Abstract
Ultraviolet B (UV-B) radiation, a very small fraction of the daylight spectrum, elicits changes in plant secondary metabolism that have large effects on plant-insect interactions. The signal transduction pathways that mediate these specific effects of solar UV-B are not known. We examined the role of jasmonate signaling by measuring responses to UV-B in wild-type and transgenic jasmonate-deficient Nicotiana attenuata plants in which a lipoxygenase gene (NaLOX3) was silenced (as-lox). In wild-type plants, UV-B failed to elicit the accumulation of jasmonic acid (JA) or the bioactive JA-isoleucine conjugate but amplified the response of jasmonate-inducible genes, such as trypsin proteinase inhibitor (TPI), to wounding and methyl jasmonate, and increased the accumulation of several phenylpropanoid derivatives. Some of these phenolic responses (accumulation of caffeoyl-polyamine conjugates) were completely lacking in as-lox plants, whereas others (accumulation of rutin and chlorogenic acid) were similar in both genotypes. In open field conditions, as-lox plants received more insect damage than wild-type plants, as expected, but the dramatic increase in resistance to herbivory elicited by UV-B exposure, which was highly significant in wild-type plants, did not occur in as-lox plants. We conclude that solar UV-B (1) uses jasmonate-dependent and -independent pathways in the elicitation of phenolic compounds, and (2) increases sensitivity to jasmonates, leading to enhanced expression of wound-response genes (TPI). The lack of UV-B-induced antiherbivore protection in as-lox plants suggests that jasmonate signaling plays a central role in the mechanisms by which solar UV-B increases resistance to insect herbivores in the field.
Peak fluence rates of solar UV-B radiation at mid latitudes rarely exceed 2 μmol m−2 s−1, which represents less than 0.5% of the total quantum flux density between 285 and 700 nm. This small fraction of solar radiation has multiple effects on plants, but none of these effects, except direct DNA damage by UV-B quanta (Britt, 2004), has been unequivocally linked with the activation of a well-defined photoreceptor molecule. In an attempt to organize what is known about UV-B responses, researchers tend to define them as “UV-B specific” and “nonspecific.” One limitation of this classification is that the definition of UV-B specific is not universal. Thus, certain authors consider that a response is UV-B specific when it is activated by signaling pathways that are not used by other environmental stressors, such as wounding or oxidative stress. This criterion is frequently used, for example, to distinguish UV-B responses elicited via the UV RESPONSE LOCUS8 (UVR8)-CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1) pathway in Arabidopsis (Arabidopsis thaliana; Brown et al., 2005; Brown and Jenkins, 2008; Favory et al., 2009), because the UVR8-COP1 pathway is not engaged by common stressors and does not lead to the expression of “general stress markers” (i.e. genes controlled by stress-related hormones such as jasmonates and ethylene; Cloix and Jenkins, 2008; Jenkins, 2009). Other authors have used a photobiological criterion (Thomas and Dickinson, 1979) to define wavelength-specific responses. According to this criterion, UV-B responses would be considered specific only if their elicitation could be shown to result from the activity of a primary receptor with maximum sensitivity in the UV-B region. This definition is independent of the biochemical pathways that are actually used to generate the final response. Based on this second criterion, all the responses to solar UV-B detected under field conditions are commonly considered as “photobiologically specific,” because they cannot be explained by the activation of photoreceptors that lack a specific sensitivity peak in the narrow UV-B region (i.e. cryptochromes, phototropins, or phytochromes). The signaling pathways that mediate these specific effects of solar UV-B are poorly understood, although it is generally accepted that multiple signaling circuits are likely to be activated to explain the diversity of UV-B responses under natural conditions (Caldwell et al., 2003, 2007; Stratmann, 2003; Jenkins, 2009).
One of the strongest and best characterized effects of ambient UV-B in terrestrial ecosystems has been documented at the interface between plants and herbivorous insects (for reviews, see Ballaré et al., 2001; Caldwell et al., 2003; Bassman, 2004; Roberts and Paul, 2006). Some of these effects are a consequence of the direct perception of UV-B radiation by insects, as demonstrated for soybean thrips by Mazza et al. (2002, 2010). However, in a large number of studies involving bioassays with animals not directly exposed to solar radiation, indirect effects have been demonstrated (i.e. UV-B effects mediated by changes in the plant host). Thus, “choice” and “no-choice” bioassays with herbivorous insects have shown that ambient UV-B can produce changes in the plant that affect insect feeding (Ballaré et al., 1996; Rousseaux et al., 1998) and oviposition choices (Caputo et al., 2006) as well as larval performance (Bergvinson et al., 1994; Zavala et al., 2001; Izaguirre et al., 2003; Kuhlmann and Müller, 2009).
The effects of UV-B on plant resistance to herbivory have been correlated with UV-B-induced variations in a number of tissue quality traits, including nitrogen content (Hatcher and Paul, 1994), leaf phenolics (McCloud and Berenbaum, 1994; Rousseaux et al., 2004; Izaguirre et al., 2007; Kuhlmann and Müller, 2009), cyanogenic compounds (Lindroth et al., 2000), and defense-related proteins such as proteinase inhibitors (Stratmann et al., 2000; Izaguirre et al., 2003; Stratmann, 2003). Increased accumulation of phenolic compounds is one of the best characterized responses to UV-B radiation; these compounds contribute to filter out UV-B photons before they reach sensitive molecules in the mesophyll (Braun and Tevini, 1993; Landry et al., 1995; Reuber et al., 1996; Bilger et al., 1997; Barnes et al., 2000; Mazza et al., 2000). Soluble phenolic compounds are often also induced in response to insect herbivory, and they are thought to play a role as direct antiherbivore defenses (Elliger et al., 1981; Stamp and Osier, 1998; Hoffland et al., 2000; Leitner et al., 2005; Izaguirre et al., 2006). In fact, UV-B and insect herbivory may trigger partially overlapping phenolic responses in some species (Izaguirre et al., 2007). This partial convergence in response has been also evidenced in studies that measured changes in the transcriptome elicited by herbivory/wounding treatments and exposure to UV-B radiation (Brosché et al., 2002; Izaguirre et al., 2003). These observations have lent support to the idea that the effects of solar UV-B on plant-insect interactions may be mediated by stimulation of the defense signaling cascades that plants activate to defend themselves against herbivore attack (Izaguirre et al., 2003, 2007; Stratmann, 2003; Caldwell et al., 2007).
Defense responses against herbivorous insects are orchestrated by a group of lipid hormones collectively known as jasmonates (for reviews, see Wasternack, 2007; Howe and Jander, 2008; Browse, 2009). Jasmonates are rapidly induced in response to herbivory and regulate the expression of many defense-related genes (Reymond et al., 2000; De Vos et al., 2005), eventually leading to the accumulation of toxic secondary metabolites like alkaloids, terpenoids, glucosinolates, phenylpropanoids, and defense-related proteins (for review, see Howe and Jander, 2008).
