Abstract
Purpose
Sleep disruption is prevalent in patients with cancer and survivors, but the prevalence of insomnia, a distressing sleep disorder, in these populations has yet to be determined in large-scale studies.
Patients and Methods
A total of 823 patients with cancer receiving chemotherapy (mean age, 58 years; 597 female patients) reported on sleep difficulties in a prospective study.
Results
During day 7 of cycle 1 of chemotherapy, 36.6% (n = 301) of the patients with cancer reported insomnia symptoms, and 43% (n = 362) met the diagnostic criteria for insomnia syndrome. Patients with cancer younger than 58 years were significantly more likely to experience either symptoms of insomnia or insomnia syndrome (χ2 = 13.6; P = .0002). Patients with breast cancer had the highest number of overall insomnia complaints. A significant positive association was found between symptoms of insomnia during cycles 1 and 2 of chemotherapy (ϕ = .62, P < .0001), showing persistence of insomnia during the first two cycles of chemotherapy. Sixty percent of the patient sample reported that their insomnia symptoms remained unchanged from cycle 1 to cycle 2. Those with insomnia complaints had significantly more depression and fatigue than good sleepers (all P < .0001).
Conclusion
The proportions of patients with cancer in this sample reporting symptoms of insomnia and meeting diagnostic criteria for insomnia syndrome during chemotherapy are approximately three times higher than the proportions reported in the general population. Insomnia complaints persist throughout the second chemotherapy cycle for the majority of patients with cancer in this study. Insomnia is prevalent, underrecognized, undermanaged, and understudied among patients with cancer receiving chemotherapy.
INTRODUCTION
Although chemotherapy is a highly effective treatment for many forms of cancer, it causes multiple side effects, including hair loss, nausea, cardiotoxicity, pain, neutropenia, depression, anxiety, cognitive dysfunction, and fatigue.1,2 Emerging evidence suggests that general sleep disruption is also common among patients receiving chemotherapy, but insomnia, a specific type of sleep disruption, has received little attention. Although general sleep disruption is frequently evaluated as part of a cluster of symptoms including fatigue and depression, insomnia is rarely examined.
In the general population, insomnia is the most common sleep disorder.3 Insomnia is defined as difficulty falling asleep, difficulty staying asleep (defined as waking up in the middle of the night with wake episodes lasting > 30 minutes) and/or early awakening (waking 30 minutes before intended wake time), or nonrestorative sleep. The criteria for an insomnia diagnosis is sleep disturbance that occurs at least three nights per week and causes significant distress or impairment of daytime functioning.4 Approximately 33% of the general population have some insomnia symptoms. When frequency of insomnia complaints is used as criteria to establish the presence of insomnia syndrome, the rates decrease into the range of 16% to 21%.4a
Insomnia can begin before cancer treatment,5,6 continue during treatment,7 and persist for years after the treatment has been completed.8 Although the prevalence of sleep disturbance varies among studies because of differences in the populations studied, disease stage, definition of sleep disturbance, and mode of assessment, most studies report that 30% to 50% of newly diagnosed patients with cancer have sleep difficulties.9
One of the first studies to compare sleep disruption in patients with cancer with healthy controls involved a small sample of patients (n = 30) with metastatic cancer of various types. In that study, the proportion of individuals who had difficulty staying asleep was significantly higher among patients with cancer (45%) than among healthy controls (14%), as was the percentage (40% v 25%) who had difficulty falling asleep.10 In a larger study,11 nearly 40% of 212 patients with cancer (mixed diagnoses) reported that they “slept less at night”; only 15% of controls reported the same level of sleep difficulty. The study included patients who had completed at least one cycle of chemotherapy or who were receiving oral therapy such as tamoxifen for at least a month.
