Abstract
The relevance of the symbiosis-induced Medicago truncatula sucrose synthase gene MtSucS1 for an efficient arbuscular mycorrhiza (AM) was studied using two independent antisense lines that displayed up to 10-fold reduced SucS1 levels in roots. Mycorrhizal MtSucS1-reduced lines exhibited an overall stunted aboveground growth under inorganic phosphorus limitation. Apart from a reduced plant height, shoot weight, and leaf development, a delayed flowering, resulting in a lower seed yield, was observed. In addition, the root-to-shoot and root weight ratios increased significantly. Gene expression studies demonstrated a major reversion of AM-associated transcription, exhibiting a significant repression of well-known plant AM marker and mycosymbiont genes, together indicating a diminished AM fungus colonization of MtSucS1-antisense lines. Concomitantly, gas chromatography-mass spectrometry-based metabolite profiling revealed that mycorrhizal MtSucS1-reduced lines were affected in important nodes of the carbon, nitrogen, and phosphorus metabolism, accentuating a physiological significance of MtSucS1 for AM. In fact, antisensing MtSucS1 provoked an impaired fungal colonization within the less abundant infected regions, evident from strongly reduced frequencies of internal hyphae, vesicles, and arbuscules. Moreover, arbuscules were early senescing, accompanied with a reduced development of mature arbuscules. This defective mycorrhiza status correlated with reduced phosphorus and nitrogen levels and was proportional to the extent of MtSucS1 knockdown. Together, our results point to an important role for MtSucS1 in the establishment and maintenance of arbuscules in the AM symbiosis.
The arbuscular mycorrhiza (AM) with endotrophic fungi (AMF; Smith and Read, 1997) of the phylum Glomeromycota (Schüßler et al., 2001) is the most important mutual symbiosis of land plants (Smith and Read, 1997). The beneficial nutrient exchange of carbon (C) for mainly phosphorus (P), nitrogen (N), and enhanced water availability at the plant/fungus interfaces is central to AM (Smith and Smith, 1990, 1997). Moreover, AM interactions support plant growth and reproduction (Valentine et al., 2001; Wu and Xia, 2006) as well as the host's resistance and tolerance to pathogens and abiotic stress conditions (Liu et al., 2007; van Wees et al., 2008). For up to 70% of the host plant P (Smith and Read, 1997), obligate biotrophic AMF receive their entire C requirement from an approximately 20% to 30% proportion of recently assimilated C (Jakobsen and Rosendahl, 1990; Douds et al., 2000; Bago et al., 2003).
In plants, a substantial portion of the photosynthetically fixed C is channeled into Suc synthesis. Suc is used for long-distance C and energy transport into diverse heterotrophic sinks and represents the preferred carbohydrate (CH) translocated to AM, which is associated with an efficient C flux into mycosymbionts (Bücking and Shachar-Hill, 2005). The symbiotic sink diverts the Suc flow to AMF-colonized sites (Blee and Anderson, 2002; Heinemeyer et al., 2006; García-Rodríguez et al., 2007), causing considerably lower total free CH contents in mycorrhizae than in nonmycorrhizal (NM) roots. Photosynthetic limitations were reported to affect mycorrhizal growth responses and inorganic P (Pi) uptake (Tester et al., 1985). Here, the CH shortage in symbiotic roots lowered or inhibited the AMF colonization (Son and Smith, 1988; Vierheilig et al., 2002) and decreased the proportion of functional arbuscules (Hayman, 1974). Consequently, photosynthetic activity, CH metabolism, and transport processes are optimized for symbiosis maintenance (Wright et al., 1998a, 1998b; Black et al., 2000), providing substrates for the higher respiratory demand of mycorrhizae (Shachar-Hill et al., 1995; Douds et al., 2000). This optimization is supported by altered expression profiles of genes involved in Suc breakdown (Blee and Anderson, 2002; Hohnjec et al., 2003; García-Rodríguez et al., 2007) and sugar transport (Harrison, 1996; García-Rodríguez et al., 2005).
So far, details on the AM-associated CH translocation at the symbiotic interface are limited (Pfeffer et al., 1999; Bago et al., 2003). It is still a matter of debate whether plants actively regulate this process (Graham et al., 1997) and whether intraradical hyphae (Solaiman and Saito, 1997; Bago et al., 2003) or arbuscules are the principal sites of fungal CH uptake (Pfeffer et al., 1999; Lammers et al., 2001). Due to the facts that intraradical fungal structures are unable to take up and utilize Suc (Shachar-Hill et al., 1995; Solaiman and Saito, 1997) and that Suc-cleaving activities are not identified in AMF yet (Schubert et al., 2003), exogenous hexoses (mainly Glc) have to be supplied to the mycosymbionts (Shachar-Hill et al., 1995; Bücking and Shachar-Hill, 2005). Interestingly, Schüßler et al. (2006) characterized the first glomeromycotan H+-monosaccharide cotransporter (GpMST1), presumably operating in the fungal arbuscular membrane of the mutual Geosiphon pyriformis/Nostoc punctiforme system.
Prior to the utilization by AMF, Suc must thus be hydrolyzed by the plant either via symplastic, cytoplasmic Suc synthases (SucS; Huber and Akazawa, 1986) and/or by different apoplastic and symplastic invertases (Inv; Sturm, 1999), particularly in the arbuscule-containing cells. In general, enhanced total Suc-cleaving activity involving different Inv and SucS followed the colonization of clover (Trifolium repens) roots with a mixed population of AMF (Wright et al., 1998a). Regarding the importance of extracellular, acidic Inv during AM, Schaarschmidt et al. (2007a) showed that, along with a root-specific inhibition of cell wall (CW)-bound, apoplastic Inv activity in transgenic tobacco (Nicotiana tabacum), the AM status was reduced. Additionally, transcriptional induction of the extracellular Inv gene LIN6 was located near AM structures and in phloem cells of mycorrhizal tomato (Solanum lycopersicum) roots (Schaarschmidt et al., 2006). However, artificially augmented hexose availability, obtained by yeast-derived, apoplastic Inv active in the arbuscule interface of transgenic mycorrhizal Medicago truncatula roots, failed to improve AM significantly (Schaarschmidt et al., 2007a; Schaarschmidt and Hause, 2008).
With respect to the involvement of cytoplasmic SucS during AM, transcript accumulation of two SucS genes was differentially affected in AMF-colonized maize (Zea mays) roots (Ravnskov et al., 2003). For the Glycine max/Glomus mosseae interaction, Schubert and coworkers (2003) showed that, along with a cytosolic alkaline Inv, also SucS activities significantly increased, while both soluble acid and apoplastic, CW-bound Inv activities diminished during AM development. On the other hand, Blee and Anderson (1998, 2002) ascertained that SucS-mediated and soluble, potentially vacuolar acid Inv-mediated C availability affected the regulation of arbuscule location and function in Phaseolus vulgaris.
Given these somewhat controversial findings regarding AM-associated Suc breakdown, we attempted to study the relevance of an efficient, SucS-mediated symplastic sink near the plant/fungus interfaces. In M. truncatula, the SucS family consists of five differentially regulated genes (Hohnjec et al., 1999, 2003). Of these, the MtSucS1 gene was most strongly expressed in root tissues, and only MtSucS1 was activated under endosymbiotic conditions. This was demonstrated by real-time reverse transcription (RT)-PCR (Hohnjec et al., 2003) and is supported by in silico expression analyses of the corresponding tentative consensus sequence TC100410 (M. truncatula Dana-Farber Cancer Institute Gene Index; http://compbio.dfci.harvard.edu/). In reporter gene assays of mycorrhizal roots, the MtSucS1 promoter was specifically activated in arbuscule-containing cells and cortical cells of the surrounding root regions (Hohnjec et al., 2003). Together, the characteristic MtSucS1 inductions in AM and root nodules suggest a physiological importance of MtSucS1 orthologues in legume/microbe interactions in general (Hohnjec et al., 2003; Baier et al., 2007).
Having shown the importance of MtSucS1 for biological N fixation in root nodules (Baier et al., 2007), we here employed the same MtSucS1-antisense (MtSucS1-as) lines to assess the function of MtSucS1 under AM conditions and investigated physiological effects occurring downstream of the reduced SucS1-mediated sink strength. Integrating physiological analyses and molecular techniques, we report a reduced growth and reproduction, photosynthetic activity, and an impaired nutritional status when MtSucS1-as lines depended on an efficient AM symbiosis. These physiological limitations were accompanied by repressions of plant and fungal AM marker genes and altered metabolite responses upon MtSucS1 reduction in endosymbiotic root tissues. Moreover, antisensing MtSucS1 in the two independent lines coincided with a defective internal AMF colonization. Apart from the reduced total root length colonization, deficiencies of the fungal occupancy of the roots were particularly evident from significantly decreased numbers of arbuscules within colonized regions and, strikingly, their handicapped development and earlier senescence. In line with our recent finding that MtSucS1 reduction affects symbiotic N fixation in M. truncatula root nodules (Baier et al., 2007), our results extend the current understanding of symbiotic legume/microsymbiont interactions toward plant/fungus nutrient exchange in AM.
RESULTS
To study the relevance of the symbiosis-induced Suc synthase MtSucS1 for the M. truncatula/G. mosseae mycorrhiza, two independent, transgenic MtSucS1-as lines (MtSucS1-as12 and -as19) were used, exhibiting approximately 10% and 20% of wild-type MtSucS1 transcript and protein levels in roots and root nodules, respectively (Baier et al., 2007). Phenotypic patterns of mycorrhizal, Pi-limited MtSucS1-as lines were compared with two mycorrhizal transgenic control lines. These independent transformation controls (subsequently named c) expressed fusions of the MtSucS1 promoter to the intron-containing gusA reporter gene (gusAint), maintained MtSucS1 wild-type levels in roots and root nodules (Baier et al., 2007), and exhibited phenotypic patterns comparable to equally treated M. truncatula R108 wild-type plants under the diverse growth conditions used in this study (data not shown).
NM growth as well as developmental patterns of MtSucS1-as lines and transformation controls were indistinguishable under Pi-deprived or fully supplemented conditions. All lines completed their life cycle in the same temporal frame, resulting in a comparable number of pods at the end of the experimental period. Variables such as seed lot vigor, plant growth and development, photosynthetic efficiency, or nutritional status (Supplemental Table S1, A–C) exceeded similar levels independent of the genetic background. Except for a tendency for an enhanced root system development in MtSucS1-as19 (Supplemental Table S1D), none of the variables reported differed significantly under nonsymbiotic growth conditions. In contrast, almost all physiological data of mycorrhizal MtSucS1-altered and control lines were significantly different under AM-dependent conditions (Supplemental Fig. S1). In the following sections, significant differences are indicated with a 0.05 > P ≥ 0.01 and b P < 0.01.
