Abstract
Purpose
Many patients with localized node-negative renal cell carcinoma (RCC) are elderly with competing comorbidities. Their overall survival benefit after surgical treatment is unknown. We reviewed cases in the Surveillance, Epidemiology, and End Results (SEER) database to evaluate the impact of kidney cancer versus competing causes of death in patients with localized RCC and develop a comprehensive nomogram to quantitate survival differences.
Methods
We identified individuals with localized, surgically treated clear-cell, papillary, or chromophobe RCC in SEER (1988 through 2003). We used Fine and Gray competing risks proportional hazards regressions to predict 5-year probabilities of three competing mortality outcomes: kidney cancer death, other cancer death, and noncancer death.
Results
We identified 30,801 cases of localized RCC (median age, 62 years; median tumor size, 4.5 cm). Five-year probabilities of kidney cancer death, other cancer death, and noncancer death were 4%, 7%, and 11%, respectively. Age was strongly predictive of mortality and most predictive of nonkidney cancer deaths (P < .001). Increasing tumor size was related to death from RCC and inversely related to noncancer deaths (P < .001). Racial differences in outcomes were most pronounced for nonkidney cancer deaths (P < .001). Men were more likely to die than women from all causes (P < .002). This nomogram integrates commonly available factors into a useful tool for comparing competing risks of death.
Conclusion
Management of localized RCC must consider competing causes of mortality, particularly in elderly populations. Effective decision making requires treatment trade-off calculations. We present a tool to quantitate competing causes of mortality in patients with localized RCC.
INTRODUCTION
The biology of renal cell carcinoma (RCC) is heterogeneous. Although metastatic RCC remains highly lethal, many small renal cancers follow a more indolent clinical course and are considerably less risky to a patient's longevity.1–3 Competing options for the management of localized RCC include excision by radical or partial nephrectomy, thermal ablation, or active surveillance (AS). A recent meta-analysis evaluating these existing treatment options failed to demonstrate a statistically significant difference in metastasis-free survival between modalities over a mean follow-up of 47.1 months.4 Moreover, despite early detection and aggressive treatments, the death rate from RCC continues to increase.2,3 These data suggest that a significant portion of localized RCC may be over-treated, because high rates of therapeutic “success” have not impacted overall RCC-related mortality. Early efforts to accurately predict the behavior of localized RCC and match biology to appropriate treatments have thus far remained elusive.4–6
Surgical resection in young healthy patients continues to be judicious, whereas these individuals have a long life expectancy, and adjuvant or salvage therapies for advanced RCC are rarely curative.7 However, management of localized RCC in elderly or comorbid patients presents a unique set of challenges. Published data report that upward of 30% of localized renal masses show zero net growth when radiographically monitored over a median follow-up of 25 months.6 Moreover, in a recent meta-analysis of AS series, the kinetics of lesions that demonstrate growth are slow, with a median rate of 3 to 4 mm per year and a metastatic progression of 1% (median follow-up, 32 months).1 These data suggest that AS with delayed intervention of growing lesions is associated with a low risk of pathologic upstaging and cancer-specific deaths, making optimal management of localized RCC in older adults or infirm particularly complex.1,4,8,9 In these populations, comorbidities compete with kidney cancer as primary causes of death. As a result, involved clinical treatment trade-off decisions are necessary, but are most often qualitative.
Given the natural history of localized kidney cancers, the long-term benefit of treatment, particularly in older adults, depends in large part on competing risks of death. Here we evaluate overall survival and competing risks of death in patients with localized kidney cancer and build a comprehensive integrated nomogram to provide the clinician with a quantitative tool to estimate a patient's probability of dying from localized RCC and compare this probability with the patient's chances of dying from competing causes.
METHODS
Using the Surveillance, Epidemiology, and End Results (SEER) registry (1988 through 2003),10 we identified 32,677 individuals ≥ 30 years of age with localized RCC ≤ 20 cm in diameter from 17 geographic regions. We excluded 1,876 patients who had SEER codes indicating that either no cancer-directed surgery was performed or it was unknown whether cancer-directed surgery was performed. The remaining 30,801 patients form our cohort. The 30,801 patients include only those with common histologic subtype codes: clear-cell (n = 27,527), papillary (n = 1,494), chromophobe (n = 712), adenocarcinoma (n = 254), or granular (n = 814). To eliminate most childhood renal tumors, we excluded individuals younger than 30 years of age. We also excluded all tumors greater than 20 cm from the analysis, given their association with metastases, unusual histologies, and local symptoms. We included individuals who under the SEER “Reason for no surgery” field were indicated to have had partial nephrectomy, nephrectomy, ablation (uncommon before 2000), or surgery not otherwise specified.