Although significant advances have been made in the last few years in mapping the interactions between perception of light signals and regulation of specific hormonal cascades (de Lucas et al., 2008; Feng et al., 2008; Tao et al., 2008; Pierik et al., 2009; Sorin et al., 2009), progress in this direction has been slow in the case of UV-B-induced responses. Jasmonates may mediate the effects of UV-B on plant defense, but the evidence for this hypothesis remains controversial. In tomato (Solanum lycopersicum), germicidal UV-C radiation or very high UV-B doses induce the expression of proteinase inhibitor genes, which are typical markers of jasmonate responses (Conconi et al., 1996; Stratmann et al., 2000). UV-C photons are not present in the solar spectrum that reaches the earth's surface, and treatment of tomato plants with more physiologically relevant UV-A/UV-B wavelengths failed to induce proteinase inhibitor expression in nonwounded leaves (Stratmann et al., 2000). However, these UV-A/UV-B treatments enhanced the proteinase inhibitor response induced by mechanical damage but without increasing jasmonic acid (JA) levels (Stratmann et al., 2000). In contrast, in Arabidopsis, controlled-environment studies have shown that UV-B can trigger JA accumulation (A-H-Mackerness et al., 1999). Unfortunately, in the Arabidopsis experiments, UV-B was delivered against a background of very low photosynthetically active radiation (PAR), which tends to exacerbate the damaging effects of UV-B radiation (Caldwell et al., 2003). Field experiments indicated that the effects of ambient solar UV-B in reducing the tissue quality of Arabidopsis plants for diamond-back moth (Plutella xylostella) oviposition is lacking in the jar1 mutant (Caputo et al., 2006). This mutant is deficient in the production of the JA-Ile conjugate (Staswick and Tiryaki, 2004), the jasmonate form considered to be biologically active (Thines et al., 2007; Fonseca et al., 2009). In Nicotiana longiflora, solar UV-B was reported to increase the expression of various genes involved in fatty acid and oxylipin metabolism and the accumulation of phenolic products that are sometimes induced by JA but not the expression of the trypsin proteinase inhibitor (TPI) gene in intact (nonwounded) plants (Izaguirre et al., 2003).
The experiments described in this paper were designed to directly test the involvement of jasmonates in the defense responses elicited by solar UV-B radiation in Nicotiana attenuata. We compared the responses elicited by UV-B with those triggered by simulated herbivory in experiments involving wild-type plants and a transgenic line impaired in JA biosynthesis by antisense expression of a specific wound- and herbivory-induced lipoxygenase gene (NaLOX3 [as-lox]; Halitschke and Baldwin, 2003). This as-lox line has been extensively tested in previous experiments, which demonstrated impaired jasmonate biosynthesis and reduced resistance to insect herbivory under field conditions (Halitschke and Baldwin, 2003; Kessler et al., 2004). Our results show that UV-B induces the accumulation of phenolic compounds using jasmonate-dependent (polyamine conjugates) and jasmonate-independent (flavonoids and chlorogenic acid) pathways. Furthermore, we found that UV-B increases the expression of some JA-inducible defenses, such as TPI, by increasing tissue sensitivity to jasmonate without altering jasmonate production or activation. Analysis of the defense phenotype of as-lox plants in the field suggests that the jasmonate-dependent effects of solar UV-B play an important role in UV-B-induced resistance to insect herbivory.
RESULTS
UV-B and Simulated Herbivory Induce the Accumulation of Phenolic Compounds
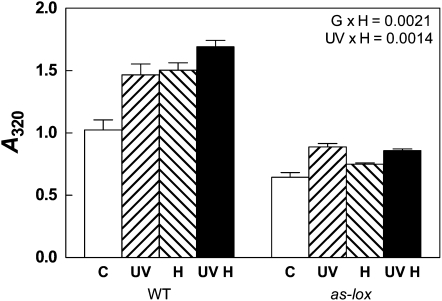
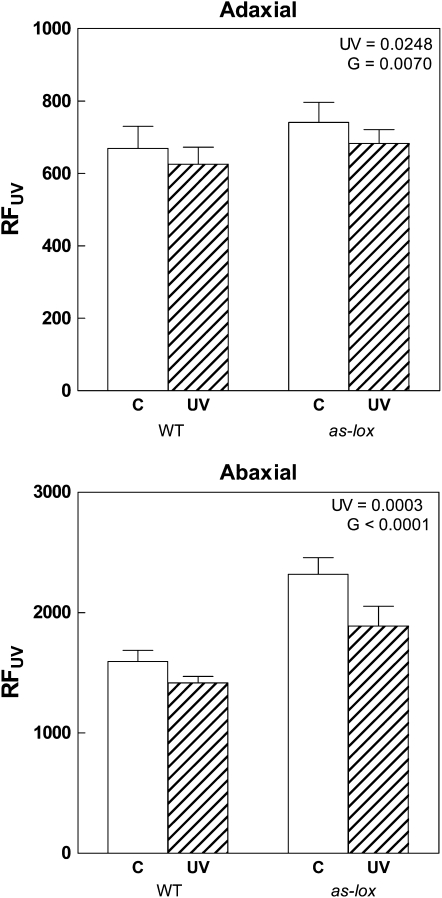
In a glasshouse experiment, plants were grown under high PAR and exposed to UV-B doses equivalent to those received in Buenos Aires during the spring (5.5 kJ m−2, biologically effective daily UV-B dose [BE-UV-B]) in factorial combination with a simulated herbivory treatment (see “Materials and Methods”). Wild-type plants responded to UV-B and simulated herbivory with the accumulation of methanol-extractable phenolic compounds (Fig. 1; for a complete description of the ANOVA results, see Supplemental Table S1). Silenced as-lox plants had lower levels of soluble leaf phenolics compared with wild-type plants; accumulation of phenolic compounds was enhanced by UV-B but not by simulated herbivory (Fig. 1, Supplemental Table S1). In a related field trial, where plants were grown under selective spectral filters, we indirectly estimated the accumulation of phenolic compounds using determinations of UV-B-excited chlorophyll fluorescence, which measures the transmittance of UV-B radiation through the epidermal tissue (Mazza et al., 2000). We found that as-lox plants had higher epidermal transmittance to UV-B than wild-type plants (i.e. lower levels of UV-B-absorbing compounds) but were still able to respond to solar UV-B with the accumulation of protective phenolic sunscreens (Fig. 2).
Figure 1.
Effects of UV-B and simulated herbivory on the accumulation of soluble phenolic compounds in N. attenuata plants grown in the glasshouse. The experimental treatments resulted from a factorial combination of UV-B and simulated herbivory: C, natural daylight; UV, natural daylight supplemented with UV-B radiation; H, natural daylight plus simulated herbivory (wounds treated with S. frugiperda regurgitate); UVH, UV radiation plus simulated herbivory (for details, see “Materials and Methods”). Leaf samples were taken 72 h after the H treatment. Each bar represents the mean + se (n = 6 individual plants). G, Genotype; WT, wild type. P values for significant interactions are shown (three-way ANOVA; for the full ANOVA table and Tukey comparisons for interaction terms, see Supplemental Table S1).
Figure 2.
Effects of solar UV-B on RFUV, which is used as an indicator of epidermal transmittance to UV radiation. Plants were grown in the field under near-ambient (UV) or attenuated (C) UV-B radiation using stretch or clear polyester filters, respectively. Each bar represents the mean + se (n = 5; each biological replicate is a pool of three individual plants). G, Genotype; WT, wild type. P values for the relevant terms are shown (two-way ANOVA; for the full ANOVA table, see Supplemental Table S2).
Some Effects of UV-B on the Accumulation of Phenolic Compounds Are LOX3 Dependent
We separated individual compounds by HPLC from methanolic extracts of glasshouse-grown plants exposed to a factorial combination of UV-B and simulated herbivory levels. We identified six prominent peaks in wild-type plants, which corresponded to free and polyamine-conjugated hydroxycinnamic acids and a flavonoid (Supplemental Fig. S1). These compounds could be divided in two groups, according to their pattern of response to UV-B and requirement for LOX3-dependent JA biosynthesis.
Group 1
Rutin (a flavonoid), chlorogenic acid, and a chlorogenic acid isomer (presumably cryptochlorogenic acid) were strongly induced by UV-B but not by simulated herbivory (except for the chlorogenic acid isomer, which was slightly induced in wild-type plants; Fig. 3; for complete ANOVA results, see Supplemental Table S3). These compounds were present, and their response to UV-B completely conserved, in as-lox plants (Fig. 3). This group of phenolics is likely responsible for the sunscreen response to UV-B detected in as-lox plants (Figs. 1 and 2).
Figure 3.