MtSucS1 Reduction Affects Plant Growth under AM-Dependent, Pi-Limited Conditions
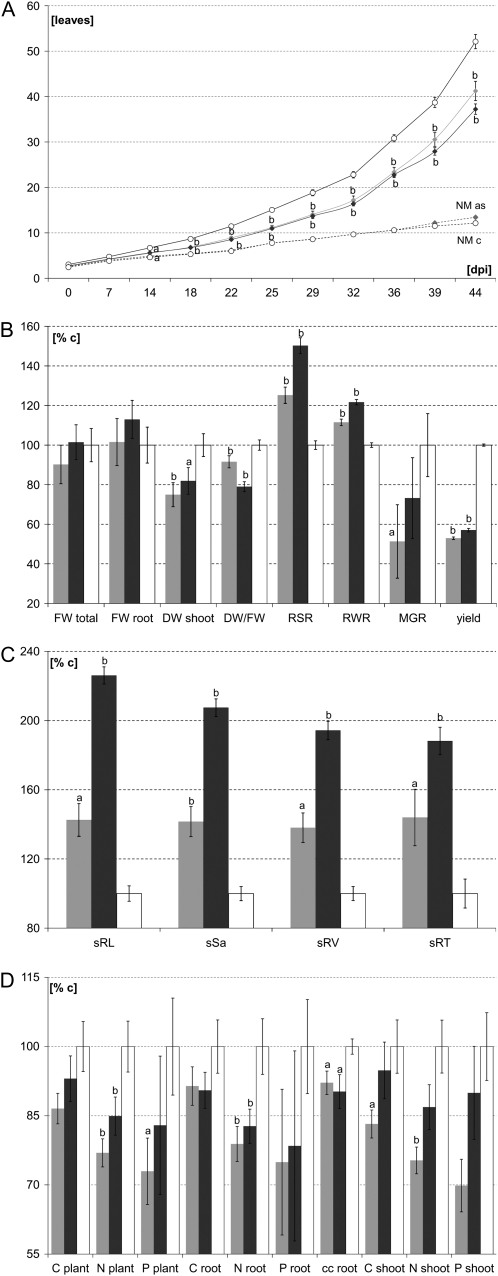
All phenotypic AM analyses were conducted on glasshouse-grown, G. mosseae-colonized plants cultured under strict Pi-deprived conditions from the day of inoculation until their final harvest at 50 d post inoculation (dpi). Depending on the symbiotic Pi supply, mycorrhizal MtSucS1-reduced plants exhibited an overall stunted vegetative growth in terms of plant height and leaf development. Along with the stronger MtSucS1 knockdown in line as12, these aboveground alterations were more pronounced here (Fig. 1). Calculated over the experimental period, phylotaxic rates such as the initiation of sequential leaf primordia (rate of leaf initiation) and the successive development of two fully expanded leaves (rate of leaf appearance) were significantly reduced in both MtSucS1-as lines (Supplemental Table S2). This led to significantly elongated plastochrons and phyllochrons of the mycorrhizal antisense lines that therefore needed more time to initiate and completely develop two successive leaves, respectively (Supplemental Table S2). Classifying four developmental stages of leaf organogenesis from leaf primordia to a fully expanded source leaf by a decimal code (Baier et al., 2007), at 14 dpi, leaf rate curves of both MtSucS1-as lines started to differentiate significantly from controls and leaf units remained at substantially lower levels throughout the experimental period (Fig. 2A). Moreover, particularly line as12 exhibited an approximately 10-d earlier senescence of older leaves. During an active AM stage (28 dpi), the C-fixing performance, measured as the photochemical quantum yield of the youngest and oldest source leaf pair, tended to decline with leaf age independently of the genetic background but biased significantly stronger mycorrhizal MtSucS1-as lines (Supplemental Fig. S2). Whereas the initiation of inflorescences commenced about 19 dpi in AM control lines, this onset was delayed by 5 d in mycorrhizal MtSucS1-as12 and by 9 d in MtSucS1-as19, resulting in considerably reduced yield at 50 dpi (Fig. 2B). Notably, MtSucS1-as and control lines exhibited equal photosynthetic performances and pod yields under nonsymbiotic growth conditions (Supplemental Table S1, A and B; Supplemental Fig. S1, A and B).
Figure 1.
Phenotypes of Pi-limited MtSucS1-as line as12 and controls colonized by G. mosseae. Phenotypes of mycorrhizal MtSucS1-as12 and corresponding mycorrhizal control lines are shown at 32 dpi (multiple plants grown in parallel; A), 32 dpi (single plant; B), 37 dpi (single plant; C), 50 dpi (multiple plants grown in parallel; D), 50 dpi (single plant; E), and 57 dpi (single plant; F). Order of plant lines, starting from the left position, is MtSucS1-as12 and control. Plant material was harvested at 50 dpi.
Figure 2.
Growth and developmental characteristics of MtSucS1-as lines. A, Leaf number development in mycorrhizal MtSucS1-as and control lines. B, Relative vegetative and reproductive characteristics of mycorrhizal MtSucS1-as and control lines at 50 dpi (n = 30 individuals per line). DW, Dry weight; FW, fresh weight; RWR, root weight ratio as DWroot per DWplant. C, Specific root parameters of mycorrhizal MtSucS1-as and control lines. The specific root length, surface area, root volume, average root diameter, and root tip number of mycorrhizal MtSucS1-as and control lines were expressed as root parameter per unit of shoot dry weight (DWshoot) at 50 dpi (nas = 9, nc = 16 mycorrhizal plants). D, Root and shoot C, N, and P contents of mycorrhizal MtSucS1-as and control lines. The mineral contents were calculated per unit dry weight in 50 dpi-old mycorrhizae (n = 8 biological replicates per line). Significantly different means ± se are indicated as follows: a 0.05 > P ≥ 0.01, b P < 0.01. Relative values are in comparison with control levels set to 100%. Gray bars, AM MtSucS1-as line as12; dark gray bars, AM MtSucS1-as line as19; white bars, AM control lines; gray squares and dashed gray line, NM MtSucS1-as; white circles and dashed black line, NM controls. Yield indicates number of pods at day of harvest. cc, Construction cost; % c, Percentage in relation to controls.
At final harvest, the total fresh weights of the mycorrhizal lines hardly varied, but 10% and 21% reduced dry weight-to-fresh weight ratios indicated altered water relations and/or cell densities in mycorrhizal MtSucS1-as12 and -as19, respectively. Deduced from the positive mycorrhizal growth responses (MGRs; growth responses to Glomus colonization as increase in aboveground dry weight; Fig. 2B), all lines benefited from mycorrhization. Nevertheless, MGRs of the antisense lines were depleted considerably, with the MGR of line as19 being reduced by 27% and that of line as12 by 49% compared with control levels. Accordingly, mycorrhizal MtSucS1-as12 developed an average shoot dry weight of 75% and as19 of 82% (Fig. 2B), reflecting the temporal aboveground development (Figs. 1 and 2, A and B). While the root fresh weights of MtSucS1-as lines were not significantly altered, the relative root growth increased in mycorrhizal MtSucS1-as lines. In fact, the root-to-shoot ratios (RSR) resulted in a ranking of as19 > as12 > c in the ratio 1.51b:1.25b:1 (Fig. 2B). In contrast, for fully supplemented MtSucS1-as12 and -as19 lines, the RSR (0.57 and 0.80, respectively) ranged near the full supplementation controls (0.60; Supplemental Table S1A) as well as near the RSR of 0.75 to 0.80 common for plants grown under nonstressed conditions (Waring et al., 1998). Compared with transformation controls, the fully supplemented line as19 already showed a tendency for an enhanced belowground allocation (Supplemental Table S1, A and D), but alterations in the biomass partitioning pattern were solely significant when depending on an efficient AM symbiosis (Supplemental Fig. S1D).
Alterations in the degree of root system plasticity are mirrored in variables of the root system architecture (RSA; Fig. 2C). Here, the total root length (TRL), root surface area (RSa), root volume (RV), and root tip numbers (RT) are sufficient parameters to describe root functions such as nutrient and water acquisition. In response to Pi limitation, according to their TRL, the ranking of AM lines was as19 > as12 > c in the ratio 1.90b:1.14:1 (Fig. 2C). Hence, MtSucS1-as19 established the largest root system and also mirrored average 1.8-fold increases on RV, RSa, and RT, depending on the symbiotic Pi acquisition (Fig. 2C). The TRL of the mycorrhizal line as12 was increased by 14%, and other absolute RSA parameters ranged between minor differences to a 24% enhancement of RTs, albeit changes were not significant. In both MtSucS1-as lines, the elevated root system growth coincided with minor but significant reductions of the average root diameter (−4% and −6% in MtSucS1-as12 and -as19 mycorrhiza, respectively). Along with altered biomass partitioning patterns, these differences in the RSA were even more dramatic when calculated with respect to the production of shoot dry weight mediated by the root system produced. Thus, mycorrhizal antisense lines differed markedly from AM controls in all the shoot mass-normalized RSA parameters tested, showing an averaged 1.4- and 2.0-fold induction in the specific RL (sRL), sRSa, sRV, and sRT of mycorrhizal MtSucS1-as12 and -as19, respectively (Fig. 2C). Thus, both antisense lines increased their relative production of root tissue under AM conditions, while their nonsymbiotic shoot mass-normalized root system growth ranged near control levels in line as19 and slightly decreased in as12 under full supplementation (Supplemental Table S1D). Based on the diverse physiological data presented, we conclude that an MtSucS1 reduction substantially affects plant growth merely under AM-dependent, Pi-limited conditions.
