Abstract
Purpose
This study was undertaken to determine the effect of treatment for childhood cancer on male fertility.
Patients and Methods
We reviewed the fertility of male Childhood Cancer Survivor Study survivor and sibling cohorts who completed a questionnaire. We abstracted the chemotherapeutic agents administered, the cumulative dose of drug administered for selected drugs, and the doses and volumes of all radiation therapy from medical records. Risk factors for siring a pregnancy were evaluated using Cox proportional hazards models.
Results
The 6,224 survivors age 15 to 44 years who were not surgically sterile were less likely to sire a pregnancy than siblings (hazard ratio [HR], 0.56; 95% CI, −0.49 to 0.63). Among survivors, the HR of siring a pregnancy was decreased by radiation therapy of more than 7.5 Gy to the testes (HR, 0.12; 95% CI, −0.02 to 0.64), higher cumulative alkylating agent dose (AAD) score or treatment with cyclophosphamide (third tertile HR, 0.42; 95% CI, −0.31 to 0.57) or procarbazine (second tertile HR, 0.48; 95% CI, −0.26 to 0.87; third tertile HR, 0.17; 95% CI, −0.07 to 0.41). Compared with siblings, the HR for ever siring a pregnancy for survivors who had an AAD score = 0, a hypothalamic/pituitary radiation dose = 0 Gy, and a testes radiation dose = 0 Gy was 0.91 (95% CI, 0.73 to 1.14; P = .41).
Conclusion
This large study identified risk factors for decreased fertility that may be used for counseling male cancer patients.
INTRODUCTION
The treatment of children and adolescents with cancer is usually successful. The majority survive for 5 years, most of whom will survive for many years after diagnosis.1 Long-term survivors are often concerned about their potential for fertility.2,3 The treatments they received may adversely affect their reproductive function directly by damaging their testes or indirectly by impairing function of the hypothalamic/pituitary axis.4,5
Byrne et al6 reported that the adjusted relative fertility of male survivors of childhood cancer who were treated between 1945 and 1975 was 0.76 (95% CI, −0.68 to 0.86). The most significant differences in the relative fertility rates were demonstrated in male survivors who had been treated with alkylating agents, with or without infradiaphragmatic irradiation.
This study was undertaken to evaluate fertility in the male participants in the Childhood Cancer Survivor Study (CCSS) and to determine risk factors for decreased fertility. The CCSS cohort is large and heterogeneous, with detailed data regarding radiation and chemotherapy exposures.
PATIENTS AND METHODS
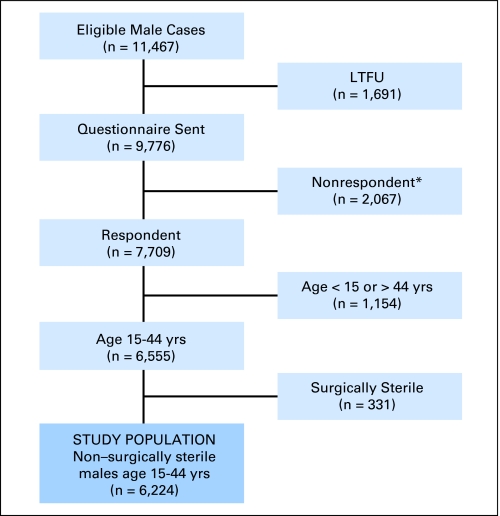
A cohort of 20,720 previously untreated patients (11,467 males) (Fig 1) who were younger than 21 years of age at diagnosis, survived for at least 5 years after the date of diagnosis, and were diagnosed with an eligible cancer between January 1, 1970, and December 31, 1986, was identified at the 26 participating institutions of the CCSS (Appendix Table A1, online only). This study was approved by the institutional review board at each participating institution. The study design, cohort characteristics, and baseline data collection are presented in detail elsewhere.7–9
Fig 1.
Flowchart of cohort subgroups for male fertility analysis. Long-term follow-up study (LTFU). (*) Includes refusals (n = 1,971) and insufficient data to define participation (n = 81).
The CCSS collected data for all surgical procedures performed for cancer treatment. Participants and siblings were asked about additional surgical procedures performed and the methods used for contraception, including vasectomy and tubal ligation. This analysis was restricted to CCSS participants who were 15 to 44 years of age at completion of the baseline questionnaire, consistent with the inclusion criteria for the National Survey of Family Growth (NSFG).10 We excluded those participants or their partners who ever underwent an operation resulting in sterilization (eg, vasectomy, tubal ligation, hysterectomy) consistent with the methods of the NSFG.10 They were classified as surgically sterile for contraceptive or noncontraceptive reasons.10 On the basis of these definitions, 331 male CCSS participants who were age 15 to 44 years at follow-up were categorized as surgically sterile (contraceptive, 288; noncontraceptive, 43). Our analysis focused on the 6,224 male survivors who were not surgically sterile (Fig 1). All reported pregnancies, including miscarriages, voluntary terminations, stillbirths, and live births were considered in the present analysis, including 43 pregnancies reported by surrogates for 474 of the 676 deceased, eligible participants. Pregnancies resulting from assisted reproductive technology were excluded from our analysis.
Permission was requested from a random sample of the cohort to contact their nearest age sibling to form a control group.9 This group was not designed for matched pair analyses but rather as a socio-demographically similar comparison population. Among 4,782 eligible siblings selected, 3,048 (80.5%) participated, of whom 1,449 were males between the ages of 15 and 44 years. One hundred fifty-seven male siblings of CCSS participants who were age 15 to 44 years at follow-up and/or the partners of the male siblings were categorized as surgically sterile (contraceptive, 157; noncontraceptive, 0) and thus were excluded from the current study, leaving a total of 1,292 in the comparison group.
