Abstract
Increasing grain sink strength by improving assimilate uptake capacity could be a promising approach toward getting higher yield. The barley (Hordeum vulgare) sucrose transporter HvSUT1 (SUT) was expressed under control of the endosperm-specific Hordein B1 promoter (HO). Compared with the wild type, transgenic HOSUT grains take up more sucrose (Suc) in vitro, showing that the transgene is functional. Grain Suc levels are not altered, indicating that Suc fluxes are influenced rather than steady-state levels. HOSUT grains have increased percentages of total nitrogen and prolamins, which is reflected in increased levels of phenylalanine, tyrosine, tryptophan, isoleucine, and leucine at late grain development. Transcript profiling indicates specific stimulation of prolamin gene expression at the onset of storage phase. Changes in gene expression and metabolite levels related to carbon metabolism and amino acid biosynthesis suggest deregulated carbon-nitrogen balance, which together indicate carbon sufficiency and relative depletion of nitrogen. Genes, deregulated together with prolamin genes, might represent candidates, which respond positively to assimilate supply and are related to sugar-starch metabolism, cytokinin and brassinosteroid functions, cell proliferation, and sugar/abscisic acid signaling. Genes showing inverse expression patterns represent potential negative regulators. It is concluded that HvSUT1 overexpression increases grain protein content but also deregulates the metabolic status of wheat (Triticum aestivum) grains, accompanied by up-regulated gene expression of positive and negative regulators related to sugar signaling and assimilate supply. In HOSUT grains, alternating stimulation of positive and negative regulators causes oscillatory patterns of gene expression and highlights the capacity and great flexibility to adjust wheat grain storage metabolism in response to metabolic alterations.
The protein content of wheat (Triticum aestivum) grains is a crucial determinant of bread and pasta quality and as such is an important economic factor. Breeding for high protein content is a main goal of wheat breeders. However, this seems to be difficult because of the well-known negative correlation between yield and grain protein content (Simmonds, 1995; Sinclair, 1998; Barneix, 2007). Because of the high starch levels of wheat grains, this compound is nearly exclusively yield determinant. Moreover, the required carbon costs per gram of seed yield is lower for starch than for protein, given that seed protein production is energetically more expensive (Vertregt and de Vries, 1987).
There is ample evidence that wheat grains grow under saturated assimilate supply, indicating that yield is mainly sink limited. Thus, grain sink strength seems to be a critical yield-limiting factor (Borrás et al., 2003; Reynolds et al., 2009). It is anticipated that modulating the balance between source and sink may result in increased yield (Reynolds et al., 2009).
Grain filling is largely dependent on assimilate supply and metabolic regulation (Weber et al., 1997b, 2005). Seeds take up Suc and nitrogen in the form of amino acids from the apoplast surrounding the filial tissue (Weber et al., 2005). While seed storage protein biosynthesis is in general dependent on nitrogen in pea (Pisum sativum), soybean (Glycine max), maize (Zea mays), and barley (Hordeum vulgare; Balconi et al., 1991; Müller and Knudsen, 1993; Salon et al., 2001; Hernández-Sebastià et al., 2005), Suc also has specific functions as a transport and nutrient sugar and as a signal molecule (Smeekens, 2000; Koch, 2004). In legume cotyledons and barley endosperm, Suc level increases at the onset of maturation, marks the switch from maternal to filial control of seed growth, and is associated with maturation (Weber et al., 2005). Suc is taken up by transporters within epidermal/endospermal transfer cells and is degraded by Suc synthase and invertases toward starch production (Weber et al., 1997a; Tegeder et al., 1999; Weschke et al., 2000). Three Suc transporter genes are expressed in the developing grain of hexaploid wheat (Aoki et al., 2002). Although gene expression was detected in the maternal nucellar projection, in the filial aleurone/subaleurone transfer cells it has been suggested that Suc transport is restricted only to filial tissues (Bagnall et al., 2000). In legume and barley seeds, Suc induces storage-associated gene expression at the transcript level and up-regulates enzymes like Suc synthase and ADP-Glc pyrophosphorylase (Heim et al., 1993; Weber et al., 1998; Weschke et al., 2000). In legume seeds, Suc feeding disrupts the meristematic state, induces cell expansion and endopolyploidization (Weber et al., 1996), and promotes cotyledonary storage activity at the transcript level (Ambrose et al., 1987; Corke et al., 1990). In Arabidopsis (Arabidopsis thaliana), Suc induces a number of storage-related genes, among them oleosins, 2S and 12S globulins, as well as leafy cotyledon (Lec)- and fusca (Fus)-like transcription factors (Tsukagoshi et al., 2007). Seed protein content is partially regulated by increased availability of carbon acceptors in the form of organic acids (Miflin and Lea, 1977), which indicates general carbon limitation for amino acid/seed protein synthesis (Weigelt et al., 2008). Taken together, Suc initiates maturation, signals the transition into the storage mode, thereby acting on transcriptional and metabolic levels, and is thus a key player within the regulatory network controlling seed maturation (Weber et al., 2005).