Antisensing MtSucS1 Leads to Reduced P and N Contents in Mycorrhizal Plants
Cellular pools of mobile cytoplasmic and vacuolar orthophosphates were measured to evaluate the Pi uptake efficiency. The P contents of the root and shoot tissues of all AMF-colonized lines were elevated compared with NM tissues, but mycorrhizal MtSucS1-as material contained considerably less P than equally treated controls (Fig. 2D). It is worth noting that P levels in shoot samples excluded the particularly Pi-demanding pods (Bryla and Koide, 1990), which were also substantially reduced in both size and number in the mycorrhizal MtSucS1-as lines (Fig. 2B). Along with the least P concentration in the aboveground material (data not shown) and a 1.33-fold lower dry weight of the MtSucS1-as12 shoot, there was a markedly decreased net P content of the shoot in this line. Together with substantial reductions on the root P pools, this also resulted in the lowest net plant P content for mycorrhizal antisense line as12 (Fig. 2D). Considering withdrawal of P from early-senescing leaves, the P concentration of the shoots of mycorrhizal antisense lines reached almost the reference level at 50 dpi but was maintained at the substantial expense of the major P storage in plant root tissue. By contrast, AM controls readily acquired sufficient P levels for the aboveground material without a withdrawal of P via augmented leaf senescence, lowered yields or seed filling (Fig. 2B), or reducing the P concentration and content in the root tissue (Fig. 2D).
Aboveground (generative organs excluded) and belowground tissues showed furthermore significantly reduced N contents (1.27- and 1.21-fold; Fig. 2D), causing a consistently increased C-N balance in both mycorrhizal MtSucS1-reduced lines. Taking the dry weight of the shoot into account, the aboveground N contents were 1.33- and 1.15-fold reduced in MtSucS1-as lines as12 and as19, respectively (Fig. 2D), also resulting in a significantly reduced whole-plant N (−1.18 and −1.30). Deduced from C and N concentrations measured, the construction costs of mycorrhizal roots (C demand per unit of root produced) were significantly reduced in MtSucS1-as lines (−1.09 and −1.11 in MtSucS1-as12 and -as19, respectively; Fig. 2D), presumably pointing to a reduced AM formation in MtSucS1-as lines. Together, these findings indicate a negative effect of MtSucS1 reduction on the P and N nutritional status of the shoot and root tissues under AM-dependent conditions.
MtSucS1 Reduction Is Accompanied by a Down-Regulation of AM Marker Genes
Since the development of an efficient AM considerably alters epidermal and cortical root cells as well as the overall root physiology (Bonfante, 2001; Genre et al., 2008; Guether et al., 2009; Pumplin and Harrison, 2009), effects of MtSucS1 reduction were expected to be mirrored in the root transcriptome. We examined transcriptional changes of 36 M. truncatula and 12 G. mosseae genes with AMF colonization (primer sets in Supplemental Table S3). Particular attention was paid to host genes primarily coding for AM-specific markers with special focus on arbuscule-related genes (Hohnjec et al., 2005, 2006; Liu et al., 2007; Gomez et al., 2009). Regarding glycoside hydrolases, we focused on the SucS family (Hohnjec et al., 1999), several Inv, as well as fructan-active enzymes (1-FEH; Wiemken et al., 1986). Glomus candidate genes had assigned functions during fungal symbiotic life.
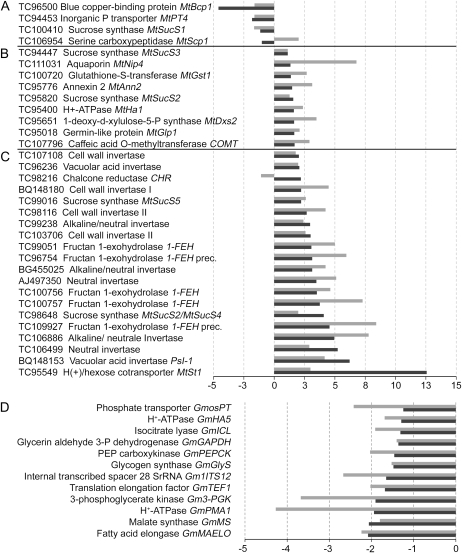
As expected, antisensing MtSucS1 significantly reduced the MtSucS1 mRNA levels in as19 and as12 mycorrhiza (20% and 13% of control levels, respectively; Table I). Subsequently, both lines displayed highly similar transcriptional changes for the candidate genes checked. As compared with mycorrhizal controls, differential transcription (2-fold threshold; P < 0.05) was monitored for 13 out of 29 plant and 10 out of 12 G. mosseae genes in at least one type of the MtSucS1-altered mycorrhiza. Common repressions were especially apparent for the set of AM marker genes (MtBcp1, MtScp1, MtPT4, MtGst1, MtAnn2, MtDsx2, MtHa1, and MtGlp) and all fungal genes measured (Table I). Particularly, the AM-specific MtPT4 expression (Harrison et al., 2002) was markedly reduced to 22% and 24% of control levels in as19 and as12, respectively (Table I), indicating extensive alterations in a reliable parameter for AM functionality and arbuscule vitality (Isayenkov et al., 2004; Javot et al., 2007).
Table I.
Ratios of relative gene expression in MtSucS1-as lines at 50 dpi
Relative expression values were calculated via MtTef1α normalization. Tentative consensus (TC), EST, and gene sequences are sorted via x-fold changes with respect to as19. Correlation analyses of genes to MtSucS1 expression are indicated by +++ (r ≥ +0.6), ++ (+0.6 > r > +0.4), + (+0.4 ≥ r > 0), and − (0 ≥ r ≥ –0.4). Relative expression for as19 and as12 denotes mean transcript ratios of MtSucS1-as lines versus control lines. Relative expression ratios exceeding the criteria of significance of both 2-fold threshold and P < 0.05 are shown in italic boldface. Ratios achieving only one criterion are marked in boldface. Columns 5 and 6 display transcript ratios of MtSucS1-as lines versus control lines per MtPT4/GmTef1 activity factor. This activity factor correlates candidate gene expression with the number of active arbuscules (measured via MtPT4) and the total presence of the AMF in the colonized root (measured via GmTef1). Means and ratios were based on five biological replicates. *, Significant difference of 0.05 > P ≥ 0.01. **, Significant difference of P < 0.01. na, Not applicable; nd, not detected.
| Group | Identifier | Putative Annotation | rMtSucS1 | Relative Expression |
Relative Expression per Activity Factor MtPT4/GmTe1 |
||
|---|---|---|---|---|---|---|---|
| as19 | as12 | as19 | as12 | ||||
| SucS | TC100410 | Sucrose synthase MtSucS1 | 1 | −4.9** | −7.8** | −1.1 | −1.7 |
| TC95820 | Sucrose synthase MtSucS2 | +* | −2.3* | −3.1** | 1.6 | 1.3 | |
| TC94447 | Sucrose synthase MtSucS3 | + | −3.7** | −3.7** | 1.1 | 1.1 | |
| TC98648 | Sucrose synthase MtSucS/MtSucS4 | +++** | −1.0 | −2.2* | 4.1 | 2.0 | |
| TC99016 | Sucrose synthase MtSucS5 | + | −1.6 | −1.6 | 2.2 | 2.6 | |
| AM marker | TC96500 | Blue copper-binding protein MtBcp1 | + | −15.6* | −11.3* | −4.6 | −1.6 |
| TC106954 | Serine carboxypeptidase MtScp1 | +++** | −4.5** | −2.6* | −1.0 | 2.0 | |
| TC94453 | Inorganic phosphorus transporter MtPT4 | +++** | −4.5** | −4.1** | −1.8 | −1.6 | |
| TC100720 | Glutathione S-transferase MtGst1 | ++* | −3.5* | −2.0* | 1.4 | 2.7 | |
| TC111031 | Aquaporin MtNip4 | + | −3.0** | 1.2 | 1.3 | 6.8 | |
| TC95776 | Annexin 2 MtAnn2 | ++* | −2.9* | −1.5 | 1.5 | 3.1 | |
| TC95651 | 1-Deoxy-d-xylulose-5-phosphate synthase MtDxs2 | ++* | −2.5* | −1.5 | 1.6 | 3.5 | |
| TC95400 | H+-ATPase MtHa1 | ++* | −2.2* | −2.1* | 1.6 | 2.4 | |
| TC95018 | Germin-like protein Glp1 | ++* | −2.2 | −2.2* | 1.7 | 2.1 | |
| TC95549 | H+/hexose cotransporter MtSt1 | − | 2.5 | −1.2 | 12.6 | 3.0 | |
| TC107796 | Caffeic acid O-methyltransferase COMT | +++** | −2.9** | −1.6** | 1.7 | 2.9 | |
| TC98216 | Chalcone reductase CHR | + | −1.4 | −2.8 | 2.2 | −1.1 | |
| Inv | BQ148180 | Cell wall invertase I | + | −3.2 | −1.3 | 2.2 | 4.5 |
| TC107108 | Cell wall invertase | ++* | −2.9 | −2.9* | 2.0 | 1.8 | |
| TC96236 | Vacuolar acid invertase | +++** | −2.3* | −2.5** | 2.1 | 2.0 | |
| TC103706 | Cell wall invertase II | +++** | −1.8 | −1.9* | 3.0 | 2.6 | |
| TC98116 | Cell wall invertase II | + | −1.7* | −1.1 | 2.7 | 4.2 | |
| TC99238 | Alkaline/neutral invertase | ++* | −1.5 | −2.0* | 3.0 | 2.4 | |
| BG455025 | Alkaline/neutral invertase | + | −1.3 | 1.0 | 3.1 | 4.2 | |
| AJ497350 | Neutral invertase | + | −1.3 | 1.0 | 3.5 | 5.1 | |
| TC106499 | Neutral invertase | + | −1.2 | −1.6* | 5.2 | 2.9 | |
| TC106886 | Alkaline/neutral Invertase | − | −1.2 | 3.9** | 5.0 | 7.8 | |
| BQ148153 | Vacuolar acid invertase PsI-1 | + | −1.1 | 1.1 | 6.2 | 4.2 | |
| TC99506 | Acid invertase | + | nd | nd | na | na | |
| 1-FEH | TC96754 | Fructan 1-exohydrolase 1-FEH precursor | + | −1.5 | 1.2 | 3.1 | 5.9 |
| TC99051 | Fructan 1-exohydrolase 1-FEH | + | −1.5 | −1.0 | 3.1 | 5.0 | |
| TC100756 | Fructan 1-exohydrolase 1-FEH | ++ | −1.3 | −1.2 | 3.5 | 4.6 | |
| TC100757 | Fructan 1-exohydrolase 1-FEH | − | −1.2 | 1.8 | 3.8 | 7.3 | |
| TC109927 | Fructan 1-exohydrolase 1-FEH precursor | – | 1.3 | 1.0 | 4.6 | 8.4 | |
| Fungal genes | AY193825 | H+-ATPase GmHA5 | +++** | −2.7** | −2.5* | −2.1 | −1.8 |
| Isocitrate lyase GmICL | +++** | −2.6** | −4.5** | −1.9 | −4.3 | ||
| Phosphoenolpyruvate carboxykinase GmPEPCK | ++** | −2.5* | −2.5* | −1.7 | −2.0 | ||
| DQ074452 | Phosphate transporter GmPT | ++** | −2.4* | −2.5* | −2.1 | −2.2 | |
| Translation elongation factor GmTEF1 | +++** | −2.4* | −2.7* | −1.5 | −2.0 | ||
| X84232 | Internal transcribed spacer 28SrRNA Gm1ITS12 | ++** | −2.2* | −2.1* | −1.5 | −1.5 | |
| Glyceraldehyde-3-phosphate dehydrogenase GmGAPDH | ++** | −1.9** | −2.9** | −1.9 | −3.7 | ||
| AF311438 | 3-Phosphoglycerate kinase Gm3-PGK | +* | −1.7* | −1.7 | −1.4 | −1.4 | |
| AY149918 | H+-ATPase GmPMA1 | + | −1.4 | −2.0* | −1.3 | −1.9 | |
| Glycogen synthase GmGlyS | ++* | −1.3 | −3.0** | −1.7 | −2.7 | ||
| Malate synthase GmMS | +* | −1.3 | −1.8 | −1.3 | −1.7 | ||
| Fatty acid elongase GmMAELO | +++** | −1.2 | −2.2** | −1.3 | −2.4 | ||
Transcripts of several Suc-hydrolyzing enzymes such as the CW Inv and vacuolar acid Inv (TC107108 and TC96236) were clearly reduced in both MtSucS1-as line mycorrhizae, while significant repressions of the CW Inv I and II (BQ148180, TC103706, and TC98116), the neutral Inv (TC106499), and the neutral/alkaline (N/A) Inv (TC99238 and TC106886) were found in one antisense line, preferentially in the stronger reduced line as12 (Table I). Differential transcriptional responses in MtSucS1-as12 and -as19 mycorrhizae were evident for the 1-FEH genes (Table I), possibly pointing to their tight dependency on the CH status of the mycorrhizal root tissue.