We used the Kaplan-Meier product-limit method to describe overall survival and the log-rank test for overall survival differences. We used the cumulative incidence function (CIF) to describe cause-specific survival and Gray's test to test for cause-specific survival differences.11 We classified cause of death as either kidney cancer related, other cancer related, or noncancer related.
We used Fine and Gray competing risks proportional hazards regressions to predict 5-year probabilities of the three competing mortality outcomes.12 The Fine and Gray model is a multivariable time-to-event model, which accounts for the fact that individuals can only have one of the three competing events. The model also accounts for censoring among those who do not have an event during follow-up.
In developing the nomogram, we used model coefficients to assign points to characteristics and predictions from the model to map cumulative point totals for each outcome to 5-year survival predictions. Prognostic markers included race, sex, histologic subtype, tumor size, and age. We did not incorporate grade into our model, because approximately 40% of the sample had missing grade data, and hence we were concerned about missing data bias.13 We accounted for year of diagnosis in models and assumed that the current year of diagnosis effect is the same as the effect of the last year of diagnosis in the data (2003). We used restricted cubic splines with three knots at the 10%, 50%, and 90% empirical quantiles to model continuous variables.14 Kattan et al15 provide additional information on competing risk nomograms. We used Wald tests of coefficients to determine statistical significance (P < .05).
To assess the predictive accuracy of our model, we adapted the calibration method of Kattan et al.15 For each individual, we predicted the probability of each outcome at 5 years after fitting the competing risk regression using data only from the other 30,800 individuals. We then averaged the model predicted probabilities within deciles defined by the magnitude of the predictions. Within each decile of individuals, we estimated the marginal cumulative incidence of death using methods described by Gray.11 We then plotted marginal estimates versus model average predictions. In a well-calibrated model, the predictions should fall on a 45-degree diagonal line.
We used R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and its cmprsk package for survival analyses.
RESULTS
Kidney Cancer Death and Competing Risk Analysis
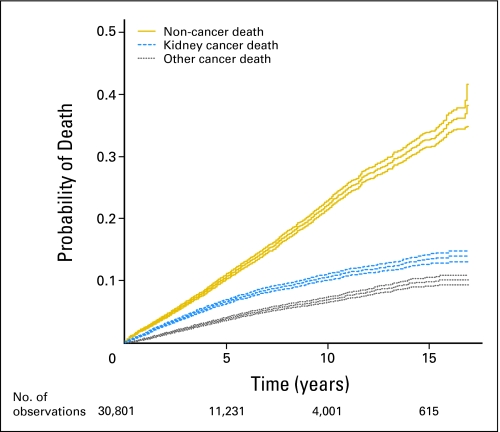
The demographics for our cohort of 30,801 patients and probabilities of death are provided in Table 1. Figure 1 depicts marginal cumulative incidence curves for the three types of death included in the analysis and is similar to a figure of Kattan et al.15 The majority of the sample were male (61%), white (84%), and had clear-cell histology (92%). The median age at diagnosis was 62 years (range, 30 to 96 years). Median tumor size was 4.5 cm (range, 0.1 to 20 cm by design). More than half the sample (53%) were diagnosed between 2000 and 2003, with 25% diagnosed between 1995 and 1999 and 22% between 1988 and 1994. The median length of follow-up until censoring or death was 3.8 years (range, 0 to 203 months); however, 9,256 individuals had 6 or more years of follow-up, demonstrating the total number of individuals with long-term follow-up to be significant. At last contact, 75% were censored, whereas 25% died, with 7% 2,149 dying from kidney cancer, 4% 1,353 dying from other cancers, and 13% 4,145 dying from other causes. Factors associated with prolonged survival included younger age, non–African American race, and smaller tumors (Table 1).
Table 1.