We have previously shown that overexpression of an amino acid permease, VfAAP1, in pea embryos increases amino acid supply and total seed nitrogen and protein content but leads to deregulation of carbon-nitrogen balance (Weigelt et al., 2008). Moreover, Suc transporter overexpression in pea cotyledons also increases seed proteins (Rosche et al., 2002, 2005). The rate-limiting role of Suc transporters has been shown for rice (Oryza sativa). Here, suppression of OsSUT1 reduces grain starch and leads to a wrinkled phenotype (Scofield et al., 2002).
Here, we present an in-depth analysis of wheat lines overexpressing the barley Suc transporter HvSUT1 (Weschke et al., 2000) under the control of an endosperm-specific promoter using phenotypical analysis together with combined transcript and metabolite profiling. The aims of this study were 2-fold: first, to evaluate the potential of improved Suc uptake capacity on wheat grain quality and yield; second, to analyze assimilate and sugar effects on grain storage metabolism. The most pronounced finding was an increase of individual grain protein content, especially of prolamins. Suc transporter overexpression obviously modified metabolic flux potential, with only subtle changes of metabolite steady-state concentrations. Such an effect could be important during specific stages when Suc uptake becomes limited due to insufficient activity of endogenous transporter. On the other hand, HvSUT1-overexpression deregulates the metabolic status of wheat grains. We found that such metabolic alterations can effectively be balanced by activation of different signaling components, having the capacity to adjust grain storage metabolism in response to metabolic alterations and showing high flexibility of wheat grain storage.
RESULTS
Generation of Wheat Lines Overexpressing HvSUT1 in the Grain
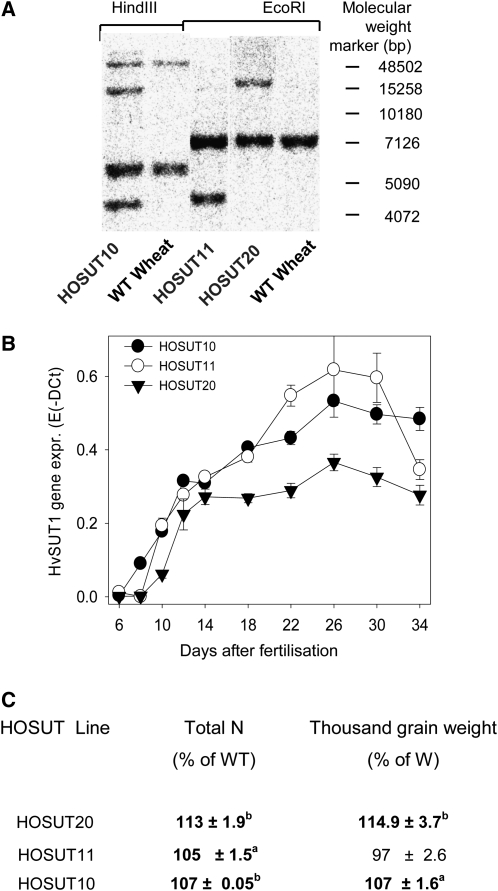
The cDNA of the barley Suc transporter HvSUT1 (Weschke et al., 2000) was fused to the Hordein B1 promoter (HO; Heim et al., 1995), which is highly active in maturing cereal endosperm. Wheat transformation was performed by biolistic (Varshney and Altpeter, 2001) and Agrobacterium tumefaciens-mediated (Hensel et al., 2009) gene transfer. Three independent, PCR-positive homozygous lines (HOSUT10, -11, and -20) were chosen and analyzed by Southern hybridization for copy number. Compared with wild-type wheat, one or two additional bands appear in lines HOSUT11, -20, and -10, indicating single insertions (Fig. 1A). Quantitative reverse transcription (RT)-PCR revealed similar transgene expression in grains of all three lines, with increasing levels between 6 and 14 d after fertilization (DAF), a plateau phase between 14 and 18 DAF, and a further increase from 18 to 26 DAF (Fig. 1B). Overexpression of HvSUT1 is directed to endosperm cells, which are normally not engaged in Suc uptake. Suc uptake is mediated by a SUT within the modified aleurone/subaleurone transfer cells (Bagnall et al., 2000). Levels of total nitrogen were found to be increased in mature seeds of all three homozygous lines of the T2 (HOSUT11 and -20) and T4 (HOSUT10) generations by 5% to 13% compared with the wild type. Thousand grain weight was significantly increased for HOSUT20 and -10 (Fig. 1C). Levels of starch were not different on a per gram basis (data not shown).
