Abstract
A high-performance liquid chromatographic (HPLC) method with diode array detection (DAD) was used to determine the total phenolics, including sinapic acid derivatives in canola. Ten Western Canadian canola seeds, six other commodity canola seeds, their corresponding press cakes and meals were analyzed. Seeds of European 00 rapeseed and Brassica Juncea (Indian mustard) were included for comparison. Phenolic compounds were separated using a gradient elution system of water–methanol-ο-phosphoric acid solution with a flow rate of 0.8 ml/min. In addition to sinapine (SP) and sinapic acid (SA), sinapoyl glucose (SG) is reported in the methanolic extracts. The detection and quantification limits of these compounds were 0.20–0.40 and 0.50–0.80 μg/ml, respectively with recovery values over 98.0%. The content of total phenolics, SP, SA and SG in canola extracts ranged from 9.16 to 16.13, 6.39 to 12.28, 0.11 to 0.59 and 1.36 to 7.50 mg/g, respectively with significant differences among varieties.
Keywords: Sinapic acid derivatives, Canola extracts, Liquid chromatography
Introduction
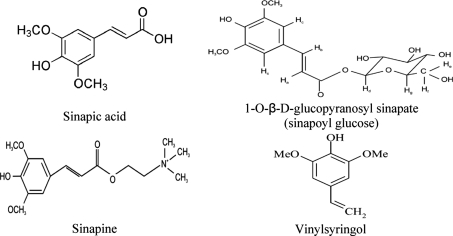
Canola seeds are much richer in phenolic compounds compared to other oilseeds [1]. Most of these phenolics remain in the meal after pressing [2]. Extracts obtained from these oil processing residues or by-products exhibit remarkable antioxidant activity, the extent of which depends on the cultivar and the extraction solvent [3]. The most significant phenolic compounds in canola seed are sinapic acid derivatives (Fig. 1). Sinapic acid constitutes over 73% of the free phenolic acids while sinapine, the choline ester of sinapic acid, is the main phenolic ester in canola seeds (about 80% of the total phenolics) [4]. The most active antioxidative component of canola meal was identified as 1-O-β-d-glucopyranosyl sinapate, a sinapic acid derivative [5]. The main compound responsible for the antioxidant activity in the polar fraction in canola oil was shown to be vinylsyringol (Fig. 1) with concentrations from 0.25 to 0.70 mg/g [2]. The amount of sinapic acid derivatives in rapeseed meal varied from 6.39 to 18.37 mg/g depending on the variety and the oil processing method [6]. Other minor phenolics in the canola and rapeseed may include p-hydroxybenzoic, vanillic, gentisic, protocatechuic, syringic, p-coumaric, ferulic, caffeic and chlorogenic acids [7].
Fig. 1.
Chemical structures of the main phenolic compounds present in canola
Phenolic compounds are readily extracted by pure or aqueous solvents such as methanol and ethanol using conventional extraction techniques. A number of analytical techniques have been reported for the identification and quantification of phenolic compounds. Of the techniques reported the most common are thin-layer chromatography, gas–liquid chromatography, gas chromatography–mass spectrometry, and capillary electrophoretic methods. In addition, High performance liquid chromatography (HPLC) is one of the most widely used quantification method [9, 10]. Data regarding the content of phenolic compounds, particularly sinapic acid derivatives such as sinapine, sinapoyl glucose, of canola seeds currently used in Canada for edible oil production or presently under field trials are limited. Thus, canola seeds were investigated for sinapic acid derivatives. In addition, the by-products or residues from oil production such as meals and press cakes were also investigated. In addition to the identification of sinapic acid and sinapine, this paper reports the presence of sinapoyl glucose.
Materials and Methods
Canola Samples
Canola seeds, meals and press cakes were procured from commercial suppliers including ADM Bunge Canada (Oakville, ON, Canada), Associated Proteins LP (Ste. Agathe, MB, Canada), and Canbra Foods Ltd. (Lethbridge, AB, Canada). One 00 rapeseed variety (S17) was acquired through collaborators in Germany. Seeds of Brassica juncea (Indian mustard) (S18) were investigated for comparison.
Phenolic Standards
Sinapic, ferulic, caffeic, t-cinnamic, syringic, p-coumaric, salicylic, vanillic, gallic acid, kaempferol and syringaldehyde were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sinapine thiocyanate and sinapoyl glucose were kindly donated by Dr. A. Baumert (retired), IPB-Halle, Germany. Sinapinaldehyde was purchased from ChromaDex, Inc. (Irvine, CA, USA).
Experimental Procedures
Sample Preparation
Samples were cleaned, ground and sieved through a 1410-μm screen and stored at 4 °C in polyethylene bags until used. Defatting of samples was conducted using a Soxtec 2050 (Foss-Tecator, Foss North America, MN, USA) according to FOSFA [11] in two cycles for seeds and one cycle for both meals and press cakes. Defatted samples were de-solventized at room temperature overnight in the fume hood.