Effects of UV-B and simulated herbivory on the accumulation of individual phenolic compounds in N. attenuata plants grown in the glasshouse. The experimental treatments resulted from a factorial combination of UV-B and simulated herbivory: C, natural daylight; UV, natural daylight supplemented with UV-B radiation; H, natural daylight plus simulated herbivory (wounds treated with S. frugiperda regurgitate); UVH, UV radiation plus simulated herbivory (for details, see “Materials and Methods”). Leaf samples were taken 72 h after the H treatment. Each bar represents the mean + se (n = 6 individual plants). G, Genotype; WT, wild type. P values for the relevant terms of the factorial model are shown (for the full ANOVA results and Tukey comparisons, see Supplemental Table S3).
Group 2
Polyamine-conjugated forms of caffeic acid (caffeoylputrescine, dicaffeoylspermidine, and a dicafeoylspermidine-related compound) were strongly induced by UV-B in wild-type plants, and the response to UV-B was very similar to the response elicited by simulated herbivory (Fig. 3). Polyamine conjugates were missing in as-lox plants (Fig. 3), which readily accumulated these compounds when they were supplied with exogenous methyl jasmonate (MeJA; Supplemental Fig. S1). Previous work has shown that JA signaling is required for the accumulation of caffeoylputrescine in response to simulated herbivory in N. attenuata (Paschold et al., 2007); our data indicate that the effect of UV-B on the accumulation of this group of phenolic conjugates has a similar jasmonate requirement. This group of phenolic compounds was most likely responsible for the differences in UV absorbance and epidermal UV transmittance detected between wild-type and as-lox plants (Figs. 1 and 2).
UV-B Increases the Expression of Herbivore-Induced Protein Defenses
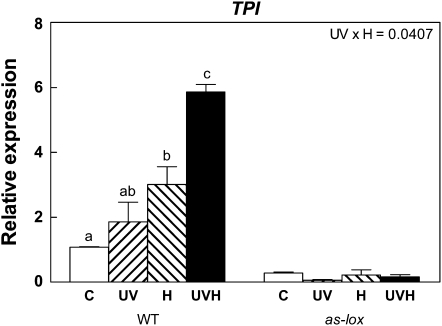
Given that LOX3-dependent JA biosynthesis was required for the effects of UV-B on the accumulation of some putative phenolic defenses, we wanted to investigate the effects of natural doses of UV-B on the expression of protein-based defenses. TPIs are important direct defenses in N. attenuata and are reliable markers of activation of the jasmonate pathway (Zavala and Baldwin, 2004; Zavala et al., 2004). We found that TPI gene expression was induced by simulated herbivory (Fig. 4), as has been found in previous studies (Horn et al., 2005; Wu et al., 2006). In agreement with the expected jasmonate requirement, TPI transcript levels were strongly reduced in as-lox plants. UV-B per se did not significantly induce TPI gene expression. Interestingly, however, UV-B significantly enhanced the TPI expression response of wild-type plants to the simulated herbivory treatment (Fig. 4). The pattern of TPI gene expression was in sharp contrast with that detected for chalcone synthase, a key enzyme in the biosynthesis of flavonoids, which was up-regulated by UV-B in both genotypes and not affected or slightly down-regulated by simulated herbivory (Supplemental Fig. S2).
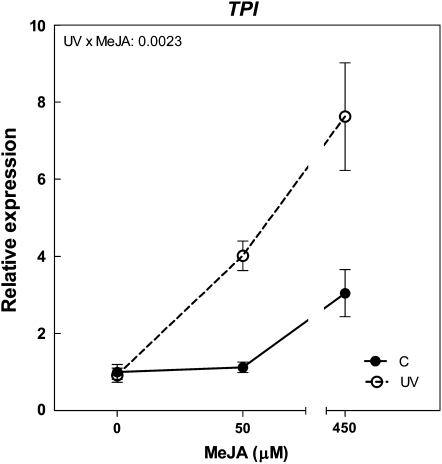
Figure 4.
Effects of UV-B and simulated herbivory on TPI gene expression in N. attenuata plants grown in the glasshouse. The experimental treatments resulted from a factorial combination of UV-B and simulated herbivory: C, natural daylight; UV, natural daylight supplemented with UV-B radiation; H, natural daylight plus simulated herbivory (wounds treated with S. frugiperda regurgitate); UVH, UV radiation plus simulated herbivory (for details, see “Materials and Methods”). TPI gene expression was measured by qPCR 24 h after the H treatment. Each bar represents the mean + se (n = 3; each biological replicate is a pool of three individual plants). WT, Wild type. The P value for the UV × H interaction term is indicated. Different letters indicate significant differences between means (P < 0.05, Tukey test). For the full ANOVA results and Tukey comparisons, see Supplemental Table S4.
UV-B Does Not Increase Jasmonate Accumulation
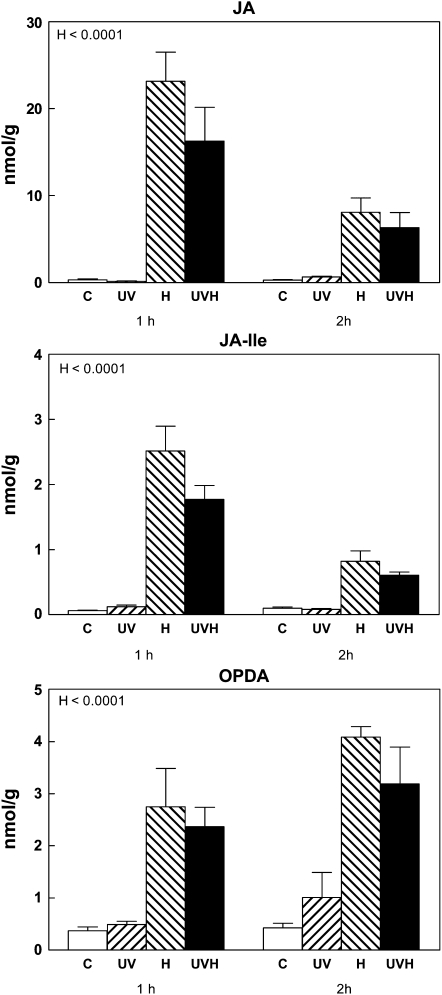
The effects of UV-B triggering the accumulation of phenolic compounds of group 2 (i.e. those that respond to both UV-B and herbivory and do not accumulate in as-lox plants; Fig. 3) and enhancing the herbivore-induced expression of TPI in wild-type plants (Fig. 4) may suggest that UV-B up-regulates JA biosynthesis or the production of bioactive JA conjugates. However, direct measurements of jasmonate contents indicated that this was not the case. Wild-type plants responded to simulated herbivory with the expected increase in the accumulation of several jasmonates, including 12-oxo-phytodienoic acid (OPDA), JA, and the JA-Ile conjugate, but jasmonate accumulation was not affected by UV-B treatment (Fig. 5; for hydroxylated derivates, see Supplemental Fig. S3).
Figure 5.
Effects of UV-B and simulated herbivory on jasmonate accumulation in N. attenuata plants grown in the glasshouse. The experimental treatments resulted from a factorial combination of UV-B and simulated herbivory: C, natural daylight; UV, natural daylight supplemented with UV-B radiation; H, natural daylight plus simulated herbivory (wounds treated with S. frugiperda regurgitate); UVH, UV radiation plus simulated herbivory (for details, see “Materials and Methods”). Samples for jasmonate determinations were obtained 1 and 2 h after the H treatment. Each bar represents the mean + se (n = 3; each biological replicate is a pool of 10 individual plants). P values for the relevant terms of the model are shown (for the full ANOVA results, see Supplemental Table S5).