Exposure Assessment
Detailed data regarding the chemotherapeutic agents administered to the patient for treatment of the original cancer and for any recurrences of the cancer, the cumulative dose of drug administered for several drugs of interest, and the doses, volumes, and dates of administration of all radiation therapy were recorded for 12,492 of those who completed the baseline questionnaire.9 The distribution of cumulative doses for each of the agents was divided into tertiles. The alkylating agent dose (AAD) score was calculated by adding the tertile score (1, 2, or 3)11 for each of the alkylating agents given to a particular patient.12 An AAD score of zero was assigned to nonexposed patients. Radiation dose to the testes and pituitary was estimated for each patient.11 Details of the dosimetry methods are described by Stovall et al.13,14
Statistical Methods
Cox proportional hazards models with age as the time scale were used to compare hazards of a pregnancy as previously described in Yasui et al.15 Participants entered the risk set for regression analyses at the age at which they entered the CCSS cohort (5 years after date of diagnosis of primary cancer) or age 15 years, as previously described.11 To create a similar age-based follow-up period, siblings were assigned a pseudo diagnosis date corresponding to the age of their survivor sibling at diagnosis of their primary cancer, and identical methods were used to define their time-to-event variables. The first pregnancies of the partners of the entire male survivor cohort were compared with those of the partners of the entire male sibling cohort. Within-family correlation for the subset of survivors with a sibling pair was accounted for by using sandwich standard-error estimates.16 Multiple-imputation methodology for event-time imputations17,18 was used for those who reported one or more pregnancies but did not report their age at first pregnancy. Age at first pregnancy was available for 81.8% (770 of 941) of the survivors who reported siring a pregnancy; of those, 61 pregnancies that occurred less than 5 years after the date of diagnosis were excluded. Similarly, age at first pregnancy was available for 83.3% (369 of 443) of the male siblings (33 pregnancies that occurred < 5 years after the date of pseudo diagnosis were excluded). Age was imputed for the remaining 18.2% (49 excluded) and 16.7% (2 excluded) of the male survivors and siblings, respectively. Analyses of treatment (exposure) variables were restricted to those male survivors for whom medical record abstraction was completed (n = 5,371), whereas analyses that required only demographic data (eg, age at completion of questionnaire, age at diagnosis, and so on) included all males age 15 to 44 years who had completed the baseline questionnaire.
Two sets of models were evaluated. The first compared fertility for survivors versus siblings, controlling for education level, marital status, and race/ethnicity. In addition, fertility among participants who did or did not receive specific treatments identified as high risk among the survivors was compared with that of the siblings. A second set of models among survivors evaluated only the impact of treatment variables while adjusting for the same variables as above and age at diagnosis. Candidate treatment variables and criteria for their inclusion have been reported previously.11 Two separate multivariable models evaluated the impact of separate chemotherapy agents and combined alkylating agents using the previously described AAD score. Interactive effects between the two radiation volumes (testes and hypothalamus/pituitary) were evaluated to the extent possible and they were not significant. Cut points for radiation categories were selected on the basis of both biologic plausibility19–22 and statistical separation of groups.
RESULTS
There were 7,709 males who returned a baseline questionnaire, of whom 6,224 were between the ages of 15 and 44 years at the time of completion of the questionnaire and were not surgically sterile. Of those, 941 indicated that they had ever sired a pregnancy 5 years or more after the date of the primary cancer diagnosis. The CCSS participants were younger (P < .001), more likely to be of minority race/ethnicity (P < .001), less likely to have a bachelor's degree or higher (P < .001), and more likely to have never been married (P < .001) (Table 1).
Table 1.
Demographic Characteristics of Male Survivors of Childhood Cancer and Male Siblings 15 to 44 Years of Age at Time of Baseline Questionnaire Who Were Not Surgically Sterile
| Characteristic | Survivors |
Siblings |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Race/ethnicity (79*) | |||||
| Non-Hispanic white | 5,172 | 83.4 | 1,279 | 92.0 | < .001 |
| Hispanic | 118 | 1.9 | 22 | 1.6 | |
| Non-Hispanic black | 298 | 4.8 | 29 | 2.1 | |
| Other | 616 | 9.9 | 60 | 4.3 | |
| Marital status (283*) | |||||
| Never married | 3,998 | 66.5 | 735 | 53.4 | < .001 |
| Currently married | 1,632 | 27.1 | 513 | 37.3 | |
| Formerly married | 383 | 6.4 | 129 | 9.4 | |
| Education level (344*) | |||||
| 1-8 years (grade school) | 1,532 | 25.8 | 288 | 20.8 | < .001 |
| 9-12 years (high school; did not graduate) | 1,238 | 20.8 | 232 | 16.8 | |
| Completed high school | 1,838 | 30.9 | 446 | 32.2 | |
| Post high school, no college | 1,338 | 22.5 | 417 | 30.2 | |
| Age at baseline, years | |||||
| 15-19 | 1,657 | 26.6 | 349 | 24.1 | < .001 |
| 20-24 | 1,622 | 26.1 | 314 | 21.7 | |