All fungal transcripts decreased sharply in MtSucS1-as mycorrhizae, with the effect being more pronounced in the strongly MtSucS1-reduced line as12. Constitutive fungal genes (GmTef1, GmITS12, Gm28sRNA; Isayenkov et al., 2004) were consistently reduced more than 2-fold in both MtSucS1-as AM lines, with GmTef1 being the most constant housekeeping gene. Using GmTef1 expression levels, endophyte concentrations in symbiotic roots diminished by 63% in as12 and by 59% in as19. This coincided with significant transcriptional decreases in six out of 12 G. mosseae genes, suggesting an MtSucS1-dependent mycosymbiont transcription (Table I). In addition, transcripts of GmPMA1 and genes involved in fungal storage processes (e.g. GmGlyS and GmMAELO) were significantly reduced at least in MtSucS1-as12 (Table I).
Applying the Bravais-Pearson coefficient of correlation r, the expression of several plant and fungal genes correlated positively with MtSucS1 transcript levels (Table I). Notably, a particularly strong correlation (rMtSucS1 ≥ 0.6) was found for plant AM marker genes (e.g. MtScp1, MtAnn2, MtPT4, MtGlp1, MtHa1, MtGst1, and MtDxs2; Table I), indicating a tight association of AM-related transcript levels with the relative MtSucS1 expression. The transcription of the 1-FEHs (TC96754 and TC109927), an N/A Inv (TC106886; Table I), and the (H+)/hexose cotransporter MtSt1 (Table I) solely correlated negatively with the MtSucS1 expression. In the case of mycosymbiont candidate gene expression, all fungal transcripts exhibited a positive interrelation with the level of MtSucS1 expression, with particularly strong correlations for GmICL, GmHA5, GmMAELO, and GmTef1 (Table I).
Since the significantly altered expression patterns in MtSucS1-limited mycorrhizae coincided with marked decreases in the fungal presence in mycorrhizal tissues, candidate gene expression values were further processed for the following reasons. Although all plant lines were treated at the same time with the same amount of inoculum, differences in transcription might be due to technical variations such as minor alterations in spore numbers and quality, affecting the fundamentals of root colonization, or to intrinsic genetic effects of the MtSucS1-as expression, causing a delayed and altered mycorrhization of the CH-limited plants. Thus, to better evaluate the efficiency of mycorrhizal infection in the symbiotic lines, to consider different levels of fungal occupation, and to account for shifts in the proportion of young and mature arbuscules and their efficiency for the symbiotic nutrient exchange, we defined an “activity factor” that correlated candidate gene expression with the number of active arbuscules (measured via MtPT4) and the total presence of the AMF in the colonized root (measured via GmTef1). Hence, transcript levels that were additionally normalized to this activity factor (MtPT4/GmTef1) are more meaningful, since gene expression is put in relation to the presence of efficient, mature arbuscules per fungal unit, thus considering fungal presence and AM developmental stage after conventional normalization via the general plant housekeeping gene MtTef1α (Table I).
It is important to note here that the MtPT4/GmTef1 ratio of the mycorrhizal control roots averaged 2.51, whereas as19 and as12 achieved significantly reduced values of 1.07 and 0.77, already implying marked reductions in the number of functional arbuscules per unit of AMF. Despite a correction via the respective line-specific activity factor, the relative expression of MtPT4 additionally normalized in this way remained at a markedly reduced level in MtSucS1-as mycorrhizae when compared with mycorrhizal controls, attaining −1.8 in MtSucS1-as19 and −1.6 in MtSucS1-as12 (Fig. 3A; Table I). The MtPT4/GmTef1-normalized values showed consistent repressions for MtSucS1 and MtBcp1 in MtSucS1-as mycorrhiza (Fig. 3A). Trends toward reduced transcriptional levels in at least one type of SucS1-reduced mycorrhiza were also monitored for the AM marker MtScp1 (Fig. 3A). Since MtBcp1, MtPT4, and MtScp1 represent well-studied AM marker genes being most active in the mature arbuscule phase, their strongly reduced expression levels in the antisense lines could point to a relevance of MtSucS1 for the development and maintenance of mature plant/fungus interfaces.
Figure 3.
Relative gene expression in mycorrhizal MtSucS1-as compared with control lines. Relative gene expression in lines as19 and as12 is given as mean MtSucS1-as/control ratios. Relative expression values were first calculated from MtTef1α-normalized values and were further normalized to the arbuscule activity-specific transcriptional coefficient MtPT4/GmTef1. Tentative consensus sequences are sorted via x-fold changes with respect to line as19. A to C, Relative quantities of mycorrhizal M. truncatula RNA per unit of MtPT4/GmTef1. D, Relative quantities of G. mosseae RNAs per unit of MtPT4/GmTef. Measurements are based upon five biological replicates. Gray bars, Mycorrhizal MtSucS1-as12; dark gray bars, mycorrhizal MtSucS1-as19. Gm, G. mosseae; TC, tentative consensus sequence.
The expression profiles of the remaining plant genes were subclassified into two clusters, applying a 2-fold threshold after normalization via the MtPT4/GmTef1 unit (Fig. 3, sixth column). The AM marker genes MtGlp1, MtDxs2, MtHa1, MtAnn2, and MtGst1 achieved expression levels between +1.4- and +1.7-fold in mycorrhizal as19 and between +2.1- and +3.5-fold in as12 (Fig. 3B). The genes activated more than 2-fold per active symbiotic interface primarily encoded Suc-degrading enzymes (Fig. 3C). In fact, MtSucS1 reduction appeared to up-regulate the arbuscule-related expression of genes encoding cytosolic glycoside hydrolases, since mRNAs from N/A Inv and neutral Inv genes showed tendencies toward an enhanced expression (Fig. 3C). Highest relative transcription in mycorrhizal MtSucS1-as root extracts was found for genes encoding 1-FEH (TC109927 and TC100757), N/A Inv (TC106886 and TC106499), and the vacuolar Inv PsI-1. Moderate reciprocal responses to MtSucS1 reduction in AM tissues also denote the relative inductions observed for MtSucS2 and MtSucS3 (Fig. 3B) as well as for MtSucS5 and MtSucS/MtSucS4 (Fig. 3C). Together, the up-regulation of genes from clusters B and C indicates an attempt of the macrosymbiont to compensate for the MtSucS1 knockdown to some extent. By contrast, since the activity of the complete set of fungal genes per active arbuscule remained clearly repressed in MtSucS1-reduced mycorrhizae (Fig. 3D), the microsymbiont transcription appears to be MtSucS1 dependent to a high degree.
MtSucS1 Reduction Is Correlated with Changes in Steady-State Pools of Polar Metabolites in AM Roots
The physiological impact of endophyte infection under MtSucS1 reduction was analyzed, monitoring differential abundances of 98 metabolic compounds in MtSucS1-as and control mycorrhizae (Supplemental Table S4). A comparison of the metabolite compositions displayed considerable alterations in 61 substances. The primary metabolic pools were mainly affected by MtSucS1 limitation, accumulating at −6.0- to +2.6-fold different levels in antisense line root extracts. Eighty-five percent of the metabolites tested, representing important nodes of the glycolysis, the tricarboxylic acid cycle, and the amino acid biosynthesis, appeared to be conjointly altered and preferentially reduced in MtSucS1-as mycorrhizae (Supplemental Table S4).
