Abstract
The biochemical function of the Laforin-like dual-specific phosphatase AtSEX4 (EC 3.1.3.48) has been studied. Crystalline maltodextrins representing the A- or the B-type allomorph were prephosphorylated using recombinant glucan, water dikinase (StGWD) or the successive action of both plastidial dikinases (StGWD and AtPWD). AtSEX4 hydrolyzed carbon 6-phosphate esters from both the prephosphorylated A- and B-type allomorphs and the kinetic constants are similar. The phosphatase also acted on prelabeled carbon-3 esters from both crystalline maltodextrins. Similarly, native starch granules prelabeled in either the carbon-6 or carbon-3 position were also dephosphorylated by AtSEX4. The phosphatase did also hydrolyze phosphate esters of both prephosphorylated maltodextrins when the (phospho)glucans had been solubilized by heat treatment. Submillimolar concentrations of nonphosphorylated maltodextrins inhibited AtSEX4 provided they possessed a minimum of length and had been solubilized. As opposed to the soluble phosphomaltodextrins, the AtSEX4-mediated dephosphorylation of the insoluble substrates was incomplete and at least 50% of the phosphate esters were retained in the pelletable (phospho)glucans. The partial dephosphorylation of the insoluble glucans also strongly reduced the release of nonphosphorylated chains into solution. Presumably, this effect reflects fast structural changes that following dephosphorylation occur near the surface of the maltodextrin particles. A model is proposed defining distinct stages within the phosphorylation/dephosphorylation-dependent transition of α-glucans from the insoluble to the soluble state.
The metabolism of starch, the most prominent storage carbohydrate in plants, is assumed to require approximately 30 to 40 distinct (iso)enzymes (Deschamps et al., 2008), but, presumably, the list of the starch-related proteins is not yet complete. Several novel proteins (and protein functions) essential for the normal starch metabolism have recently been identified among which are two α-glucan phosphorylating dikinases. One dikinase (glucan, water dikinase [GWD], EC 2.7.9.4) utilizes ATP as dual phosphate donor and esterifies the C6 position of amylopectin-related glucosyl residues, whereas the other dikinase (phosphoglucan, water dikinase [PWD], EC 2.7.9.5) selectively transfers the β-phosphate group from ATP to the C3 position of glucosyl residues (Ritte et al., 2006).
Two other previously unknown starch-related enzymes were designated as SEX4 protein (EC 3.1.3.48; At3g52180; previous designations PTPKIS1 and DSP4) and as Like Sex Four1 (LSF1) protein (At3g01510; previously named PTPKIS2; Comparot-Moss et al., 2010). Both proteins are predicted to contain a noncatalytic carbohydrate-binding module (CBM; Boraston et al., 2004; Shoseyov et al., 2006) and a catalytic dual-specificity phosphatase (DSP) domain. The latter is shared by the large family of DSPs that dephosphorylate distinct target phosphoproteins both at phosphotyrosine and phosphoserine/phosphothreonine residues. Some DSPs also act on various nonproteinaceous substrates, such as phospholipids or phosphorylated polyglycans (Pulido and Hooft van Huijsduijnen, 2008).
Arabidopsis (Arabidopsis thaliana) mutants lacking a functional SEX4 protein contain both elevated starch levels and significant amounts of soluble phosphooligoglucans that are below the limit of detection in wild-type plants and probably originate from starch. However, the precise biochemical function of SEX4 is far from being clear (Kötting et al., 2009). The phenotype of the SEX4-deficient mutant is complex: Transitory starch possesses an elevated amylose-to-amylopectin ratio but the phosphate content of amylopectin is not increased. It has been hypothesized that SEX4 and LSF1 selectively hydrolyze C6- and C3-phosphate esters, respectively, but experimental evidence is lacking (Kötting et al., 2009). Likewise, it is unknown whether SEX4 preferentially acts on particulate starch or on soluble phosphoglucans.
Crystalline maltodextrins (MDcryst) have recently been introduced as a model mimicking some structural features of the native starch granules (Hejazi et al., 2008). They can be crystallized as either the A- or the B-type allomorph (Gallant et al., 1997; Gérard et al., 2001). Recombinant StGWD phosphorylates both maltodextrin allomorphs with a far higher rate than native starch granules and thereby initiates solubilization of both phosphorylated and nonphosphorylated maltodextrins. In vitro both allomorphs act also as substrate for PWD provided a prephosphorylation by GWD (Hejazi et al., 2009).
In this study, we used the prephosphorylated A- and B-type allomorphs of MDcryst to study biochemical functions of AtSEX4. As highly ordered α-glucans are the preferred sites of the dikinase-mediated phosphorylation, we designed experiments to answer the following questions: Does SEX4 preferentially act on phosphorylated insoluble or soluble glucans? If insoluble α-glucans are the preferred substrate, does the phosphatase distinguish between the A- and the B-type allomorph? Does SEX4 preferentially or selectively hydrolyze C6-phosphate esters? Does SEX4 also interact with nonphosphorylated oligoglucans? Finally, assuming that SEX4 acts on insoluble phosphoglucans, does the removal of phosphate esters affect the phase transition and/or the physical order of the glucans?
RESULTS
SEX4 Acts on C6-Phosphate Esters of Both Crystalline and Solubilized Maltodextrins But the Mode of Action Differs
The physical and physicochemical properties of the two crystalline maltodextrin preparations representing the A- or the B-type allomorph (A-MDcryst and B-MDcryst, respectively) used in this study were indiscernible from those previously described (Hejazi et al., 2009).
For the determination of kinetic constants of AtSEX4, both MDcryst preparations were prephosphorylated for 60 min with recombinant StGWD and [β-33P]ATP. Subsequently, the glucosyl and the glucosyl 6-P contents of the pelletable (phospho)glucans were quantified. The water-insoluble preparations derived from the A- or the B-type allomorph differed by less than 10% in the ratio of glucosyl 6-P residues and glucosyl moieties (Table I). As phosphorylation of the two MDcryst preparations is restricted to the surface of the particles, the surface-near degree of phosphorylation is likely to be higher as the average values given in Table I.
Table I.
Kinetic parameters of SEX4 acting on prephosphorylated A- and B-type MDcryst (STA)
Vmax and S0.5 values were determined according to Hanes (1932). A- and B-type MDcryst were prephosphorylated using StGWD and [β-33P]ATP for 60 min. Subsequently, varying amounts of both prelabeled water-insoluble MD were incubated with AtSEX4 (25 ng). At 1 min intervals, aliquots of the reaction mixtures were heat inactivated and the [33P]orthophosphate was quantified by TLC and phosphor imaging (n = 3; ±sd). G6P, Glc 6-P.
| Allomorph | Degree of Phosphorylation | S0.5 | Vmax | Vmax/S0.5 |
|---|---|---|---|---|
| nmol G6P/mmol Glc | mg | nmol min−1 mg−1 SEX4 | nmol min−1 mg−1 SEX4/mg MD | |
| A type | 0.56 | 2.4 ± 0.1 | 30 ± 1.1 | 12.5 |
| B type | 0.52 | 3.6 ± 0.2 | 49 ± 2.1 | 13.6 |
Varying amounts of the two prelabeled MD preparations were incubated with wild-type AtSEX4 using the short-term assay (STA). Under these conditions, less than 4% of the glucosyl 6-P esters were hydrolyzed but the hydrolysis rates were constant. The maximal hydrolytic activity (Vmax) and the half-saturating MD levels (S0.5 values) were determined according to Hanes (1932; Table I). For the B-type derived substrate, both the Vmax and the S0.5 values were slightly higher than those for the A-type counterpart. The first-order rate constants (Vmax/S0.5), i.e. the rates at limiting substrate levels, were very similar and, therefore, AtSEX4 does not exert any obvious allomorph preference. However, the GWD-mediated prephosphorylation initiates transition from the highly ordered to the soluble state (Hejazi et al., 2009). Therefore, the actual substrates of AtSEX4 (i.e. phosphoglucans) are unlikely to represent the original A- or B-type allomorph but rather intermediate structures.