| 25-29 | 1,495 | 24.0 | 291 | 20.1 | |
| 30-34 | 953 | 15.3 | 275 | 19.0 | |
| 35-39 | 378 | 6.1 | 147 | 10.1 | |
| 40-44 | 119 | 1.9 | 73 | 5.0 | |
| Age at diagnosis, years | |||||
| 0-4 | 1,944 | 31.2 | NA | NA | |
| 5-9 | 1,638 | 26.3 | NA | ||
| 10-14 | 1,470 | 23.6 | NA | ||
| 15-19 | 1,057 | 17.0 | NA | ||
| > 20 | 115 | 1.8 | NA | ||
| Primary diagnosis | |||||
| Leukemia | 1,976 | 31.7 | NA | NA | |
| CNS | 837 | 13.4 | NA | ||
| Hodgkin's lymphoma | 963 | 15.5 | NA | ||
| Non-Hodgkin's lymphoma | 684 | 11.0 | NA | ||
| Kidney (Wilms disease) | 429 | 6.9 | NA | ||
| Neuroblastoma | 283 | 4.5 | NA | ||
| Soft tissue sarcoma | 506 | 8.1 | NA | ||
| Bone cancer | 546 | 8.8 | NA | ||
| Testicular radiation dose, Gy (1,230*) | |||||
| No dose | 1,497 | 30.0 | NA | NA | |
| 0.001-3.99 | 3,137 | 62.8 | NA | ||
| 4.00-4.99 | 62 | 1.2 | NA | ||
| 5.00-5.99 | 45 | 0.9 | NA | ||
| 6.00-14.99 | 116 | 2.3 | NA | ||
| 15.00-23.99 | 137 | 2.7 | NA | ||
| Hypothalamic/pituitary radiation dose, Gy (1,140*) | |||||
| No dose | 1,508 | 29.7 | NA | NA | |
| 0.001-3.99 | 1,544 | 30.4 | NA | ||
| 4.00-4.99 | 14 | 0.3 | NA | ||
| 5.00-5.99 | 23 | 0.5 | NA | ||
| 6.00-14.99 | 137 | 2.7 | NA | ||
| 15.00-23.99 | 1,022 | 20.1 | NA | ||
| ≥ 24.00 | 836 | 16.4 | NA | ||
| Summed alkylating agent dose (1,642*)†‡ | |||||
| 0 | 2,270 | 49.5 | NA | NA | |
| 1 | 483 | 10.5 | NA | ||
| 2 | 570 | 12.4 | NA | ||
| 3 | 724 | 15.8 | NA | ||
| 4 | 234 | 5.1 | NA | ||
| 5 | 138 | 3.0 | NA | ||
| 6-11 | 163 | 3.6 | NA | ||
| Dactinomycin (980*) | |||||
| No | 4,269 | 81.4 | NA | NA | |
| Yes | 975 | 18.6 | NA | ||
| Cytarabine (980*) | |||||
| No | 3,966 | 75.6 | NA | NA | |
| Yes | 1,278 | 24.4 | NA | ||
| Daunomycin (980*) | |||||
| No | 4,562 | 87.0 | NA | NA | |
| Yes | 682 | 13.0 | NA | ||
| Vinblastine (980*) | |||||
| No | 4,933 | 94.1 | NA | NA | |
| Yes | 311 | 5.9 | NA | ||
| Vincristine (980*) | |||||
| No | 1,465 | 27.9 | NA | NA | |
| Yes | 3,779 | 72.1 | NA | ||
| Teniposide (980*) | |||||
| No | 5,013 | 95.6 | NA | NA | |
| Yes | 231 | 4.4 | NA | ||
| Lomustine (980*) | |||||
| No | 4,999 | 95.3 | NA | NA | |
| Yes | 245 | 4.7 | NA | ||
| Nitrogen mustard (980*) | |||||
| No | 4,838 | 92.3 | NA | NA | |
| Yes | 406 | 7.7 | NA | ||
| Procarbazine, tertile (1,119*)‡ | |||||
| 0 | 4,616 | 90.4 | NA | NA | |
| First | 138 | 2.7 | NA | ||
| Second | 175 | 3.4 | NA | ||
| Third | 176 | 3.5 | NA | ||
| Cyclophosphamide, tertile (1,243*)‡ | |||||
| 0 | 2,811 | 56.4 | NA | NA | |
| First | 597 | 12.0 | NA | ||
| Second | 760 | 15.3 | NA | ||
| Third | 813 | 16.3 | NA | ||
Abbreviation: NA, not applicable.
Number of missing, in both survivors and siblings.
See section Exposure Assessment.
“Yes” with no dose information was set to missing.
The hazard ratio (HR) for a CCSS participant ever siring a pregnancy was 0.56 (95% CI, 0.49 to 0.63; P < .001) compared with the siblings of CCSS participants, adjusted for marital status, race/ethnicity, and educational attainment. The HR for ever siring a pregnancy, adjusted for marital status, race/ethnicity, and educational attainment, for CCSS participants who had an AAD score of 0, a hypothalamic/pituitary radiation dose of 0 Gy, and a testes radiation dose of 0 Gy was 0.91 (95% CI, 0.73 to 1.14; P = .41) (Table 2). Those with a diagnosis of Hodgkin's lymphoma were least likely to sire a pregnancy (HR, 0.34; 95% CI, 0.28 to 0.41; P < .001), whereas those with diagnoses of Wilms tumor (HR, 0.99; 95% CI, 0.73 to 1.34; P = .95) or neuroblastoma (HR, 0.97; 95% CI, 0.63 to 1.49; P = .88) were as likely as the siblings to sire a pregnancy (Table 2).
Table 2.
RR of Fertility Among of Male Survivors of Childhood Cancer Age 15 to 44 Years at Time of Baseline Questionnaire Who Were Not Surgically Sterile Compared With Male Siblings
| Characteristic | RR* | 95% CI | P |
|---|---|---|---|
| Siblings | 1.00 | ||
| Alkylating agent dose = 0 Gy, hypothalamic/pituitary radiation = 0 Gy, or testes radiation dose = 0 Gy | 0.91 | 0.73 to 1.14 | .41 |
| Alkylating agent dose ≥ 1 Gy, hypothalamic/pituitary radiation > 0 Gy, or testes radiation dose > 0 Gy | 0.52 | 0.45 to 0.60 | < .001 |
| Hypothalamic/pituitary radiation dose, Gy | |||
| No dose | 0.72 | 0.45 to 0.86 | < .001 |
| > 0-40.0 | 0.50 | 0.43 to 0.58 | < .001 |
| > 40.0 | 0.31 | 0.19 to 0.50 | < .001 |
| Testicular radiation dose, Gy | |||
| No dose | 0.73 | 0.61 to 0.87 | < .001 |
| > 0-7.5 | 0.53 | 0.46 to 0.62 | < .001 |
| > 7.5 | 0.05 | 0.02 to 0.14 | < .001 |
| Summed alkylating agent dose score† | |||
| 0 | 0.71 | 0.61 to 0.83 | < .001 |
| 1 | 0.77 | 0.56 to 1.04 | .085 |
| 2 | 0.56 | 0.44 to 0.72 | < .001 |
| 3 | 0.37 | 0.28 to 0.49 | < .001 |
| 4 | 0.26 | 0.17 to 0.40 | < .001 |
| 5 | 0.30 | 0.17 to 0.50 | < .001 |
| 6-11 | 0.11 | 0.06 to 0.22 | < .001 |
| Diagnosis | |||
| Leukemia | 0.70 | 0.59 to 0.84 | < .001 |
| CNS | 0.58 | 0.45 to 0.76 | < .001 |
| Hodgkin's lymphoma | 0.34 | 0.28 to 0.41 | < .001 |
| Non-Hodgkin's lymphoma | 0.60 | 0.48 to 0.74 | < .001 |
| Wilms disease | 0.99 | 0.73 to 1.34 | .95 |
| Neuroblastoma | 0.97 | 0.63 to 1.49 | .88 |
| Soft tissue sarcoma | 0.52 | 0.41 to 0.68 | < .001 |
| Bone cancer | 0.47 | 0.37 to 0.59 | < .001 |
NOTE. Each row represents a separate multivariable regression model adjusted for education, marital status, and race.
Abbreviation: RR, relative risk.
RR compared with siblings controlled for education, race/ethnicity, and marital status.
See section Exposure Assessment.