In the general population, insomnia is associated with significant impairments in quality of life,12 almost a two-fold increase in the number of accidents,13 increased days spent in bed (even after controlling for comorbid disease severity and chronicity), and decreased activity.14 Insomnia is associated with psychiatric illness such as major depression and anxiety and increases the risk for the development of depression. Some data show that sleep disruption and sleep loss are associated with dysregulated immune functioning as measured by increased production of interleukin-6 (IL-6),15 tumor necrosis factor,16,17 and C-reactive protein.18 Several studies19,20 have found that insomnia disrupts immune functioning to a greater extent than depression, thus this increase cannot be explained by the comorbid depression. To date, the consequences of insomnia in patients with cancer and survivors have not been fully examined; however, recent research suggests that sleep disturbances and/or circadian dysregulation might be adversely related to cardiac morbidity,21 immune functioning,22 cancer-stimulating cytokines,22 and psychiatric morbidity.23
A recent study7 specifically evaluated sleep disruption during chemotherapy in 115 patients with lung cancer. Sleep disturbance was measured by the Pittsburgh Sleep Quality Index. Fifty-two percent of patients were rated as “poor sleepers,” with 49% reporting waking up in the middle of the night or waking up earlier in the morning than intended, and 50% reporting inability to fall asleep within 30 minutes. Patients reported significantly more disturbance in subjective sleep quality, sleep duration, total sleep time, and daytime functioning during days of chemotherapy compared with rest periods. A study conducted by Savard et al8 examined the prevalence, clinical characteristics, and risk factors for insomnia among breast cancer survivors after treatment. These authors surveyed 300 breast cancer survivors (median time since diagnosis of 49 months) and reported that approximately 51% described insomnia symptoms, and 19% of their total sample met diagnostic criteria for an insomnia disorder.
The largest and most comprehensive study of insomnia among patients with cancer was conducted by Davidson et al.24 In this study, 982 patients and survivors with various types of cancer were assessed for insomnia using a questionnaire developed by the authors. The sample consisted of patients who had either no recent treatment or who had received treatment within the last 6 months. In the study, 300 patients (30.5%) reported insomnia. Of these 300 patients, the majority (76%) reported the most difficulty with frequent awakening. Forty-four percent reported difficulty falling asleep, 35% reported waking up for long periods of time in the middle of the night, and 33% complained of waking up too early.
To date, no studies have specifically evaluated the prevalence of insomnia in a large diverse national sample of patients with cancer undergoing chemotherapy. Emerging evidence suggests that chemotherapy results in more insomnia complaints. The aims of this study were to determine prospectively the prevalence of insomnia among patients with cancer receiving chemotherapy, as compared with the prevalence found in the general population, and describe the influence of age, sex, and race on insomnia prevalence during chemotherapy.
PATIENTS AND METHODS
This was a post-hoc analysis of data collected for a large randomized clinical trial examining the effects of paroxetine versus placebo on fatigue in patients with cancer undergoing chemotherapy. Patients received study medication approximately 10 days after their second cycle of chemotherapy. The institutional review board of the University of Rochester and each participating site approved the protocol. All participants provided written consent.
Patients with any diagnosis of cancer who were beginning chemotherapy were recruited from 18 oncology private practice groups around the United States that were part of the National Cancer Institute Community Clinical Oncology Program. All the data for this report were collected before randomization. The enrollment of patients occurred between 1997 and 1999. To be eligible, participants had to be at least 18 years old and scheduled for at least four cycles of chemotherapy with cycles separated by at least 3 weeks, without concurrent radiation therapy or interferon. The primary findings of the randomized clinical trial and a full description of the study have been published.25 Patients were instructed to complete questionnaires at home on day 7 of cycles 1 and 2 and were given reminder phone calls between days 5 and 7 of their chemotherapy cycles 1 and 2.
Assessments
Demographics.
Participants reported age, sex, race/ethnicity, level of education, relationship status, and medical variables such as treatment and diagnosis.
Insomnia.
The Hamilton Depression Inventory (HDI)26 was used to assess sleep disruption at each cycle. The HDI consists of 38 questions designed to evaluate 23 symptom domains. There are six questions assessing frequency and duration of sleep problems. It measures severity of symptoms in the last 2 weeks.
Statistical Methods
Data reduction.