Extraction of Phenolic Compounds from Canola Seed Fractions
Defatted samples (1 g) were extracted three times with 9 ml of one of the following: 70% methanol, 70% iso-propanol or 70% ethanol using ultra-sonication (1 min) followed by centrifugation under refrigerated conditions (10 min at 5,000×g) and filtered through Whatman No. 1 filter paper. The filtrates from the three extractions were combined and made up to a total volume of 25 ml with the respective extraction solution. These extractions were carried out in duplicates.
Total Phenolics
Total phenolic content was determined using the Folin–Ciocalteu reagent according to Swain and Hillis [12] with some modifications as described. Aliquots (0.2 ml) of extracts were diluted to 0.5 ml with distilled water, and Folin–Ciocalteau’s phenol reagent (0.5 ml) was added. After 3 min, 19% sodium carbonate (1 ml) was added. After 60 min, the absorption was measured at 750 nm using the DU 800 UV/Visible Spectrophotometer (Beckman Coulter Inc., Mississauga, ON, Canada). Sinapic acid (Sigma) was used for the calibration, and the results of duplicate analyses were expressed as sinapic acid equivalents (SAE).
Characterisation of Phenolic Compounds in Canola Extracts using HPLC–DAD
Canola extracts were analysed by reversed-phase HPLC-DAD (Ultimate 3000; Dionex, Sunnyvale, CA, USA) equipped with on-line degasser, binary pump, auto sampler, column heater and diode array detector. A gradient elution was performed using water/methanol (90:10) with 1.2% o-phosphoric acid as solvent A, and methanol (100%) with 0.1% o-phosphoric acid as solvent B [10], using two C18 columns; Gemini 3μm 110 Å; 150 × 4.6 mm and Synergi 4μ Fusion-RP 80 Å; 150 × 4.0 mm 4 micron (Phenomenex, Canada) at 0, 7, 20, 25, 28, 31 and 40 min with 10, 20, 45, 70, 100, 100 and 10% B. Solvent A and B as well as canola extracts were filtered through a 0.45 μm filter. The column was maintained at 25 °C and different flow rates of the mobile phase (0.6–1.0 ml/min) were tested seeking for the optimum separation efficiency. Chromatograms were acquired at 230, 275 and 330 nm and data were analysed using the Chromeleon software (Version 6.8). Peaks were identified by comparing their relative retention times with those of the authentic standards previously mentioned. Each extract was injected two times in two different vials and results of duplicate analyses from each extract were used for quantification.
Calibration Curves
The three phenolics; sinapine thiocyanate, sinapoyl glucose, and sinapic acid were used in the preparation of the calibration curves by plotting the concentration of each compound against the area.
Calculation of Sinapine, Sinapic Acid and Sinapoyl Glucose Contents
Sinapine, sinapoyl glucose, and sinapic contents of the canola extracts were calculated using the following equations: Sinapine thiocyanate = (a + bA) (V c.V t/V s.W); Sinapine = sinapine thiocyanate (310.4/368.45); sinapoyl glucose = (a + bA) (V c.V t/V s.W); Sinapic acid = (a + bA) (V c.V t/V s.W); where a and b = y-intercept and slope of the standard curves for sinapine thiocyanate, sinapoyl glucose, or sinapic acid, respectively, A = peak area, V c = injection volume for calibration (μl), V S = injection volume for sample (μl), V t = volume of solvent added to sample (ml), W = weight of the canola sample (mg), 310.4 = molecular weight of sinapine, and 368.45 = molecular weight of sinapine thiocyanate. “Sum of phenolic acids” was estimated as sinapic acid equivalents (SAE) from the total area of all peaks including the unidentified ones based on the sinapic acid calibration curve.
Method Validation
Validation of this method was performed according to the ICH guidelines [13]. Linearity of the detector responses and the detection limits were tested for the phenolic standards over a 0.05–200 μg/ml range. Reliability of the method was established through repeatability tests. For the phenolic standards as well as the extracts, each sample was run five times during the course of this work. Identity and purity of the phenolic compounds peaks were monitored (in addition to monitoring retention times and the symmetry of the peak), comparing the spectral data of the sample peaks with that obtained for the phenolic standards.
Spiking experiments were carried out to evaluate the recovery of sinapic acid during the extraction. Thus during each time of the three time extraction, spiking with 100 μl of sinapic acid stock solution (1.0 mg/ml) was done.
Statistical Analysis
Data were analysed using a one factor analysis of variance (ANOVA) and Tukey mean separation for multiple comparisons with the Statistical Analysis System (SAS) Program (SAS Institute, Carey, NC). Significance was accepted at p ≤ 0.05.
Results and Discussion
Extraction of Phenolic Compounds from Defatted Canola Seeds
Total phenolic content of defatted canola fractions were extracted using different solvents; 70% methanol, 70% ethanol and 70% iso-propanol. The efficiency of these solvents to extract phenolic compounds from canola was tested using HPLC-DAD. Methanol (70%) was the most efficient solvent in extracting phenolic compounds from canola seed as it gave the highest total phenolic content as well as the highest contents for the major phenolics (sinapine, sinapoyl glucose and sinapic acid) compared to either 70% ethanol or 70% iso-propanol (Table 1). The range in sinapine was greatest (~2–3 mg/g) when these three solvents were compared, while sinapic acid did not show any significant differences. Thus, 70% methanol was used in the phenolic extraction during the whole work.