For a more quantitative hydrolysis of the phosphate esters, incubation with AtSEX4 was extended to 60 min. Furthermore, the prephosphorylated maltodextrins were applied either in an insoluble or a heat-solubilized state (long-term assay [LTA]). The four labeled samples were incubated with equal amounts of recombinant AtSEX4. At intervals, the content of [33P]orthophosphate, as percentage of the total label, was determined. The solubilized phosphoglucans, derived from either the A- or B-type allomorph are not necessarily identical as they may differ in the intramolecular position of the phosphate esters.
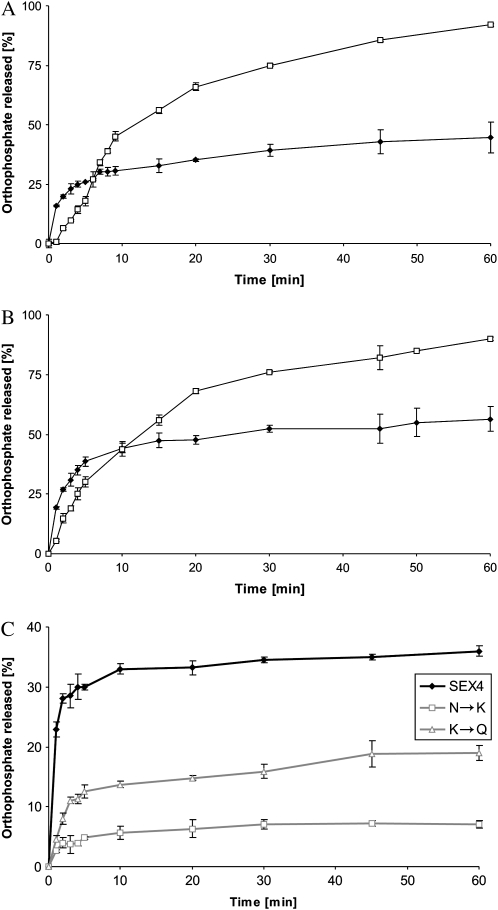
Recombinant AtSEX4 dephosphorylated both the solubilized and the water-insoluble prephosphorylated MD but the mode of action differed (Fig. 1). Dephosphorylation of the solubilized maltodextrins proceeded with time and after 60 min the majority of the total 33P label was recovered as orthophosphate. By contrast, the initial rates of dephosphorylation of the insoluble phosphoglucans were higher but, subsequently, strongly declined. After 60 min incubation, dephosphorylation was far from being complete. Essentially the same mode of action was found for both the prephosphorylated A- and B-MDcryst but the dephosphorylation of the A-type allomorph tended to be less complete as compared to the B-type allomorph. When the period of the GWD-mediated prephosphorylation was varied between 5 and 60 min, similar results were obtained (data not shown). Likewise, the incomplete dephosphorylation was observed in a relatively wide range of AtSEX4 levels (Table II).
Figure 1.
AtSEX4 dephosphorylates glucosyl 6-P residues of both the prephosphorylated A- and B-type MDcryst. Wild-type AtSEX4 was incubated with the prelabeled A-type (A) or the B-type (B) MD in the insoluble (black symbols) or solubilized (white symbols) state using the LTA (see “Materials and Methods”). In C, two mutated AtSEX4 proteins (N333K [N→K] and K307Q [K→Q]) and the wild-type AtSEX4 protein (see insert) were incubated with the prelabeled insoluble B-type allomorph. The three phosphatases were balanced to equal p-nitrophenyl phosphate hydrolyzing activity. In A to C, prephosphorylation (and prelabeling) was performed for 60 min. At intervals, aliquots of each of the four reaction mixtures were heat treated and subjected to TLC. Radioactivity was quantified by phosphor imaging (n = 3; ±sd).
Table II.
Incomplete dephosphorylation of insoluble phosphoglucans using varying levels of AtSEX4
The B-type allomorph of MDcryst, prephosphorylated with StGWD and [β-33P]ATP for 60 min, was incubated with either 25 or 100 ng of AtSEX4. At intervals, the levels of [33P]orthophosphate were determined. [33P]Orthophosphate released is given as percentage of the total 33P content of the prephosphorylated MD. Mean values n = 3; ±sd.
| Time |
33P-Orthophosphate Released |
|
|---|---|---|
| 25 ng SEX4 | 100 ng SEX4 | |
| min | % | |
| 5 | 30 ± 3 | 33 ± 2 |
| 10 | 36 ± 2 | 36 ± 4 |
| 30 | 38 ± 3 | 41 ± 4 |
| 60 | 40 ± 3 | 46 ± 3 |
In other experiments, two mutated SEX4 proteins (N333K and K307Q) were used in which a single amino acid residue within the putative CBM (family 48; Christiansen et al., 2009) is exchanged. Both mutations diminish functionality of the CBM (Gentry et al., 2007). The two mutated AtSEX4 proteins and the wild-type phosphatase were adjusted to equal phosphatase activity as revealed by the p-nitrophenyl phosphate assay. With both mutated AtSEX4 proteins, the rate of the orthophosphate release from the prephosphorylated B-type allomorph was diminished but the time dependencies were similar to that of the wild-type AtSEX4. Following a relatively fast hydrolysis at the onset of the incubation, dephosphorylation gradually ceased and following 45 min, no further increase in the orthophosphate formed was detectable (Fig. 1). Thus, a functional CBM appear to be essential for the efficient (yet incomplete) dephosphorylation of insoluble phosphomaltodextrins.
AtSEX4 Acts on Singly, Doubly, and Triply Phosphorylated α-Glucans
As the incomplete hydrolysis shown in Figure 1 could be due to a selective action of AtSEX4 on distinct phosphate esters, we analyzed the ratio of singly, doubly, and triply phosphorylated glucan chains in the water-insoluble MD before and after the AtSEX4 action. Both allomorphs were prephosphorylated for 60 min using StGWD and [β-33P]ATP. By increasing the total amount of the insoluble MD analyzed (compare with Hejazi et al., 2009), the 33P label of singly, doubly, and triply phosphorylated maltodextrins could be quantified. For both the A- and B-type allomorphs, the majority of the 33P was recovered in monophosphorylated α-glucans (Table III).
Table III.
Patterns of singly, doubly, and triply phosphorylated maltodextrins following the action of recombinant StGWD on insoluble MD
Both A- and B-type MDcryst were prephosphorylated for 60 min. Subsequently, the distribution of 33P between singly, doubly, and triply phosphorylated maltodextrins was determined. The 33P content of each of the three phosphoglucan species is calculated as percentage of the total 33P labeling (100%).
| MDcryst Allomorph |
33P Distribution of Phosphorylated MD |
||
|---|---|---|---|
| Singly | Doubly | Triply | |
| % | |||
| A type | 68.7 | 28.3 | 3.0 |
| B type | 59.0 | 36.2 | 4.8 |
Following both 30 and 60 min of incubation, the 33P labeling of singly, doubly, and triply phosphorylated glucans of the residual pelletable MD were quantified. Values are given as percentage of the respective phosphoglucan before the incubation with AtSEX4 (Table IV).
Table IV.