Responses to six sleep-related questions from the HDI were used to classify patients into three groups (insomnia syndrome, insomnia symptoms, and good sleepers).27 Patients who reported difficulty falling asleep, difficulty staying asleep (waking up in the middle of the night), and/or early morning awakenings for at least 3 days a week for 2 weeks, with each episode lasting at least 30 minutes, were coded as meeting criteria for insomnia syndrome. This definition of insomnia is used in clinical research and represents the combined criteria described in the International Classification of Sleep Disorders and the Diagnostic and Statistical Manual IV.28,29 We were unable to assess the impact of insomnia on daytime functioning. Those with some sleep disturbance but not meeting criteria for frequency or duration were coded as having insomnia symptoms. Patients were considered good sleepers if they reported no sleep difficulties on any of the HDI sleep items (score of “zero” on six sleep items).
Data analyses.
Means and standard deviations or frequencies and percentages were reported. Pearson χ2 tests were used to compare insomnia groups of the subjects in this study with the general population4a and to test the significance of associations of these rates with demographic characteristics. Ordinal logistic regression using the three levels of insomnia severity (good sleepers, insomnia symptoms, insomnia syndrome) at cycle 1 as the response was used to investigate the dependency of insomnia rates on age, sex, race (white v nonwhite) and their interactions. The proportional odds model assumption of common slopes was violated (χ2 = 31.9; df = 3; P < .0001) meaning that the effects of age, sex, and race on the transition from good sleepers to insomnia symptoms were different than their effects on the transition from insomnia symptoms to insomnia syndrome. A continuation ratio model30,31 that is robust to the proportional odds model assumption was used instead, and it clearly showed no dependency of age, sex, and race on the transition from insomnia symptoms to syndrome. That allowed us to merge insomnia syndrome with insomnia symptoms into a single category (v good sleepers), creating a binary response that could be analyzed with logistic regression. Likelihood ratio tests were used to assess the significance of the model terms and to guide selection of the final terms in the model. All P values reported are two-sided; P < .05 is considered statistically significant. All computations were conducted using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient Sample
Data are from 823 patients with a median age of 58 years (range, 22 to 93 years). Seventy-two percent were female. Sixty-eight percent reported having previous surgery for cancer, 15% had previous chemotherapy, 11% had radiation therapy, and 6% had another type of cancer therapy. Eighty-eight percent of the patients were white. Table 1 provides additional demographics and patient characteristics.
Table 1.
Participant Demographics and Medical Characteristics
| Characteristic | No Symptoms |
Some Insomnia Symptoms |
Clinical Insomnia |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| Mean | 61.3 | 57 | 55.9 | |||
| SE | 0.08 | 0.04 | 0.03 | |||