Figure 1.
Characterization of HvSUT1-overexpressing wheat lines. A, Independent lines HOSUT10 (T4 generation), -11, and -20 (T2 generation) were analyzed for transgene integration by DNA gel-blot analysis. WT, Wild type. B, Quantitative real-time PCR analysis of HOSUT caryopses for transgene expression. Values are means of three biological replicates ± sd. C, Total nitrogen (N) and thousand grain weight of mature HOSUT grains (greenhouse grown). n = 5 to 10 ± sd. Significant differences according to t test are as follows: aP < 0.05, bP < 0.01. W, Wild type.
HvSUT1 Overexpression Increases Suc Uptake into Grains
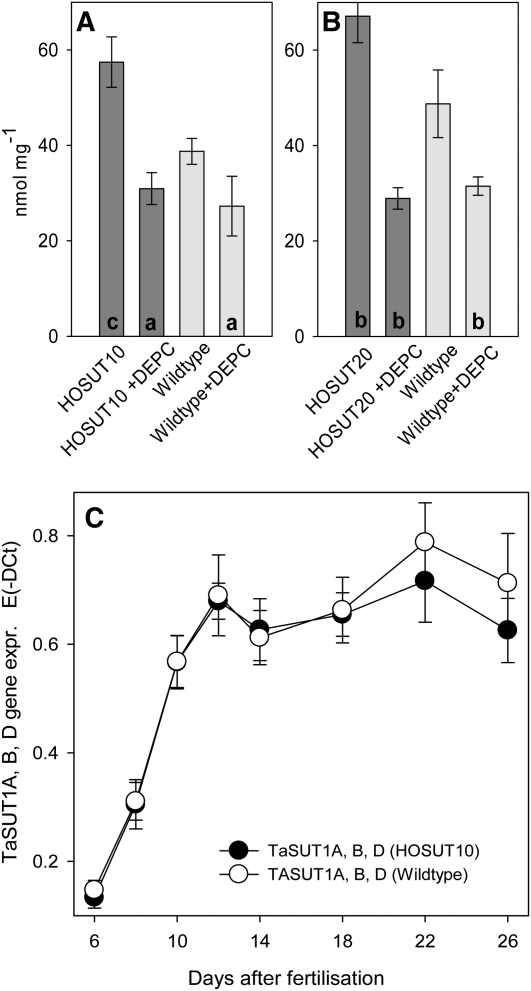
To prove the function of HvSUT1 overexpression, we measured 14C label incorporation after short-term labeling with [14C]Suc of immature grains of wild-type wheat and lines HOSUT10 and -20 at 19 DAF. Preincubation with the covalent protein modifier diethylpyrocarbonate, which is known to inhibit proton-coupled symport (Bush, 1989), led to 30% to 50% inhibition of label incorporation. Compared with the wild type, HOSUT10 (Fig. 2A) and HOSUT20 (Fig. 2B) grains incorporated about 30% to 40% more 14C, indicating that endosperm-specific HvSUT1 expression increased Suc uptake into grains.
Figure 2.
Suc uptake and TaSUT gene expression. A and B, 14C label incorporation was measured after 10 min of labeling with [14C]Suc of immature grains of wild-type Certo and lines HOSUT10 (A) and HOSUT20 (B) at 19 DAF. Thirty-minute preincubation was done with the covalent protein modifier diethylpyrocarbonate (DEPC), known to inhibit proton-coupled symport. Significant differences according to t test are as follows: aP < 0.05, bP < 0.01, cP < 0.001. C, Quantitative RT-PCR of gene expression of endogenous wheat Suc transporters in HOSUT10 and wild-type endosperm. Values shown are means of at least three replicates ± sd.
HvSUT1 Seeds Have Higher Grain Nitrogen and Prolamin Contents
Line HOSUT10 (T4 generation) was chosen for further analysis. To study whether ectopic expression of HvSUT1 changes gene expression of endogenous Suc transporters, we performed quantitative real-time PCR on growing endosperms, showing that gene expression of the three endogenous wheat transporters TaSUT1A, -1B, and -1D (Aoki et al., 2002) was not different between HOSUT10 and the wild type (Fig. 2C).