Accumulated Suc levels reciprocally correlated with the extent of MtSucS1 expression (1.40- and 1.46-fold increased in as19 and as12, respectively; subsequently +1.40/+1.46), indicating reduced sink strength in, but also a sufficient Suc supply to, the MtSucS1-altered mycorrhizae. Subsequently, the major hexoses and immediate Suc cleavage products Glc (−1.20/−1.08) and Fru (−1.24/+1.08) tended toward lower abundances in MtSucS1-as extracts. Moreover, MtSucS1-reduced mycorrhizae were associated with significant decreases in the hexose/Suc ratio, yielding 68% of control levels in as12 and 62% in as19. Concerning the soluble sugar content, pool sizes were either concomitantly reduced as for cellobiose, trehalose, and raffinose or enhanced as for maltose, Gal, rhamnose, and melibiose. The gross of sugar acids derived from Glc such as glycerate or glucuronate as well as dicarboxylic or hydroxy acids such as maleate and 2-hydroxyglutarate decreased in symbiotic MtSucS1-altered roots. The abundance of the endofungal, nonmetabolizable sugar alcohol mannitol (Rasmussen et al., 2008) was clearly reduced in both MtSucS1-as AM extracts (−2.30a/−2.31a). According to the reduced tissue P content in MtSucS1-as material (Fig. 2D), the phosphorylated metabolic pool also markedly decreased with an MtSucS1 knockdown (−1.40a/−1.35a). Beyond that, with direct linkage to a functional AM, the fungal C pool trehalose (−1.43b/−1.72b) as well as the plant N storage and transport compound Asn (−6.00a/−3.65a) were significantly reduced in MtSucS1-as mycorrhizae. With respect to fungus-mediated biotic stress or AM-mediated plant protection (Conrath et al., 2006; Pozo et al., 2009), the levels of γ-aminobutyrate (−2.33/−1.78) and the classical defense-related plant compound salicylic acid (−1.12/−1.38) also showed tendencies for reduction in mycorrhizal MtSucS1-as extracts, together indicating a reduced establishment and performance of AM in the MtSucS1-limited root material.
Glc reductions were accompanied by lowered pool sizes of almost all glycolytic intermediates, including Glc-6-P, Fru-6-P, dihydroxyacetone phosphate, 3- and 2-phosphoglycertae, and phosphoenolpyruvate, which implies a reduced catabolic activity in the glycolytic payoff phase upon MtSuc1 knockdown. Subsequently, compounds involved in the tricarboxylic acid cycle such as pyruvate, α-ketoglutarate, and succinate were reduced. Citrate, cis-aconitate, isocitrate, and malate were considerably more abundant in MtSucS1-as mycorrhiza (average +1.31 in as19 and +1.25 in as12), suggesting an adjusted glyoxylate pathway under conditions of limited Suc breakdown.
Marked reductions were found in the content of 13 out of the 20 proteinogenic amino acids, being most apparent for Asn. Reduced pool sizes were prominent for the major amino acids Ala, Arg, Asp, Lys, Ser, Thr, and Tyr, exhibiting average metabolic changes of −1.86 in as19 and −1.44 in as12. The minor amino acids Cys, Gly, Ile, Lys, Pro, Tyr, Trp, and Val, the aromatic amino acid precursor shikimate, as well as several intermediates and derivatives of the amino acid biosynthesis were also reduced in both antisense lines (Supplemental Table S4). By contrast, Glu and Gln showed average 1.3-fold increases in MtSucS1-as mycorrhiza. Nevertheless, along with approximately 1.2- to 1.3-fold reduced N concentrations and contents of mycorrhizal MtSucS1-as roots (Fig. 2D), the summarized content of all proteinogenic amino acids in line as19 comprised 74% and that in line as12 81% of control levels. Furthermore, N-containing metabolites representing the urea cycle as well as uncommon and nonproteinogenic amino acids (e.g. pyroglutamate and β-Ala) showed marked reductions. The latter is in agreement with likewise repressed pantothenate, the essential precursor of CoA (Raman and Rathinasabapathi, 2004). Intriguingly, classical markers of senescence processes such as RNA degradation are increased, since the free nucleobases adenine, cytosine, uracil, and thymine accumulated in MtSucS1-as mycorrhizae (average +1.22/+1.24), in concert with an enhanced level of Rib at least in line as12.
In conclusion, several molecular symbiosis markers such as the AM-related plant and fungal transcriptome and important nodes of the C, P, and N metabolism of mycorrhizal roots were significantly altered by an MtSucS1 reduction.
Antisensing MtSucS1 Leads to a Higher Proportion of Senescing Arbuscules
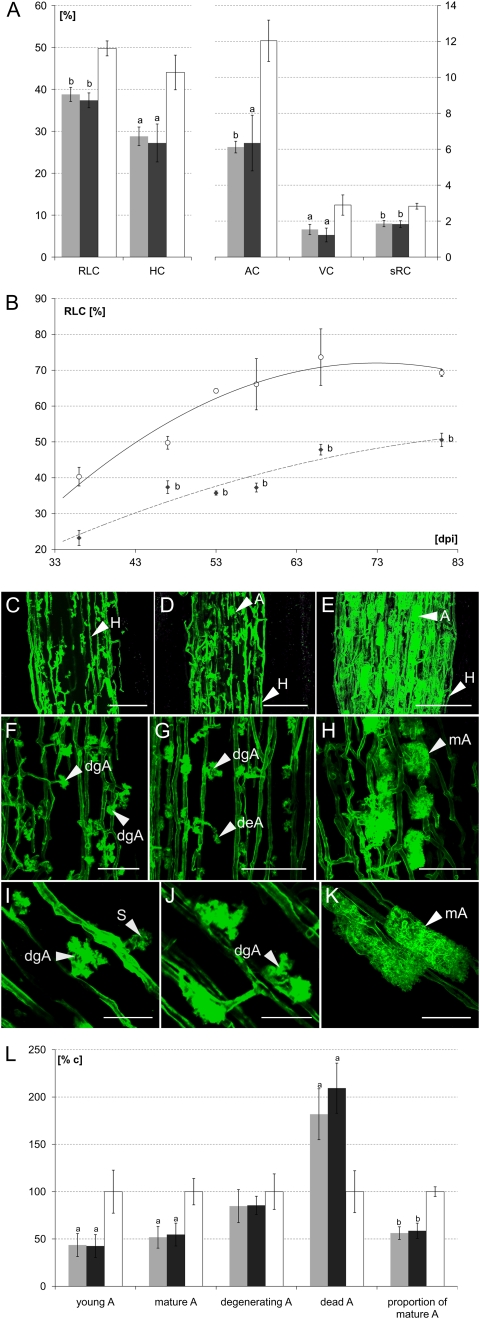
Although at 50 dpi the MtSucS1-as and control lines displayed Arum-type mycorrhizae with G. mosseae, a detailed investigation revealed a considerably lower mycorrhizal status with a reduced internal colonization upon MtSucS1 reduction. Controls achieved a uniform root length colonization (RLC) of 49.8%, whereas RLC reached 37.4% in line as19 and 38.8% in line as12 (Fig. 4A). Moreover, clear differences in the colonization pattern were found in transverse sections within a given colonized region. Independent of the total colonization, the successive fungal progression within the root cortex differed significantly, evidenced by densities of the intraradical hyphae, arbuscule, and vesicle colonization per root intersect. In fact, these intracellular fungal structures appeared 40% to 60% less numerous in MtSucS1-reduced mycorrhizae. In MtSucS1-as19, hyphal densities were less than in as12 and controls, coinciding with rarely produced vesicles (Fig. 4A), an effect most likely related to the enhanced root system partitioning. Despite an increased relative root system growth in MtSucS1-as plants (Fig. 2, B and C), the total amount of root length colonized by the fungus never exceeded the AMF occupancy observed in control mycorrhizae. Moreover, the specific root colonization (accentuated value of AMF per total root fresh weight) showed significant 1.52- and 1.55-fold reductions in as12 and as19, respectively (Fig. 4A). In general, using 35 individually analyzed mycorrhizae formed by the different plant lines, a positive correlation of the visually estimated RLC and parameters of plant fitness (e.g. generative yield and fresh weight of the shoot; data not shown) were evident. Furthermore, MtSucS1 expression and transcripts of plant AM marker genes strongly correlated with the reduced colonization (0.49 < rRLC < 0.74), an effect most prominent for MtPT4.
Figure 4.
Mycorrhiza phenotypes of MtSucS1-as and control lines. A, G. mosseae root length colonization and specific internal colonization of intraradical hyphae, arbuscules, and vesicles in mycorrhizal regions at 50 dpi. sRC, Specific root colonization. B, Root length colonization time course of mycorrhizal MtSucS1-as19 and control lines as revealed from sequentially harvested plants. Each point represents the mean ± se of at least three plants. Regression curves for mycorrhizal lines are shown. C to K, Representative fluorescence images of AlexaFluor488-stained mycorrhizal root segments at 50 dpi. C to E, MtSucS1-as12 (C), MtSucS1-as19 (D), and control lines (E) depicting the significantly reduced mycorrhiza status in both MtSucS1-as lines. Bars = 100 μm. F to K, Pronounced arbuscule senescence in MtSucS1-as12 and -as19 segments displayed by high proportions of degenerating (dgA) and dead (deA) arbuscules. Bars = 75 μm (F–H) and 30 μm (I–K). L, Distribution of the arbuscule developmental stages in MtSucS1-as lines at 50 dpi compared with controls set to 100%. Significantly different means ± se are indicated as follows: a 0.05 > P ≥ 0.01, b P < 0.01. Gray bars, MtSucS1-as line as12; dark gray bars, MtSucS1-as line as19; white bars, controls; gray squares and dashed gray line, mycorrhizal MtSucS1-as19; white circles and solid line, controls. Relevant morphological fungal structures such as intraradical hyphae (H), arbuscules (A), and septae (S) are highlighted with arrowheads. AC, Arbuscule colonization; HC, intraradical hyphae; mA, mature arbuscule; % c, percentage in relation to controls; VC, vesicle.
In a separate approach, RLC of MtSucS1-as19 and control mycorrhizae was monitored in a time course up to 81 dpi (Fig. 4B). Mycelium was visible on and inside control roots at 16 dpi. Intercellular and intracellular hyphal growth was not yet spacious, coinciding with some young arbuscules in cortical cells. Data from regular harvests exhibited the establishment of a mature reference AM within 36 dpi. The progression of the RLC in controls reached a plateau level of 65% to 70%, starting at 53 dpi (Fig. 4B) with numerous well-developed arbuscules, which were by now either mature or already degenerating (Fig. 4L). By contrast, AM development in MtSucS1-as19 was initially delayed and proceeded slower, resulting in a lower plateau than controls by more than 60 dpi. Until the end of the experimental period at 81 dpi, the mycorrhiza status of line as19 remained at an approximately −1.54-fold proportion of the RLC observed in reference mycorrhiza.