Probability of Death
| Characteristic | No. | Noncancer |
Kidney Cancer |
Other Cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 Years (%) | 10 Years (%) | P | 5 Years (%) | 10 Years (%) | P | 5 Years (%) | 10 Years (%) | P | ||
| All patients | 30,801 | 11 | 22 | 4 | 7 | 7 | 11 | |||
| Race | < .001 | .137 | .002 | |||||||
| Black | 3,199 | 15 | 30 | 6 | 8 | 4 | 7 | |||
| White | 26,009 | 10 | 21 | 7 | 11 | 4 | 7 | |||
| Other | 1,593 | 9 | 19 | 6 | 10 | 2 | 4 | |||
| Sex | < .001 | .003 | < .001 | |||||||
| Male | 18,773 | 11 | 22 | 7 | 11 | 4 | 8 | |||
| Female | 12,028 | 9 | 22 | 6 | 10 | 3 | 6 | |||
| Age at diagnosis, years | < .001 | < .001 | < .001 | |||||||
| < 50 | 5,822 | 3 | 7 | 4 | 7 | 1 | 2 | |||
| 50-64 | 11,180 | 7 | 14 | 6 | 10 | 3 | 5 | |||
| 65-74 | 8,448 | 12 | 27 | 7 | 11 | 6 | 10 | |||
| 75-84 | 4,841 | 21 | 45 | 9 | 13 | 6 | 11 | |||
| 85+ | 510 | 37 | 66 | 11 | 16 | 8 | 9 | |||
| Size, cm | < .001 | < .001 | .006 | |||||||
| < 4 | 12,503 | 11 | 24 | 3 | 5 | 4 | 7 | |||
| 4-7 | 12,570 | 11 | 23 | 7 | 11 | 4 | 7 | |||
| > 7 | 5,728 | 8 | 16 | 13 | 21 | 3 | 6 | |||
NOTE. Patient characteristics and predicted probabilities of death as calculated from cumulative incidence functions. P values11 correspond to comparisons among groups within outcomes of the underlying subdistribution hazards used to estimate the probabilities.
Fig 1.
Marginal cumulative incidence curves with 95% CIs for the three types of death included in the predictive model.
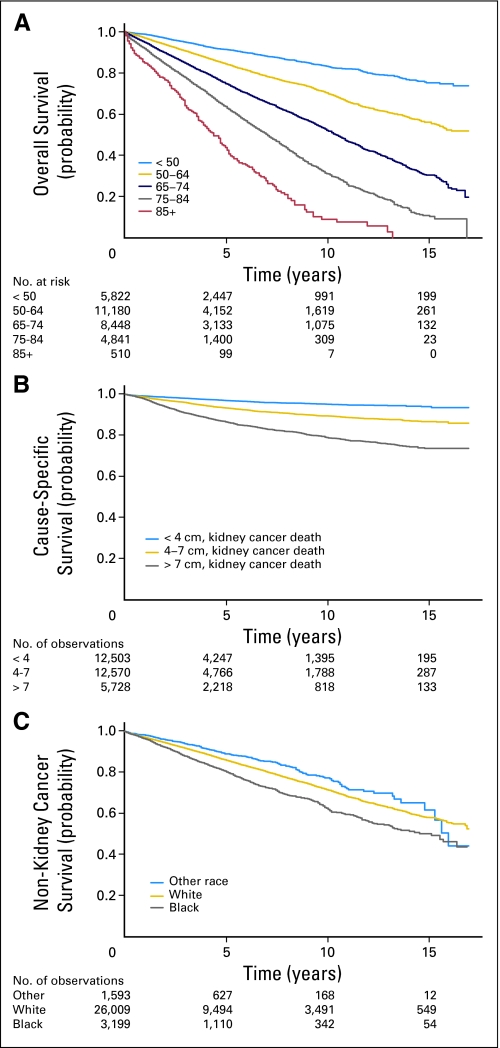
Age was strongly predictive of mortality and most predictive of nonkidney cancer deaths (P < .0001 for the first spline term for all three outcomes). Increasing tumor size was related to death from kidney cancer and inversely related to death from other causes (P < .04 for the first spline coefficient for all three outcomes). Racial differences in outcomes were more pronounced for nonkidney cancer deaths (P < .002 for a test of equality of the race coefficients only for the two nonkidney cancer death outcomes; Fig 2). Men were more likely to die than women from all causes (P < .002 for all outcomes).
Fig 2.
Predicted probability of (A) overall survival by age shown using Kaplan- Meier curve, (B) kidney cancer–specific survival (determined using codes 29010, 29020, 29030, and 29040) by tumor size shown using (1−) cumulative incidence function, and (C) non–kidney cancer specific survival by race shown using (1−) cumulative incidence function.