Table 1.
Effect of different solvents on the phenolic contents of canola seeds
| Solvents | Content of phenolic compounds (mg/g) | |||
|---|---|---|---|---|
| SP | SG | SA | TP* | |
| 70% Iso-propanol | 7.06 ± 0.00c | 0.93 ± 0.00b | 0.12 ± 0.00a | 7.73c |
| 70% Ethanol | 8.01 ± 0.00b | 0.92 ± 0.01b | 0.13 ± 0.00a | 8.75b |
| 70% Methanol | 10.28 ± 0.00a | 1.19 ± 0.00a | 0.12 ± 0.00a | 11.95a |
SP Sinapine, SA sinapic acid, SG sinapoyl glucose, TP total phenolics
Values in the same column followed by the same superscript letter are not significantly different (p ≤ 0.05)
*Estimated as sinapic acid equivalent (SAE) from the total area of all peaks including SP, SG, SA and the other unidentified peaks
Total phenolics for the canola samples are shown in Table 3. The total phenolics were 16.16–22.54, 17.73–19.71 and 14.56–16.66 mg/g in the defatted canola seeds, press cakes and meals, respectively. Sum of the phenolic acids estimated by HPLC-DAD were found to be lower than total phenolics. The corresponding values of sum of phenolic acids were 11.95–16.13, 13.10–15.00 and 9.16–14.55 mg/g, respectively. These results are in agreement with other published results. The total phenolic content was reported to be 15.42–18.07 mg/g [14], and 18.00 mg/g [15] in canola defatted meals and 10.80–12.81 mg/g in the dehulled flour [7]. Kozlowzka et al. [4] reported that the total phenolic acids content of canola meal, to vary between 6.40 and 18.40 mg/g depending on the variety of the plant and oil processing method. Canola flour methanolic extract estimated for total phenolics using Folin–Ciocalteu method and HPLC methods were reported to be 22.90 and 22.58 mg/g, respectively [9]. The variation in the total phenolic contents could be attributed to the variety, growing conditions and the degree of maturation which affect the composition of phenolic compounds.
Table 3.
Phenolic contents (mg/g) of different canola fractions
| Sinapine (SP) | Sinapoyl glucose (SG) | Sinapic acid (SA) | SP + SG +SA | Total phenolics (SAE)* | ||
|---|---|---|---|---|---|---|
| HPLC** | Folin–Ciocalteu | |||||
| Seeds | ||||||
| S1 | 11.89 ± 0.36a | 3.04 ± 0.06c | 0.20 ± 0.01g | 15.13 | 13.79b | 17.77 ± 0.07c |
| S2 | 9.58 ± 0.00b | 6.65 ± 0.00a | 0.20 ± 0.00g | 16.43 | 15.33a | 18.46 ± 0.71b |
| S3 | 11.73 ± 0.32ab | 1.13 ± 0.03f | 0.11 ± 0.00k | 12.97 | 12.55d | 16.30 ± 0.50c |
| S4 | 10.12 ± 0.00b | 3.01 ± 0.00c | 0.25 ± 0.00f | 13.38 | 13.14c | 19.39 ± 0.70b |
| S5 | 9.16 ± 0.19c | 2.96 ± 0.07c | 0.10 ± 0.00k | 12.22 | 12.53c | 17.58 ± 0.00c |
| S6 | 10.42 ± 0.07a | 2.65 ± 0.04c | 0.17 ± 0.00hi | 13.24 | 12.08d | 16.16 ± 0.01c |
| S7 | 9.20 ± 0.38b | 3.46 ± 0.16c | 0.10 ± 0.00k | 12.76 | 13.40c | 17.95 ± 0.31c |
| S8 | 8.59 ± 0.23c | 4.86 ± 0.12b | 0.15 ± 0.01j | 13.60 | 12.28c | 16.58 ± 0.41c |
| S9 | 10.06 ± 0.99b | 3.54 ± 0.30b | 0.19 ± 0.02gh | 13.73 | 12.85c | 17.55 ± 0.82c |
| S10 | 9.11 ± 0.17b | 4.75 ± 0.04b | 0.16 ± 0.00ij | 14.02 | 12.69c | 17.34 ± 0.41c |
| S11 | 8.68 ± 0.24c | 2.45 ± 0.06d | 0.19 ± 0.01g | 11.32 | 11.63d | 16.96 ± 0.25c |
| S12 | 8.65 ± 0.48c | 3.04 ± 0.16c | 0.15 ± 0.01ij | 11.84 | 13.18c | 17.95 ± 0.34c |