| Diagnosis | ||||||
| Alimentary tract | 27 | 36 | 30 | 40 | 18 | 24 |
| Brain and peripheral | 1 | 33.3 | 1 | 33.3 | 1 | 33.3 |
| Breast | 61 | 15.3 | 156 | 39 | 183 | 45.8 |
| Genitourinary tract | 6 | 21.4 | 7 | 25 | 15 | 53.6 |
| Gynecologic | 11 | 17.2 | 25 | 39.1 | 28 | 43.8 |
| Hematologic | 23 | 19.5 | 44 | 37.3 | 51 | 43.2 |
| Lung | 24 | 20 | 35 | 29.2 | 61 | 50.8 |
| Melanoma | 0 | 0 | 0 | 0 | 1 | 100 |
| Neck and head | 3 | 75 | 0 | 0 | 1 | 25 |
| Soft tissue sarcoma | 1 | 33.3 | 1 | 33.3 | 1 | 33.3 |
| Unknown primary | 3 | 42.9 | 2 | 28.6 | 2 | 28.6 |
| Marital status | ||||||
| Married | 106 | 18.2 | 220 | 37.7 | 257 | 44.1 |
| Divorced | 16 | 18.6 | 25 | 29.1 | 45 | 52.3 |
| Separated | 3 | 30 | 1 | 10 | 6 | 60 |
| Single | 14 | 23 | 22 | 36.1 | 25 | 41 |
| Widowed | 21 | 25.3 | 33 | 39.8 | 29 | 34.9 |
| Race | ||||||
| American Indian | 4 | 66.7 | 0 | 0 | 2 | 33.3 |
| Black | 16 | 28.1 | 17 | 29.8 | 24 | 42.1 |
| Hawaiian/Pacific Islander | 4 | 19 | 10 | 47.6 | 7 | 33.3 |
| Non-Hispanic | 2 | 14.3 | 3 | 21.4 | 9 | 64.3 |
| Unknown | 0 | 0 | 0 | 0 | 3 | 100 |
| White | 134 | 18.6 | 271 | 37.5 | 317 | 43.9 |
| Ethnicity | ||||||
| Nonwhite | 26 | 25.7 | 30 | 29.7 | 45 | 44.6 |
| White | 134 | 18.6 | 271 | 37.5 | 317 | 43.9 |
| Sex, female | 106 | 17.7 | 229 | 38.3 | 263 | 44.0 |
| Previous treatment | ||||||
| Surgery | 95 | 17.9 | 204 | 38.3 | 233 | 43.8 |
| Chemotherapy | 21 | 17.9 | 56 | 47.9 | 40 | 34.2 |
| Radiation | 21 | 24.4 | 28 | 32.6 | 37 | 43.0 |
| Other | 147 | 20.2 | 271 | 37.3 | 309 | 42.5 |
| Other disease characteristics | ||||||
| Adjuvant | 94 | 17.8 | 186 | 35.3 | 247 | 46.9 |
| Metastatic | 60 | 22.7 | 100 | 37.9 | 104 | 39.4 |
| Neoadjuvant | 3 | 33.3 | 6 | 66.7 | 0 | 0 |
| Other | 2 | 15.4 | 5 | 38.5 | 6 | 46.2 |
| Recurrent | 1 | 14.3 | 3 | 42.9 | 3 | 42.9 |
Insomnia During Chemotherapy
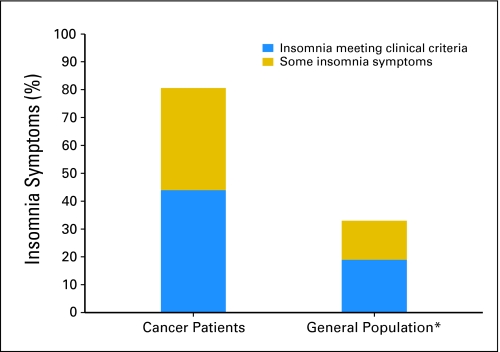
During cycle 1, 301 (36.6%) of the patients reported insomnia symptoms, 362 (43%) met the criteria for insomnia syndrome, and 160 (20.4%) were good sleepers. The insomnia rates for these patients were significantly higher (χ2 = 847.8; df = 2; P < .0001; Fig 14a) than insomnia rates in the general population (14% having some symptoms and 19% meeting clinical criteria).4a At cycle 2, 272 (33.1%) of the patients reported insomnia symptoms, 290 (35.2%) of patients met criteria for insomnia syndrome, and 173 (31.7%) were good sleepers.
Fig 1.
Insomnia symptoms in patients after first cycle of chemotherapy (N = 823) versus general population. (*) From multiple epidemiologic studies summarized in Ohayon.4a
Insomnia by Diagnosis
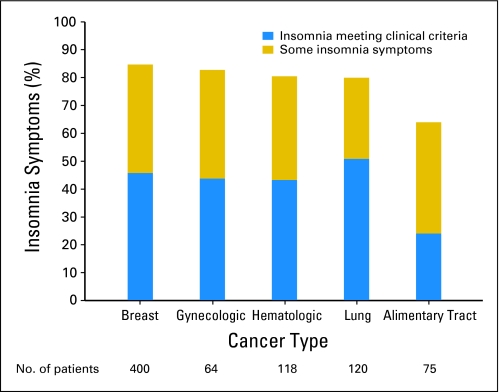
To study the prevalence of insomnia with respect to diagnosis, only diagnoses for which the sample size was greater than 50 were considered, specifically, lung, gynecologic, breast, hematologic, and alimentary tract cancers. There were significant differences in the insomnia rates across these diagnoses (χ2 = 24.85; df = 8; P = .0017). Patients with lung cancers had the highest prevalence of insomnia syndrome, whereas patients with alimentary cancers had the lowest prevalence. Patients with breast cancer had the highest number of overall insomnia complaints (Fig 2).