The histologic subtype effect was not statistically significant or clinically relevant in the models (P > .05 for all outcomes). Hence we left histologic subtype out of the final nomogram model.
Nomogram
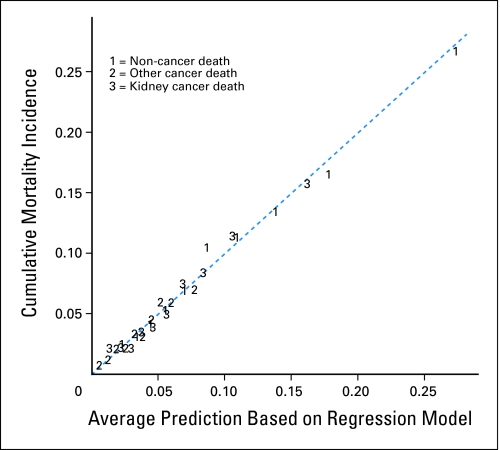
We constructed a nomogram to facilitate simultaneous integration of the previously mentioned factors in the calculation of competing risks of death into a useful clinical tool. We did not use model selection techniques but investigated a full model, comparable to the work of Kattan et al.15 Instead of model selection, we used restricted cubic splines to flexibly model continuous variables. We used deciles for calibration (rather than quintiles used by Kattan et al), because we had enough outcome data. Figure 3 presents the results of the model calibration. The model is well calibrated, because the points are close to the 45-degree line.
Fig 3.
Calibration after grouping individuals by decile of regression predicted 5-year probabilities.
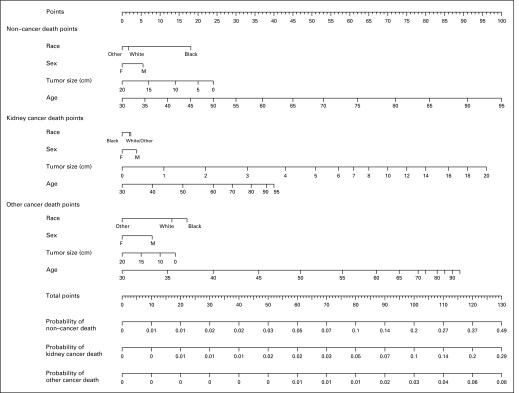
The full nomogram is presented in Figure 4 and can help physicians identify patients with localized node-negative kidney cancer who may have a high risk of competing causes of death. As such, patients and physicians may use these data to “trade-off ” the risk of surgery. For example, using the nomogram, a 75-year-old white male with a 4-cm tumor would have a 5-year mortality rate of 5% (80 points) from RCC versus 5% (115 points) from other cancers and 14% (91 points) from noncancerous causes. Meanwhile, a 65-year-old white male with an 8.5-cm malignancy is predicted to have a 5-year mortality rate of 10% (98 points) from RCC, 4% (106 points) from other cancers, and 6% (68 points) from noncancerous causes.
Fig 4.
Nomogram evaluating 5-year competing risks of death in patients with localized renal cell carcinoma. Total point values are independently calculated for each cause of death and then applied to the corresponding probability scale at the bottom of the figure. For example, a 75-year old white male with a 4-cm tumor would have a 5-year mortality of 5% (80 points) from RCC versus 4.5% (114 points) from other cancers and 14% (91 points) from noncancerous causes.
DISCUSSION
We demonstrate that patients with localized node-negative kidney cancer not only have an excellent 5- (96%) and 10-year (93%) cancer-specific survival, but a significant 5- and 10-year overall risk of death from other cancer deaths (7%, 11%) and non–cancer-related mortality (11%, 22%).
This multivariable model is based on more than 30,000 patients from the SEER database who underwent surgical treatment for localized RCC. The nomogram affords the clinician and patient an opportunity to quantitate three competing 5-year mortality outcomes: 1 death from RCC, 2 death from other (non-RCC) malignancies, and 3 noncancer death. The value of this model is its ability to help guide management decisions in the preoperative setting. We believe the model can be used both for clinical and research purposes. Risk estimates provided by the model can be extremely useful in patient counseling, especially when discussing less aggressive treatment options with elderly or comorbid patients. Moreover, the nomogram can be used in clinical trials designed to evaluate AS protocols for RCC.