| S13 | 9.73 ± 0.15b | 4.54 ± 0.02b | 0.28 ± 0.00e | 14.55 | 13.76b | 19.22 ± 0.01b |
| S14 | 9.82 ± 0.41b | 1.26 ± 0.04f | 0.09 ± 0.00k | 11.17 | 11.36d | 16.58 ± 0.90c |
| S15 | 9.08 ± 0.22c | 6.54 ± 0.11a | 0.17 ± 0.00ij | 15.79 | 14.82a | 18.15 ± 0.12b |
| S16 | 10.25 ± 0.00a | 1.30 ± 0.00f | 0.10 ± 0.00k | 11.65 | 11.82c | 16.09 ± 0.26c |
| S17 | 10.70 ± 0.00a | 5.82 ± 0.00a | 0.59 ± 0.00a | 17.11 | 15.16a | 22.54 ± 0.46a |
| S18 | 10.94 ± 0.06a | 0.50 ± 0.01g | 0.15 ± 0.00ij | 11.59 | 11.00e | 16.20 ± 0.44c |
| Press cakes | ||||||
| C1 | 11.17 ± 0.27a | 2.58 ± 0.06d | 0.29 ± 0.01e | 14.04 | 12.73c | 17.73 ± 0.31c |
| C2 | 9.90 ± 0.02a | 4.01 ± 0.02b | 0.44 ± 0.00b | 14.35 | 13.71b | 19.71 ± 0.00b |
| C3 | 11.31 ± 0.20ab | 2.37 ± 0.03d | 0.35 ± 0.00d | 14.03 | 14.47b | 18.01 ± 0.59b |
| Meals | ||||||
| M1 | 10.11 ± 0.10b | 1.37 ± 0.01e | 0.33 ± 0.00d | 11.80 | 11.19e | 16.66 ± 0.43c |
| M2 | 6.11 ± 0.10e | 1.81 ± 0.02e | 0.41 ± 0.00c | 8.33 | 9.19f | 14.56 ± 0.37d |
| M3 | 9.39 ± 0.09c | 1.35 ± 0.01e | 0.35 ± 0.00d | 11.09 | 11.67e | 16.26 ± 0.22c |
| M4 | 8.80 ± 0.00c | 1.65 ± 0.00e | 0.39 ± 0.00c | 10.84 | 10.99e | 16.10 ± 0.69c |
| M5 | 9.22 ± 0.29c | 1.70 ± 0.05e | 0.32 ± 0.01d | 11.24 | 10.97b | 15.43 ± 0.39d |
| M6 | 7.66 ± 0.16d | 1.58 ± 0.03e | 0.38 ± 0.01cd | 9.62 | 9.57f | 15.42 ± 0.10d |
| M7 | 8.76 ± 0.08c | 1.99 ± 0.01d | 0.41 ± 0.00c | 11.16 | 11.16e | 16.40 ± 0.24c |
Values are means ± the standard deviation (SD)
S Seed, C Cake, M Meal
Values in the same column followed by similar superscript letters are not significantly different at p ≤ 0.05
*SAE = sinapic acid equivalents
**Estimated from the total area of all the peaks based on the sinapic acid calibration curve
Quantification of Canola Phenolics using the HPLC–DAD
Method Evaluation
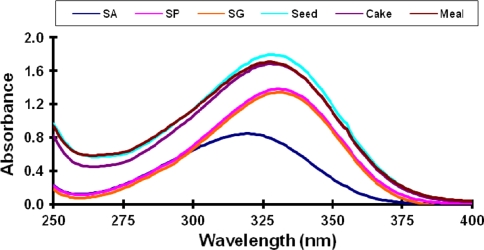
Pure phenolic compounds namely sinapic, ferulic, caffeic, t-cinnamic, syringic, p-coumaric, salicylic, vanillic, gallic aid, syringaldehyde, sinapine, sinapoyl glucose as well as the canola extracts were spectrophotometrically scanned to investigate their absorbance spectra. These spectra were used as initial indicators for the phenolic profile of canola extracts. It was found that canola extracts showed maximum absorbance at 330 nm. This indicates that the majority of phenolics in the canola extracts absorbs at 330 nm. The UV absorbance maxima for sinapine thiocyanate, sinapoyl glucose, and sinapic acid (Fig. 2) were ~329, ~329 and 322 nm, respectively. As sinapine is known to be the major phenolic component of canola, the detector wavelength was set to the sinapine maximum (330 nm) as the primary wavelength and data were mainly analysed at this wavelength. Furthermore, this wavelength has been previous used in determinations of sinapine [9, 10, 16] and sinapic acid [9, 10, 17]. It was confirmed from the authentic phenolic standards and their spiking with the canola extracts that the major phenolic compounds found in all canola extracts are sinapine, sinapoyl glucose and sinapic acid along with some other unknown components which may be other sinapic acid derivatives.
Fig. 2.