Fig 2.
Prevalence of insomnia symptoms by diagnosis at cycle 1.
Insomnia at Cycles 1 and 2
A significant positive association was found between insomnia at cycle 1 and cycle 2 (ϕ = 0.62; χ2 = 278.32; df = 4; P < .0001; Table 2). On average, 60% of patients reported that their sleep complaints remained unchanged from cycle 1 to cycle 2. Nearly two thirds of patients who reported insomnia meeting clinical criteria after cycle 1 continued to meet that classification after cycle 2. Ten percent of good sleepers after cycle 1 developed insomnia syndrome, and an additional 24.6% developed insomnia symptoms by cycle 2.
Table 2.
Proportion of Patients With Insomnia Symptoms at Cycle 2 Categorized by Level of Symptom at Cycle 1
| Insomnia Levels at Cycle 1 | Insomnia Levels at Cycle 2 |
||
|---|---|---|---|
| Insomnia Syndrome (%) | Insomnia Symptoms (%) | Good Sleepers (%) | |
| Insomnia syndrome (n = 317) | 65.3 | 28.4 | 6.3 |
| Insomnia symptoms (n = 276) | 25.0 | 53.3 | 21.7 |
| Good sleepers (n = 142) | 9.9 | 24.6 | 65.5 |
NOTE. Numbers in cells are smaller as a result of missing data.
Sex
Male patients had a lower rate of insomnia complaints than female patients, but the difference was not significant (χ2 = 5.1; df = 2; P = .08).
Age
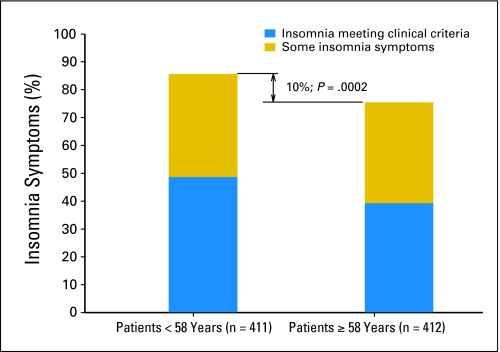
There was a statistically significant association of overall insomnia complaints and prevalence of insomnia syndrome with age. By splitting the age data at the median (58 years) and examining the association of insomnia versus no symptoms, we found that patients 58 years of age or older compared with younger patients had a significantly lower rate of insomnia (χ2 = 13.6; df = 1; P = .0002; Fig 3). There was no significant interaction between sex, age, and insomnia.
Fig 3.
Prevalence of insomnia symptoms by age. Note: Among patients who were 58 years of age or younger, 85.6% had insomnia, whereas among those who were 58 years of age or older, 75.5% had insomnia.
Sex, Age, and Race
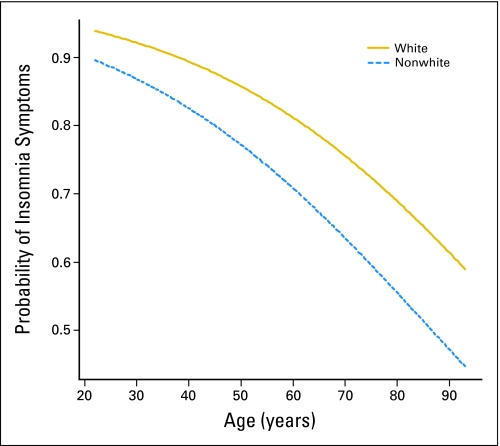
The logistic regression of insomnia with age, sex, race, and all possible interactions (age by sex, age by race, race by sex, and age by race by sex) produced a significant overall model (χ2 = 29.3; df = 7; P < .0001), but a comparison of the overall model with a main effects model (ie, no interactions) showed that the interactions were not significant (χ2 = 3.8; df = 4; P = .43). The main effects model showed that the effect of sex was not significant (χ2 = 1.1; df = 1; P = .29), and this term was dropped from the model. The final model with age and race was highly significant (χ2 = 24.4; df = 2; P < .0001), with both age and race being significant (age: χ2 = 20.57, df = 1, P < .0001; race: χ2 = 3.74, df = 1, P = .0015). The odds ratio associated with an increase in 10 years of age was 0.71, with a 95% CI ranging from 0.62 to 0.82. Hence a 10-year increase in age is associated with a 29% decrease in reporting insomnia relative to reporting none. The odds ratio associated with race was 0.56, with a 95% CI (profile likelihood) ranging from 0.34 to 0.92. Thus nonwhites were 44% less likely to report insomnia than whites. Figure 4 is a plot of the probability of reporting insomnia versus age, broken down by race.