We used Fine and Gray competing risks proportional hazards regressions to model the CIF.12 The CIF for a specific outcome describes the probability of having that outcome over time. For our study, the probability of dying at any time point was the sum of the three probabilities of dying from cause-specific events as estimated by the CIF. A difference between estimators of the CIF compared with the Kaplan-Meier estimator is the accounting of censoring. In Kaplan-Meier estimation, those who have competing events are censored, and such censoring is considered noninformative about the competing outcomes. Such noninformative censoring is an unrealistic assumption, because those who die from one cause will never be able to die from another cause. Estimation of the CIF assumes that those who die from one cause will never die from a competing cause. Heuristically, Cox regressions are multivariable models akin to Kaplan and Meier curves in the same way that Fine and Gray proportional hazards regressions are akin to CIFs.
In our model, age was a strong predictor of overall mortality and most predictive of non-RCC deaths. Men were more likely to die from all causes, and tumor size was directly related to death secondary to RCC and inversely related to death from other causes.
Incidentally detected kidney cancer is common, particularly in older adults.16,17 Many have concurrent comorbidities, which must be considered when developing a treatment plan.18 Despite increasing early detection of RCC and high rates of extirpative surgery, mortality rates from RCC have continued to increase,2,3 suggesting that despite the aggressive and lethal nature of some renal tumors, some localized masses pose little risk to longevity in the short or intermediate terms.1,2,19 Moreover, depending on tumor size, 20% to 40% of renal masses less than 4 cm may be histologically indolent.20 In a recent SEER study of more than 18,000 patients with localized RCC, 86% (< 4 cm) and 70% (> 7 cm) of tumors exhibited low nuclear grade.21 Moreover, a radiographically unidentifiable proportion will be benign. Lane recently published a preoperative nomogram evaluating the clinical aggressiveness of renal masses ≤ 7 cm. In 863 patients, they defined “aggressive” as all grade 3 clear-cell RCCs, grade 4 tumors of any type, and any tumor with vascular, fat, or urothelial invasion. Using a multiple logistic regression model, they determined that only 30% of localized renal cancers (24% of those < 4 cm) were “potentially aggressive.” Only advanced age was independently significant on multivariate analysis (P < .005) as a differentiating factor predicting indolence.5
Accurate prediction of an individual's renal mass biology is highly desirable. This is especially critical in elderly or comorbid patients in whom surgery poses significant risks. Although the use of ablation has been suggested in these individuals, long-term data are lacking, with a cumulative median follow-up of 15 to 18 months.1,4,22 Unfortunately, it seems that critical decision making regarding risks and trade-offs in managing elderly or ill patients with RCC remains very qualitative.
Most predictive models for RCC combine pathologic variables such as grade, presence of tumor necrosis, vascular invasion, and, in more recent studies, molecular markers.23–32 Although limited grade information exists in SEER, none of the other commonly used clinical prognostic variables such as performance status or comorbid indices are available. Moreover, existing RCC models are useful guides after surgery but are of limited value preoperatively. Only a handful of preoperative models that risk-stratify patients before surgery are published.33–35 Although these models estimate recurrence risk based on variables available to the clinician before resection, they permit limited risk stratification.33–35
Given the long natural history of localized prostate cancer, competing risks of death and treatment trade-off calculations are more common. Models predicting life expectancy of patients with stratified-risk prostatic cancer have been developed.36–38 Conversely, most renal tumors were historically considered more rapidly progressive and were believed to pose a greater risk of mortality. Therefore, competing risk analyses for localized RCC have not been undertaken. Instead, as with many solid tumors deemed “high risk,” treatment trade-off calculations remain qualitative and subject to practitioner biases. Although attempts to stratify by performance status or comorbidity indices are valuable, they fail to quantitate competing risks of death versus the index cancer.