Confocal laser-scanning microscopy of fluorescence-labeled fungal structures demonstrated significant alterations in the morphology and life span of arbuscules in both mycorrhizal antisense lines. Here, a sharp reduction in the total number and proportion of mature arbuscules was evident (Fig. 4, C–K). In mycorrhizal controls, the distribution of the stages young, mature, degenerating, and dead arbuscules (Floß et al., 2008) was 1:2.3:1.4:1.1 (Fig. 4L), depicting a successive colonization process with balanced rates of arbuscule decay and reemergence. In contrast, MtSucS1-altered lines exhibited a significantly different proportioning of arbuscule developmental stages of 1:2.8:2.8a:4.6b in as12 and of 1:3.0:2.9a:5.5b in as19. Together, these figures clearly show that not only the total arbuscule formation was reduced but also the number of degenerating and dead arbuscules increased considerably at the expense of mature symbiotic interfaces in MtSucS1-reduced lines (Fig. 4L). In line with this observation, the arbuscules often exhibited a lowered branching, resulting in a reduced size of the mature plant/fungus interface, often showing a high degree of septation (Fig. 4, I and J), possibly caused by a block of development at a premature stage in MtSucS1-reduced mycorrhizae. Ultimately, the arbuscule development observed indicates negative effects of an MtSucS1 knockdown on arbuscule development, AM formation, and maintenance.
DISCUSSION
The key result of our study is a significantly affected internal colonization of mycorrhizal MtSucS1-reduced plants. Here, the generally highly branched intraradical hyphal network is less differentiated, accompanied by alterations in fungal root occupancy, the timing of infection, fungal development, and, most strikingly, in the longevity of the asynchronous AM symbiosis. This arbuscule phenotype matches observations for M. truncatula plants depleted in the AM marker genes MtPT4 (Javot et al., 2007) and MtDsx2 (Floß et al., 2008). Since during nodulation, MtSucS1-as plants also exhibited early symbiosome senescence (Baier et al., 2007), MtSucS1 is likely involved in the C supply to microsymbionts and in the sufficient development of endosymbiotic interfaces in general.
The defective internal mycorrhization phenotype of MtSucS1-reduced plants implies a distinct symbiotic function for an MtSucS1-dependent Suc breakdown, serving various metabolic purposes particularly in the arbuscule-containing and -adjacent cells in addition to the known function of apoplastic Inv for fungal hexose supply (Schaarschmidt et al., 2007a). Along with an impaired symbiotic Suc utilization in MtSucS1-as AM, several lines of evidence point to a diminished C transfer to the AMF. Marked reductions of the main Glomus hyphal biomass located inside the root (Maherali and Klironomos, 2007) indicate a generally hindered total fungal growth and an insufficient C utilization for the mycosymbiont in MtSucS1-as AM. Moreover, the strongly reduced fungal structures (particularly fewer vesicles) and characteristic interim fungal C pools linked to a functional AM (trehalose and mannitol) as well as the repression of the glycogen and fatty acid synthesis clearly highlight fungal C limitations. In addition, reduced mycorrhiza construction costs mirrored an impaired symbiosis formation and maintenance, since the extraradical mycelium and frequently developed spores directly contribute to the C budget of the plant (Peng et al., 1993; Olsson and Johansen, 2000). Comparable colonization and C starvation phenotypes were observed in Inv-modified mycorrhizal tobacco, deficient in the direct fungal hexose supply that was altered either by the expression of an apoplastic Inv inhibitor under the control of an inducible promoter (Schaarschmidt et al., 2007a) or by the activity of a p35S-driven apoplastic Inv in leaves (Schaarschmidt et al., 2007b). The phenotypes recorded resembled plants with reduced Suc translocation to AM due to an impaired photoassimilation (Tester et al., 1985; Son and Smith, 1988; Vierheilig et al., 2002), one factor also reducing the proportion of functional arbuscules (Hayman, 1974). Similarly, the incomplete and delayed fungal colonization demonstrates an effect on AM formation, maintenance, and efficiency by a limited MtSucS1-mediated C availability at plant/fungus interfaces. Extending the model that merely apoplastic Inv activity increases apoplastic sugars during AM (Bago et al., 2000, Schaarschmidt et al., 2006), our findings support the assumption that cytoplasmic MtSucS1 participates in C efflux toward the arbuscule apoplast. It should be noted here that the SucS-dependent Suc breakdown requires half the net energy of the Inv-mediated pathway, allowing SucS to operate under conditions of lower oxygen (Koch, 2004). Hence, cytoplasmic MtSucS1 could meet the CH requirements during AM at times of high glycolytic and C demands (e.g. under increasing anaerobiosis in maturing arbuscule-containing cells). MtSucS1 expression in these and in arbuscule-adjacent cells (Hohnjec et al., 2003), in concert with a symplastic cell-to-cell movement of Suc cleavage products and/or an AM-induced expression of hexose transporters in cortical cells of colonized regions, could support the enhanced plant and fungal metabolism in active arbuscules. Notably, hexose transporter genes were induced in MtSucS1-as mycorrhizae (e.g. MtSt1; Harrison, 1996). Together with the enhanced expression of genes encoding N/A Inv as well as other Suc- and fructan-hydrolyzing enzymes, the up-regulation of hexose transporter genes could thus sustain a basic hexose supply to MtSucS1-as arbuscules.
Interestingly, we observed limited increases of symbiotic root Suc pools in antisense lines compared with controls, while the levels of hexoses were hardly changed. Given that the metabolic pathways by which C is handled in AM underlie multilevel regulations (Bago et al., 2000) and that Suc breakdown is also essential for the initiation of hexose-based sugar signals in Suc-importing structures (Koch, 2004), MtSucS1-reduction could cause posttranscriptional and posttranslational regulatory steps that affect MtSucS1 activity. Apart from development-, tissue-, and cell-specific transcriptional control (Fu and Park, 1995; Zrenner et al., 1995; Barratt et al., 2001), SucS expression is modulated by various stimuli such as the tissue CH status (Koch et al., 1992), environmental conditions or anaerobiosis (Shaw et al., 1994), while the steady-state level of SucS is posttranscriptionally regulated via the cellular redox state (Marino et al., 2008). Considering a significantly reduced MtSucS1 expression in antisense mycorrhizae, posttranslational regulations, such as by reversible seryl-phosphorylation (Carlson and Chourey, 1996; Winter et al., 1997, 1998; Wienkoop et al., 2008), represent possible strategies to sustain residual MtSucS1 activity in colonized antisense roots. Additionally, a variety of regulatory stimuli and mechanisms to modulate Inv at the transcriptional and posttranscriptional level (Sturm, 1999; Roitsch and Ehne, 2000; Roitsch et al., 2003; Koch, 2004) could further have a compensatory impact on Suc utilization in mycorrhizal antisense roots. Nevertheless, the phenotypic traits displayed by our mycorrhizal antisense lines suggest that the above-mentioned possibilities to bypass hexose limitations were apparently not sufficient to allow the formation of a more complex hyphal network in antisense roots and, moreover, to support the establishment of normal numbers of mature arbuscules in colonized regions under MtSucS1 limitation.
Most likely, SucS and Inv act synergistically in AM. Their concerted activities were particularly evident during the highly C-demanding AM initiation (Schaarschmidt and Hause, 2008). For the final AM stages, when arbuscules are purged from root cells, Blee and Anderson (2002) postulated that the actual SucS function is related to the reestablishment of precolonization conditions (e.g. the synthesis of starch and cellulose). In addition, they assumed a regulation of arbuscule formation and location in cortical cells by C availability (Blee and Anderson, 1998). In MtSucS1-altered mycorrhizae, the formation and proportion of mature arbuscules were indeed reduced, coinciding with rising numbers of degenerating and dead arbuscules. This points to a degradation of insufficiently formed, presumably less functional arbuscules prior to their maturation, an effect that was also observed for transgenic lines where only the apoplastic Suc metabolism was manipulated (Schaarschmidt et al., 2007a, 2007b).
One drawback of visual colonization estimations is that these include both living and dead fungal material and largely exclude the plasticity of functional mycorrhizae (Burleigh et al., 2002). The substantial repression of Glomus and plant AM marker genes observed here, on the other hand, represent principal measures of a reduced functional colonization intensity and symbiotic performance, resulting from lower colonization of MtSucS1-altered roots by living fungal biomass. Moreover, our attempt to evaluate AM marker gene expression in relation to the presence of functional, mature arbuscules revealed substantial down-regulation, particularly for MtBcp1, MtPT4, MtScp1, and MtHa1 (Harrison et al., 2002; Krajinski et al., 2002; Liu et al., 2003; Hohnjec et al., 2005). This points to reductions of the encoded proteins that most likely function in the periarbuscular membrane itself or in the case of MtBcp1 in the periarbuscular membrane domain surrounding arbuscule trunks (Pumplin and Harrison, 2009), again indicating that arbuscules were declining in their development and function under MtSucS1 limitation.
The multistep processes of fungal invasion and, in particular, arbuscule accommodation, accompanied by massive reorganization of the host cell morphology involving intracellular structures (Bonfante and Perotto, 1995; Genre et al., 2005), might constitute additional explanations for the effect of MtSucS1 reduction. Several studies demonstrated profound cytoskeletal rearrangements before hyphae invade cortical cells (Genre et al., 2005) and from the early stages of arbuscule development until the formation of senescing arbuscules (Genre and Bonfante, 1998; Blancaflor et al., 2001). In addition, reassembly of actin filaments supported organelle positioning near the periarbuscular membrane (Genre and Bonfante, 1998; Lohse et al., 2005; Pumplin and Harrison, 2009). Apart from these extensive cytoskeleton reorganizations, the differentiation and de novo synthesis of perifungal plasma membrane and CW extracellular matrix material from the beginning of AMF colonization until the branching hyphae in arbuscule-containing cells (Bonfante-Fasolo, 1984; Balestrini et al., 1996; Balestrini and Bonfante, 2005) are major and costly events, possibly involving an MtSucS1-mediated Suc breakdown already during the earliest phases of AM formation.
Apart from the possibility that MtSucS1 reduction could affect intraradical fungal growth already prior to arbuscule formation, our arbuscule-related phenotype points to the importance of MtSucS1 for the accommodation of fungal structures via transient cytoskeletal rearrangements in arbuscule-containing and -adjacent cells as well as membrane deposition and/or cellulose synthesis relevant for invading hyphae. Moreover, the limited but frequently replicated cycle of arbuscule growth, septation, collapse, and senescence in successive fungal colonizations (Alexander et al., 1989; Javot et al., 2007) could impose a repeated need for a continuous MtSucS1-mediated C supply, contributing to the activated metabolism of nascent arbuscules. Consequently, the reduced colonization in MtSucS1-altered plants, the lower and occasionally arrested arbuscule formation, as well as their premature degeneration can also be interpreted as a secondary effect of an MtSucS1 knockdown that results from a decreased plant/fungus contact area.