UV-B Exposure Increases Jasmonate Sensitivity
Since jasmonate accumulation was not affected by UV-B, we hypothesized that the effects of UV-B on TPI expression in wild-type plants could be mediated by an increase in sensitivity to jasmonates. We tested this hypothesis with exogenous applications of MeJA. Jasmonate treatment increased TPI expression in a dose-dependent manner, and there was a highly significant effect of UV-B increasing transcript levels in MeJA-treated plants (Fig. 6). These results indicate that UV-B increases plant sensitivity to jasmonates.
Figure 6.
Effects of UV-B on plant sensitivity to jasmonate. The experimental treatments resulted from a factorial combination of UV-B and MeJA applications: C, natural daylight; UV, natural daylight supplemented with UV-B radiation (for details, see “Materials and Methods”). Samples for qPCR analysis were obtained 24 h after MeJA application. Expression data are normalized to the expression level detected in the control × 0 μm MeJA combination. Thin bars indicate ±1 se (n = 3; each biological replicate is a pool of three individual plants). The P value for the UV × MeJA interaction is indicated (for the full ANOVA results, see Supplemental Table S6).
Functional Significance
UV-B Protection
In a glasshouse experiment with supplemental UV-B radiation, plants of the as-lox line were more susceptible to high UV-B doses than wild-type plants (Supplemental Fig. S4). This observation suggests that the phenolic compounds that are lacking in as-lox plants (group 2; Fig. 3) and the associated differences in UV-B screening (Figs. 1 and 2) or some other JA-dependent defense trait not measured in these experiments are functionally significant in UV-B protection. The daily UV-B dose in this experiment (BE-UV-B = 20 kJ m−2) was deliberately chosen to induce visible symptoms of leaf damage (such as glazing and leaf curling) in as-lox plants under glasshouse conditions; the impacts of UV-B on as-lox plant morphology were less pronounced in field trials with natural UV-B (data not shown), suggesting that other protective mechanisms may be activated under field conditions that partially compensate for the deficiency in jasmonate-dependent acclimation responses.
Antiherbivore Defense
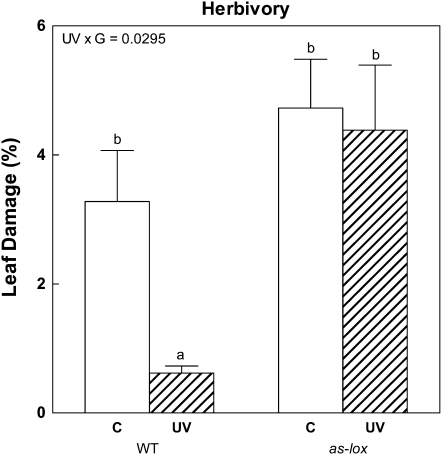
In a field trial, wild-type and as-lox plants were exposed to natural herbivory, which was predominantly caused by two species of thrips (Thrips tabaci and Frankliniella spp.; Thysanoptera: Thripidae). Silenced as-lox plants had more insect damage than wild-type plants (Fig. 7), confirming the critical role of jasmonates in defense activation against a wide spectrum of herbivores, including thrips (Abe et al., 2009). Solar UV-B reduced herbivore damage in wild-type plants, as expected on the basis of previous field experiments involving thrips (Mazza et al., 1999) and herbivory bioassays using N. attenuata plants (Izaguirre et al., 2003). Importantly, in our experiment, the strong UV-B effect of suppressing insect herbivory was completely lacking in as-lox plants (Fig. 7). This result demonstrates that those effects of ambient UV-B on plant defense that require LOX3-dependent jasmonate biosynthesis are significant for antiherbivore protection.
Figure 7.
UV-B exposure in the field reduces natural herbivory in wild-type plants but not in as-lox plants. Plants were grown in the field under near-ambient (UV) or attenuated (C) UV-B radiation using stretch or clear polyester filters, respectively. Leaf damage in the field was predominantly caused by thrips (T. tabaci and Frankliniella spp.) and is expressed as a percentage of total leaf area. Each bar represents the mean + se (n = 4 independent blocks of three plants each). WT, Wild type. Different letters indicate significant differences between means (P < 0.05, Tukey test). For the full ANOVA results and Tukey comparisons, see Supplemental Table S7.
DISCUSSION
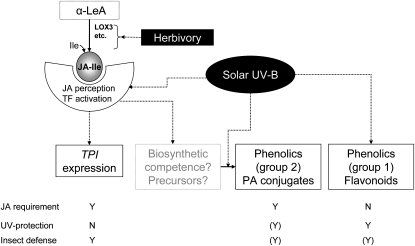
This study revealed that ecologically meaningful doses of UV-B radiation affect the expression of functionally important plant defenses in N. attenuata through jasmonate-dependent and -independent mechanisms (Fig. 8). The jasmonate dependence was established by comparing UV-B responses between wild-type plants and plants silenced in the LOX3 gene, which are deficient in the production of jasmonates (Halitschke and Baldwin, 2003). The responses investigated were those that previous work had identified as potentially important for the effects of UV-B on antiherbivore defense (Izaguirre et al., 2003, 2007). Discussed separately below are the interactions between UV-B and jasmonate signaling for induction of (1) flavonoids and chlorogenic acid, (2) polyamine conjugates, and (3) TPI gene expression.
Figure 8.
Model of the proposed interaction between UV-B and jasmonate signaling in the induction of plant defenses. UV-B induces accumulation of flavonoids through a mechanism that does not require jasmonate synthesis and is not activated in response to herbivory or jasmonate treatment. In contrast, UV-B induces the accumulation of polyamine (PA) adducts via a mechanism that is completely dependent on LOX3 activity and is also activated by MeJA. UV-B does not promote TPI gene expression, which is induced by jasmonate and herbivory, but greatly enhances the response to herbivory by increasing plant sensitivity to jasmonate. The jasmonate-dependent effects of solar UV-B on plant defense are functionally significant, determining the antiherbivore effects of UV-B radiation in the field. Solid arrows indicate synthesis or transformation, and dotted arrows indicate controls. TF, Transcription factors.
Flavonoids and Chlorogenic Acid
The accumulation of colorless flavonoids is a typical response to UV-B irradiation (Ballaré et al., 1995; Lavola et al., 1998; Ryan et al., 1998; Wilson et al., 1998; Olsson et al., 1999; De La Rosa et al., 2001; Hofmann et al., 2003; Tegelberg et al., 2004; Izaguirre et al., 2007; Kuhlmann and Müller, 2009), and our study clearly demonstrates that accumulation of these compounds in response to ambient doses of UV-B does not require jasmonate signaling (Fig. 3). This finding is consistent with previous studies in N. attenuata in which rutin accumulation was not induced by jasmonate treatment (Keinänen et al., 2001; Lou and Baldwin, 2003) or insect herbivory (Kessler and Baldwin, 2004; Izaguirre et al., 2007; Wu et al., 2008). Flavonoid accumulation is likely limited by chalcone synthase activity (Strid et al., 1994), and CHS gene expression has been shown to be induced by UV-B through the UVR8-COP1 pathway in Arabidopsis, which is generally thought to be independent of jasmonate signaling (Brown et al., 2005). Rutin plays an important role in UV-B screening and accounts for a significant fraction of the increase in UV-B absorbance of the leaf extracts of plants exposed to UV-B (data not shown). Rutin may also have antiherbivore functions (Elliger et al., 1981; Stamp and Yang, 1996; Hoffland et al., 2000; Hoffmann-Campo et al., 2001). Chlorogenic acid in our experiment was also induced by UV-B but not (or only slightly) induced by simulated herbivory (Fig. 3). The apparent lack of effect of simulated herbivory is in contrast to some previous observations (Kranthi et al., 2003; Kessler and Baldwin, 2004; Izaguirre et al., 2007) but agrees with results of other studies (Steppuhn et al., 2004; Wu et al., 2008). We speculate that the variation in the chlorogenic acid response to simulated herbivory may be related to differences in the induction protocol (e.g. Spodoptera frugiperda versus Manduca sexta oral secretions), which may influence the level of polyphenol oxidase (PPO) induction in the treated leaves, as chlorogenic acid is the preferred substrate of this enzyme (Li and Steffens, 2002; but see Keinänen et al., 2001). Chlorogenic acid and feruloyl quinic acid can be important resistance factors against thrips (Leiss et al., 2009). In our experiments, chlorogenic acid was induced by UV-B both in wild-type and as-lox lines (Fig. 3), but UV-B-increased resistance against thrips was only observed in wild-type plants (Fig. 7). Because the biological effect of chlorogenic acid depends on its transformation to reactive quinones by plant PPOs and PPO gene expression was very low in the as-lox line (and increased by UV-B in wild-type plants; data not shown), we hypothesize that reduced PPO activity could be another factor contributing to the loss of UV-B-induced antiherbivore resistance in as-lox plants (Fig. 7).