We evaluated two multivariable models within the survivor cohort. The first considered the AAD summed score. No effect of pituitary irradiation on fertility was observed after adjusting for other risk factors and confounders (Table 3). CCSS participants who received testicular radiation at a dose ≤ 7.5 Gy were not less likely to sire a pregnancy compared with patients who received no testicular radiation (HR, 1.62; 95% CI, 0.39 to 6.71; P = .51). Those who received a testicular radiation dose of more than 7.5 Gy were less likely to sire a pregnancy compared with those who did not receive testicular radiation (HR, 0.12; 95% CI, 0.02 to 0.64; P = .012).
Table 3.
HR of Fertility Among Male Survivors of Childhood Cancer Age 15 to 44 Years at Time of Baseline Questionnaire Who Were Not Surgically Sterile (multivariate model)
| Characteristic | Summed Alkylating Agent Dose Score |
Individual Chemotherapy |
||||
|---|---|---|---|---|---|---|
| HR* | 95% CI | P | HR | 95% CI | P | |
| Age at diagnosis, years | ||||||
| 0-4 | 1.80 | 1.31 to 2.47 | < .001 | 1.75 | 1.28 to 2.39 | < .001 |
| 5-9 | 1.16 | 0.89 to 1.50 | .27 | 1.11 | 0.86 to 1.44 | .41 |
| 10-14 | 0.95 | 0.76 to 1.18 | .65 | 0.92 | 0.74 to 1.14 | .45 |
| 15-20 | 1.00 | 1.00 | ||||
| Education | ||||||
| No high school/GED | 1.00 | 1.00 | ||||
| High school/GED | 0.75 | 0.55 to 1.02 | .069 | 0.70 | 0.52 to 0.95 | .022 |
| Some college | 0.69 | 0.52 to 0.91 | .01 | 0.64 | 0.49 to 0.84 | .001 |
| Bachelor's degree or higher | 0.53 | 0.40 to 0.71 | < .001 | 0.48 | 0.36 to 0.63 | < .001 |
| Race/ethnicity | ||||||
| White | 1.00 | 1.00 | ||||
| Hispanic | 1.13 | 0.53 to 2.41 | .75 | 1.01 | 0.47 to 2.15 | .98 |
| Black | 1.91 | 1.29 to 2.84 | .001 | 1.96 | 1.35 to 2.86 | < .001 |
| Other | 1.35 | 1.02 to 1.78 | .035 | 1.32 | 1.00 to 1.73 | .05 |
| Marital status | ||||||
| Never married | 1.00 | 1.00 | ||||
| Currently married | 9.64 | 7.23 to 12.85 | < .001 | 9.57 | 7.24 to 12.65 | < .001 |
| Formerly married | 6.36 | 4.40 to 9.20 | < .001 | 5.89 | 4.10 to 8.45 | < .001 |
| Hypothalamic/pituitary radiation dose, Gy | ||||||
| No dose | 1.00 | 1.00 | ||||
| > 0-40.0 | 0.52 | 0.13 to 2.16 | .37 | 0.50 | 0.12 to 2.07 | .34 |
| > 40.0 | 0.29 | 0.06 to 1.28 | .1 | 0.25 | 0.06 to 1.13 | .072 |
| Testicular radiation dose, Gy | ||||||
| No dose | 1.00 | 1.00 | ||||
| > 0-7.5 | 1.62 | 0.39 to 6.71 | .51 | 1.79 | 0.43 to 7.41 | .42 |
| > 7.5 | 0.12 | 0.02 to 0.64 | .012 | 0.14 | 0.03 to 0.71 | .018 |
| Summed alkylating agent dose score† | ||||||
| 0 | 1.00 | |||||
| 1 | 0.95 | 0.68 to 1.33 | .77 | |||
| 2 | 0.67 | 0.51 to 0.88 | .004 | |||
| 3 | 0.48 | 0.36 to 0.65 | < .001 | |||
| 4 | 0.34 | 0.22 to 0.52 | < .001 | |||
| 5 | 0.38 | 0.22 to 0.66 | < .001 | |||
| 6-11 | 0.16 | 0.08 to 0.32 | < .001 | |||
| Dactinomycin | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.97 | 0.76 to 1.23 | .78 | 0.94 | 0.74 to 1.20 | .62 |
| Cytarabine | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.80 | 1.35 to 2.40 | < .001 | 1.54 | 1.14 to 2.08 | .005 |
| Daunomycin | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.10 | 0.79 to 1.52 | .58 | 0.89 | 0.64 to 1.23 | .47 |
| Vinblastine | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.11 | 0.59 to 2.08 | .75 | 1.35 | 0.79 to 2.31 | .28 |
| Vincristine | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.07 | 0.87 to 1.32 | .53 | 1.05 | 0.85 to 1.29 | .68 |
| Teniposide | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.05 | 0.62 to 1.79 | .84 | 1.34 | 0.80 to 2.27 | .27 |
| Lomustine | ||||||
| No | 1.00 | |||||
| Yes | 0.67 | 0.33 to 1.33 | .25 | |||
| Nitrogen mustard | ||||||
| No | 1.00 | |||||
| Yes | 0.69 | 0.40 to 1.21 | .20 | |||
| Cyclophosphamide tertiles, mg/m2 | ||||||
| 0 | 1.00 | |||||
| First | 1.03 | 0.76 to 1.39 | .84 | |||
| Second | 0.82 | 0.63 to 1.07 | .14 | |||
| Third | 0.42 | 0.31 to 0.57 | < .001 | |||
| Procarbazine tertiles, mg/m2 | ||||||
| 0 | 1.00 | |||||
| First | 0.56 | 0.29 to 1.11 | .096 | |||
| Second | 0.48 | 0.26 to 0.87 | .016 | |||
| Third | 0.17 | 0.07 to 0.41 | < .001 | |||
NOTE. All factors displaying estimates for a specific column are included together in that multivariate model.
Abbreviations: HR, hazard ratio; GED, general equivalency diploma.
HR is controlled for age at diagnosis, education, race/ethnicity, and marital status.
See section Exposure Assessment.
