Abstract
Type 1 diabetes is considered as Th1 cell mediated autoimmune disease and the suppression of Th1 cells or the activation of Th2 cells has been regarded as a plausible immunologic intervention for the prevention of type 1 diabetogenesis in a rodent model. CpG ODN is an immunostimulatory sequence primarily present in bacterial DNA, viral DNA and BCG. CpG ODN is conventionally classified as a Th1 cell activator, which has been clinically applied to cancer, allergy and infectious disease. Recently, there was a promising report of that CpG ODN administration suppressed the development of type 1 diabetes in NOD mice by inducing Th2 cell mediated cytokine. However, the antidiabetogenic effect of CpG ODN on NOD mice is controversial. Thus, two studies were serially undertaken with various kinds of CpG motif to find a more optimal sequence and administration method. In the first study, CpG ODN was vaccinated four times and pancreatic inflammation and the quantity of serum insulin subsequently evaluated. In the second study, the amounts of IFN γ and IL-4 in sera were measured as representative cytokines of Th1 and Th2 cells, respectively. As a result, vaccination or continuous injection of CpG ODN failed to show a preventive effect on type 1 diabetogenesis in NOD mice. Structural differences of CpG ODN also had no affect on the result. CpG ODN also consistently showed affect on the pancreatic pathology. The productions of IFN and IL-4 were γ detected only in the K and D type CpG ODN administration groups. Comparison of the two cytokines leads to the conclusion that CpG ODN generated a Th1-weighted response in both study groups. It was assumed that CpG ODN failed to produce Th2-weighted cytokine milieu, which can overcome the genetically determined phenotype of NOD mice. Given these results, it was concluded that the immunotherapeutic application of CpG ODN on Type 1 diabetes had clear limitations.
Keywords: Type 1 diabetes, CpG ODN, NOD mice, Th1/Th2 balance
INTRODUCTION
Type 1 diabetes is a metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion.1,2 Type 1 diabetes is caused by the progressive destruction of the insulin-producing beta cells through an autoimmune process.3 It has been suggested that T helper 1 (Th1) cells play a pathogenic role in the autoimmune destruction of beta cells, whereas T helper 2 (Th2) cells play a nondestructive or preventive role in type 1 diabetes due to the cytokine network they produce.4
Th1 cells secrete interleukin (IL)-2, interferon (IFN) γ and tissue necrotic factor (TNF) α, and predominantly support cell-mediated immunity. Th2 cells secrete interleukins IL-4, IL-5, IL-10 and IL-13, and primarily support humoral factor-mediated immunity.5 Based on the immunopathogenesis of type 1 diabetes, its immunotherapy has been targeted to the immunosuppression of autoimmune diseases, deletion of selective T cell subsets and the induction of immune tolerance to islet protein.6-8 Numerous immunostimulatory protocols have been designed to down-regulate the development of T cell mediated autoimmune diabetes in rodent models, such as NOD (nonobese diabetic) mice and BB (biobreeding) rats.9-11 As an effective immune modulator, BCG (Bacile Calmette-Guerin) therapy has been well documented. However, the underlying immunologic mechanism of BCG therapy in NOD mice is obscure.12-14
Cytosine-phosphorous-Guanine (CpG) oligonucleotide (ODN) is unmethylated base pairs present in bacterial DNA, viral DNA and BCG.15,16 CpG ODN can be classified into the K and D types according to the phosphorous backbone structure.17 K type CpG ODN, with phosphorothioate backbones, triggers the maturation of plasmacytoid dendritic cells and stimulate the production of immunoglobulin M and IL-6. Whereas, D type CpG ODN, with a phosphodiester/phosphorothioate backbone, triggers the maturation of antigen presenting cells, preferentially inducing the secretion of IFN α and IFN γ.18,19 CpG ODN also can be classified into the human and mouse types according to the motif contained. Although there is some cross-stimulation, the human or murine type CpG ODN has an ability to activate human or murine leukocytes, respectively.16,20 Such a CpG ODN is known to possess immunomodulatory activity in the same way as other DNA vaccines.15,16 Many experimental and clinical studies have manifested the CpG motif activate Th1-like immune response that can be harnessed as immune therapies for cancer, allergies and infectious diseases.12,14,21,22 A recent report suggested CpG ODN treatment significantly reduces the cumulative incidence of diabetes in NOD mice.23 However, the effect of CpG ODN on NOD mice is still controversial. Besides, effective CpG sequence, dose and administration methods have, as yet, not been elucidated. To clarify these issues, two studies were serially performed using different types of CpG ODN with different administration methods. Thus, this study was designed to evaluate the effect of CpG ODN in reducing the incidence of type 1 diabetes, and compare the effects of species-specific CpG ODN on a rodent model. Second, an attempt was made to evaluate the effect of a continuous injection of CpG ODN in reducing the development of type 1 diabetes using different structural of CpG ODN types.
