcγRIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–1474. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 41.Treon SP, Hansen M, Branagan AR, et al. Polymorphisms in FcγRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, Jung HD, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 43.Mitrovic Z, Aurer I, Radman I, et al. FcγRIIIA and FcγRIIA polymorphisms are not associated with response to rituximab and CHOP in patients with diffuse large B cell lymphoma. Haematologica. 2007;92:998–999. doi: 10.3324/haematol.10327. [DOI] [PubMed] [Google Scholar]
- 44.Carlotti E, Palumbo GA, Oldani E, et al. FcgRIIIA and FcgRIIA polymorphisms do not predict clinical outcome of follicular non-Hodgkin's lymphoma patients treated with sequential CHOP and rituximab. Haematologica. 2007;92:1127–1130. doi: 10.3324/haematol.11288. [DOI] [PubMed] [Google Scholar]
- 45.Hagberg H, Gisselbrecht C. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: An update of the CORAL study. Ann Oncol. 2006;17(suppl 4):iv31–iv32. doi: 10.1093/annonc/mdj996. [DOI] [PubMed] [Google Scholar]