Fig 4.
Probability of reporting insomnia symptoms by age and by race.
Insomnia by Fatigue and Depression
Patients with insomnia had significantly more symptoms of depression and fatigue compared with patients without insomnia, as assessed by all four measures of fatigue (ie, Profiles of Mood States [POMS]32 Fatigue-Inertia Subscale, POMS Vigor Subscale, Fatigue Symptom Checklist,33 Multidimensional Assessment of Fatigue34) and two measures of depression (ie, Center for Epidemiologic Studies Depression Scale35 and POMS–Depression). Patients with insomnia syndrome had significantly more fatigue and depression compared with patients with insomnia symptoms, and both insomnia groups had significantly more symptoms than good sleepers (all P values <.0001; Appendix Table A1, online only).
DISCUSSION
This study found a high prevalence of both insomnia symptoms and insomnia syndrome in patients with cancer undergoing chemotherapy. We found rates of insomnia in patients with cancer to be nearly three times higher than the rates in the general population.4a Moreover, insomnia is prevalent in patients undergoing chemotherapy for a variety of cancer diagnoses. We also found that insomnia symptoms persist from cycle 1 to 2 for the majority of patients, supporting the view that insomnia does not resolve on its own.
Surprisingly, we failed to find a significant difference in insomnia rates between men and women, and we did find that younger patients reported more symptoms of insomnia than older patients. These findings are noteworthy because in the general population, both female sex and older age are associated with greater risk for developing insomnia.36 Nevertheless, these findings are in line with a recent study37 that describes symptom burden in cancer survivors. They reported that younger patients (age < 50 years) suffered a greater symptom burden, including insomnia, than older patients (age > 64 years), with an adjusted odds ratio for insomnia in the two patient groups of 2.7 and 1.44, respectively. The authors speculated, and we agree based on our clinical experience, that this result might derive from expectations for better health among younger patients with cancer and from the fact that younger patients may have had more aggressive tumors and may have received more aggressive treatments than older patients.
We found that white patients reported significantly more insomnia symptoms than nonwhite patients. Although some literature suggests that nonwhite patients, black patients in particular, might have more sleeping problems than white patients,38 evidence from large epidemiologic studies indicates that black participants are less likely to complain of sleep disturbances than white participants.39,40 The mechanism for these differences is unknown and can possibly be explained either by true biologic differences or a response bias. Previous research has shown that black patients with prostate cancer, for example, have more distress and psychological concerns and overall lower quality of life postdiagnosis41,42 than whites. However, minorities may underreport their concerns due to social desirability or for other psychological reasons, such as a tendency not to encode a negative event; in other words, they might experience an equal or perhaps even higher number of insomnia episodes but not remember them. Jean-Louis et al43 suggest, however, that nonwhite participants in their study experienced and reported fewer insomnia symptoms because they were able to engage in a type of a positive coping that allowed them to either block or better regulate negative emotions.
We found significant differences in insomnia symptoms and prevalence by cancer diagnosis. Patients with breast cancer had the highest number of overall insomnia complaints. Lung cancers reported the highest insomnia syndrome prevalence, whereas patients with alimentary cancers had the lowest prevalence. Savard et al8 identified risk factors for the development of insomnia in patients with breast cancer. Being on sick leave, being unemployed, and being widowed were each significant contributors to the development of insomnia in women with breast cancer. The authors hypothesized that the spike in symptoms during chemotherapy might be explained by antiemetic drugs or by the onset of menopause brought on by chemotherapy. Although we are unable to determine the precise reason behind those differences, we can speculate that differences in insomnia could be affected by tumor biology, cancer treatments, and/or side effects associated with treatments.