To analyze the impact of Suc transporter overexpression on seed quality characteristics, plants were grown in greenhouses, in soil beds under semicontrolled conditions, and in field trials. Compositional analysis of mature HOSUT10 grains demonstrated that compared with wild-type seeds, total nitrogen content, which reflects grain protein content, was increased. To analyze the accumulation of different storage protein classes, seed protein was extracted from mature grains and separated into the fractions albumins/globulins and the prolamin classes gliadins and glutenins. Under all growth conditions, there was a significant increase of gliadins and glutenins, whereas the albumin/globulin fraction was unchanged, indicating a specific stimulation of prolamin synthesis in SUT1-overexpressing grains (Table I). There was a slight but significant increase in total seed carbon, whereas starch content was unchanged under semicontrolled and field conditions and slightly decreased for the greenhouse-grown material. Compared with the wild type, thousand grain weight was significantly higher in HOSUT10 by 10% and 6.8% under semicontrolled and field conditions. Overall, from measured parameters, an increase was observed in relative yield (seed mass per plant) and in protein yield in HOSUT10 grains. However, this was significant only when plants were grown under semicontrolled conditions (Table I).
Table I.
Compositional analysis of mature grains of line HOSUT10 grown under different conditions
Values are means ± se. Statistically significant differences according to Student's t test are as follows: aP < 0.05, bP < 0.01, cP < 0.001.
| Variable | Field Trial 2006/2007 |
Semicontrolled 2006/2007 |
Greenhouse 2006 |
|||
|---|---|---|---|---|---|---|
| HOSUT10 | Wild Type | HOSUT10 | Wild Type | HOSUT10 | Wild Type | |
| Nitrogen (% of total mass) | 2.62 ± 0.05a | 2.45 ± 0.04 | 2.73 ± 0.01b | 2.60 ± 0.03 | 3.62 ± 0.05c | 3.12 ± 0.05 |
| Albumins/globulins (%) | 100.87 ± 1.82 | 100 | 98.68 ± 1.26 | 100 | 99.61 ± 1.02 | 100 |
| Gliadins (%) | 112.67 ± 2.64b | 100 | 109.61 ± 2.24b | 100 | 115.26 ± 2.77c | 100 |
| Glutenins (%) | 106.32 ± 2.04a | 100 | 105.98 ± 1.88a | 100 | 123.93 ± 4.38c | 100 |
| Carbon (% of total mass) | 44.81 ± 0.01b | 44.67 ± 0.03 | 44.78 ± 0.01c | 44.58 ± 0.03 | 45.06 ± 0.03b | 44.88 ± 0.01 |
| Nitrogen-carbon ratio | 0.058 ± 0.001a | 0.055 ± 0.001 | 0.061 ± 0.0003b | 0.059 ± 0.001 | 0.080 ± 0.001c | 0.07 ± 0.001 |
| Grain protein (% of total mass) | 14.95 ± 0.29a | 13.96 ± 0.2 | 15.54 ± 0.07b | 14.81 ± 0.18 | 20.66 ± 0.3c | 17.79 ± 0.28 |
| Starch (% of total mass) | 62.5 ± 0.8 | 64.1 ± 0.8 | 62.8 ± 0.2 | 64.3 ± 0.7 | 60.3 ± 0.5a | 64.1 ± 0.8 |
| Thousand grain weight (g) | 44.0 ± 1.6 | 41.2 ± 2 | 51.0 ± 0.4a | 46.4 ± 1.7 | 58.6 ± 0.5 | 57.4 ± 0.5 |
| Yield impact (%) | 112.1 ± 19.1 | 100 | 129 ± 6.2b | 100 | 100.21 ± 20.8 | 100 |
| Protein yield impact (%) | 120.6 ± 21 | 100 | 136.3 ± 5.2c | 100 | 116.4 ± 24.2 | 100 |
HOSUT10 Grains Display Altered Development