Labeling of the singly, doubly, and triply phosphorylated glucans remaining in the insoluble MD after the action of recombinant AtSEX4
Following 30 or 60 min of incubation with AtSEX4, the residual insoluble phosphoglucans were isolated. The 33P content of each of the three phosphoglucan species is given as percentage of the initial 33P labeling of the respective species (100%).
| MDcryst Allomorph |
33P Labeling of Phosphorylated MD after SEX4 Incubation |
||
|---|---|---|---|
| Singly | Doubly | Triply | |
| % | |||
| 30 min incubation with SEX4 | |||
| A type | 57.3 | 18.5 | 10.8 |
| B type | 46.1 | 15.9 | 9.4 |
| 60 min incubation with SEX4 | |||
| A type | 49.1 | 13.6 | 6.8 |
| B type | 41.3 | 13.8 | 6.2 |
In both MD preparations, the labeling patterns of the residual pelletable phosphoglucans were very similar. The 33P content of the triply phosphorylated compounds is decreased by approximately 90% during 30 min AtSEX4 action and subsequently it decreased only slightly. Doubly phosphorylated glucans were diminished by more than 80% during 30 min with very little change during the next 30 min. The 33P content of the residual monophosphorylated glucans was approximately half of that at the onset of the AtSEX4 action. Thus, the phosphoglucan patterns of residual insoluble (phospho)glucans derived from both the A- and the B-type allomorphs are deprived of multiply phosphorylated glucans but enriched in monophosphorylated compounds.
Nonphosphorylated Soluble Maltodextrins Inhibit AtSEX4
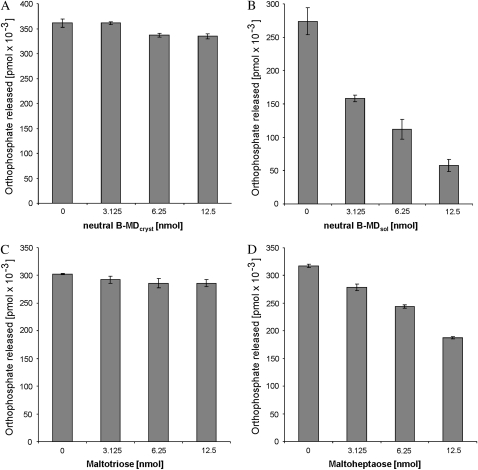
Nonphosphorylated rather than phosphorylated glucans are predicted to bind to CBM48 and, therefore, we hypothesized that nonphosphorylated α-glucans can also interact with AtSEX4. If so, they possibly could interfere with the phosphatase activity. To test this hypothesis, an aliquot of the B-type MDcryst preparation was untreated (nonphosphorylated MD), whereas another aliquot was prephosphorylated for 60 min by using recombinant StGWD and [β-33P]ATP and, subsequently, served as substrate (500 μg each) of recombinant AtSEX4. The nonphosphoryated MD were either solubilized by heating (B-MDsol) or remained water insoluble (B-MDcryst). Varying amounts of either B-MDsol or B-MDcryst nonphosphorylated MD were preincubated for 10 min with recombinant AtSEX4 (25 ng each). The phosphatase reaction was started by adding prephosphorylated insoluble MD and, at intervals, aliquots of the complete reaction mixtures were heat treated. Finally, the [33P]orthophosphate released was quantified by thin-layer chromatography (TLC) and phosphor imaging. In Figure 2 the amount of orthophosphate formed in the various reaction mixtures during 5 min is given. For more detailed kinetics, see Supplemental Figure S1.
Figure 2.
Nonphosphorylated solubilized maltodextrins inhibit the phosphatase activity of AtSEX4. B-type MDcryst was prephosphorylated (and prelabeled) for 60 min using StGWD. Equal amounts of AtSEX4 were preincubated with varying amounts of nonphosphorylated B-MDcryst (A), nonphosphorylated B-MDsol (B), maltotriose (C), or maltoheptaose (D). Subsequently, equal amounts of the prephosphorylated insoluble MD were added and the orthophosphate released after 5 min was determined as in Figure 1. n = 3; ±sd.
Varying amounts of nonphosphorylated B-MDcryst had very little effect on the release of orthophosphate but nonphosphorylated B-MDsol were efficient inhibitors, reducing the phosphatase activity by up to 80%.
The maltodextrins used in these experiments consist of a degree of polymerization (DP) ranging from 6 to approximately 30 (Hejazi et al., 2009). Maltodextrins extracted from leaves rarely exceed a DP of 7 (Critchley et al., 2001) and, therefore, it is uncertain whether they can be considered as potential inhibitors of SEX4. To clarify this question, we included two commercially available maltodextrins with a defined DP, maltotriose and maltoheptaose. When applied in micromolar concentrations, maltotriose had almost no effect on the AtSEX4 activity. By contrast, maltoheptaose did significantly inhibit the phosphatase (Fig. 2). Both DP3 and DP7 were applied in the same range of molar concentrations as B-MDsol if the calculation is based on the molar mass of the most frequently occurring α-glucan, DP15. Thus, soluble maltodextrins having a physiologically relevant size are potential inhibitors of AtSEX4.
AtSEX4 Hydrolyzes C3-Phosphate Esters of Both Insoluble and Solubilized Phosphomaltodextrins and of Native Prephosphorylated Granules as Well
Recombinant AtPWD is unable to introduce phosphate esters at the C3 position in the A- or B-type allomorph of the crystalline maltodextrin preparations except the two types have been prephosphorylated by StGWD (Hejazi et al., 2009). To specifically analyze any action of AtSEX4 on the phosphate esters at C3, a three-step procedure was chosen: Crystalline maltodextrins representing either the A- or the B-type allomorph were first phosphorylated at the C6 position using recombinant StGWD and unlabeled ATP. Subsequently, they were esterified at C3 by recombinant AtPWD and [β-33P]ATP. Following the second phosphorylating reaction, the insoluble maltodextrins were freed from AtPWD and [β-33P]ATP and each of the two insoluble maltodextrin samples was then divided into two equal parts, one of which remained water insoluble, whereas the other one was solubilized by heat treatment. The four prelabeled maltodextrin preparations were then incubated with AtSEX4 and, at intervals, the content of [33P]orthophosphate was determined. Thus, all four (phospho)maltodextrins are phosphorylated both at the C6 and the C3 position but only those at C3 contain 33P.
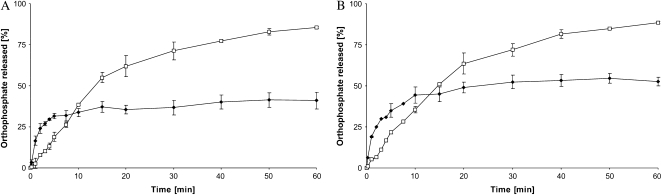
AtSEX4 hydrolyzed phosphate esters at the C3 position in all four (phospho)maltodextrin samples (Fig. 3). However, with both insoluble maltodextrins (derived from either the A- or the B-type allomorph) orthophosphate formation was fast during the first 10 min of incubation and, subsequently, the rate of hydrolysis ceased. By contrast, hydrolysis of the C3 esters from the solubilized (phospho)glucans proceeded with time and after 60 min incubation the amount of orthophosphate released was approximately 2-fold higher than that from the insoluble (phospho)glucans. Thus, the mode of the AtSEX4 action on C3 esters closely resembles the hydrolysis of C6 phosphates (Fig. 1).
Figure 3.