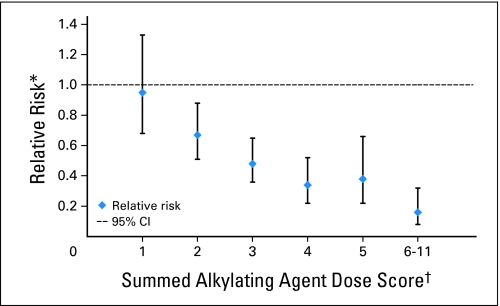
The HR of siring a pregnancy was inversely related to the summed AAD score (P for linear trend ≤ .001). Those who had a summed AAD score of 2 (HR, 0.67; 95% CI, 0.51 to 0.88; P = .004), 3 (HR, 0.48; 95% CI, 0.36 to 0.65; P < .001), 4 (HR, 0.34; 95% CI, 0.22 to 0.52; P < .001), 5 (HR, 0.38; 95% CI, 0.22 to 0.66; P < .001), or 6 to 11 (HR, 0.16; 95% CI, 0.08 to 0.32; P < .001) were also less likely to ever sire a pregnancy compared with those who did not receive any alkylating agents (Fig 2 and Table 3).
Fig 2.
Relationship between summed alkylating agent dose score and the relative risk for siring a pregnancy. (*) Relative risk is adjusted for age at diagnosis, race/ethnicity, marital status, educational attainment, nonalkylating chemotherapy drugs, hypothalamic/pituitary radiation dose, and testicular radiation dose. (†) Referent group = 0 score.
Several individual chemotherapeutic agents demonstrated a significant association with impaired fertility in univariate models (data not shown) and were included in the multivariable model (Table 3). CCSS participants who received a cumulative procarbazine dose in the second tertile (4,201 to 6,999 mg/m2; HR, 0.48; 95% CI, 0.26 to 0.87) or third tertile (7,000 to 58,680 mg/m2; HR, 0.17; 95% CI, 0.07 to 0.41) were less likely to sire a pregnancy compared with those who did not receive procarbazine. Similarly, those exposed to a cumulative cyclophosphamide dose in the third tertile (9,360 to 143,802 mg/m2; HR, 0.42; 95% CI, 0.31 to 0.57) were less likely to ever sire a pregnancy compared with those who did not receive cyclophosphamide.
In both models (Table 3), those who received cytarabine were more likely to ever sire a pregnancy than those who were not exposed to this agent. In addition, those who were 0 to 4 years of age at diagnosis were more likely to ever sire a pregnancy than those 15 to 20 years of age at diagnosis. We did not observe differences in the likelihood of siring a pregnancy for those 5 to 9 or 10 to 14 years of age, relative to the 15- to 20-year-old group. The models yielded qualitatively identical results whether constructed with or without the inclusion of those for whom age at first pregnancy was imputed.
DISCUSSION
Previous studies investigated the fertility of childhood cancer survivors, frequently in convenience samples, populations with poorly quantified exposures and/or infrequent treatment with those agents most likely to adversely impact fertility such as alkylating agents. The current study provides new information by estimating risk for siring a pregnancy 5 years or more after diagnosis and with specific quantified exposures. The results can inform guideline-based long-term follow-up recommendations.
Compared with the siblings, survivors were approximately half as likely to sire a pregnancy. Byrne et al6 reported that the unadjusted relative risk for fertility was 0.83 (95% CI, 0.74 to 0.93) among the male childhood cancer survivors included in the Five Center Study. This study excluded from the analysis eligible participants who were never married, married before the diagnosis of cancer, had/sired their first pregnancy before the diagnosis of cancer, or who reported no pregnancies and said they did not want children or that they wanted to adopt children. Few participants in the Five Center Study were exposed to gonadotoxic therapies, particularly alkylating agents. In another study of survivors of acute lymphoblastic leukemia, the relative risk for fertility was 0.80 (P = .47) for survivors who were 18 to 21 years of age when they first sired a pregnancy compared with sibling controls; the relative risk was 1.02 (P = .93) for survivors who were older than age 21 years at the time they first sired a pregnancy.23
In our treatment models, prior radiation therapy to the testes of more than 7.5 Gy, a summed alkylating agent dose score ≥ 2, treatment with procarbazine, or treatment with higher doses of cyclophosphamide were the major factors that decreased the HR of a CCSS survivor siring a pregnancy. Importantly, those who did not have any of these high-risk exposures were as likely as the siblings to sire a pregnancy.
Previous studies reported that recovery of spermatogenesis was unlikely after single-dose exposures exceeding 4.0 Gy19 or low-dose fractionated exposures.20–22 Loss of both spermatogenesis and androgen secretion occurred at high doses (≥ 24.0 Gy);20–22,24 lower-dose exposures may not produce azoospermia.25,26 Leydig cell function may be preserved when the testicular dose is ≤ 20.0 Gy.27
Severe damage to the testicular germinal epithelium frequently follows treatment which includes an alkylating agent and procarbazine.25,26,28–38 Azoospermia was present in all men by the start of the third cycle of nitrogen mustard, vinblastine, procarbazine, and prednisone chemotherapy.33 Less than 20% had recovery of spermatogenesis when evaluated 37 to 48 months after treatment.32 The chemotherapy regimen originally reported by DeVita et al39 had an AAD score of 6.11 Azoospermia occurred less frequently following treatment with two (AAD score = 2), rather than six (AAD score = 6), cycles of nitrogen mustard, vincristine, procarbazine, and prednisone.38 The combination of doxorubicin, bleomycin, vinblastine, and dacarbazine produced oligo- or azoospermia frequently during the course of treatment, but recovery of spermatogenesis occurred after treatment was completed.34
The administration of cyclophosphamide has been associated with impaired spermatogenesis after treatment of children for nonmalignant40–45 and malignant46–51 diseases. Azoospermia was reported after cumulative cyclophosphamide doses as low as 6.0 g/m249 whereas spermatogenesis was preserved after cumulative doses as high as 16 g/m2.48 Impaired spermatogenesis was more likely after cumulative doses exceeding 7.5 to 9.5 g/m2.46,47
Cumulative cyclophosphamide doses used in contemporary regimens for Hodgkin's disease (3.2 to 4.8 g/m2),52 and rhabdomyosarcoma (4.8 to 16.8 g/m2) (S. Spunt, personal communication, May 2008) correspond to AAD scores of 1 to 3. Current regimens for Ewing sarcoma include cyclophosphamide (8.4 g/m2) and ifosfamide in combination (63 g/m2),53 resulting in an AAD score of 6.