In conclusion, wild-type MtSucS1 levels apparently are directly or indirectly prerequisite not to induce but to sustain normal arbuscule maturation and lifetime. Although arbuscules are initiated under MtSucS1 reduction, they cannot properly mature, indicating that Inv cannot fully substitute for SucS during later stages of arbuscule development.
MATERIALS AND METHODS
Plant Material, Conditions of Growth, and Fungal Inoculation
Two independent Medicago truncatula p35S-MtSucS1-as lines (as12 and as19) were compared with two independently transformed, homozygous control lines expressing pMtSucS1-gusAint fusions (Hohnjec et al., 2003; Baier et al., 2007). Seeds were surface sterilized, scarified (Baier et al., 2007), initially grown in 27-cm3 pots, and fertilized with half-strength Hoagland solution twice a week containing 50 μmol Pi until at least two trifoliate leaves developed. Afterward, plantlets were transferred into 600-cm3 pots, inoculated with a defined quantum of a granule inoculum (Glomus mosseae BEG12; Biorize R&D), and supplemented with half-strength Hoagland solution twice a week. Growth conditions were set to a 16-h-light (24°C)/8-h-dark (18°C) cycle, providing a photosynthetically active radiation of 500 μE m−2 s−1 at 60% relative humidity.
Plant Phenotyping, Tissue Nutrient Content Analyses, and Metabolite Profiling
Measurements of aboveground development, photosynthetic performance, root parameters, and the gas chromatography-mass spectrometry-based profiling of polar metabolites were described by Baier et al. (2007). Plant material was harvested in the middle of the light cycle at 50 dpi. Upon microscopy and dry weight measurements, aliquots of 1- to 2-cm-long root subsamples were separated. Remaining mycorrhizal root material was immediately frozen in liquid N2 and stored at −80°C. MGR were obtained as MGR = 100 × [(AM − NM)/NM], using the shoot dry weights of individual AM plants and the mean shoot dry weight of corresponding NM lines (Smith et al., 2003). The scaling of biomass relationships [dry weight of shoot (y) and dry weight of root (x)] assessed grade shifts on allometry. Using logey = klogex + bloge, the slope of the best-fit linear regression defines the allometric coefficient k. Tissue construction costs (mmol C g−1 dry weight) were calculated according to Mortimer et al. (2008). RSR were based on fresh weights. Root weight ratios were measured as dry weight of root per dry weight of plant. Dry weight of root was calculated via line-specific dry weight/fresh weight ratios, based on individual root subsamples. Dry matter was obtained by freeze or oven drying (70°C). Mineral contents were calculated per unit of dry weight of 50-dpi mycorrhizal plants. N and C contents were measured via gas chromatography as described by Baier et al. (2007). Using gas chromatography-mass spectrometry, P accumulation was determined via integration of the peak area of the characteristic fragment ion of mass-to-charge ratio 299 according to Schliemann et al. (2008).
Total RNA Isolation and Quantitative RT-PCR
Mycorrhiza samples were taken from frozen stocks, pooled, and ground in an MM100 mixer mill (Retsch) prior to metabolite and RNA extractions. Total RNA isolation and DNase I on-column digestion were performed according to the manufacturer's instructions (Qiagen). RNA preparations were electrophoretically and spectrophotometrically checked for yield and quality (NanoDrop ND-1000; NanoDrop Technologies). Applying the QuantiTect Reverse Transcription kit (Qiagen) and 500 ng of total template RNA (final volume = 20 μL), a first-strand cDNA synthesis was performed, according to the supplier's protocol. Via in silico analyses of the M. truncatula Gene Index (http://compbio.dfci.harvard.edu/), tentative consensus sequences, representing genes for AM or sink metabolism, were selected. The unique, gene-specific PCR primer sets (Supplemental Table S3) displayed the following parameters: melting temperature of 53°C ± 0.5°C, primer sequence of 21 bp, amplification product of 315 ± 35 bp. Primers were tested on plant and fungal DNA/cDNA for specificity. Length and melting behavior of the PCR product were analyzed. RT-PCR was performed with the QuantiTect SYBR Green PCR Kit (Qiagen), using 1/20 volume of first-strand cDNA derived from 25 ng of total RNA (final volume = 25 μL). Glomus genes were profiled in a one-step RT-PCR apparatus using the Quantace SensiMix One-Step Kit (Quantace). Expression results were averaged over six biological and two technical replicates using the constitutive translational elongation factor gene MtTef1α (TC106485) for internal normalization. The CTMtTef1α was analyzed at least twice per run, and average values were used to calculate relative gene expression levels (2−ΔCT; ΔCT = CTgene − CTMtTef1α).
Mycorrhizal Assessment
Light microscopy was performed on cleared, Shaeffer ink-stained root fragments (Vierheilig et al., 1998) using an Olympus BH-2 light microscope with an Olympus C-2000Z digital camera (Olympus Optical). RLC was assessed by the grid line intersect method (Giovannetti and Mosse, 1980) using a Zeiss stereomicroscope (Carl Zeiss) recording more than 1,000 intersects per pooled biological replicate. Hyphal, arbuscular, and vesicular colonization were determined in colonized regions by the magnified (200×) line intersect method (McGonigle et al., 1990). Arbuscule developmental stages (Floß et al., 2008) were studied on eight samples per transgenic line on WGA-AlexaFluor488-stained root segments (Javot et al., 2007) by a Leica SP2 laser-scanning microscope (Leica Microsystems) recording more than 190 arbuscules per biological replicate (c, 2,160; as12, 1,800; as19, 1,900 arbuscules). Image processing was carried out with Adobe Photoshop 7.0 (Adobe Systems) and Leica LCSlight.
Statistical Analyses
Parameters/variables were calculated as means ± se and analyzed for significance by Student's t test with the following P levels: a 0.05 > P ≥ 0.01; b P < 0.01. Coefficiencies of Bravais-Pearson product-moment correlation r > |0.6| were defined as strong, and |0.3| > r < |0.6| were defined as weak. Critical r values depended on the independent replicate number n. All statistical tools used were incorporated in MS Excel 2007 (Microsoft).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Attributes of the physiology fitness of MtSucS1-as and control lines cultured under nonsymbiotic, Pi-deprived, and fully supplemented conditions compared to growth patterns observed under mycorrhizal conditions.
Supplemental Figure S2. Photochemical yield of MtSucS1-as and control lines at a mature mycorrhizal stage (28 dpi).
Supplemental Table S1. Phenotypic variables of nonsymbiotic MtSucS1-as and corresponding control lines cultured under NM, Pi-deprived, or fully supplemented growth conditions.
Supplemental Table S2. Leaf developmental attributes of mycorrhizal MtSucS1-as and control lines.
Supplemental Table S3. Primer sequences used in real-time RT-PCR experiments on total RNA derived from mycorrhizal root tissue.
Supplemental Table S4. Ratios of polar metabolite steady-state levels in MtSucS1-as and control lines at 50 dpi.
Supplementary Material
Acknowledgments
We thank Susana Galvez, Martin Crespi, and Florian Frugier (Institut des Sciences du Végétales, Centre National de la Recherche Scientifique, Gif sur Yvette, France) for advice in plant transformation and for the supply of binary vector MF2.
This work was supported by the European Union projects FIXNET (grant no. CT97–2319) and GRAIN LEGUMES (grant no. FOOD–CT–2004–506223) as well as by the Deutsche Forschungsgemeinschaft (grant no. SPP1084 “MolMyk”).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Helge Küster (helge.kuester@genetik.uni-hannover.de).
The online version of this article contains Web-only data.