Two studies attempt to quantitate competing risk of death for patients with RCC.39,40 Arrontes et al39 report a single-institution retrospective competing risk analysis in 192 patients with clear-cell RCC. After a median follow-up of less than 4 years, there were 72 patient deaths, with 45 (62.5%) secondary to clear-cell RCC. Patients with localized RCC and a Charlson comorbidity index (CCI) of more than 2 showed a statistically significant reduction in overall survival compared with patients with similar renal masses but a CCI of ≤ 2. They conclude that patients with localized RCC and CCI more than 2 do not gain a survival advantage from renal surgery.39 CCI did not influence survival in patients with locally advanced or metastatic clear-cell RCC, a finding consistent with a previous report.41 Hollingsworth et al40 performed a competing risk analysis for patients with RCC undergoing surgery. The authors examined a SEER cohort (n = 26,618) and stratified into 20 groups by tumor size and age at presentation. Five-year mortality estimates from RCC and other causes were generated for each group of patients. Nearly 30% of patients older than 70 years with tumors ≤ 4 cm died from causes unrelated to RCC. Given the similar data set, variables predicting outcome in this report were consistent with our data. The authors conclude that older patients with small renal masses benefited least from surgery.40
Although our nomogram is based on a postoperative data set, we believe it can be used for clinical purposes in the preoperative setting. As such, this model affords a quantitative scaffold on which one can base clinical decisions. Nevertheless, clinicians and patients should note that our model yields a 5-year probability of death from competing causes only if surgery is pursued. Hence appropriate caution is advised when predictions of this model are extrapolated to patients contemplating AS. Moreover, no predictive model can identify all critical variables important for judicious clinical decision making.
Several other limitations exist. The nomogram excludes the effect that benign renal masses have on competing risks of mortality and therefore may overestimate RCC death rates when tumor type is not known. Furthermore, individuals who did not undergo surgical management (n = 1,876) were excluded. Although indications for surveillance are not available through SEER, it is assumed that these patients were poor surgical candidates. Indeed, only 25% of these patients were registered alive in the data set. Therefore, one has to be cognizant that this model's prediction estimates are biased toward patients who are acceptable surgical candidates.
The nomogram estimates risk of non-RCC death from variables that include race, sex, tumor size, and age, but does not incorporate patients' comorbidities due to limitations of SEER. Nevertheless, even if comorbidities were available, their use may be inappropriate in the context of a competing risk analysis, as has been argued by Hollingsworth et al.40 Another potential limitation is the model's reliance on the SEER's cause-of-death item, which is based on death certificate reporting. Although death certificate validity is known to be imperfect, it is considered to be relatively robust in patients with malignancy.40,42 Finally, our nomogram estimates risks within 5 years from diagnosis of RCC. Although death may occur at a later time point, our model is not intended for estimation of these long-term competing risks of death.
In conclusion, here we evaluate overall survival and competing risks of death in patients with localized renal cancer. We present the first comprehensive nomogram to estimate competing risks of death for an index abdominal cancer (kidney) versus other cancers versus noncancer deaths in a population-based cohort. This tool can serve to quantitate treatment trade-off assumptions and guide management of elderly or infirm patients with localized RCC. It helps address ubiquitous qualitative biases in clinical decision making regarding whether to treat solid renal masses in patients with short- and intermediate-term competing risks of death. This nomogram is an important step toward the goal of matching surgical treatment options to the biology of RCC, while accounting for competing survival risks of an individual.
Acknowledgment
We thank the High Performance Computing Facility at Fox Chase Cancer Center for conducting the calculations that appear in this publication.
Footnotes
Supported in part by Fox Chase Cancer Center via institutional support of the Kidney Cancer Keystone Program; by National Institutes of Health Grant No. P30 CA 06927; and an appropriation from the Commonwealth of Pennsylvania.
Presented in part at the Annual Meeting of the American Urological Association, May 17-22, 2008, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brian L. Egleston, Yu-Ning Wong, Robert G. Uzzo
Administrative support: Robert G. Uzzo
Collection and assembly of data: Alexander Kutikov, Brian L. Egleston, Robert G. Uzzo
Data analysis and interpretation: Alexander Kutikov, Brian L. Egleston, Yu-Ning Wong, Robert G. Uzzo
Manuscript writing: Alexander Kutikov, Brian L. Egleston, Yu-Ning Wong, Robert G. Uzzo
Final approval of manuscript: Alexander Kutikov, Brian L. Egleston, Yu-Ning Wong, Robert G. Uzzo
REFERENCES
- 1.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: Meta-analysis and review of the world literature. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: Casting doubt on the efficacy of early intervention. Urology. 2001;57:1013–1015. doi: 10.1016/s0090-4295(01)00991-8. [DOI] [PubMed] [Google Scholar]