Polyamine Conjugates
Polyamine conjugates in wild-type plants were clearly induced by both UV-B and simulated herbivory (Fig. 3), in agreement with previous observations (Izaguirre et al., 2007; Paschold et al., 2007). The results with the as-lox line indicate that jasmonate signaling is required not only for the accumulation of these conjugates in response to herbivory, as shown previously (Paschold et al., 2007), but also for the response to ambient doses of UV-B radiation. In Nicotiana tabacum BY2 cell cultures, MeJA promoted the synthesis of several phenylpropanoid conjugates, including polyamine adducts, and this effect was mediated through a MeJA-inducible MYB-type transcription factor (NtMYBBJS1; Gális et al., 2006). In N. attenuata, a MYB gene was reported to be induced after plant exposure to natural UV-B in the field, but this MYB was related to rutin and chlorogenic acid accumulation (Pandey and Baldwin, 2008). Natural doses of UV-B do not induce jasmonate accumulation in N. attenuata (Fig. 5), and UV-B induces polyamine conjugates in wild-type but not in as-lox plants (Fig. 3). Therefore, we speculate that basal levels of jasmonate are somehow necessary to generate the biosynthetic competence or the precursors for conjugate biosynthesis and that UV-B only acts by increasing the sensitivity to jasmonate, as shown for TPI gene expression (Fig. 6), or on a later step in the biosynthesis of the conjugates (Fig. 8). The functional roles of these UV-B- and herbivory-induced caffeoyl-polyamine conjugates are not known, but they are likely to contribute to UV-B screening, as deduced from the differences in UV-B absorbance (Fig. 1) and epidermal transmittance (Fig. 2) between wild-type and as-lox plants. Polyamines and their conjugates have been implicated in MeJA-induced systemic resistance against plant pathogens (Walters, 2003) and resistance to abiotic stress (Walters, 2000; Edreva et al., 2007). Plants damaged by herbivore attack may benefit from the expression of MeJA-induced systemic resistance, as wounds are a primary site of pathogen penetration to plant tissue.
Proteinase Inhibitors
UV-B up-regulated the expression of TPI, but only in plants where the jasmonate cascade was activated by herbivory (Fig. 4) or MeJA treatment (Fig. 6). These observations are in contrast with the results of experiments using germicidal UV-C or unbalanced UV-B doses (Conconi et al., 1996; Stratmann et al., 2000), where a strong direct effect of the radiation treatments on proteinase inhibitors was observed, but are generally consistent with the results of studies that used UV-B/UV-A mixtures (Stratmann et al., 2000) or ambient doses of UV-B radiation (Izaguirre et al., 2003), which have shown modest or negligible direct responses to UV-B. Since TPI is a typical marker of jasmonate response, the lack of a direct effect of UV-B on TPI expression in our experiments is consistent with the observation that UV-B by itself failed to activate JA-Ile accumulation (Fig. 5). Our MeJA dose-response experiment demonstrates that natural UV-B levels increase jasmonate sensitivity (Fig. 6), which provides a potential mechanistic explanation for the enhanced expression of TPI in the plants induced by simulated herbivory (Fig. 4). This enhanced expression of TPI, mediated by increased sensitivity to jasmonates, is likely to play a significant role in the mechanism that explains the increased resistance to herbivory in plants exposed to solar UV-B, an effect that is missing in as-lox plants (Fig. 7).
Implications for Modulation of Plant Defense by Canopy Light Signals
Effects of light quality on plant sensitivity to jasmonates have been recently reported (Moreno et al., 2009) and may be important to adjust the extent of the defense response that the plant activates to cope with herbivory as a function of the energy demands placed by other biotic stressors (Ballaré, 2009). Far-red radiation (FR), a light signal used by plants to detect the proximity of neighbors, down-regulates jasmonate sensitivity, presumably to conserve resources that the plant will need to invest in growth responses associated with competition (Cipollini, 2004; McGuire and Agrawal, 2005; Izaguirre et al., 2006; Moreno et al., 2009). Based on our results showing that, in contrast with FR, UV-B enhances jasmonate sensitivity, we speculate that UV-B acts as a canopy signal that conveys the opposite information to that conveyed by FR (i.e. a signal of abundant light and, therefore, no limitation to the investment of resources in antiherbivore defense).
CONCLUSION
Some effects of UV-B on plant defense in N. attenuata require LOX3-dependent jasmonate biosynthesis, while others do not. Solar UV-B does not affect jasmonate accumulation in N. attenuata but increases jasmonate sensitivity, presumably via regulation of the expression or stability of some of the downstream components involved in jasmonate signaling. Furthermore, the defense phenotypes of as-lox plants under UV-B, along with the results of field experiments in the Arabidopsis/Plutella system (Caputo et al., 2006), suggest that those effects of UV-B radiation that require jasmonate signaling play a central role in the mechanisms whereby solar UV-B increases plant resistance to herbivorous insects.
MATERIALS AND METHODS
Plant Material
Nicotiana attenuata seeds were germinated as described by Krugel et al. (2002). Wild-type and as-lox (A300-1) lines were those described by Halitschke and Baldwin (2003). After germination, plants were grown under glasshouse conditions between spring and summer for 3 weeks in individual pots (0.25 L) with a perlite:soil (1:1) mixture and watered every 2 d with Hakaphos Rojo solution 18-18-18 (Compo). Peak levels of PAR were between 800 and 1,000 μmol m−2 s−1. Rosette plants of similar age and size (typically 3 to 4 weeks old with seven to 10 mature leaves) were selected for glasshouse experiments and randomly assigned to the treatments. For the field experiment, plants were grown in the glasshouse in 0.5-L individual pots for 3 weeks before being transferred to the field in the spring and summer of 2008.
Glasshouse Experiments
The experimental setup under glasshouse conditions was similar to that described by Izaguirre et al. (2007). UV-B radiation was provided by fluorescent lamps (UV313; Q-Panel). Radiation from the lamps was filtered through clear cellulose diacetate film (simulated ambient UV-B) or polyester film (no UV-B). Plants were irradiated for 6 h each day, with the irradiation period centered at solar noon; plants were randomly rotated within the irradiation area every 2 d to minimize position effects. The BE-UV-B in all the elicitation experiments was 5.5 kJ m−2 (calculated using the plant action spectrum normalized at 300 nm; Caldwell, 1971). The unweighted irradiance integrated between 290 and 315 nm was 1.1 μmol m−2 s−1. Plants were randomly assigned to the various elicitation treatments (MeJA or simulated herbivory) after 10 to 11 d of irradiation. For acute UV-B exposure experiments (Supplemental Fig. S4), the BE-UV-B was 20 kJ m−2 (approximately 4 μmol m−2 s−1 UV-B).