AtSEX4 dephosphorylates glucosyl 3-P residues of both the prelabeled A- and B-type allomorphs. Both types were subjected to a two-step procedure. First, both allomorphs were prephosphorylated (but unlabeled) in C6 using StGWD and unlabeled ATP (60 min incubation). Second, both water-insoluble MD were phosphorylated (and labeled) using recombinant AtPWD and [β-33P]ATP. Subsequently, a part of the prelabeled insoluble MD solubilized by heating, whereas the other part remained untreated. All four (phospho)glucans, solubilized (white symbols) and insoluble (black symbols) of A-type (A) or B-type allomorph (B), were incubated with 100 ng AtSEX4. At intervals, aliquots of each reaction mixture were withdrawn and processed as in Figure 1 (n = 3; ±sd).
Similar results were obtained when native starch granules prelabeled in either the C6 or the C3 position were incubated with AtSEX4 (Table V). Hydrolysis of the C6 phosphate ester appears to be faster as compared to that of the C3 counterpart. This difference may, however, be not relevant as in the prephosphorylated native starch granules C3 esters occurred less frequently than C6 phosphates (Table V). Furthermore, AtSEX4 is likely to act simultaneously on both the labeled C3 and the unlabeled C6 esters but, if so, hydrolysis of the latter remains unnoticed.
Table V.
AtSEX4-mediated hydrolysis of C6 and C3 phosphate esters at native starch granules
Native sex1-3 starch granules were prelabeled with 33P at either C6 or C3 positions and were then incubated with AtSEX4 (see “Materials and Methods”). The released radioactive label was quantified by scintillation counting. Phosphatase activity is given as specific enzyme activity (nmol P mg−1 SEX4 min−1) or as the relative rate of 33P release in the reaction mixture; the total 33P content of the starch added to the reaction mixture is taken as 100% (% P min−1). Mean values ±se are given (n = 3).
| Position of 33P-Labeled Phosphate | Phosphate Content | Phosphatase Activity | |
|---|---|---|---|
| pmol P mg−1 starch | nmol P mg−1 SEX4 min−1 | % P min−1 | |
| C6 | 90.169 | 3.675 ± 0.037 | 0.509 ± 0.005 |
| C3 | 32.615 | 0.643 ± 0.012 | 0.247 ± 0.005 |
AtSEX4 Does Not Hydrolyze the Catalytically Essential Phosphohistidine Residue of StGWD and Very Poorly Acts on ATP
For a more detailed analysis of the dephosphorylation of MD we intended to study a simultaneous action of both AtSEX4 and StGWD. This experiment is, however, only meaningful if two prerequisites are given: AtSEX4 should neither dephosphorylate the essential intermediary state of GWD, i.e. GWD-P, nor should it hydrolyze ATP.
Based on sequence analysis, SEX4 is predicted to be a DSP acting on both proteinaceous and nonproteinaceous substrates but so far any potential protein target is unknown. Therefore, we tested whether or not AtSEX4 is capable of hydrolyzing the catalytically active state of StGWD (designated as GWD-P). Although phosphohistidine residues do not belong to the predicted targets of any known DSPs (see above), this possibility needed to be disproven as otherwise a short yet nonproductive reaction sequence [β-33P]ATP → GWD-33P → 33Pi could occur that bypasses any glucan phosphorylation.
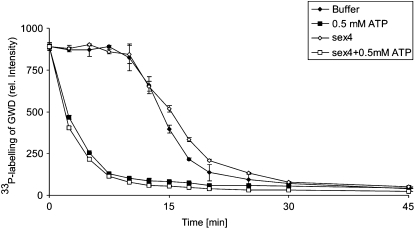
The stability of GWD-P in the presence of SEX4 was tested using two approaches (procedures A and B, see “Materials and Methods”). In both procedures, recombinant StGWD was prephosphorylated for 3 min with [β-33P]ATP in the absence of any α-glucan.
In procedure A, incubation of StGWD was continued in the presence of [β-33P]ATP and either the absence or presence of AtSEX4. At intervals, the alkaline-stable 33P content of StGWD was determined. In both reaction mixtures the 33P content of the dikinase remained constant for 10 min and, subsequently, decreased but the recombinant AtSEX4 did not affect this process (Fig. 4). When in the prephosphorylation mixture a higher concentration of ATP was used (10 μm instead of 5 μm), the period of the maximal 33P content of GWD was extended to 20 min but, subsequently, the labeling decreased strongly without any noticeable enhancing effect of AtSEX4 (data not shown). In procedure B, a pulse-chase experiment was performed. Following 3 min incubation, a large excess of unlabeled ATP (500 μm) was added and incubation of StGWD was continued. Under these conditions, the 33P content of StGWD immediately declined. Again, the presence of AtSEX4 did not enhance the loss of the label in StGWD-P.
Figure 4.
AtSEX4 does not hydrolyze the StGWD-P. A total of 100 μg of recombinant StGWD was incubated for 3 min with 5 μm ATP and 15 μCi [β-33P]ATP in the absence of α-glucan (see “Materials and Methods”). Following prephosphorylation, aliquots were further incubated with either 15 μg of recombinant AtSEX4 or, as a control, in the absence of the phosphatase (Buffer; see insert). For pulse-chase experiments, two aliquots were mixed with 0.5 mM unlabeled ATP in the presence (sex4 + 0.5 mm ATP; see insert) or absence (0.5 mm ATP; see insert) of 15 μg of recombinant AtSEX4. At intervals, aliquots (10 μL each) were mixed with equal volume of 0.2 n NaOH and 2 μL each of the samples were spotted onto nitrocellulose. The membrane was washed and the radioactivity was quantified by phosphor imaging. n = 3; ±sd.
Thus, in the absence of any α-glucan, the recombinant StGWD mediates a transfer of a phosphate group from ATP to the conserved His residue and from there to a so-far-unknown endogenous acceptor. Subsequently, the dephosphorylated His residue of StGWD returns into the phosphorylated state. If either the concentration or the specific radioactivity of the [β-33P]ATP is significantly decreased, the 33P label of StGWD disappears but AtSEX4 does not enhance this process.
AtSEX4 acts very poorly on ATP. [β-33P]ATP was also incubated with AtSEX4 in the absence of StGWD and the release of orthophosphate was determined. In this particular experiment, 75% and 25% of the [33P]ATP contained the radioactivity in the β- and γ-position, respectively. For comparison, a commercially available phosphatase, the Antarctic phosphatase was included. The activity of both phosphatases was balanced using the p-nitrophenyl phosphate assay. The Antarctic phosphatase hydrolyzed ATP efficiently, whereas almost no [33P]orthophosphate release was observed during 60 min incubation with AtSEX4 (Table VI). Thus, the entire catalytic activity of StGWD is unaffected by AtSEX4 and, therefore, the analysis of the simultaneous action of both enzymes is meaningful.
Table VI.
Orthophosphate release from ATP by AtSEX4 and Antarctic phosphatase
Both phosphatases were balanced to equal enzyme activity using p-nitrophenyl phosphate as substrate. [33P]Orthophosphate, as determined by TLC and phosphor imaging, is given as percentage of the total 33P content of the reaction mixture (n = 3; ±sd). n.d., Below the limit of detection.
| Time |
33P-Orthophosphate Released |
|
|---|---|---|
| SEX4 | Antarctic Phosphatase | |
| min | % | |
| 1 | n.d. | 15 ± 2 |
| 2 | n.d. | 29 ± 0.4 |
| 5 | 1 ± 0.2 | 65 ± 4.8 |
| 10 | 3 ± 0.1 | 86 ± 2.5 |
| 30 | 6 ± 0.4 | 88 ± 1.2 |
| 60 | 6 ± 0.8 | 96 ± 0.2 |
AtSEX4 Counteracts the GWD-Mediated Solubilization of Insoluble (Phospho)glucans
The B-type allomorph of crystalline maltodextrins was prephosphorylated (and prelabeled) for either 5 or 60 min with StGWD and [β-33P]ATP. Following termination of the phosphorylation and washing, unlabeled ATP was added to the suspension of the insoluble (phospho)glucans and the resulting mixtures were incubated with either StGWD or AtSEX4 or with both the dikinase and the phosphatase. At intervals, aliquots of the reaction mixtures were centrifuged and the supernatants were analyzed for the contents of [33P]orthophosphate, 33P-phosphoglucans, and total glucosyl residues (Table VII
Table VII.