References
- Alexander T, Toth R, Meier R, Weber H (1989) Dynamics of arbuscule development and degeneration in onion, bean, and tomato with reference to vesicular-arbuscular mycorrhizae in grasses. Can J Bot 67 2505–2513 [Google Scholar]
- Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, Douds DD, Lammers PJ, Shachar-Hill Y (2003) Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol 131 1496–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago B, Pfeffer PE, Shachar-Hill Y (2000) Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol 124 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier MC, Barsch A, Küster H, Hohnjec N (2007) Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiol 145 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini R, Bonfante P (2005) The interface compartment in arbuscular mycorrhizae: a special type of plant cell wall? Plant Biosyst 139 8–15 [Google Scholar]
- Balestrini R, Hahn MG, Faccio A, Mendgen K, Bonfante P (1996) Differential localization of carbohydrate epitopes in plant cell walls in the presence and absence of arbuscular mycorrhizal fungi. Plant Physiol 111 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C (2001) Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 127 655–664 [PMC free article] [PubMed] [Google Scholar]
- Black KG, Mitchell DT, Osborne BA (2000) Effect of mycorrhizal-enhanced leaf phosphate status on carbon partitioning, translocation and photosynthesis in cucumber. Plant Cell Environ 23 797–809 [Google Scholar]
- Blancaflor EB, Zhao L, Harrison MJ (2001) Microtubule organization in root cells of Medicago truncatula during development of an arbuscular mycorrhizal symbiosis with Glomus versiforme. Protoplasma 217 154–165 [DOI] [PubMed] [Google Scholar]
- Blee KA, Anderson AJ (1998) Regulation of arbuscule formation by carbon in the plant. Plant J 16 523–530 [Google Scholar]
- Blee KA, Anderson AJ (2002) Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Mol Biol 50 197–211 [DOI] [PubMed] [Google Scholar]
- Bonfante P (2001) At the interface between mycorrhizal fungi and plants: the structural organization of cell wall, plasma membrane and cytoskeleton. In K Esser, B Hock, eds, The Mycota IX. Springer-Verlag, Berlin, pp 45–61
- Bonfante P, Perotto S (1995) Strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol 130 3–21 [Google Scholar]
- Bonfante-Fasolo P (1984) Anatomy and morphology of VA mycorrhizae. In CL Powell, DJ Bagyaraj, eds, VA Mycorrhizas. CRC Press, Boca Raton, FL, pp 5–33
- Bryla DR, Koide RT (1990) Regulation of reproduction in wild and cultivated Lycopersicon esculentum Mill. by vesicular-arbuscular mycorrhizal infection. Oecologia 84 74–81 [DOI] [PubMed] [Google Scholar]
- Bücking H, Shachar-Hill Y (2005) Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol 165 899–912 [DOI] [PubMed] [Google Scholar]
- Burleigh SH, Cavagnaro T, Jakobsen I (2002) Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J Exp Bot 53 1593–1601 [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS (1996) Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet 252 303–310 [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, et al (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19 1062–1071 [DOI] [PubMed] [Google Scholar]
- Douds DD, Pfeffer PE, Shachar-Hill Y (2000) Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant Soil 226 255–261 [Google Scholar]
- Floß DS, Hause B, Lange PR, Küster H, Strack D, Walter MH (2008) Knock-down of the MEP pathway isogene 1-deoxy-D-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes. Plant J 56 86–100 [DOI] [PubMed] [Google Scholar]
- Fu H, Park WD (1995) Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell 7 1369–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez S, Azcón-Aguilera C, Ferrol N (2007) Transcriptional regulation of host enzymes involved in the cleavage of sucrose during arbuscular mycorrhizal symbiosis. Physiol Plant 129 737–746 [Google Scholar]
- García-Rodríguez S, Pozo MJ, Azcón-Aguilera C, Ferrol N (2005) Expression of a tomato sugar transporter is increased in leaves of mycorrhizal or Phytophthora parasitica-infected plants. Mycorrhiza 15 489–496 [DOI] [PubMed] [Google Scholar]
- Genre A, Bonfante P (1998) Actin versus tubulin configuration in arbuscule-containing cells from mycorrhizal tobacco roots. New Phytol 140 745–752 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Faccio A, Barker DG, Bonfante P (2008) Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell 20 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG (2005) Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17 3489–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84 489–500 [Google Scholar]
- Gomez SK, Javot H, Deewatthanawong P, Torres-Jerez I, Tang Y, Blancaflor EB, Udvardi MK, Harrison MJ (2009) Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol 9 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JH, Duncan LW, Eissenstat DM (1997) Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol 135 335–343 [Google Scholar]
- Guether M, Balestrini R, Hannah M, He J, Udvardi MK, Bonfante P (2009) Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol 182 200–212 [DOI] [PubMed] [Google Scholar]
- Harrison M, Dewbre G, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14 2413–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ (1996) A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J 9 491–503 [DOI] [PubMed] [Google Scholar]
- Hayman DS (1974) Plant growth responses to vesicular-arbuscular mycorrhiza. VI. Effect of light and temperature. New Phytol 73 71–80 [Google Scholar]
- Heinemeyer A, Ineson P, Ostle N, Fitter AH (2006) Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol 171 159–170 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Becker JD, Pühler A, Perlick AM, Küster H (1999) Genomic organization and expression properties of the MtSucS1 gene, which encodes a nodule-enhanced sucrose synthase in the model legume Medicago truncatula. Mol Gen Genet 261 514–522 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Henckel K, Bekel T, Gouzy J, Dondrup M, Goesmann A, Küster H (2006) Transcriptional snapshots provide insights into the molecular basis of arbuscular mycorrhiza in the model legume Medicago truncatula. Funct Plant Biol 33 737–748 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Perlick AM, Pühler A, Küster H (2003) The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Mol Plant Microbe Interact 16 903–915 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H (2005) Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol 137 1283–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Akazawa T (1986) A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol 81 1008–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov S, Fester T, Hause B (2004) Rapid determination of fungal colonization and arbuscule formation in roots of Medicago truncatula using real-time (RT) PCR. J Plant Physiol 161 1379–1383 [DOI] [PubMed] [Google Scholar]
- Jakobsen I, Rosendahl L (1990) Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol 115 77–83 [Google Scholar]
- Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K (2004) Sucrose metabolism, regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT (1992) Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell 4 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajinski F, Hause B, Gianinazzi-Pearson V, Franken P (2002) Mtha1, a plasma membrane H+-ATPase gene from Medicago truncatula, shows arbuscule-specific induced expression in mycorrhizal tissue. Plant Biol 4 754–761 [Google Scholar]
- Lammers PJ, Jun J, Abubaker J, Arreola R, Gopalan A, Bago B, Hernandez-Sebastià C, Allen JW, Douds DD, Pfeffer PE, et al (2001) The glyoxylate cycle in an arbuscular mycorrhizal fungus: carbon flux and gene expression. Plant Physiol 127 1287–1298 [PMC free article] [PubMed] [Google Scholar]
- Liu J, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ (2007) Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J 50 529–544 [DOI] [PubMed] [Google Scholar]
- Liu JY, Blaylock LA, Endre G, Cho J, Town C, VandenBosch K, Harrison M (2003) Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell 15 2106–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse S, Schliemann W, Ammer C, Kopka J, Strack D, Fester T (2005) Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol 139 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316 1746–1748 [DOI] [PubMed] [Google Scholar]
- Marino D, Hohnjec N, Küster H, Moran JF, Gonzalez EM, Arrese-Igor C (2008) Evidence for transcriptional and posttranscriptional regulation of sucrose synthase in pea nodules by the cellular redox state. Mol Plant Microbe Interact 21 622–630 [DOI] [PubMed] [Google Scholar]
- McGonigle T, Miller M, Evans D, Fairchild D, Swan J (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115 495–501 [DOI] [PubMed] [Google Scholar]
- Mortimer PE, Pérez-Fernández MA, Valentine AJ (2008) The role of arbuscular mycorrhizal colonization in the carbon nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol Biochem 40 1019–1027 [Google Scholar]
- Olsson PA, Johansen A (2000) Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol Res 104 429–434 [Google Scholar]
- Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC (1993) Growth depression in mycorrhizal citrus at high-phosphorus supply: analysis of carbon costs. Plant Physiol 101 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PE, Douds DD, Bécard G, Shachar-Hill Y (1999) Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol 120 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MJ, Verhage A, García-Andrade J, García JM, Azcón-Aguilar C (2009) Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In C Azcón-Aguilar, JM Barea, S Gianinazzi, V Gianinazzi-Pearson, eds, Mycorrhizas: Functional Processes and Ecological Impact. Springer-Verlag, Heidelberg, pp 123–135
- Pumplin N, Harrison MJ (2009) Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol 151 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman SB, Rathinasabapathi B (2004) Pantothenate synthesis in plants. Plant Sci 167 961–968 [Google Scholar]
- Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol 146 1440–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnskov S, Wu Y, Graham JH (2003) Arbuscular mycorrhizal fungi differentially affect expression of genes coding for sucrose synthases in maize roots. New Phytol 157 539–545 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54 513–524 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Ehne R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32 359–367 [Google Scholar]
- Schaarschmidt S, Gonzalez MC, Roitsch T, Strack D, Sonnewald U, Hause B (2007. a) Regulation of arbuscular mycorrhization by carbon: the symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity. Plant Physiol 143 1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt S, Hause B (2008) Apoplastic invertases: multi-faced players in the arbuscular mycorrhization. Plant Signal Behav 3 317–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt S, Kopka J, Ludwig-Müller J, Hause B (2007. b) Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J 51 390–405 [DOI] [PubMed] [Google Scholar]
- Schaarschmidt S, Roitsch T, Hause B (2006) Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J Exp Bot 57 4015–4023 [DOI] [PubMed] [Google Scholar]
- Schliemann W, Ammer C, Strack D (2008) Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 69 112–146 [DOI] [PubMed] [Google Scholar]
- Schubert A, Allara P, Morte A (2003) Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol 161 495–501 [DOI] [PubMed] [Google Scholar]
- Schüßler A, Martin H, Cohen D, Fitz M, Wipf D (2006) Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature 444 933–936 [DOI] [PubMed] [Google Scholar]
- Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105 1413–1421 [Google Scholar]
- Shachar-Hill Y, Pfeffer PE, Douds DD, Osman SF, Doner LW, Ratcliffe RG (1995) Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhiza leek. Plant Physiol 108 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JR, Ferl RJ, Baier J, St. Clair D, Carson C, McCarty DR, Hannah LC (1994) Structural features of the maize sus1 gene and protein. Plant Physiol 106 1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FA, Smith SE (1997) Structural diversity in (vesicular)-arbuscular mycorrhizal symbioses. New Phytol 137 373–388 [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ (1997) Mycorrhizal Symbioses, Ed 2. Academic Press, London
- Smith SE, Smith FA (1990) Structure and function of the interfaces in biotrophic symbioses as they relate to nutrient transport. New Phytol 114 1–38 [DOI] [PubMed] [Google Scholar]
- Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133 16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman MZ, Saito M (1997) Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol 136 533–538 [DOI] [PubMed] [Google Scholar]
- Son CL, Smith SE (1988) Mycorrhizal growth response: interactions between photon irradiance and phosphorus nutrition. New Phytol 108 305–314 [DOI] [PubMed] [Google Scholar]
- Sturm A (1999) Invertases: primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Smith FA, Smith SE (1985) Phosphate inflow into Trifolium subterraneum L.: effects of photon irradiance and mycorrhizal infection. Soil Biol Biochem 17 807–809 [Google Scholar]
- Valentine AJ, Osborne BA, Mitchell DT (2001) Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci Hortic (Amsterdam) 88 177–189 [Google Scholar]
- van Wees SCM, van der Ent S, Pieterse CMJ (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11 443–448 [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Bago B, Lerat S, Piché Y (2002) Shoot-produced, light-dependent factors are partially involved in the expression of the arbuscular mycorrhizal (AM) status of AM host and non-host plants. J Plant Nutr Soil Sci 165 21–25 [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular mycorrhizal fungi. Appl Environ Microbiol 64 5004–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring RH, Landsberg JJ, Williams M (1998) Net primary production of forests: a constant fraction of gross primary production? Tree Physiol 18 129–134 [DOI] [PubMed] [Google Scholar]
- Wiemken A, Frehner M, Keller F, Wagner W (1986) Fructan metabolism, enzymology, and compartmentation. Curr Top Plant Biochem Physiol 5 17–37 [Google Scholar]
- Wienkoop S, Larrainzar E, Glinski M, González EM, Arrese-Igor C, Weckwerth W (2008) Absolute quantification of Medicago truncatula sucrose synthase isoforms and N-metabolism enzymes in symbiotic root nodules and the detection of novel nodule phosphoproteins by mass spectrometry. J Exp Bot 59 3307–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1997) Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett 420 151–155 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1998) Identification of sucrose synthase as an actin-binding protein. FEBS Lett 430 205–208 [DOI] [PubMed] [Google Scholar]
- Wright DP, Read DJ, Scholes JP (1998. a) Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ 21 881–891 [Google Scholar]
- Wright DP, Scholes JD, Read DJ (1998. b) Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ 21 209–216 [Google Scholar]
- Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163 417–425 [DOI] [PubMed] [Google Scholar]
- Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U (1995) Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants. Plant J 7 97–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.