Absorbance spectra of sinapic acid, sinapine, sinapoyl glucose and canola extracts. SA sinapic acid, SP sinapine, SG sinapoyl glucose
Comparing the spectral data of the canola extracts with those obtained for phenolic standards (Fig. 2) confirmed the reliability of quantifying sinapine, sinapoyl glucose, and sinapic acid. Therefore, calibration curves using different concentrations of pure sinapine, sinapic acid and sinapoyl glucose were prepared and used to calculate the actual concentrations of those components in canola extracts. The retention time, peak area and separation quality were studied at different solvent flow rates; 0.6, 0.7, 0.8 and 1.0 ml/min. A strong (R 2 = 0.99) negative linear correlation was found between the flow rate and the retention time of the individual components. The 0.8 ml/min flow rate gave the highest real area percent as well as highly smooth and symmetric peaks. So, it was chosen for the separation of the phenolic compounds during this study. Both the columns, gemini and synergi showed good and consistent results however in the case of gemini, sinapine and sinapoyl glucose eluted adjacently making it erroneous in interpreting the two peaks for quantification. Thus we proceeded with the synergi column for this study.
Method Validation
Detection limits, quantification limits, linearity ranges, and recovery values were evaluated using authentic standards (Table 2). Detection and quantification limits were 0.20, 0.40 and 0.20 μg/ml for sinapine, sinapoyl glucose, and sinapic acid, respectively. However, quantification limits were 0.50, 0.80 and 0.50 μg/ml. Linearity was evaluated over the range of 0.20–200.00 μg/ml with five measurements for each calibration point. All the analyses exhibited excellent linearity over the evaluated range with correlation coefficients between 0.99 and 1.00.
Table 2.
Parameters of the calibration curves for different phenolic standards
| Phenolics | Linearitya | Detection limit (μg/ml)b | Quantification limit (μg/ml)b | Recovery (%)c |
|---|---|---|---|---|
| Sinapic acid | 1.00 | 0.20 | 0.50 | 98.98 ± 0.21 |
| Sinapine | 0.99 | 0.20 | 0.50 | 98.80 ± 0.22 |
| Sinapoyl glucose | 0.99 | 0.40 | 0.80 | 99.60 ± 0.16 |
aLinearity was expressed as the correlation coefficients of each calibration curve which was determined by five calibration points
bMeans of triplicate measurements ± standard deviation; quantification limit is the concentration at which the determination was most accurate with recovery over 98.00%
cRecovery values are expressed as mean ± standard deviation conducted in canola extracts through spiking
The retention time and area for each peak were almost the same with intra- and inter-day standard deviation (SD) values less than 0.10 and 1.80 for the retention time and area, respectively.
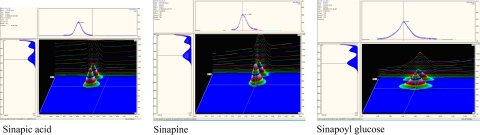
To demonstrate the method specificity, the peak purity tests were performed and the diode array analysis confirmed that each chromatographic peak was attributable to a single component (Fig. 3). To verify its accuracy, recovery tests were carried out through spiking known amounts of the standards into the canola extract. During this, the samples’ extracts were spiked with all standards at 10, 50 and 100 μg/ml either separately or as a combination. The recovery values of all compounds were more than 98.0% (Table 2). To confirm the repeatability of the method, seeds extracts were analysed repeatedly five times a day for five consequent days. The variation coefficients of all measurements were less than 1.2%. In all cases, peak purity was carefully examined by tracing the spectra. High elution purity was observed for all the peaks with match factor always around 1000. The strong linear correlation (R 2 > 0.99) between the peak area and the concentration advocated the possibility of using peak area in the determination of the concentration of those compounds within the sample extracts. The following regression equations generated from the calibration curves were used to calculate the actual amount of sinapine (Y = 1217x − 0.0205), sinapoyl glucose (Y = 681.71x + 1.1741), and sinapic acid (Y = 1817.4x + 0.1903); where Y is the area (mAU* min) and x is the concentration (mg/ml).
Fig. 3.
Purity of the phenolic standards used in this study
Analysis of Canola Extracts
To achieve better separation of phenolics, canola extracts were diluted (1:1 v/v) in different solvents including 70% methanol, 70% acidified methanol (1.2% o-phosphoric acid), and mobile phase A. Those extracts were injected either without dilution (5 μl) or after dilution (10 μl). This was conducted in order to get pH values for the extracts similar to that of the mobile phase to provide the maximum compatibility between the sample solution with the mobile phase and thus achieving the best separation. The pH values were ~5.6, 2.2, 1.5 in 70% methanol, 70% acidified methanol, and mobile phase A, respectively. It was found that diluting the extracts 1:1 (v/v) with the mobile phase achieved the highest separation and revealed the highest contents of the individual phenolics as well as the total phenolic content. So, this procedure was followed during this study.