Simultaneous action of AtSEX4 and StGWD on prelabeled insoluble (phospho)glucans
The B-type allomorph of MDcryst was prephosphorylated (and prelabeled) for 5 or 60 min using StGWD and [β-33P]ATP. Subsequently, the insoluble MD were incubated for 60 min with StGWD, AtSEX4, or StGWD plus AtSEX4. At intervals, aliquots were centrifuged and the supernatants were collected. The content of [33P]orthophosphate, 33P-phosphoglucans, and of the total glucosyl residues was determined. n.d., Below the limit of detection. Mean values are given (n = 3; ±sd).
| Incubation Time | Preincubation |
||||||
|---|---|---|---|---|---|---|---|
| 5 min |
60 min |
5 min |
60 min |
5 min |
60 min |
||
| 33P-Orthophosphate Released [pmol] | 33P in Soluble Phosphoglucans [pmol] | Glc Residues Released [nmol] | |||||
| min | |||||||
| StGWD | 0 | n.d. | n.d. | n.d. | 0.8 | n.d. | 0.5 |
| 10 | n.d. | n.d. | 0.043 | 9.2 | n.d. | 6.1 | |
| 20 | n.d. | n.d. | 0.081 | 18.0 | n.d. | 2.2 | |
| 30 | n.d. | n.d. | 0.090 | 26.4 | 0.22 | 44.4 | |
| 40 | n.d. | n.d. | 0.105 | 27.2 | 45.5 | 44.4 | |
| 50 | n.d. | n.d. | 0.189 | 30.4 | 46.6 | 50.0 | |
| 60 | n.d. | n.d. | 0.234 | 31.2 | 55.5 | 55.0 | |
| AtSEX4 | 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | 0.08 | 8.4 | n.d. | n.d. | n.d. | n.d. | |
| 20 | 0.16 | 13.6 | n.d. | n.d. | n.d. | n.d. | |
| 30 | 0.20 | 17.6 | n.d. | n.d. | n.d. | n.d. | |
| 40 | 0.22 | 18.4 | n.d. | n.d. | n.d. | n.d. | |
| 50 | 0.22 | 19.2 | n.d | n.d | n.d | n.d | |
| 60 | 0.23 | 19.2 | n.d. | n.d. | n.d. | n.d. | |
| StGWD + AtSEX4 | 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d |
| 10 | 0.55 | 21.6 | n.d. | n.d. | n.d. | n.d. | |
| 20 | 0.75 | 29.6 | n.d. | n.d. | n.d. | n.d. | |
| 30 | 0.80 | 32.4 | n.d. | n.d. | n.d. | n.d. | |
| 40 | 0.84 | 34.0 | n.d. | n.d. | 0.13 | 0.33 | |
| 50 | 0.86 | 35.2 | n.d. | n.d. | 0.27 | 0.50 | |
| 60 | 0.86 | 35.2 | n.d. | n.d. | 0.27 | 0.55 | |
). As a control, the recombinant enzymes were inactivated by heating, were then incubated with the insoluble glucans, and the resulting incubation mixtures were processed as described. The supernantants from the control mixtures contained very little orthophosphate, phosphoglucans, and total glucosyl residues. The values obtained for the controls were subtracted from the data obtained with the functional enzymes. Due to the StGWD-mediated phosphorylation, both phosphoglucans and nonphosphorylated glucans are solubilized during 60 min incubation. In the presence of AtSEX4 [33P]orthophosphate was liberated but no release of nonphosphorylated nor of phosphorylated glucans could be detected. In the presence of both StGWD and AtSEX4, more [33P]orthophosphate was released but the solubilization of nonphosphorylated glucans was (at most) 1% of that observed if AtSEX4 was omitted. Essentially the same results were obtained when the amount of the recombinant StGWD was increased up to 30-fold but the level of AtSEX4 remained unchanged (data not shown).
DISCUSSION
In many plant systems, starch turnover includes the phosphorylation of relatively few amylopectin-related glucosyl residues at the C6 and C3 position. Leaf cells usually do not accumulate phosphorylated oligoglucans (Kötting et al., 2009) and, therefore, the normal starch turnover is likely to include reactions, allowing starch-derived phosphoglucosyl residues to be quantitatively introduced into the central carbon metabolism. As α-glucan-hydrolyzing enzymes, such as β-amylases, are unable to cleave interglucose bonds in close vicinity of phosphoglucosyl residues (Takeda and Hizukuri, 1981), phosphate esters at both the C6 and the C3 position are expected to be hydrolyzed before degradation of a phosphoglucan chain is completed.
In this study we have identified AtSEX4 as the plastidial phosphatase capable of efficiently hydrolyzing both phosphate esters (Figs. 1 and 3). The substrate specificity of AtSEX4 appears to be relatively low. While the enzyme very poorly hydrolyzes ATP (Table VI) and does not noticeably act on GWD-P (Fig. 4), it hydrolyzes singly, doubly, and triply phosphorylated α-glucans (Table IV). Likewise, AtSEX4 dephosphorylates both soluble and insoluble phosphoglucans (Figs. 1 and 3). In the latter case, insoluble phosphoglucans derived from either the A- or the B-type allomorph are utilized as substrate with similar kinetic constants (Table I). However, the actual substrate of AtSEX4 is unlikely to be the highly ordered crystalline A- or B-type allomorph, but rather the structurally altered (phospho)maltodextrins at the surface of the particles.
Presumably, one important feature defining a molecule to be a carbohydrate substrate of AtSEX4 is that it can interact with the functional CBM of the phosphatase (Fig. 1). Possibly the same characteristics also allow nonphosphorylated α-glucans to be a potential inhibitor of AtSEX4 (Fig. 2). This effect also explains the lower activity of AtSEX4 when acting on solubilized (phospho)glucans (Figs. 1 and 3). Under these conditions, a mixture consisting of both the actual substrate and the inhibitor of AtSEX4 are presented. As opposed to soluble nonphosphorylated glucans, highly ordered MDcryst almost completely lack an inhibitory effect and, therefore, the interaction between AtSEX4 and carbohydrates appears to be restricted to the less ordered or even soluble state of α-glucans.
The maltodextrins used in most of the experiments of this study comprise a relatively wide range of DPs. Furthermore, even monophosphorylated maltodextrins represent a rather complex mixture of molecules varying both in size and in the intramolecular position of the phosphate ester. Therefore, detailed kinetical analyses of the phosphatase activity and of the inhibitory effects of nonphosphorylated maltodextrins are almost impossible. Nevertheless, the significant inhibition of AtSEX4 by micromolar levels of maltoheptaose (Fig. 2) clearly indicates that in vivo the plastidial pool of soluble maltodextrins contains potential regulators of AtSEX4 that probably prevent an efficient dephosphorylation of soluble phosphoglucans by AtSEX4. By contrast, inhibitory effects are likely to be minor (or even absent) if AtSEX4 acts on phosphoglucans located at the surface of native starch granules. Thus, despite the relatively low substrate specificity, the surface of native starch granules is likely to be the preferred in vivo substrate of AtSEX4. If so, mechanisms have to be postulated that regulate the targeting of AtSEX4 to the surface of starch granules. Furthermore, control mechanisms are needed that prevent any interference with the phosphorylating activity of both GWD and PWD as they also use the interface between the starch granule and the stromal space. In the absence of any α-glucan, the autophosphorylated intermediate of GWD (i.e. GWD-P) appears to be turned over, resulting in a permanent consumption of ATP. Therefore, targeting of GWD to the granule surface and regulation of its autophosphorylating activity appear to be closely linked. The mechanisms involved are currently being investigated in our laboratory.