Our study demonstrated that young males (0 to 4 years of age at diagnosis) were more likely to sire a pregnancy than those who were 15 to 20 years of age at diagnosis. Some reports suggested that the prepubertal testis was less sensitive than the postpubertal testis to damage by chemotherapy,31,54 but others have questioned this observation.25,48,55–57
This study has a number of strengths. The CCSS is the largest, most thoroughly characterized cohort of survivors of cancer diagnosed during childhood or adolescence. Thus, important questions regarding the frequency of outcomes that may be modified by treatment exposures, as well as the relationship of these exposures to significant, though uncommon, late events can be evaluated with substantial statistical power.
There are also limitations that must be taken into account. The participants were ascertained retrospectively. Fifteen percent of those eligible were lost to follow-up and 16% declined participation. Participants did not differ from nonparticipants with regard to demographic or cancer characteristics.9 Radiation dosimetry was performed using the data supplied by the participating institutions. No independent quality control was performed to determine whether significant data, such as the use of a testicular shield (which can reduce the testicular dose to approximately 1% of the prescription dose),58 were omitted.
The CCSS used self-administered questionnaires for ascertainment of outcomes. Approximately 22% of pregnancies were not recognized clinically.59 Comparison of the number of births reported by NSFG participants 15 to 19 years of age to those ascertained by vital records suggests that 15- to 19-year-old males are not informed of all pregnancies by their partners.10 Information relating to adjustment variables (eg, education and so on) was derived from a single point in time (ie, at baseline questionnaire), was a surrogate for the presence or absence of particular factors at the time of pregnancy, and did not directly measure their influence over time.
We did not evaluate fertility in light of personal choices made by the male CCSS survivors. Some may have chosen not to attempt to sire a pregnancy because they were concerned that they might transmit a trait that would predispose their children to cancer.2 Others may have thought or been told that they were or might be infertile. Factors that may influence their ability to form or maintain an intimate heterosexual relationship include appearance, sexual preference, and neurocognitive function.2,3,60,61 Some of these factors may be related to the therapeutic exposures considered in this analysis and may have confounded the results of our study. Our finding that the HR for siring a pregnancy was not significantly decreased in CCSS participants who experienced none of the high-risk exposures for decreased fertility suggests that these factors may not influence decisions regarding paternity significantly in this group.
We demonstrated that the fertility of male childhood cancer survivors is impaired. Men age 15 to 44 years, who received a testicular radiation dose of more than 7.5 Gy, were treated with procarbazine or cyclophosphamide, or had a summed alkylating agent dose score of ≥ 2, or were less likely to sire a pregnancy. Men diagnosed in early childhood were more likely to sire a pregnancy than those diagnosed in adolescence. These data may be used to counsel patients and their parents before initiation of treatment regarding their future fertility.
Appendix
This research used the Childhood Cancer Survivor Study (CCSS), a resource supported by National Cancer Institute Grant No. CA 55727, to promote and facilitate research on long-term survivors of cancer diagnosed in childhood and adolescence. Investigators may apply to use the CCSS by proposing an analysis of existing data or proposing initiatives that would use the cohort. Interested investigators are encouraged to visit the CCSS Web site at www.stjude.org/ltfu to learn more about this unique resource.
Table A1.
CCSS Institutions and Investigators
| CCSS Institutions | Investigators |
|---|---|
| St Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhD*†, Melissa Hudson, MD†‡, Greg Armstrong, MD†, Daniel M. Green, MD† |
| Children's Healthcare of Atlanta/Emory University, Atlanta, GA | Lillian Meacham, MD‡, Ann Mertens, PhD† |
| Children's Hospitals and Clinics of Minnesota Minneapolis, St. Paul, MN | Joanna Perkins, MD‡ |
| Children's Hospital and Medical Center, Seattle, WA | Douglas Hawkins, MD‡, Eric Chow, MD† |
| Children's Hospital, Denver, CO | Brian Greffe, MD‡ |
| Children's Hospital, Los Angeles, CA | Kathy Ruccione, RN, MPH‡ |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MD† |
| Children's Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD‡, Anna Meadows, MD† |
| Children's Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD‡ |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MD‡, Roger Packer, MD† |
| Cincinnati Children's Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD† |
| City of Hope Medical Center, Duarte, CA | Smita Bhatia, MD†‡ |
| Dana-Farber Cancer Institute/Children's Hospital, Boston, MA | Lisa Diller, MDठ|
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD†‡ |
| Hospital for Sick Children, Toronto, ON, Canada | Mark Greenberg, MBChB‡, Paul C. Nathan, MD†‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD† |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD‡ |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD†‡, Kevin Oeffinger, MD† |
| Miller Children's Hospital, Long Beach, CA | Jerry Finklestein, MD§ |
| National Cancer Institute, Bethesda, MD | Roy Wu, PhD§, Nita Sibel, MD§ |
| Preetha Rajaraman, PhD§ | |
| Nationwide Children's Hospital, Columbus, OH | Amanda Termuhlen, MD‡, Sue Hammond, MD† |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD‡ |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, MD‡ |
| St Louis Children's Hospital, St. Louis, MO | Robert Hayashi, MD‡ |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD‡, Sarah S. Donaldson, MD† |
| Texas Children's Hospital, Houston, TX | Zoann Dreyer, MD‡ |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH‡ |
| University of Alberta, Edmonton, AB, Canada | Yutaka Yasui, PhD† |
| University of California at Los Angeles, Los Angeles, CA | Jacqueline Casillas, MD, MSHS‡, Lonnie Zeltzer, MD†§ |
| University of California at San Francisco, San Francisco, CA | Robert Goldsby, MD‡ |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD‡ |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH†‡ |
| University of Southern California, Los Angeles, CA | Dennis Deapen, DrPH† |
| University of Texas-Southwestern Medical Center, Dallas, TX | Dan Bowers, MD‡ |
| University of Texas M. D. Anderson Cancer Center, Houston, TX | Louise Strong, MD†‡, Marilyn Stovall, MPH, PhD† |
NOTE. The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project funded as a resource by the National Cancer Institute (NCI) consisting of individuals who survived 5 years or more after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and a randomly selected subset of siblings of survivors who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by NCI Resource Grant No. U24 CA55727 awarded to St Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and use the CCSS resource, Visit www.stjude.org/ccss/.
Project principal investigator (U24 CA55727).
Member CCSS Steering Committee.
Institutional principal investigator.
Former institutional principal investigator.