MATERIALS AND METHODS
NOD mice
Five week-old female NOD/LtJ mice, from Jackson laboratory (Bar Harbor, ME, USA), were bred in the animal laboratory of Wonju College of Medicine, Yonsei University. In study one, 40 mice were divided into 3 groups. Twenty were used as a control group and ten each were used as mouse and human CpG ODN groups. In study two, 40 mice were divided into 4 groups; ten each were used as the control, K, D and Cohen's types CpG ODN groups. NOD mice were maintained in autoclaved cages, with sterile water. Rat diet Pico 5053 (Nutritional International Inc., Brentwood, USA) was given as food. Food and water was given without restrictions.
CpG preparation and injection
All the CpG ODNs were synthesized at Metabion GmbH (Tlanegg/Martinsried, Germany). The mouse and human type CpG ODN sequences were as follows:
Mouse type CpG ODN: 5'-TCC ATG ACG TTC CTG ACG TT-3'
Human type CpG ODN: 5'-TCG TCG TTT TGT CGT TTT GTC GTT-3'
K, D and Cohen's type CpG ODN sequences were as follows:
The K type CpG ODN was the same as that of the mouse type CpG ODN: 5'-TCC ATG ACG TTC CTG ACG TT-3'
D type CpG ODN: 5'-GGT GCA TCG ATG CAG GGG GG-3'
Cohen's type CpG ODN: 5'-TCC ATA ACG TTG CAA ACG TTT TG-3'
In study one, 100µL (10µg) of mouse type CpG ODN, human type CpG ODN and normal saline were subcutaneously vaccinated to each group in the inguinal area. Vaccination was started during the 5 week-old period, and repeated 7, 14 and 28 days later. In study two, 100 µL (10 µg) of the K, D and Cohen's CpG ODN types and normal saline were given weekly from the 5 week-old to the 24 week-old periods, as long as the NOD mice were not diabetic.
Blood glucose
The blood glucose was measured using a Surestep glucometer (Lifescan, California, USA).
Animals were considered diabetic when two consecutive measurements were greater than 13.75 mM/L (250 mg/dL), and sacrificed when considered diabetic. The mice were otherwise sacrificed at 24 weeks of age in both studies.
Pancreatic histology
The pancreata were fixed with 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) and embedded in paraffin. The paraffin blocks were serially sectioned into thickness of 5µm and three hematoxylin-eosin (H-E) stained slides made for each block. Of the three H-E slides from each block, the one with the most evenly distributed islets with regard to size was selected. All the H-E pancreata sections were evaluated for insulitis. Pancreata were graded for insulitis on the basis of the most severely involved islet in each pancreas. Each pancreas was graded, and placed into one of the following categories: Grade G0 when no infiltrate was detected, G1 when peri-insulitis or an intraislet infiltrate occupied < 25% of the islet, G2 when 25-50% of the islet was occupied by intraislet inflammatory cells and G3 when more than 50% of the islet was occupied by lymphocytes.