An important result of this study is the incomplete hydrolysis of the phosphate esters formed by the prephosphorylation of MDcryst. This effect is robust and observed under a wide range of experimental conditions such as with both the A- and the B-type allomorph (Fig. 1), the prelabeling of the C6 or C3 position (Fig. 3), varying degrees of prephosphorylation, varying AtSEX4 levels (Table II), and with the mutated AtSEX4 proteins (Fig. 1C) as well.
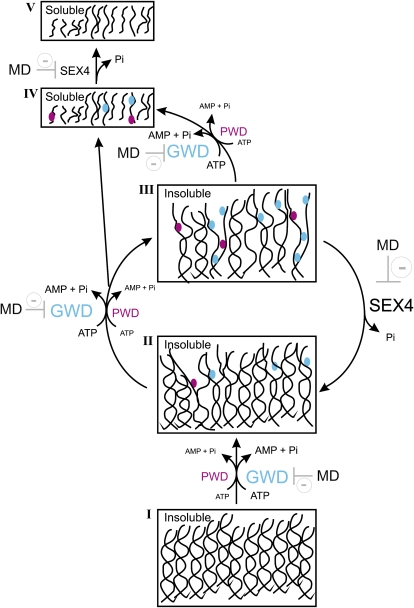
Probably, the incomplete dephosphorylation of insoluble phosphoglucans cannot be explained exclusively on the basis of kinetic properties of AtSEX4 but rather reflect a close interaction between the phosphatase activity and dynamic maltodextrin structures as affected by phosphorylation status. The structural dynamics appear to determine the accessibility of phosphate esters for AtSEX4. We propose that several steps within the de- and reorganization of the highly ordered maltodextrins structure can be distinguished (Fig. 5).
Figure 5.
Proposed steps for the transition of crystallized (phospho)maltodextrins to solution as affected by the two starch-related dikinases and SEX4. The phase transition is initiated with the GWD-mediated phosphorylation of highly ordered insoluble α-glucans (I). In starches from some mutant lines, PWD can functionally replace GWD. Phosphorylation results in a less-ordered yet insoluble state (II). By further phosphorylation reactions state (III) is reached that immediately allows solubilization of both nonphosphorylated and phosphorylated glucans. State (III) can also function as substrate of SEX4. If so, partial dephosphorylation results in a structural reorganization preventing the solubilization of nonphosphorylated glucans and limits the accessibility of residual phosphate esters to SEX4. Following solubilization (state IV) phosphoglucans can be converted by SEX4 into nonphosphorylated chains that also inhibit the phosphatase and GWD activity.
Phase transition is initiated by the GWD-mediated phosphorylation of C6 positions of glucosyl residues in close vicinity of the surface of a highly ordered MDcryst, representing either the A- or the B-type allomorph. In most native starches, this process takes place at the semicrystalline regions of the granule and appears to be specific for plants (Deschamps et al., 2008).
In MDcryst (and in wild-type starches as well) the GWD-mediated phosphorylation at the C6 position leads to a partial structural change that converts both nonphosphorylated and phosphorylated glucans from a PWD-inaccessible state into a potential substrate. This conversion is supported by the fact that PWD, to a significant extent, utilizes nonphosphorylated glucans and thereby forms monophosphorylated maltodextrins (Hejazi et al., 2009). In addition to PWD, GWD also can act on the structurally altered glucans and catalyzes further phosphorylations. In either case a glucan structure is reached that allows an immediate transition of both neutral glucans and phosphoglucans to the soluble state. This structure defines also the actual substrate of AtSEX4 (and of other hydrolyzing enzymes as well; Edner et al., 2007) when acting on insoluble (phospho)glucans. By doing so, AtSEX4 removes less than 50% of the phosphate esters introduced by GWD (and PWD), which allows a partial reorganization of the insoluble (phospho)glucans. This reorganization has two consequences: First, the residual phosphate esters are inaccessible to the phosphatase and retained in the insoluble phase. Second, the transition of nonphosphorylated glucans into solution is almost completely impeded. It is likely that the reorganization occurs fast as the simultaneous action of both StGWD and AtSEX4 results in a continuous release of [33P]orthophosphate but almost no solubilization of nonphosphorylated maltodextrins (Table VII). This effect was observed in a relatively wide range of StGWD-to-AtSEX4 ratios. It is, however, reasonable to assume that massively altered ratios of the two recombinant proteins will result in different product patterns.
AtSEX4 acts on both insoluble and solubilized phosphoglucans but the latter enzymatic process is less efficient due to the presence of inhibitory nonphosphorylated maltodextrins. For SEX4-deficient Arabidopsis mutants an accumulation of soluble phosphoglucans has been described (Kötting et al., 2009). The results presented in this study do not favor the assumption that the pool of these phosphoglucans represents the actual in vivo substrate of SEX4.
The MDcryst used here somehow reflect structural properties of the native starch granules as both share the highly ordered structure of double-helix formation. However, in MDcryst, the branches (i.e. the less-ordered regions) do not occur and, therefore, the release of glucans and (phosphor)glucans without debranching is possible.
As opposed to GWD and PWD, the occurrence of proteins similar to SEX4 appears not to be restricted to starch-metabolizing organisms. In the mammalian system, the protein most closely related to SEX4 is laforin, a DSP containing a functionally indispensable CBM (CBM20). Laforin is essential for the normal mammalian glycogen metabolism. Mutations in the gene encoding the human laforin (EPM2A) are often associated with a progressive neurological deterioration designated as epilepsy of the Lafora type. At the cellular level, Lafora disease is accompanied by the accumulation of a modified glycogen termed polyglucosan. It is a less-frequently branched, highly phosphorylated, and insoluble Glc polymer that is deposited in Lafora bodies and accumulates with time (Minassian et al., 1998, 2000; Ganesh et al., 2006). The formation of polyglucosan seems to reflect a disturbed regulation of the glycogen metabolism and is, to some extent, consistent with the absence of a functional phosphatase acting on phosphorylated glycogen. Furthermore, functional laforin has been reported to partially complement a SEX4-deficient Arabidopsis mutant (Gentry et al., 2007), suggesting a close functional similarity between this type of plant and mammalian DSPs. However, laforin is known to exert phosphatase activity on various phosphoproteins and to undergo additional protein-protein interactions such as homodimerization (Vilchez et al., 2007; Worby et al., 2008; Puri et al., 2009; Vernia et al., 2009). Currently, it is totally unknown whether or not the mammalian laforin and the plant SEX4 protein share functional similarities even in some of these (phospho)protein-related actions.
MATERIALS AND METHODS
Chemicals and Materials
[β-33P]ATP (10 mCi mL−1; 3,000 mCi mmol−1) was purchased from Hartmann Analytic (http://www.hartmann-analytic.de). Maltooligosaccharides (EC 232-940-4) used for crystallization, maltotriose (EC 214-174-2) and maltoheptaose (EC 252-119-4), were obtained from Sigma-Aldrich (http://www.sigma-aldrich.com) and PEI-cellulose F plates from Merck (http://www.merck-chemicals.com). The starch kit was purchased from Roche Diagnostics (http://www.roche-applied-science.com). A-MDcryst and B-MDcryst were prepared and characterized as previously described (Hejazi et al., 2009).