Footnotes
Supported by the National Institutes of Health/National Cancer Institute Grant No. U24 CA55727 (L.L.R., Principal Investigator), by the Children's Cancer Research Fund, and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel M. Green, Toana Kawashima, Wendy Leisenring, Charles A. Sklar, Ann C. Mertens, Sarah S. Donaldson, Julianne Byrne, Leslie L. Robison
Financial support: Leslie L. Robison
Administrative support: Ann C. Mertens, Leslie L. Robison
Provision of study materials or patients: Daniel M. Green, Marilyn Stovall, Sarah S. Donaldson, Leslie L. Robison
Collection and assembly of data: Wendy Leisenring, Ann C. Mertens, Leslie L. Robison
Data analysis and interpretation: Daniel M. Green, Toana Kawashima, Marilyn Stovall, Wendy Leisenring, Charles A. Sklar, Ann C. Mertens, Leslie L. Robison
Manuscript writing: Daniel M. Green, Toana Kawashima, Wendy Leisenring, Charles A. Sklar, Ann C. Mertens, Sarah S. Donaldson, Julianne Byrne, Leslie L. Robison
Final approval of manuscript: Daniel M. Green, Toana Kawashima, Marilyn Stovall, Wendy Leisenring, Charles A. Sklar, Ann C. Mertens, Sarah S. Donaldson, Julianne Byrne, Leslie L. Robison
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review: 1975-2005. [Google Scholar]
- 2.Oosterhuis BE, Goodwin T, Kiernan M, et al. Concerns about infertility risks among pediatric oncology patients and their parents. Pediatr Blood Cancer. 2008;50:85–89. doi: 10.1002/pbc.21261. [DOI] [PubMed] [Google Scholar]
- 3.Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–699. doi: 10.1002/pon.784. [DOI] [PubMed] [Google Scholar]
- 4.Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33:2–8. doi: 10.1002/(sici)1096-911x(199907)33:1<2::aid-mpo2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Sklar CA, Constine LS. Chronic neuroendocrinological sequelae of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:1113–1121. doi: 10.1016/0360-3016(94)00427-M. [DOI] [PubMed] [Google Scholar]
- 6.Byrne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317:1315–1321. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 7.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 10.Martinez GM, Chandra A, Abma JC, et al. Hyattsville, MD: National Center for Health Statistics; 2006. Fertility, Contraception, and Fatherhood: Data on Men and Women from the 2002 National Survey of Family Growth. [PubMed] [Google Scholar]
- 11.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer. A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker MA, Meadows AT, Boice JD, Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 13.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: Gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 15.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 16.Therneau TM, Grambsch PM. New York, NY: Springer-Verlag; 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 17.Rubin DB. New York, NY: John Wiley & Sons; 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 18.Taylor JM, Muñoz A, Bass SM, et al. Estimating the distribution of times for HIV seroconversion to AIDS using multiple imputation: Multicentre AIDS Cohort Study. Stat Med. 1990;9:505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 19.Rowley M, Leach D, Warner G, et al. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665–678. [PubMed] [Google Scholar]
- 20.Blatt J, Sherins RJ, Niebrugge D, et al. Leydig cell function in boys following treatment for testicular relapse of acute lymphoblastic leukemia. J Clin Oncol. 1985;3:1227–1231. doi: 10.1200/JCO.1985.3.9.1227. [DOI] [PubMed] [Google Scholar]
- 21.Shalet SM, Horner A, Ahmed SR, et al. Leydig cell damage after testicular irradiation for lymphoblastic leukemia. Med Pediatr Oncol. 1985;13:65–68. doi: 10.1002/mpo.2950130204. [DOI] [PubMed] [Google Scholar]
- 22.Brauner R, Czernichow P, Cramer P, et al. Leydig-cell function in children after direct testicular irradiation for acute lymphoblastic leukemia. N Engl J Med. 1983;309:25–28. doi: 10.1056/NEJM198307073090106. [DOI] [PubMed] [Google Scholar]
- 23.Byrne J, Fears TR, Mills J, et al. Fertility of long-term male survivors of acute lymphoblastic leukemia diagnosed during childhood. Pediatr Blood Cancer. 2004;42:364–372. doi: 10.1002/pbc.10449. [DOI] [PubMed] [Google Scholar]
- 24.Leiper AD, Grant DB, Chessels JM. Gonadal function after testicular radiation for acute lymphoblastic leukemia. Arch Dis Child. 1986;61:53–56. doi: 10.1136/adc.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark ST, Radford JA, Crowther D, et al. Gonadal function following chemotherapy for Hodgkin's disease: A comparative study of MVPP and a seven-drug hybrid regimen. J Clin Oncol. 1995;13:134–139. doi: 10.1200/JCO.1995.13.1.134. [DOI] [PubMed] [Google Scholar]
- 26.Shafford EA, Kingston JE, Malpas JS, et al. Testicular function following the treatment of Hodgkin's disease in childhood. Br J Cancer. 1993;68:1199–1204. doi: 10.1038/bjc.1993.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sklar CA, Robison LL, Nesbit ME, et al. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the Children's Cancer Study Group. J Clin Oncol. 1990;8:1981–1987. doi: 10.1200/JCO.1990.8.12.1981. [DOI] [PubMed] [Google Scholar]
- 28.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin's disease. Med Pediatr Oncol. 1996;27:74–78. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.DeVita VT, Arseneau JC, Sherins RJ, et al. Intensive chemotherapy for Hodgkin's disease: Long-term complications. Natl Cancer Inst Monogr. 1973;36:447–454. [PubMed] [Google Scholar]
- 30.Asbjornsen G, Molne K, Klepp O, et al. Testicular function after combination chemotherapy for Hodgkin's disease. Scand J Haematol. 1976;16:66–69. doi: 10.1111/j.1600-0609.1976.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 31.Sherins RJ, Olweny CL, Ziegler JL. Gynecomastia and gonadal dysfunction in adolescent boys treated with combination chemotherapy for Hodgkin's disease. N Engl J Med. 1978;299:12–16. doi: 10.1056/NEJM197807062990103. [DOI] [PubMed] [Google Scholar]
- 32.Chapman RM, Sutcliffe SB, Rees LH, et al. Cyclical combination chemotherapy and gonadal function: Retrospective study in males. Lancet. 1979;1:285–289. doi: 10.1016/s0140-6736(79)90701-3. [DOI] [PubMed] [Google Scholar]