This study has several important strengths and limitations. Among the study limitations: use of measure designed to assess depression and not sleep, inability to assess impact of insomnia on daytime functioning (although we demonstrated that the rates of depression and fatigue were significantly higher in patients with insomnia), use of retrospective self-report, and heterogeneity among patients with cancer. In addition, patients were recruited nearly a decade ago, and chemotherapy agents have changed significantly, limiting the reliability of our findings. On the plus side, this study is one of the first to examine insomnia in a large diverse sample of patients with cancer during chemotherapy treatment longitudinally. Given the high rate of insomnia in patients with cancer, more investigation into its prevalence in specific cancer populations is needed. Additional research is likely to uncover cancer-specific physiological, psychological, and behavioral factors that contribute to the development of insomnia during chemotherapy. Future research should examine insomnia in other populations experiencing stress and major health issues (eg, myocardial infarction) to improve our understanding of the causes of insomnia. And most importantly, interventions to prevent and treat insomnia in patients with cancer must be developed and tested.
Supplementary Material
Appendix
Table A1.
Prevalence of Depression and Fatigue by Insomnia Symptoms
| Scale | Insomnia Syndrome |
Insomnia Symptoms |
Good Sleepers |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| FSCL | 59.34 | 19.55 | 46.77 | 15.53 | 43.55 | 17.40 |
| POMS-Total | 20.48 | 18.55 | 7.82 | 15.36 | 2.85 | 15.35 |
| POMS-V | 3.84 | 3.80 | 5.51 | 4.42 | 6.02 | 4.65 |
| POMS-F | 9.38 | 5.43 | 5.85 | 4.71 | 4.65 | 5.38 |
| POMS TA | 5.60 | 4.07 | 3.27 | 3.06 | 2.12 | 2.80 |
| POMS-DD | 4.21 | 4.15 | 2.32 | 2.94 | 1.35 | 2.32 |
| POMS-AH | 3.70 | 3.90 | 1.86 | 2.70 | 1.13 | 2.03 |
| POMS-CB | 1.43 | 3.34 | 0.03 | 2.56 | −.37 | 2.86 |
| MAF | 5.89 | 2.55 | 4.55 | 2.67 | 3.87 | 2.97 |
| CES-D | 18.69 | 10.51 | 11.32 | 8.46 | 7.59 | 7.84 |
NOTE. All P values < .0001.
Abbreviations: SD, standard deviation; FSCL, Fatigue Symptom Checklist; POMS, Profiles of Mood States; POMS-V, vigor-activity; POMS-F, fatigue-inertia; POMS-TA, tension-anxiety; POMS-DD, depression-dejection; POMS-AH, anger-hostility; POMS-CB, confusion-bewilderment; MAF, Multidimensional Assessment of Fatigue Question 1, “To what degree have you experienced fatigue?”; CES-D, Center for Epidemiologic Studies Depression Scale.
Footnotes
Supported by National Cancer Institute Grants No. 1K07CA132916 (O.G.P.), R25CA10618 (G.R.M.), U10-CA37420 (G.R.M.), and CA112035 (S.A.-I.).