Enzymes
Recombinant GWD from Solanum tuberosum (StGWD), PWD from Arabidopsis (Arabidopsis thaliana; AtPWD), and wild-type or two mutated (N333K; K307Q) SEX4 proteins (AtSEX4) were generated as described elsewhere (Ritte et al., 2002; Kötting et al., 2005; Gentry et al., 2007). Antarctic phosphatase was purchased from New England Biolabs (product no. M0289S; http://www.neb-on-line.de).
Enzyme-Catalyzed Reactions
Except where stated, all reaction mixtures were kept for 1 h at 30°C and were continuously agitated.
MDcryst Prephosphorylation by StGWD
Except where stated the reaction mixture (1 mL final volume) contained 50 mg A-MDcryst or B-MDcryst, 50 mm HEPES-KOH (pH 7.4), 6 mm MgCl2, 1 mm EDTA, 0.4 mg bovine serum albumin (BSA), 25 μm ATP, 20 μCi [β-33P]ATP, and 2.5 μg purified recombinant StGWD. Reaction was terminated by adding SDS dissolved in water (final concentration 2% [w/v]). The insoluble maltodextrins were washed five times with water and were then used as substrate for recombinant phosphatases. For 33P quantification by liquid scintillation counting, aliquots of the (phospho)glucans were solubilized by heat treatment.
MDcryst Prephosphorylation by AtPWD
A-MDcryst and B-MDcryst were prephosphorylated by GWD (and unlabeled ATP) as described above except [β-33P]ATP was omitted. Following termination of the reaction and washing (see above), the prephosphorylated (but unlabeled) insoluble MD were resuspended in a reaction mixture (as above) but containing 2.5 μg purified recombinant AtPWD instead of StGWD. Samples were processed as described above.
Prephosphorylation of Native Starch Granules by StGWD
Starch was isolated from leaves of a GWD-deficient Arabidopsis mutant (sex1-3) essentially as described elsewhere (Kötting et al., 2005). Prelabeling at the C6 position was performed in a mixture (10 mL final volume; 2.5 h at room temperature) containing 50 mm HEPES-KOH (pH 7.5), 200 mg native starch granules, 1 mm EDTA, 6 mm MgCl2, 0.05% (w/v) Triton X-100, 10 mg BSA, 1 mm dithiothreitol, 50 μm ATP plus 200 μCi [β-33P]ATP, and 100 μg recombinant StGWD. Following the addition of SDS (as above), starch granules were washed six times with a mixture containing 2% (w/v) SDS and 2 mm ATP and then four times with water. 33P-labeled starch granules were resuspended in water and immediately used for the phosphatase assay or were stored at −20°C until use.
Prephosphorylation of Native Starch Granules by AtPWD
Starch granules were phosphorylated at C6 as described above except that [β-33P]ATP was omitted and the concentration of the unlabeled ATP was 10 μm. Following termination of the reaction and washing (see above), native starch granules were labeled by recombinant AtPWD using the same reaction mixture as described above except that AtPWD (300 μg) replaced StGWD and incubation was extended to 16.25 h. Fresh AtPWD (300 μg each) was added after 45 min and after 14 h. All subsequent steps were performed as described for the GWD-mediated starch labeling.
p-Nitrophenyl Phosphate-Based Phosphatase Activity Assay
The assay mixture (final volume 50 μL) contained 100 mm bis-Tris-HCl (pH 6.0), 2 mm dithioerythritol (DTE), 50 mm p-nitrophenyl phosphate, and 10 to 100 ng purified recombinant phosphatase protein. At 1 min intervals, 600 μL 0.25 n NaOH was added and absorbance was measured at 410 nm.
Enzymatic Phosphomaltodextrin Dephosphorylation
STA
The assay mixture (final volume 50 μL) contained 50 mm bis-Tris-HCl (pH 6.0), 2 mg insoluble prelabeled MDcryst derived from the A- or B-type allomorph, 2 mm DTE, and 20 μg BSA. Recombinant AtSEX4 was added (25–100 ng; as indicated) and aliquots of the reaction mixtures were heated (5 min at 95°C). Aliquots (2 μL each) were spotted onto a PEI-cellulose F plate. Following chromatography, the 33P content of the labeled compounds was quantified using a phosphor imager.
LTA
The assay mixture was as described for STA, except that the prephosphorylated maltodextrins were applied both in the insoluble and in the heat-solubilized state. Dephosphorylation was initiated as indicated. At intervals, aliquots were processed as described above.
Enzymatic Dephosphorylation of Soluble or Insoluble (Phospho)maltodextrins in the Presence of Nonphosphorylated Glucans
In a final volume of 50 μL, varying amounts of nonphosphorylated α-glucans (as indicated) were preincubated with 25 ng of recombinant AtSEX4, 100 mm bis-Tris-HCl (pH 6.0), 2 mm DTE, 20 μg BSA for 10 min. To start the reaction, 0.5 mg of 33P-labeled B-MDcryst was added. At intervals, aliquots of the reaction mixtures were processed as described above.
Simultaneous Action of StGWD and AtSEX4
B-MDcryst was prelabeled using StGWD and [β-33P]ATP for 5 or 60 min as described above. Subsequently, 1.5 mg each of the insoluble and prelabeled MD was incubated in a reaction mixture (50 μL) containing 50 mm HEPES-KOH (pH 7.4), 2 mm DTE, 6 mm MgCl2, 20 μg BSA, 25 μm ATP (unlabeled), and either 0.25 μg StGWD or 0.1 μg AtSEX4 or 0.25 μg StGWD plus 0.1 μg AtSEX4. As a control, heat-inactivated StGWD and/or AtSEX4 were added. At intervals, reaction mixtures were centrifuged (1 min at 10,000g, room temperature). The pellet was washed twice with water (100 μL each) and resuspended in 50 μL water. The supernatants were combined. Aliquots of the combined supernatants and the resuspended pellets were heated for 5 min at 95°C and the 33P content was determined by TLC and phosphor imaging. For quantification of the soluble α-glucans, 20 μL of the combined supernatant fractions were hydrolyzed in 0.7 n HCl (2 h at 95°C). Following neutralization with 0.7 n NaOH, Glc was quantified using the starch kit.
Enzymatic Starch Dephosphorylation
The assay volume (50 μL) contained native starch granules (200 μg each) prelabeled at either the C6 or the C3 position (see above), 100 mm sodium acetate, 50 mm bis-Tris, 50 mm Tris-HCl (pH 6.0), 0.025% (v/v) Triton X-100, 50 μg BSA, and 2 mm dithiothreitol. Reaction was initiated by adding 25 ng AtSEX4. After 5 min incubation at room temperature 20 μL 10% (w/v) SDS was added and the starch granules were pelleted by centrifugation. Radioactivity in the supernatant (20 μL each) was determined by liquid scintillation counting.
Enzymatic ATP Hydrolysis
In a final volume of 50 μL, recombinant SEX4 protein (0.1 μg) or Antarctic phosphatase (50 mU) was incubated with 25 μm ATP (including 2 μCi [β-33P]ATP) in 50 mm bis-Tris, pH (6.0). At intervals, aliquots were heated for 5 min at 95°C. Subsequently, 2 μL of each heat-treated mixture was spotted onto the TLC plates, chromatographed, and radioactivity was quantified by phosphor imaging.