Insulin immunoassay
The serum insulin level was checked at the time of sacrifice, and was measured in the fasting state using a radioimmunoassay commercial Kit (Linco Research Inc. St. Charles, Missouri, USA).
Cytokine assay
The IFN γ and IL-4 in the sera were measured at the time of sacrifice. Serum cytokines were measured with an ELISA at the cytokine bank, Chonbuk National University (Chonbuk, Korea).
Statistical significance
Prism 3.0 (San Diego, USA) and SPSS10 (SPSS Inc, Chicago, USA) were used for statistical analyses. Kaplan-Meier, chi-square, one-way ANOVA and repeated measures ANOVA tests were performed to clarify the differences between the experimental and control groups. P values below 0.05 were considered statistically significant.
RESULTS
Study One
CpG ODN vaccination is not protective on type 1 diabetogenesis in NOD mice
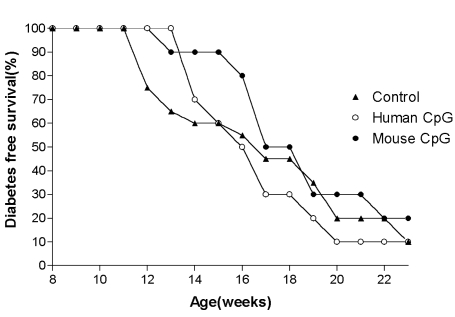
The diabetes free survival was 20% in the mouse CpG ODN type, 10% in human CpG ODN type and controls by 24 weeks of age. No statistical difference was found between the groups by the end or during the course of the study (Fig. 1).
Fig. 1.
Diabetes free survival of female NOD mice (Study one).
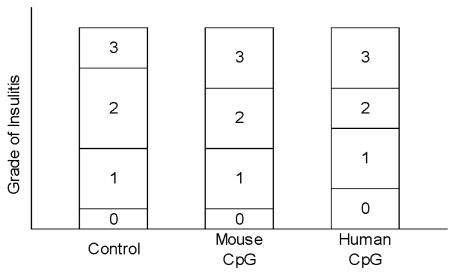
CpG ODN vaccination had no effect on the degree of insulitis
No difference in insulitis was found between the three groups. The mouse and human CpG ODN types did not protect or aggravate insulitis in NOD mice (Fig. 2).
Fig. 2.
Grade of insulitis in NOD mice.
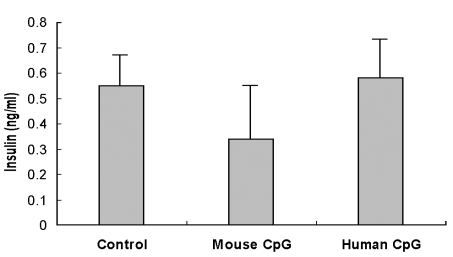
Quantification of insulin
There were no statistical differences in levels of insulin between the three groups (Fig. 3).
Fig. 3.
Insulin radioimmunoassay in NOD mice.
Study Two
Continuous injection of CpG ODN is not protective on type 1 diabetogenesis in NOD mice
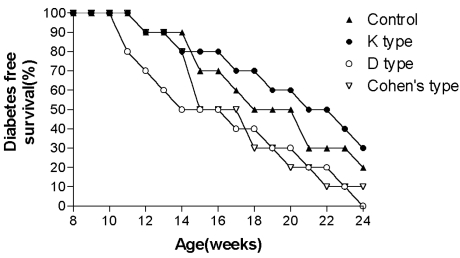
The diabetes free survivals were 30, 20, 10 and 0% in the K, control, Cohen's and D CpG ODN type groups, respectively, by the end of study period. No statistical differences were observed between the groups during the course of or at the end of the study period (Fig. 4).
Fig. 4.
Diabetes free survival of female NOD mice (Study two).