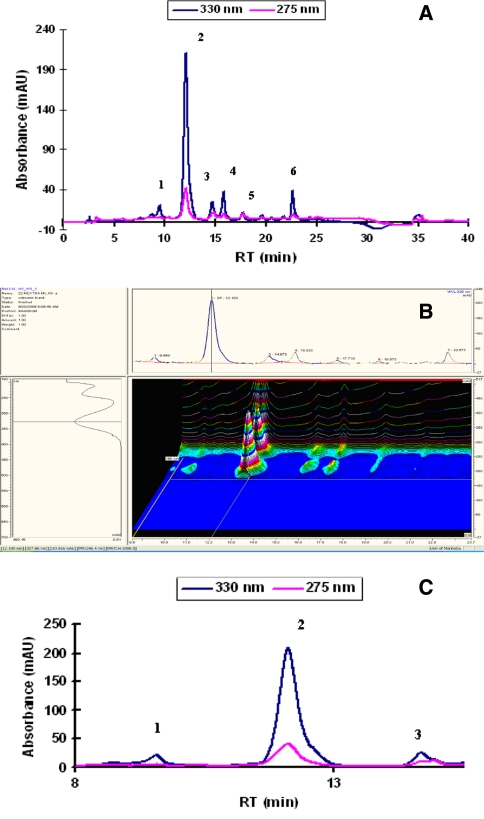
HPLC-DAD chromatograms of canola seed extract are shown in Fig. 4. The chromatograms of the canola extracts were monitored at 230, 275 and 330 nm. The content of the sinapic acid derivatives were calculated at 330 nm. Contents of the major phenolics in canola extracts (shown in Fig. 4) are illustrated in Table 3. It was found that sinapine (the choline ester of sinapic acid) was the major phenolic constituent in canola seeds, meals and press cakes’ extracts representing 69.76–87.15% of the total phenolic content of different canola fractions. This was also true for canola grown under field trials. Its contents were 8.65–11.89, 9.90–11.31 and 6.11–10.11 mg/g of the defatted canola seeds, press cakes and meals, respectively. Furthermore, sinapic acid; the most known free phenolic acid in canola seed constituted 0.86–3.76% of the total phenolics reaching 0.09–0.59 mg/g defatted canola. Sinapine was also the principal component of the “00” rapeseed used in this study.
Fig. 4.
HPLC chromatogram of canola extract: 1- sinapoyl glucose, 2-sinapine, 3- sinapic acid, 4, 5 and 6- other unidentified sinapic acid derivatives. a 2D chromatogram, b 3D chromatogram, c 2D chromatogram of the major phenolics
Similar results were reported by Cai and Arntfield [9] who stated that the 70% methanolic extract of canola flour had 11.74 and 0.40 mg/g sinapine and sinapic acid contents representing 51.99 and 1.77% of the canola total phenolics, respectively. The present results also conform to those reported by Kozlowzka et al. [18] and Shahidi and Naczk [19]. The other identified phenolic compound in this study; sinapoyl glucose ranged from 1.13 to 6.65, 2.37 to 4.01 and 1.35 to 1.99 mg/g in the extracts of canola seeds, press cakes and meals. This finding supports the possibility of using the methanolic extracts of the defatted canola residues as a source of natural antioxidants especially sinapoyl glucose to be used to enhance the stability of lipids and lipid-containing systems. Wanasundara et al. [5] studied the antioxidant effect of different TLC fractions of canola extract. The main compound in the fraction with the highest antioxidant activity was 1-O-β-d-glucopyranosyl sinapate. Seeds of Brassica juncea showed significantly lower contents of sinapoyl glucose (0.50 mg/g or 4% of the total phenolics) and total phenolics than those of canola (Brassica napus), however there were no significant differences in both sinapine and sinapic acid contents.
Evaluation of Unidentified Peaks of the Canola Extracts
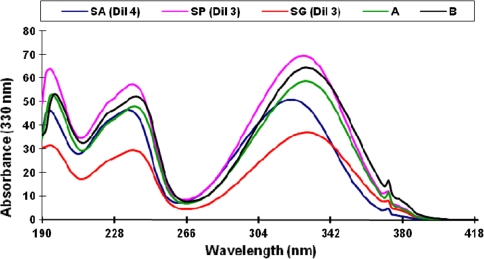
Attempts were made to identify the unknown peaks other than sinapine, sinapoyl glucose, and sinapic acid. The unknown phenolic compounds could be other sinapic acid derivatives. The amount of these unidentified compounds ranged from 2.60 to 5.00 mg/g (SAE). From these unknowns, the two major peaks eluting at 17.35 (A) and 23.30 (B) min represented about 90% of the total area of the unknown peaks. The absorbance maxima of all the unknown compounds ranged from 321 to 329 nm supporting that they are members of the cinnamic acid derivatives [20]. The absorbance spectra of the two major unknown compounds as well as those of sinapine and sinapoyl glucose are close to 330 nm (Fig. 5).
Fig. 5.
Ultraviolet spectra of the two major unidentified compounds (A and B) as compared to those of sinapine thiocyanate (SP), sinapic acid (SA) and sinapoyl glucose (SG)
The presence of other unknown components in the canola extract rather than SP, SG and SA agrees with previous findings. Fenton et al. [21] identified seven other compounds in the acetone extract of rapeseed that, upon hydrolysis, produced sinapic acid. Furthermore, it was reported that the methanolic extracts of rapeseed showed other 31 compounds which could contribute to the sinapic acid ester fraction [22]. In addition to the dominating sinapic acid derivatives, syringic, ferulic and p-coumaric acids were also detected in rapeseed [7, 18].