Presented in part at the Annual Meeting of the American Society of Preventive Oncology, March 16-18, 2008, Bethesda, MD, and Associated Professional Sleep Societies/SLEEP 2008 meeting, June 7-12, 2008, Baltimore, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Sonia Ancoli-Israel, Ferring Pharmaceuticals (C), Pfizer (C), Respironics (C), sanofi-aventis (C), Sepracor (U) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Oxana G. Palesh, Joseph A. Roscoe, Gary R. Morrow
Financial support: Gary R. Morrow
Administrative support: Gary R. Morrow
Data analysis and interpretation: Oxana G. Palesh, Joseph A. Roscoe, Thomas Roth, Charles Heckler, Gary R. Morrow
Manuscript writing: Oxana G. Palesh, Joseph A. Roscoe, Karen M. Mustian, Thomas Roth, Josée Savard, Sonia Ancoli-Israel
Final approval of manuscript: Oxana G. Palesh, Joseph A. Roscoe, Karen M. Mustian, Thomas Roth, Josée Savard, Sonia Ancoli-Israel, Charles Heckler, Jason Q. Purnell, Michelle C. Janelsins, Gary R. Morrow
REFERENCES
- 1.Hamilton A, Hortobagyi G. Chemotherapy: What progress in the last 5 years? J Clin Oncol. 2005;23:1760–1775. doi: 10.1200/JCO.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Hofman M, Morrow GR, Roscoe JA, et al. Cancer patients' expectations of experiencing treatment-related side effects: A University of Rochester Cancer Center–Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004;101:851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Kales A, Soldatos CR, et al. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–1262. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. ed 4, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 4a.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Berger AM, Farr LA, Kuhn BR, et al. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008;62:391–400. doi: 10.1016/j.lungcan.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Savard J, Simard S, Blanchet J, et al. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 9.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 10.Kaye J, Kaye K, Madow L. Sleep patterns in patients with cancer and patients with cardiac disease. J Psychol. 1983;114:107–113. doi: 10.1080/00223980.1983.9915403. [DOI] [PubMed] [Google Scholar]
- 11.Malone M, Harris AL, Luscombe DK. Assessment of the impact of cancer on work, recreation, home management and sleep using a general health status measure. J R Soc Med. 1994;87:386–389. doi: 10.1177/014107689408700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth T. Insomnia: Definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 13.Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53(suppl):34–39. [PubMed] [Google Scholar]
- 14.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 15.Redwine L, Hauger RL, Gillin JC, et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: Physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 18.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Cover H, Irwin M. Immunity and depression: Insomnia, retardation, and reduction of natural killer cell activity. J Behav Med. 1994;17:217–223. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- 20.Savard J, Miller SM, Mills M, et al. Association between subjective sleep quality and depression on immunocompetence in low-income women at risk for cervical cancer. Psychosom Med. 1999;61:496–507. doi: 10.1097/00006842-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Palesh O, Zeitzer JM, Conrad A, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–449. [PMC free article] [PubMed] [Google Scholar]
- 22.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson JR, MacLean AW, Brundage MD, et al. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 25.Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center–Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin CM, Belanger L, Leblanc M, et al. The natural history of insomnia: A population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.American Sleep Disorders Association. Rochester, MN: American Sleep Disorders Association; 1997. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. [Google Scholar]
- 30.Bender R, Grouven U. Using binary logistic regression models for ordinal data with non-proportional odds. J Clin Epidemiol. 1998;51:809–816. doi: 10.1016/s0895-4356(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer D, Lemeshow S. Applied Logistic Regression. ed 2. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 32.McNair DM, Lorr M, Droppelman LF. San Diego, CA: Educational and Industrial Testing Service; 1971. Manual for the Profile of Mood States. [Google Scholar]
- 33.Yoshitake H. Three characteristic patterns of subjective fatigue symptoms. Ergonomics. 1978;21:231–233. doi: 10.1080/00140137808931718. [DOI] [PubMed] [Google Scholar]
- 34.Tack B. San Francisco, CA: University of California; 1991. Dimensions and correlates of fatigue in older adults with rheumatoid arthritis [doctoral dissertation, nursing] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depressive scale for research in the general population. J Applied Psychol Measur. 1977;1:385–401. [Google Scholar]
- 36.Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–755. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 37.Mao J, Armstrong K, Bowman M. Symptom burden among cancer survivors: Impact of age and comorbidity. JABFM. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 39.Blazer DG, Hays JC, Foley DJ. Sleep complaints in older adults: A racial comparison. J Gerontol A Biol Sci Med Sci. 1995;50:M280–M284. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- 40.Whitney CW, Enright PL, Newman AB, et al. Correlates of daytime sleepiness in 4578 elderly persons: The Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: An examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92:1451–1459. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubeck DP, Kim H, Grossfeld C, et al. Health related quality of life differences between black and white men with prostate cancer: Data from the Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2001;166:2281–2285. [PubMed] [Google Scholar]
- 43.Jean-Louis G, Magai C, Consedine NS, et al. Insomnia symptoms and repressive coping in a sample of older Black and White women. BMC Womens Health. 2007;7:1. doi: 10.1186/1472-6874-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.