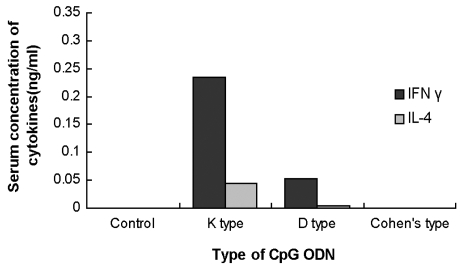
Serum cytokine concentration
IFN γ and IL-4 were measured as representative cytokines of Th1 and Th2 cells, respectively. The concentrations of IFN γ and IL-4 were below the limits of detection in the control and Cohen's type CpG ODN groups. The mean values of IFN γ and IL-4 were 0.235 and 0.045 ng/mL, and 0.052 and 0.004 ng/mL in the K and D type CpG ODN groups, respectively (Fig. 5).
Fig. 5.
Comparative Th1 and Th2 cytokine releases in the sera of the different CpG vaccinated mice types.
DISCUSSION
The two serially performed studies showed that CpG ODN has no protective effect against type 1 diabetogenesis in NOD mice. Our rationale for CpG ODN adoption in the rodent model of autoimmune diabetes was based on the facts that BCG may have the potential to suppress the development of type 1 diabetes, or attenuate insulitis in NOD mice.12,24,25 Tokunaga demonstrated that the effective fraction of BCG contain unmethylated CpG ODN or CC-dinucleo-typed ODNs.26 Now, biologically active CpG ODN is commonly found in bacterial, viral and vaccine plasmid DNA.15,16 Despite much controversy relating to the Th1/Th2 balance in type 1 diabetes, Cohen's group reported a promising result in the suppression of type 1 diabetes in NOD mice with their CpG motif by inducing more of IL-10 than IFN γ.23 Bioactive CpG ODN is classified into the K or D types, as well as the mouse or human types. Cohen's motif can be classified as non-K and non-D types.
In study one, species-specific K type CpG ODN was used, although this kind of discrimination is not absolute.18,20 Before the in vivo administration of human type CpG ODN, it was confirmed that the human type CpG motif could stimulate innate immune cells (mainly macrophage) in mice (unpublished data). The potency of the human type CpG motif has been proved in a rodent model, as it has cross-reactivity with mouse type CpG ODN. In study two, the K, D and Cohen's type CpG ODN were used. Theoretically, using K type CpG ODN in NOD mice has the advantage of modulating the autoimmune process due to its known adjuvant potency, while the function of the D type CpG motif is unclear. The Cohen's CpG motif was adopted in our therapeutic protocol to see whether it could reproduce the promising result, as well as to evaluate the uniqueness of this sequence in causing different results.
As shown Fig. 1 and 4, the diabetes free survivals of NOD mice were not statistically significant, and rather consistent with the natural incidence of diabetes in NOD mice. The results of Cohen's type CpG ODN, once assumed to be a significant suppressor of type 1 diabetes, were no better than any other CpG sequences. In study one, the peri-insulitis and insulin concentration were evaluated. Mouse and human CpG ODN had no protective effects against inflammation of pancreatic islet cells. Also, the quantity of serum insulin was no different between the study groups. In study two, IFN γ and IL-4 secretions were only shown in the K and D type CpG ODN groups, and the level of secretion was lower than the limits of detection in the control and Cohen's CpG ODN groups. Cohen's study explained their result in terms of the Th1/Th2 balance, with relatively a higher level of IL-10 to IFN γ causing such a consequence. Although the vaccination schedule, including the timing and dose, was the same as in Cohen's study, in our first experiment Cohen's CpG ODN failed to secrete Th1 and Th2 cell mediated cytokines. Thus, it is strange that in both experiments and in nature the same results are not reproducible. Our hypothesis is, "such a discrepancy might be due to the difference in CpG potencies". Cohen's CpG ODN may in fact not be active enough to induce apoptosis of pathogenic autoreactive T cells. Overall, it was assumed that CpG ODN produced Th1-weighted cytokine milieu and the genetically determined phenotype of NOD mice was not affected by CpG ODN as a consequence.