- 4.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: The small renal mass dilemma—A meta-analysis and review. J Urol. 2008;179:1227–1233. doi: 10.1016/j.juro.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:429–434. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 6.Kunkle DA, Crispen PL, Chen DY, et al. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177:849–853. doi: 10.1016/j.juro.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 7.Kunkle DA, Haas NB, Uzzo RG. Adjuvant therapy for high-risk renal cell carcinoma patients. Curr Urol Rep. 2007;8:19–30. doi: 10.1007/s11934-007-0017-5. [DOI] [PubMed] [Google Scholar]
- 8.Crispen PL, Viterbo R, Fox EB, et al. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112:1051–1057. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 9.Kouba E, Smith A, McRackan D, et al. Watchful waiting for solid renal masses: Insight into the natural history and results of delayed intervention. J Urol. 2007;177:466–470. doi: 10.1016/j.juro.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute, Division of Cancer Control and Population Sciences Surveillance Research Program Cancer Statistics Branch: Surveillance, Epidemiology, and End Results (SEER) Program Limited Use Data (1973-2004): Released April 2007 (revised September 2007), based on the November 2006 submission. http://seer.cancer.gov/data/
- 11.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Egleston B, Wong YN. Sensitivity analysis to investigate the impact of a missing covariate on survival analyses using cancer registry data. Stat Med. 2009;28:1498–1511. doi: 10.1002/sim.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell FE. New York, NY: Springer; 2001. Regression Modeling Strategies: General Aspects of Fitting Regression Models. [Google Scholar]
- 15.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 16.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 17.Lamb GWA, Bromwich EJ, Vasey P, et al. Management of renal masses in patients medically unsuitable for nephrectomy: Natural history, complications, and outcome. Urology. 2004;64:909–913. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 19.Frank I, Blute ML, Leibovich BC, et al. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173:1889–1892. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 20.Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: An analysis of pathological features related to tumor size. J Urol. 2003;170:2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 21.Rothman J, Egleston B, Wong, et al. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: A SEER analysis. J Urol. 2009;181:29–33. doi: 10.1016/j.juro.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: A meta-analysis. Cancer. 2008;113:2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HL, Seligson D, Liu X, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10:5464–5471. doi: 10.1158/1078-0432.CCR-04-0488. [DOI] [PubMed] [Google Scholar]
- 24.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 25.Karakiewicz PI, Briganti A, Chun FKH, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 26.Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 27.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 28.Zisman A, Pantuck AJ, Dorey F, et al. Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol. 2002;20:1368–1374. doi: 10.1200/JCO.2002.20.5.1368. [DOI] [PubMed] [Google Scholar]
- 29.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 30.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 31.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173:1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RH, Leibovich BC, Lohse CM, et al. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: The D-SSIGN score. J Urol. 2007;177:477–480. doi: 10.1016/j.juro.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 33.Cindolo L, De La Taille A, Messina G, et al. A preoperative clinical prognostic model for non-metastatic renal cell carcinoma. BJU Int. 2003;92:901–905. doi: 10.1111/j.1464-410x.2003.04505.x. [DOI] [PubMed] [Google Scholar]
- 34.Yaycioglu O, Roberts WW, Chan T, et al. Prognostic assessment of nonmetastatic renal cell carcinoma: A clinically based model. Urology. 2001;58:141–145. doi: 10.1016/s0090-4295(01)01207-9. [DOI] [PubMed] [Google Scholar]
- 35.Raj GV, Thompson RH, Leibovich BC, et al. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. J Urol. 2008;179:2146–2151. doi: 10.1016/j.juro.2008.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albertsen PC, Hanley JA, Gleason DF, et al. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280:975–980. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 37.Cowen ME, Halasyamani LK, Kattan MW. Predicting life expectancy in men with clinically localized prostate cancer. J Urol. 2006;175:99–103. doi: 10.1016/S0022-5347(05)00018-2. [DOI] [PubMed] [Google Scholar]
- 38.Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25:3576–3581. doi: 10.1200/JCO.2006.10.3820. [DOI] [PubMed] [Google Scholar]
- 39.Arrontes DS, Acenero MJ, Gonzalez JI, et al. Survival analysis of clear cell renal carcinoma according to the Charlson comorbidity index. J Urol. 2008;179:857–861. doi: 10.1016/j.juro.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 40.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: A population-based competing risk analysis. Cancer. 2007;109:1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 41.Gettman MT, Boelter CW, Cheville JC, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol. 2003;169:1282–1286. doi: 10.1097/01.ju.0000049093.03392.cc. [DOI] [PubMed] [Google Scholar]
- 42.Kircher T, Nelson J, Burdo H. The autopsy as a measure of accuracy of the death certificate. N Engl J Med. 1985;313:1263–1269. doi: 10.1056/NEJM198511143132005. [DOI] [PubMed] [Google Scholar]