Simulated Herbivory and MeJA Treatments
For simulated herbivory treatments, the two youngest fully expanded leaves were damaged longitudinally at both sides of the midvein with a razor blade. The wounds were treated with 10 μL of an aqueous dilution of Spodoptera frugiperda oral secretion (1:5, v/v). Undisturbed leaves of equivalent nodal positions from independent plants were used as controls. For MeJA treatments, plants were sprayed with 1 mL of distilled water or aqueous solutions of MeJA (Sigma-Aldrich).
UV-B Effects on Natural Herbivory under Field Conditions
The experiment was carried out during the summer at the experimental field site of Instituto de Investigaciones Fisiológicas y Ecológicas vinculadas a la Agricultura in Buenos Aires. The experiment consisted of a randomized block design with four replicates. In each block, two light treatments were represented: attenuated UV-B (daily BE-UV-B approximately 0 kJ m−2) and near-ambient UV-B (daily BE-UV-B approximately 8 kJ m−2). UV-B attenuation was obtained by covering the plots with clear polyester (which filtered out more than 90% of ambient UV-B); near-ambient UV-B was obtained by covering the plots with clear stretch film (more than 80% transmittance in the solar UV region; Mazza et al., 2002). Wild-type and as-lox plants grown in the glasshouse as described were transferred to the experimental plots and randomly assigned to the light treatments. Four days after transfer to the experimental plots, and every 2 d after, plants were inspected for signs of herbivore damage. Once plants terminated the vegetative phase, all leaves were collected and scanned with a HP Scanjett 4500c (Hewlett-Packard). Total leaf area and damaged areas were measured using Adobe Photoshop software (version 7.0; Adobe Systems).
Measurement of Jasmonates
Leaves of plants from glasshouse experiments were collected at various time points after simulated herbivory treatment and immediately frozen in liquid N2. About 250 mg of freeze-dried material without the midvein was used for analysis. Plant material was homogenized with 10 mL of methanol and 50 ng of [2H6]JA, 50 ng of [2H5]OPDA, and 50 ng of [2H3]JA-Ile as internal standards. The methanolic extracts were prepurified by chromatographic steps (Andrade et al., 2005).
HPLC was used to separate JA, OPDA, and JA-Ile. An Alliance 2695 separation module (Waters) equipped with a 100-mm × 2.1-mm, 3-μm Restek C18 column (Restek) was used to maintain performance of the analytical column. Fractions were separated using a gradient of increasing methanol concentrations, constant glacial acetic acid concentration of 0.2% (v/v) in water, and initial flow rate of 0.2 mL min−1. The gradient was increased linearly from 40% (v/v) methanol-60% (v/v) water-acetic acid at 25 min to 80% (v/v) methanol-20% (v/v) water-acetic acid. After 1 min, initial conditions were restored and the system was allowed to equilibrate for 10 min. Mass spectrometry analysis was performed on a quadruple tandem mass spectrometer (Quattro Ultima; Micromass) fitted with an electrospray ion source (electrospray-ion negative mode). A mixture containing all unlabeled compounds and internal standards was separated by HPLC and analyzed by tandem mass spectrometry with single-ion recording to determine retention times for all compounds. The spectrometer software MassLynx version 4.1 (Micromass) was used. Analysis of the compounds was based on appropriate multiple reaction monitoring of ion pairs for labeled and endogenous JA, OPDA, and JA-Ile using the following mass transitions: [2H6]JA, 215 > 59; JA, 209 > 59; [2H5]OPDA, 298 > 230; OPDA, 293 > 225; [2H3]JA-Ile, 326 > 131; JA-Ile, 323 > 128, with retention times of 15, 18, and 22 min for JA, OPDA, and JA-Ile, respectively. The optimized conditions were as follows: collision energy used was 5 eV for all compounds, cone voltage was 35 V, ion spray voltage energy was 3.25 kV, desolvation temperature was 350°C, and source temperature was 120°C. Response was calculated as product ion peak area × (IS concentration/IS product ion peak area), where IS concentration is the known amount of the internal standard added.
Leaf Phenolics, HPLC Analysis, and Determination of Phenolic Sunscreens
For determination of total soluble leaf phenolics, leaf samples (one 1-cm diameter leaf disc; youngest fully expanded leaves) were placed in 1.4 mL of a methanol:HCl solution (99:1, v/v) and allowed to extract for 48 h at −20°C (Mazza et al., 2000). Absorbance of extracts was read in a spectrophotometer (UV-1700 series; Shimadzu). The remaining leaf tissue was freeze dried and stored in a container with silica gel until HPLC analysis.
Individual leaf phenolics were determined as described by Keinänen et al. (2001) with slight modifications. Briefly, about 10 to 15 mg of freeze-dried tissue without the midvein was ground in a mortar and transferred to an Eppendorf with 1.5 mL of a methanol:0.25% acetic acid mixture (2:3, v/v). Samples were vortexed for 45 s and centrifuged at 12,000 rpm for 20 min. The supernatant was filtered through a 45-μm syringe filter and kept at −20°C until use. Phenolics were separated by HPLC (Knauer Euroline) on a Restek Pinnacle II C18 (5.0 μm, 4.6 × 150 mm) column with solvents A (0.25% aqueous H3PO4) and B (acetonitrile), eluted with a gradient of 8% B at 0 min, 12% B at 6 min, 20% B at 10 min, 50% B at 23 min to 30 min, with an equilibration time of 10 min and a flow rate of 1 mL min−1. The injection volume was 20 μL, and elution was monitored with a diode array detector at 230, 305, and 320 nm. The retention times and UV-visible spectra of chlorogenic acid and rutin were compared with those of true standards, and amounts were calculated on the basis of a calibration curve. Caffeoyl-polyamine conjugates were quantified as chlorogenic acid equivalents. Solvents used for determinations of leaf phenolics were purchased from Sintorgan. True standards used in HPLC analysis were from Sigma-Aldrich.
The accumulation of phenolic sunscreens in leaf tissue was estimated indirectly by measuring UV-induced chlorophyll fluorescence (RFUV) essentially as described by Mazza et al. (2000).
RNA Isolation and Real-Time PCR
Total RNA was extracted from 100 mg of frozen tissue using the LiCl-phenol/chloroform method as described by Izaguirre et al. (2003). Purified fractions of total RNA were subjected to RQ1 (RNase-free) DNase treatment (Promega) to avoid contamination with genomic DNA. For cDNA synthesis, fractions of 1 μg of RNA were reverse transcribed using oligo(dT) as primer and Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's instructions. The obtained cDNA samples were diluted 1:10 before use. Quantitative real-time PCR (qPCR) was performed in a 7500 Real Time PCR System (Applied Biosystems) following the manufacturer's standard method for absolute quantification using FastStart Universal SYBR Green Master Mix (Roche Applied Science) and primers at a final concentration of 500 nm. Primer pairs are listed in Supplemental Table S1. N. attenuata ACTIN gene was used to normalize for differences in concentrations of cDNA samples. Normalized gene expression levels were expressed as fold change relative to the control treatment.
Statistical Analyses
Statistical analyses were carried out using INFOSTAT software (professional version 1.1). Data on soluble phenolic compounds were analyzed using a three-way ANOVA with UV-B, simulated herbivory, and genotype as factors, except for polyamines, where only wild-type plants were analyzed using a two-way ANOVA. Data on jasmonate accumulation were analyzed using a three-way ANOVA with UV-B, simulated herbivory, and time as factors. For gene expression analyses, CHS was analyzed using a three-way ANOVA, whereas TPI gene expression data were analyzed only for wild-type plants using a two-way ANOVA. Data from the field experiments (RFUV and herbivory) were analyzed using a two-way ANOVA following a randomized block design. When interaction terms were significant, differences between means were analyzed using Tukey comparisons. Full ANOVA tables and mean comparisons are presented in the Supplemental Data. Appropriate transformations of the primary data were used when needed to meet the assumptions of the analysis.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. HPLC profiles of methanolic leaf extracts obtained from glasshouse-grown wild-type and as-lox N. attenuata plants exposed to control conditions, simulated herbivory, UV-B radiation, or MeJA.