Our findings indicate that CpG ODN can not confer significant relief of type 1 diabetogenesis in NOD mice. In conclusion, this result indicates that CpG ODN has clear limitations when applied as a immunotherapeutic for type 1 diabetes.
References
- 1.Schuster DP, Duvuuri V. Diabetes mellitus. Clin Podiatr Med Surg. 2002;19:79–107. doi: 10.1016/S0891-8422(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 2.Scott A, Donnelly R. Improving outcomes for young people with diabetes: use of new technology and skills-based training approach is urgently needed. Diabet Med. 2001;18:861–863. doi: 10.1046/j.1464-5491.2001.00627.x. [DOI] [PubMed] [Google Scholar]
- 3.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 4.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune disease. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 5.Tisch R, McDevitt HO. Insulin dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SS, White TC, DeLuca D. Therapeutic alteration of insulin-dependent diabetes mellitus progression by T cell tolerance to glutamic acid decarboxylase 65 peptides in vitro and in vivo. J Immunol. 2001;167:569–577. doi: 10.4049/jimmunol.167.1.569. [DOI] [PubMed] [Google Scholar]
- 7.Palmer JP. Immunomodulatory therapy of human type 1 diabetes: lessons from the mouse. J Clin Invest. 2001;108:31–33. doi: 10.1172/JCI13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simone EA, Wegmann DR, Eisenbarth GS. Immunologic "vaccination" for the prevention of autoimmune diabetes (type 1A) Diabetes Care. 1999;22:7–15. [PubMed] [Google Scholar]
- 9.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: Sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: As good as it gets? Nature Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 11.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: Immune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch A. Immunoregulatory and cytokine imbalance in the pathogenesis of IDDM. Diabetes. 1994;43:613–621. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- 14.Tascon RE, Colston MJ, Ragno S, Stavropoulous E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nature Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM, Kline JN. Immune effect and therapeutic applications of CpG motif in bacterial DNA. Immunopharmacology. 2000;48:303–305. doi: 10.1016/s0162-3109(00)00228-9. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM, Wagner H. Causing a commotion in the blood: immunotherapy progresses from bacteria to bacterial DNA. Immunol Today. 2000;21:521–526. doi: 10.1016/s0167-5699(00)01719-9. [DOI] [PubMed] [Google Scholar]
- 17.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Lkinman DM. CpG oligodeoxynucleotides as vaccine adjuvant in primates. J Immunol. 2002;168:1659–1663. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 18.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD 11c-type II dendritic precursor and CD 11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–2295. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Shoda LK, Brayton KA, Estes DM, Palmer GH, Brown WC. Induction of interleukin-6 and interleukin-12 in bovine B lymphocytes, monocytes, and macrophages by a CpG oligonucleotide (ODN 2059) containing the GTC GTT motif. J Interferon Cytokine Res. 2001;21:871–881. doi: 10.1089/107999001753238123. [DOI] [PubMed] [Google Scholar]
- 21.Kim SK, Ragupatihi G, Musselli C, Choi S, Park YS, Livingston PO. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MVC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine. 1999;18:597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 22.Juffermans NP, Leemans JC, Florquin S, Verbon A, Kolk AH, Speelman P. CpG oligonucleotides enhances host defense during murine tuberculosis. Infect Immune. 2002;70:147–152. doi: 10.1128/IAI.70.1.147-152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: Modulation of spontaneous 60-k Da heat shock protein autoimmunity. J Immunol. 2000;165:6148–6155. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 24.Kim YU, Kang HS, Hwang TS, Chung CH. Prevention of insulitis by BCG administration in NOD mice of the neonatal period. J Wonju Coll Med. 1998;11:97–105. [Google Scholar]
- 25.Hwang TS, Kim HS, Kim YU, Chung CH. Prevention of overt diabetes and insulitis by BCG administration in the NOD mice in the breast-fed period. Korean J Anat. 2000;33:49–54. [Google Scholar]
- 26.Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Jpn J Infect Dis. 1999;52:1–11. [PubMed] [Google Scholar]