Autophosphorylation and Stability of the Autophosphorylated StGWD
In a final volume of 1 mL, StGWD (100 μg) was incubated for 3 min in a mixture containing 50 mm HEPES-KOH (pH 7.4), 6 mm MgCl2, 1 mm EDTA, 2 mm DTE, 5 μm ATP including 15 μCi [β-33P]ATP. Following autophosphorylation, the 33P content of StGWD was followed by either of two procedures. Procedure A: Aliquots (150 μL each) of the prephosphorylation mixture were further incubated either in the presence of 15 μg recombinant AtSEX4 or, as a control, in the absence of the recombinant phosphatase. At intervals, 10 μL each of the reaction mixtures were mixed with an equal volume of 0.2 n NaOH and 4 × 2 μL each of the samples were spotted onto nitrocellulose. The membrane was washed twice (10 min each; room temperature) with 1 mm unlabeled ATP dissolved in 10 mm Tris-HCl (pH 8.2), and then five times (10 min each) with the same buffer lacking ATP. Finally, radioactivity was quantified by phosphor imaging. Procedure B (pulse chase): Following 3 min prephosphorylation, two aliquots (150 μL each) were mixed with 0.5 mm unlabeled ATP in the presence of 15 μg AtSEX4 or, alternatively, in the absence of the phosphatase. Incubation, sampling, and sample processing were performed as outlined for procedure A.
Analytical Techniques
The total glucosyl content of (phospho)glucans was quantified enzymatically (Hejazi et al., 2008). TLC and the quantification of the 33P content were performed as described elsewhere (Hejazi et al., 2008). Phosphomaltodextrins and nonphosphorylated maltodextrins were separated by high-performance anion exchange chromatography coupled to pulsed amperometric detection. The 33P content of the singly, doubly, and triply phosphorylated glucans was determined by liquid scintillation counting.
Protein Quantification
Buffer-soluble proteins were quantified using the microversion of the Bio-Rad protein assay kit (Bradford, 1976) with BSA serving as standard.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of neutral maltodextrins on the activity of AtSEX4.
Supplementary Material
Acknowledgments
The SEX4 expression vector was a kind gift of Matthew S. Gentry (University of Kentucky). We thank Simona Eicke (ETH Zurich) for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB–429–TP–B2 to M.S. and J.F.), the Bundesministerium für Bildung und Forschung systems biology project GoFORSYS (to M.S.), the Swiss-South African Joint Research Programme (grant no. IZ–LS–Z3122916 to S.C.Z.), and the ETH Zurich (to O.K. and S.C.Z.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Joerg Fettke (fettke@uni-potsdam.de).
The online version of this article contains Web-only data.
References
- Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Christiansen C, Hachem MA, Janecek S, Vikso-Nielsen A, Blennow A, Svenssen B (2009) The carbohydrate-binding module family 20—diversity, structure, and function. FEBS J 276 5006–5029 [DOI] [PubMed] [Google Scholar]
- Comparot-Moss S, Kötting O, Stettler M, Edner C, Graf A, Weise SE, Streb S, Lue W-L, MacLean D, Mahlow S, et al (2010) A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol 152 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Tahaka T, Smith AM, Smith SM (2001) A critical role of disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26 89–100 [DOI] [PubMed] [Google Scholar]
- Deschamps P, Colleoni C, Nakamura Y, Sizuki E, Putaux JL, Buléon A, Haebel S, Ritte G, Steup M, Falcón LI, et al (2008) Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol 25 536–548 [DOI] [PubMed] [Google Scholar]
- Edner C, Li J, Albrecht T, Mahlow S, Hejazi M, Hussain H, Kaplan F, Guy C, Smith SM, Steup M, et al (2007) Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol 145 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant DJ, Bouchet B, Baldwin P (1997) Microscopy of starch: evidence for a new level of granule organization. Carbohydr Polym 32 177–191 [Google Scholar]
- Ganesh S, Puri R, Singh S, Mittal S, Dubey D (2006) Recent advances in the molecular basis of Lafora's progressive myoclonus epilepsy. J Hum Genet 51 1–8 [DOI] [PubMed] [Google Scholar]
- Gentry MS, Dowen RH, Worby CA, Mattoo S, Ecker JR, Dixon JE (2007) The phosphatase laforin crosses evolutionary boundaries and links carbohydrate metabolism to neutral disease. J Cell Biol 178 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C, Colona P, Buléon A, Planchot V (2001) Amylolysis of maize mutant starches. J Sci Food Agric 81 1281–1287 [Google Scholar]
- Hanes CS (1932) Studies on plant amylases: the effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J 26 1406–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Haebel S, Edner C, Paris O, Frohberg C, Steup M, Ritte G (2008) Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J 55 323–334 [DOI] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Paris O, Steup M (2009) The two plastidial starch-related dikinases sequentially phosphorylate glucosyl residues at the surface of both the A- and the B-type allomorphs of crystallized maltodextrins but the mode of action differs. Plant Physiol 150 962–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G (2005) Identification of a novel enzyme required for starch metabolism in Arabidopsis: the phosphoglucan, water dikinase (PWD). Plant Physiol 137 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O, Santelia D, Edner C, Eicke S, Marthaler T, Gentry MG, Comparot-Moss S, Chen J, Smith AM, Steup M, et al (2009) STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana L. Plant Cell 21 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian BA, Ianzano L, Meloche M, Andermann E, Rouleau GA, Delgado-Escueta AV, Scherer SW (2000) Mutation spectrum and predicted function of laforin in Lafora's progressive myoclonus epilepsy. Neurology 55 341–346 [DOI] [PubMed] [Google Scholar]
- Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, Dunham I, Gardner R, Fong CY, Carpenter S, et al (1998) Mutations in a gene encoding a novel protein tyrosine phosphatise cause progressive myoclonus epilepsy. Nat Genet 20 171–174 [DOI] [PubMed] [Google Scholar]
- Pulido R, Hooft van Huijsduijnen R (2008) Protein tyrosine phosphatases: dual-specificity phosphatases in health and disease. FEBS J 275 848–866 [DOI] [PubMed] [Google Scholar]
- Puri R, Suzuki T, Yamakawa K, Ganesh S (2009) Hyperphosphorylation and aggregation of Tau in laforin-deficient mice, an animal model for the Lafora disease. J Biol Chem 284 22657–22663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Heydenreich M, Mahlow S, Haebel S, Kötting O, Steup M (2006) Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett 580 4872–4876 [DOI] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an α-glucan, water dikinase. Proc Natl Acad Sci USA 99 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O, Shani Z, Levy H (2006) Carbohydrate-binding modules: biochemical properties and novel applications. Microbiol Mol Biol Rev 70 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Hizukuri S (1981) Studies on starch phosphate. Part 5. Reexamination of the action of sweet-potato beta-amylases on phosphorylated (1->4)-α-D-glucan. Carbohydr Res 89 174–178 [Google Scholar]
- Vernia S, Solaz-Fuster MC, Gimeno-Alcaniz JV, Rubio T, Garcia-Haro L, Foretz M, de Córdoba SR, Sanz P (2009) AMP-activated protein kinase phosphorylates R5/PTG, the glycogen targeting subunit of the R5/PTG-protein phosphatase 1 holoenzyme, and accelerates its down-regulation by the laforin-malin complex. J Biol Chem 284 8247–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallés J, García-Fojeda B, Criado-García O, Fernández-Sánchez E, Medrano-Fernández I, Domínguez J, et al (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10 1407–1413 [DOI] [PubMed] [Google Scholar]
- Worby CA, Gentry MS, Dixon JE (2008) Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J Biol Chem 283 4069–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.