Supplemental Figure S2. Effect of UV-B and simulated herbivory on CHS gene expression in N. attenuata plants grown in the glasshouse.
Supplemental Figure S3. Effect of UV-B and simulated herbivory on accumulation of hydroxylated jasmonates.
Supplemental Figure S4. Effect of high UV-B doses on leaf morphology in wild-type and as-lox plants.
Supplemental Table S1. ANOVA results for methanol-extractable phenolic compounds.
Supplemental Table S2. ANOVA results for leaf epidermal transmittance.
Supplemental Table S3. ANOVA results for individual phenolic compounds.
Supplemental Table S4. ANOVA results for TPI gene expression responses to UV-B and simulated herbivory.
Supplemental Table S5. ANOVA results for JA, JA-Ile, and OPDA.
Supplemental Table S6. ANOVA results for TPI gene expression responses to UV-B and MeJA.
Supplemental Table S7. ANOVA results for UV-B effects on natural herbivory.
Supplemental Table S8. ANOVA results for CHS gene expression responses to UV-B and simulated herbivory.
Supplemental Table S9. ANOVA results for hydroxylated derivatives of jasmonate.
Supplemental Table S10. Primer sequences used for qPCR assays.
Supplementary Material
Acknowledgments
We thank Miriam Izaguirre, Carlos Mazza, Javier Moreno, Miriam Cargnel, Mercedes Keller, and Amy Austin for discussions, Matthias Schöttner for help with phenolic determinations, and Fabiana Ojeda for thrips determinations.
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica and Universidad de Buenos Aires (grant nos. PICT–04 1798, PICT–06 1296, and UBACyT–08–10 G034 to C.L.B.) and by the Max Planck Gesellschaft (to I.T.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Carlos L. Ballaré (ballare@ifeva.edu.ar).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe H, Shimoda T, Ohnishi J, Kugimiya S, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M (2009) Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol 9 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A-H-Mackerness S, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22 1413–1423 [Google Scholar]
- Andrade A, Vigliocco A, Alemano S, Miersch O, Botella MA, Abdala G (2005) Endogenous jasmonates and octadecanoids in hypersensitive tomato mutants during germination and seedling development in response to abiotic stress. Seed Sci Res 15 309–318 [Google Scholar]
- Ballaré CL (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32 713–725 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Barnes PW, Flint SD (1995) Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings. 2. Time-course, comparison with flavonoid responses and adaptive significance. Physiol Plant 93 593–601 [Google Scholar]
- Ballaré CL, Rousseaux MC, Searles PS, Zaller JG, Giordano CV, Robson MT, Caldwell MM, Sala OE, Scopel AL (2001) Impacts of solar ultraviolet-B radiation on terrestrial ecosystems of Tierra del Fuego (southern Argentina): an overview of recent progress. J Photochem Photobiol B Biol 62 67–77 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Stapleton AE, Yanovsky MJ (1996) Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiol 112 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PW, Searles PS, Ballaré CL, Ryel RJ, Caldwell MM (2000) Non-invasive measurements of leaf epidermal transmittance of UV radiation using chlorophyll fluorescence: field and laboratory studies. Physiol Plant 109 274–283 [Google Scholar]
- Bassman JH (2004) Ecosystem consequences of enhanced solar ultraviolet radiation: secondary plant metabolites as mediators of multiple trophic interactions in terrestrial plant communities. Photochem Photobiol 79 382–398 [DOI] [PubMed] [Google Scholar]
- Bergvinson DJ, Arnason JT, Hamilton RI, Tachibana S, Towers GHN (1994) Putative role of photodimerized phenolic acids in maize resistance to Ostrinia nubilalis (Lepidoptera: Pyralidae). Environ Entomol 23 1516–1523 [Google Scholar]
- Bilger W, Veit M, Schreiber L, Schreiber U (1997) Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant 101 754–763 [Google Scholar]
- Braun J, Tevini M (1993) Regulation of UV-protective pigment synthesis in the epidermal layer of rye seedlings (Secale cereale L. cv. Kustro). Photochem Photobiol 57 318–323 [Google Scholar]
- Britt AB (2004) Repair of DNA damage induced by solar UV. Photosynth Res 81 105–112 [Google Scholar]
- Brosché M, Schuler MA, Kalbina I, Connor L, Strid A (2002) Gene regulation by low level UV-B radiation: identification by DNA array analysis. Photochem Photobiol Sci 1 656–664 [DOI] [PubMed] [Google Scholar]
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA 102 18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, Jenkins GI (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60 183–205 [DOI] [PubMed] [Google Scholar]
- Caldwell MM (1971) Solar UV irradiation and the growth and development of higher plants. In AC Giese, ed, Photophysiology, Vol VI: Current Topics in Photobiology and Photochemistry. Academic Press, New York, pp 131–177
- Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, Kulandaiveli G, Tevini M (2003) Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem Photobiol Sci 2 29–38 [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Bornman JF, Ballaré CL, Flint SD, Kulandaivelu G (2007) Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem Photobiol Sci 6 252–266 [DOI] [PubMed] [Google Scholar]
- Caputo C, Rutitzky M, Ballaré CL (2006) Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia 149 81–90 [DOI] [PubMed] [Google Scholar]
- Cipollini D (2004) Stretching the limits of plasticity: can a plant defend against both competitors and herbivores? Ecology 85 28–37 [Google Scholar]
- Cloix C, Jenkins GI (2008) Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Mol Plant 1 118–128 [DOI] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA (1996) The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 383 826–829 [DOI] [PubMed] [Google Scholar]
- De La Rosa TM, Julkunen-Tiitto R, Lehto T, Aphalo PJ (2001) Secondary metabolites and nutrient concentrations in silver birch seedlings under five levels of daily UV-B exposure and two relative nutrient addition rates. New Phytol 150 121–131 [Google Scholar]
- de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451 480–484 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18 923–937 [DOI] [PubMed] [Google Scholar]
- Edreva AM, Velikova VB, Tsonev TD (2007) Phenylamides in plants. Russ J Plant Physiol 54 287–301 [Google Scholar]
- Elliger CA, Wong Y, Chan BG, Waiss AC Jr (1981) Growth inhibitors in tomato (Lycopersicon) to tomato fruitworm (Heliothis zea). J Chem Ecol 7 753–758 [DOI] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SH, Martinez C, Gusmaroli G, Wang Y, Zhou JL, Wang F, Chen LY, Yu L, Iglesias-Pedraz JM, Kircher S, et al (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-Iso-jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5 344–350 [DOI] [PubMed] [Google Scholar]
- Gális I, Åimek P, Narisawa T, Sasaki M, Horiguchi T, Fukuda H, Matsuoka K (2006) A novel R2R3 MYB transcription factor NtMYBJS1 is a methyl jasmonate-dependent regulator of phenylpropanoid-conjugate biosynthesis in tobacco. Plant J 46 573–592 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36 794–807 [DOI] [PubMed] [Google Scholar]
- Hatcher PE, Paul ND (1994) The effect of elevated UV-B radiation on herbivory of pea by Autographa gamma. Entomol Exp Appl 71 227–233 [Google Scholar]
- Hoffland E, Dicke M, Van Tintelen W, Dijkman H, Van Beusichem ML (2000) Nitrogen availability and defense of tomato against two-spotted spider mite. J Chem Ecol 26 2697–2711 [Google Scholar]
- Hoffmann-Campo CB, Harborne JB, McCaffery AR (2001) Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl 98 181–194 [Google Scholar]
- Hofmann RW, Campbell BD, Bloor SJ, Swinny EE, Markham KR, Ryan KG, Fountain DW (2003) Responses to UV-B radiation in Trifolium repens L: physiological links to plant productivity and water availability. Plant Cell Environ 26 603–612 [Google Scholar]
- Horn M, Patankar AG, Zavala JA, Wu J, Doleckova-Maresova L, Vujtechova M, Mares M, Baldwin IT (2005) Differential elicitation of two processing proteases controls the processing pattern of the trypsin proteinase inhibitor precursor in Nicotiana attenuata. Plant Physiol 139 375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59 41–66 [DOI] [PubMed] [Google Scholar]