- 33.Chapman RM, Sutcliffe SB, Malpas JS. Male gonadal dysfunction in Hodgkin's disease. JAMA. 1981;245:1323–1328. [PubMed] [Google Scholar]
- 34.Viviani S, Santoro A, Ragni G, et al. Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol. 1985;21:601–605. doi: 10.1016/0277-5379(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 35.Charak BS, Gupta R, Mandrekar P, et al. Testicular dysfunction after cyclophosphamide-vincristine-procarbazine-prednisolone chemotherapy for advanced Hodgkin's disease. A long-term follow-up study. Cancer. 1990;65:1903–1906. doi: 10.1002/1097-0142(19900501)65:9<1903::aid-cncr2820650905>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Dhabhar BN, Malhotra H, Joseph R, et al. Gonadal function in prepubertal boys following treatment for Hodgkin's disease. Am J Pediatr Hematol Oncol. 1993;15:306–310. [PubMed] [Google Scholar]
- 37.Heikens J, Behrendt H, Adriaanse R, et al. Irreversible gonadal damage in male survivors of pediatric Hodgkin's disease. Cancer. 1996;78:2020–2024. doi: 10.1002/(sici)1097-0142(19961101)78:9<2020::aid-cncr25>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.da Cunha MF, Meistrich ML, Fuller LM, et al. Recovery of spermatogenesis after treatment for Hodgkin's disease: Limiting dose of MOPP chemotherapy. J Clin Oncol. 1984;2:571–577. doi: 10.1200/JCO.1984.2.6.571. [DOI] [PubMed] [Google Scholar]
- 39.DeVita VT, Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970;73:881–895. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- 40.Watson AR, Rance AP, Bain J. Long term effects of cyclophosphamide on testicular function. Br Med J (Clin Res Ed) 1985;291:1457–1460. doi: 10.1136/bmj.291.6507.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etteldorf JN, West CD, Pitcock JA, et al. Gonadal function, testicular histology and meiosis following cyclophosphamide therapy in patients with nephrotic syndrome. J Pediatr. 1976;88:206–212. doi: 10.1016/s0022-3476(76)80983-3. [DOI] [PubMed] [Google Scholar]
- 42.Kirkland RT, Bongiovanni AM, Cornfield D, et al. Gonadotropin responses to luteinizing releasing factor in boys treated with cyclophosphamide for nephrotic syndrome. J Pediatr. 1976;89:941–944. doi: 10.1016/s0022-3476(76)80600-2. [DOI] [PubMed] [Google Scholar]
- 43.Penso J, Lippe B, Ehrlich R, et al. Testicular function in prepubertal and pubertal male patients treated with cyclophosphamide for nephrotic syndrome. J Pediatr. 1974;84:831–836. doi: 10.1016/s0022-3476(74)80758-4. [DOI] [PubMed] [Google Scholar]
- 44.Qureshi MS, Pennington JH, Goldsmith HJ, et al. Cyclophosphamide therapy and sterility. Lancet. 1972;2:1290–1291. doi: 10.1016/s0140-6736(72)92657-8. [DOI] [PubMed] [Google Scholar]
- 45.Lentz RD, Bergstein J, Steffes MW, et al. Postpubertal evaluation of gonadal function following cyclophosphamide therapy before and during puberty. J Pediatr. 1977;91:385–394. doi: 10.1016/s0022-3476(77)81305-x. [DOI] [PubMed] [Google Scholar]
- 46.Pryzant RM, Meistrich ML, Wilson G, et al. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin's lymphomas. J Clin Oncol. 1993;11:239–247. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- 47.Meistrich ML, Wilson G, Brown B, et al. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer. 1992;70:2703–2712. doi: 10.1002/1097-0142(19921201)70:11<2703::aid-cncr2820701123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 48.Aubier F, Flamant F, Brauner R, et al. Male gonadal function after chemotherapy for solid tumors in childhood. J Clin Oncol. 1989;7:304–309. doi: 10.1200/JCO.1989.7.3.304. [DOI] [PubMed] [Google Scholar]
- 49.Kenney LB, Laufer MR, Grant FD, et al. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 50.Garolla A, Pizzato C, Ferlin A, et al. Progress in the development of childhood cancer therapy. Reprod Toxicol. 2006;22:126–132. doi: 10.1016/j.reprotox.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Relander T, Cavallin-Stahl E, Garwicz S, et al. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol. 2000;35:52–63. doi: 10.1002/1096-911x(200007)35:1<52::aid-mpo9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz CL, Constine LS. Current protocol and concepts for classical Hodgkin disease (HD) in the USA: A Children's Oncology Group (COG) report. 7th International Symposium on Hodgkin Lymphoma; November 3-7, 2007; Cologne, Germany. abstr 1017. [Google Scholar]
- 53.Womer RB, West DC, Krailo MD, et al. Randomized comparison of every-two-week v. every-three-week chemotherapy in Ewing sarcoma family tumors (ESFT) J Clin Oncol. 2008;26(suppl):554s. abstr 10504. [Google Scholar]
- 54.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced damage. JAMA 1988; 1988;259:2123–2125. [PubMed] [Google Scholar]
- 55.Green DM, Brecher ML, Lindsay AN, et al. Gonadal function in pediatric patients following treatment for Hodgkin's disease. Med Pediatr Oncol. 1981;9:235–244. doi: 10.1002/mpo.2950090306. [DOI] [PubMed] [Google Scholar]
- 56.Whitehead E, Shalet SM, Morris-Jones PH, et al. Gonadal function after combination chemotherapy for Hodgkin's disease in childhood. Arch Dis Child. 1982;47:287–291. doi: 10.1136/adc.57.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaffe N, Sullivan MP, Ried H, et al. Male reproductive function in long-term survivors of childhood cancer. Med Pediatr Oncol. 1988;16:241–247. doi: 10.1002/mpo.2950160404. [DOI] [PubMed] [Google Scholar]
- 58.Fraass BA, Kinsella TJ, Harrington FS, et al. Peripheral dose to the testes: The design and clinical use of a practical and effective gonadal shield. Int J Radiat Oncol Biol Phys. 1985;11:609–615. doi: 10.1016/0360-3016(85)90196-8. [DOI] [PubMed] [Google Scholar]
- 59.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 60.Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer. A pilot survey of survivors' attitudes and experiences Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 61.Copeland DR, Fletcher JM, Pfefferbaum-Levine B, et al. Neuropsychological sequelae of childhood cancer in long-term survivors. Pediatrics. 1985;75:745–753. [PubMed] [Google Scholar]