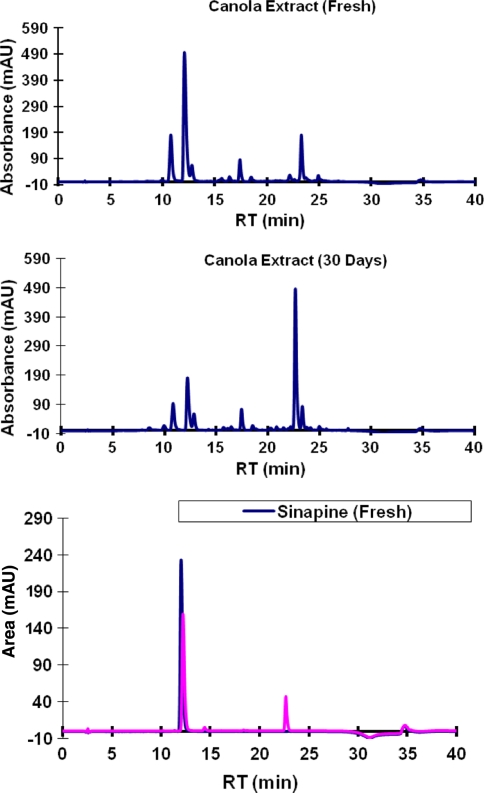
In a further attempt to identify other peaks, a 70% methanolic extract stored at room temperature (25.0 ± 2.0 °C) for 30 days (Fig. 6) showed that there was a significant increase in an unknown compound eluting at 22.6 min. The increase in this unknown compound was accompanied by a decline in sinapine. To confirm that this compound is a degradation product of sinapine, a solution of pure sinapine was stored under similar conditions. Data (Fig. 6) indicates that this compound was likely generated from sinapine. The degradation rate of sinapine standard solution at room temperature was 6.97, 20.95, 40.39 and 84.92% after 1, 2, 3 and 30 days, respectively. There was no significant change in either the extracts or the pure standards when kept frozen for 30 days as compared to the fresh extracts.
Fig. 6.
Degradation of canola extracts and pure sinapine during storage at room temperature for 30 days
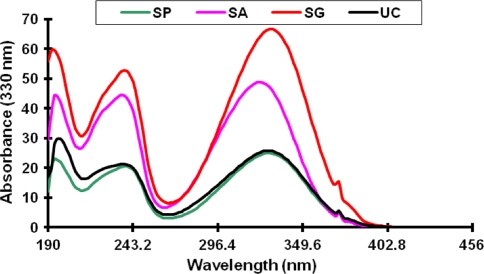
Comparing the absorbance spectrum of this unknown compound with those of sinapine thiocyanate, sinapoyl glucose, and sinapic acid (Fig. 7), the unknown compound showed an identical spectrum to sinapine.
Fig. 7.
Ultraviolet spectra of the unidentified compound (UC) comparing to sinapine thiocyanate (SP), sinapic acid (SA) and sinapoyl glucose (SG)
These results suggest that one of the unknown compounds could be a degradation product of sinapine. These results also suggest that canola extracts should be kept frozen to avoid misinterpretation of compounds in fresh and stored extracts. Further work is needed to study the effect of different storage conditions on the phenolic profile of canola either as a whole or defatted seeds as well as its extracts.
Conclusions
The maximum extraction of phenolic compounds from canola was achieved by using 70% methanol. Extracts of canola seed and its by-products contains significant amounts of phenolics, mainly sinapic acid and its choline ester; sinapine. All substrates showed the content of high amounts of sinapine, sinapoyl glucose and sinapic acid (in this order). This profile was also demonstrated in the residues of the edible oil processing. Seeds grown for field trials also indicated this pattern. Furthermore, the study also indicates that sinapine could be degraded to produce another phenolic compound and that phenolic extracts of canola should be kept frozen to maintain the profile of their active components. Further studies to understand the mechanism of degradation and structural changes for sinapine and other sinapic acid derivatives are needed.