- Izaguirre MM, Mazza CA, Biondini M, Baldwin IT, Ballaré CL (2006) Remote sensing of future competitors: impacts on plants defenses. Proc Natl Acad Sci USA 103 7170–7174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Mazza CA, Svatos A, Baldwin IT, Ballaré CL (2007) Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann Bot (Lond) 99 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL (2003) Convergent responses to stress: solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132 1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GI (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60 407–431 [DOI] [PubMed] [Google Scholar]
- Keinänen M, Oldham NJ, Baldwin IT (2001) Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem 49 3553–3558 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38 639–649 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305 665–668 [DOI] [PubMed] [Google Scholar]
- Kranthi S, Kranthi KR, Wanjari RR (2003) Influence of semilooper damage on cotton host-plant resistance to Helicoverpa armigera (Hub). Plant Sci 164 157–163 [Google Scholar]
- Krugel T, Lim M, Gase K, Halitschke R, Baldwin IT (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12 177–183 [Google Scholar]
- Kuhlmann F, Müller C (2009) Development-dependent effects of UV radiation exposure on broccoli plants and interactions with herbivorous insects. Environ Exp Bot 66 61–68 [Google Scholar]
- Landry LG, Chapple CC, Last RL (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavola A, Julkunen-Tiitto R, Roininen H, Aphalo P (1998) Host-plant preference of an insect herbivore mediated by UV-B and CO2 in relation to plant secondary metabolites. Biochem Syst Ecol 26 1–12 [Google Scholar]
- Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PGL (2009) Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol 150 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner M, Boland W, Mithöfer A (2005) Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol 167 597–606 [DOI] [PubMed] [Google Scholar]
- Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215 239–247 [DOI] [PubMed] [Google Scholar]
- Lindroth RL, Hofmann RW, Campbell BD, McNabb WC, Hunt DY (2000) Population differences in Trifolium repens L. response to ultraviolet-B radiation: foliar chemistry and consequences for two lepidopteran herbivores. Oecologia 122 20–28 [DOI] [PubMed] [Google Scholar]
- Lou Y, Baldwin IT (2003) Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proc Natl Acad Sci USA 100 14581–14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL (2000) Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol 122 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Izaguirre MM, Curiale J, Ballaré CL (2010) A look into the invisible: ultraviolet-B sensitivity in an insect (Caliothrips phaseoli) revealed through a behavioural action spectrum. Proc R Soc B 277 367–373 [DOI] [PMC free article] [PubMed]
- Mazza CA, Izaguirre MM, Zavala J, Scopel AL, Ballaré CL (2002) Insect perception of ambient ultraviolet-B radiation. Ecol Lett 5 722–726 [Google Scholar]
- Mazza CA, Zavala J, Scopel AL, Ballaré CL (1999) Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proc Natl Acad Sci USA 96 980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud ES, Berenbaum MR (1994) Stratospheric ozone depletion and plant-insect interactions: effects of UVB radiation on foliage quality of Citrus jambhiri for Trichoplusia ni. J Chem Ecol 20 525–539 [DOI] [PubMed] [Google Scholar]
- McGuire R, Agrawal AA (2005) Trade-offs between the shade-avoidance response and plant resistance to herbivores? Tests with mutant Cucumis sativus. Funct Ecol 19 1025–1031 [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballaré CL (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson LC, Veit M, Bornman JF (1999) Epidermal transmittance and phenolic composition in leaves of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiol Plant 107 259–266 [Google Scholar]
- Pandey SP, Baldwin IT (2008) Silencing RNA-directed RNA polymerase 2 increases the susceptibility of Nicotiana attenuata to UV in the field and in the glasshouse. Plant J 54 845–862 [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin I (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51 79–91 [DOI] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LACJ (2009) Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol 149 1701–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber S, Bornman JF, Weissenbäck G (1996) Phenylpropanoid compounds in primary leaf tissues of rye (Secale cereale): light response of their metabolism and the possible role in UV-B protection. Physiol Plant 97 160–168 [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Paul ND (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170 677–699 [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Ballaré CL, Scopel AL, Searles PS, Caldwell MM (1998) Solar ultraviolet-B radiation affects plant-insect interactions in a natural ecosystem of Tierra del Fuego (southern Argentina). Oecologia 116 528–535 [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Julkunen-Tiitto R, Searles PS, Scopel AL, Aphalo PJ, Ballaré CL (2004) Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus antarctica. Oecologia 138 505–512 [DOI] [PubMed] [Google Scholar]
- Ryan KG, Markham KR, Bloor SJ, Bradley JM, Mitchell KA, Jordan BR (1998) UVB radiation induced increase in quercetin:kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem Photobiol 68 323–330 [Google Scholar]
- Sorin C, Salla-Martret M, Bou-Torrent J, Roig-Villanova I, Martinez-Garcia JF (2009) ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J 59 266–277 [DOI] [PubMed] [Google Scholar]
- Stamp NE, Osier TL (1998) Response of five insect herbivores to multiple allelochemicals under fluctuating temperatures. Entomol Exp Appl 88 81–96 [Google Scholar]
- Stamp NE, Yang YL (1996) Response of insect herbivores to multiple allelochemicals under different thermal regimes. Ecology 77 1088–1102 [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine's defensive function in nature. PLoS Biol 2 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J (2003) Ultraviolet-B radiation co-opts defense signaling pathways. Trends Plant Sci 8 526–533 [DOI] [PubMed] [Google Scholar]
- Stratmann JW, Stelmach BA, Weiler EW, Ryan CA (2000) UVB/UVA radiation activates a 48 kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochem Photobiol 71 116–123 [DOI] [PubMed] [Google Scholar]
- Strid Å, Chow WS, Anderson JM (1994) UV-B damage and protection at the molecular level in plants. Photosynth Res 39: 475–489 [DOI] [PubMed]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ (2004) Red:far-red light ratio and UV-B radiation: their effects on leaf phenolics and growth of silver birch seedlings. Plant Cell Environ 27 1005–1013 [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448 661–665 [DOI] [PubMed] [Google Scholar]
- Thomas B, Dickinson HG (1979) Evidence for two photoreceptors controlling growth in de-etiolated seedlings. Planta 146 545–550 [DOI] [PubMed] [Google Scholar]
- Walters DR (2000) Polyamines in plant-microbe interactions. Physiol Mol Plant Pathol 57 137–146 [Google Scholar]
- Walters DR (2003) Polyamines and plant disease. Phytochemistry 64 97–107 [DOI] [PubMed] [Google Scholar]
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KE, Wilson MI, Greenberg BM (1998) Identification of the flavonoid glycosides that accumulate in Brassica napus L. cv. Topas specifically in response to ultraviolet B radiation. Photochem Photobiol 67 547–553 [Google Scholar]
- Wu J, Hettenhausen C, Baldwin I (2006) Evolution of proteinase inhibitor defenses in North American allopolyploid species of Nicotiana. Planta 224 750–760 [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Schuman MC, Baldwin IT (2008) A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore Manduca sexta. Plant Physiol 146 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT (2004) Fitness benefits of trypsin proteinase inhibitor expression in Nicotiana attenuata are greater than their costs when plants are attacked. BMC Ecol 4 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui D, Baldwin IT (2004) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Scopel AL, Ballaré CL (2001) Effects of ambient UV-B radiation on soybean crops: impact on leaf herbivory by Anticarsia gemmatalis. Plant Ecol 156 121–130 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.