Acknowledgements
This study was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canola Council of Canada, and Syngenta Inc, Canada. Sinapine thiocyanite and sinapoyl glucose were generously contributed by Dr. A. Baumert (retired), IPB-Halle, Germany.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Naczk M, Amarowicz R, Sullivan A, Shahidi F. Current research developments on polyphenolics of rapeseed/canola: a review. Food Chem. 1998;62:489–502. doi: 10.1016/S0308-8146(97)00198-2. [DOI] [Google Scholar]
- 2.Koski A, Pekkarinen S, Hopia A, Wahala K, Heinonen M. Processing of rapeseed oil: effects on sinapic acid derivative content and oxidative stability. Eur Food Res Technol. 2003;217:110–114. doi: 10.1007/s00217-003-0721-4. [DOI] [Google Scholar]
- 3.Amarowicz R, Naczk M, Shahidi F. Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J Agric Food Chem. 2000;48:2755–2759. doi: 10.1021/jf9911601. [DOI] [PubMed] [Google Scholar]
- 4.Kozlowska H, Naczk M, Shahidi F, Zadernowski R. Phenolic acids and tannins in rapeseed and canola. In: Shahidi F, editor. Canola and rapeseed; production, chemistry, nutrition and processing technology. USA: Van Nostrand Reinhold; 1990. pp. 193–210. [Google Scholar]
- 5.Wanasundara U, Amarowicz R, Shahidi F. Isolation and identification of an antioxidative component in canola meal. J Agric Food Chem. 1994;42:1285–1290. doi: 10.1021/jf00042a006. [DOI] [Google Scholar]
- 6.Baumert A, Milkowski C, Schmidt J, Nimtz M, Wray V, Strack D. Formation of a complex pattern of sinapate esters in Brassica napus seeds catalyzed by enzymes of serine carboxypeptidase-like acyltransferase family. Phytochem. 2005;66:1334–1345. doi: 10.1016/j.phytochem.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Krygier K, Sosulski F, Hogge L. Free, esterified and insoluble phenolic acids. II. Composition of phenolic acids in rapeseed flour and hulls. J Agric Food Chem. 1982;30:334–336. doi: 10.1021/jf00110a029. [DOI] [Google Scholar]
- 8.Amarowicz R, Raab B, Shahidi F. Antioxidant activity of phenolic fractions of rapeseed. J Food Lipids. 2003;10:51–62. doi: 10.1111/j.1745-4522.2003.tb00005.x. [DOI] [Google Scholar]
- 9.Cai R, Arntfield S. A rapid high-performance liquid chromatographic method for the determination of sinapine and sinapic acid in canola seed and meal. JAOCS. 2001;78:903–910. doi: 10.1007/s11746-001-0362-4. [DOI] [Google Scholar]
- 10.Thiyam U, Stöckmanna H, Feldeb TZ, Schwarza K. Antioxidative effect of the main sinapic acid derivatives from rapeseed and mustard oil by-products. Eur J Lipid Sci Technol. 2006;108:239–248. doi: 10.1002/ejlt.200500292. [DOI] [Google Scholar]
- 11.FOSFA International Official Method (1998) Determination of oil content in oilseeds: solvent extraction (reference method) FOSFA International Manual, Part 2, Standard Contractual Method 45–50, pp 283–288
- 12.Swain T, Hillis WE. The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- 13.CH I. International conference on harmonization: guidance for industry: text on the validation of analytical procedures availability. Fed Regist. 1995;60:11260–11262. [Google Scholar]
- 14.Shahidi F, Naczk M. An overview of phenolics of canola and rapeseed: chemical, sensory and nutritional significance. JAOCS. 1992;69:917–924. doi: 10.1007/BF02636344. [DOI] [Google Scholar]
- 15.Thiyam U, Kuhlmann A, Stockmann H, Schwarz K. Prospects of rapeseed oil by-products with respect to antioxidative potential. C. R. Chimie. 2004;7:611–616. [Google Scholar]
- 16.Wang SX, Oomah BD, McGregor DI. Application and evaluation of ion-exchange UV spectrophotometric method for determination of sinapine in Brassica seeds and meals. J Agric Food Chem. 1998;46:575–579. doi: 10.1021/jf9705577. [DOI] [PubMed] [Google Scholar]
- 17.Hagerman AE, Nicholson RL. High-performance liquid chromatographic determination of hydroxycinnamic acids in the maize mesocotyl. J Agric Food Chem. 1982;30:1098–1102. doi: 10.1021/jf00114a023. [DOI] [Google Scholar]
- 18.Kozlowska H, Rotkiewicz DA, Zadernowski RL, Sosulskik FW. Phenolic acids in rapeseed and mustard. JAOCS. 1983;60:1119–1123. doi: 10.1007/BF02671339. [DOI] [Google Scholar]
- 19.Shahidi F, Naczk M. Food phenolics: sources, chemistry, effects, applications. Lancaster: Technomic Publishing; 1995. [Google Scholar]
- 20.Harbome JB. Ultraviolet spectroscopy of polyphenols. In: Pridham JB, editor. Methods in polyphenol chemistry. New York: Macmillan; 1964. pp. 13–36. [Google Scholar]
- 21.Fenton TW, Leung J, Clandinin DR. Phenolic components of rapeseed meal. J Food Sci. 1980;45:1702–1705. doi: 10.1111/j.1365-2621.1980.tb07592.x. [DOI] [Google Scholar]
- 22.Lacki K, Duvnjak Z. Comparison of three methods for the determination of sinapic acid ester content in enzymatically treated canola meals. Appl Microbiol Biotechnol. 1996;45:530–537. doi: 10.1007/BF00578467. [DOI] [PubMed] [Google Scholar]