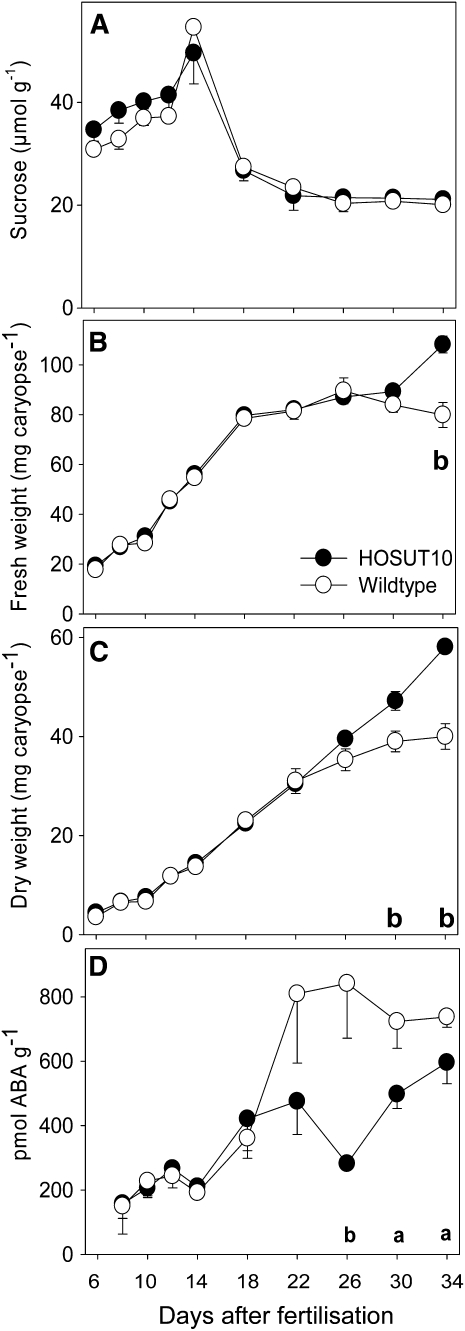
Grain developmental parameters were analyzed for line HOSUT10 and wild-type grains. Unexpectedly, there was no change in Suc levels between 6 and 34 DAF (Fig. 3A) and in mature grains (data not shown). Fresh and dry weight accumulation of HOSUT10 and wild-type caryopses was similar only at early and mid development. However, at later stages, from 26 DAF onward, accumulation in HOSUT10 grains further increased. In contrast, the wild-type fresh and dry weight accumulation curve became flattened between 26 and 30 DAF (Fig. 3, B and C). This indicates a prolonged filling phase due to HvSUT1 overexpression. However, there was no apparent difference between the timing when HOSUT10 and wild-type grains reached maturity.
Figure 3.
Growth parameters of HOSUT10 grains. A, Suc concentration in caryopses ± sd (n = 5). B, Fresh weight accumulation (n = 100). C, Dry weight accumulation (n = 100). D, ABA concentration (pmol g−1 fresh weight) ± sd (n = 5). Significant differences according to t test are as follows: aP < 0.05, bP < 0.01.
Abscisic acid (ABA) is the major hormone required for the initiation of seed maturation as well as for seed dormancy and desiccation tolerance (Finkelstein et al., 2002). ABA levels, measured on a fresh weight basis, during caryopses development were similar between 8 and 22 DAF but significantly lower in HOSUT10 grains between 26 and 34 DAF (Fig. 3D), which may also indicate altered maturation behavior of HOSUT10 grains, as seen from the dry/fresh weight accumulation curve.
Transcriptional Changes in HOSUT10 Grains Compared with the Wild Type
In order to compare gene expression between HOSUT10 and wild-type grains during development, we isolated mRNA from grains at 6, 8, 10, 12, 14, 18, 22, and 26 DAF for hybridization to an Affymetrix GeneChip Wheat Genome Array representing 55,052 transcripts from the hexaploid wheat genome (http://www.affymetrix.com/products_services/arrays/specific/wheat.affx). In a first screen, 1,700 sequences were selected with altered gene expression between HOSUT10 and wild-type grains. Approximately 50% of these candidates revealed no annotation and were designated as “unknown.” A subset of 109 sequences was further selected with clear annotations that represent members of functional groups: amino acid metabolism, cell proliferation, nitrogen-carbon signaling, transcription factors, mitochondrial metabolism, hormone functions and development, primary and secondary metabolism, seed maturation, and transport (Supplemental Table S1). From this collection, altered gene expression between HOSUT10 and wild-type grains was verified by quantitative RT-PCR at 10 stages between 6 and 34 DAF using three biological replicates. Supplemental Table S2 shows those genes that are differentially expressed, based on quantitative RT-PCR in HOSUT10 grains (P < 0.05) in at least one developmental stage.
HvSUT1 Overexpression Preferentially Stimulates Prolamin Gene Expression
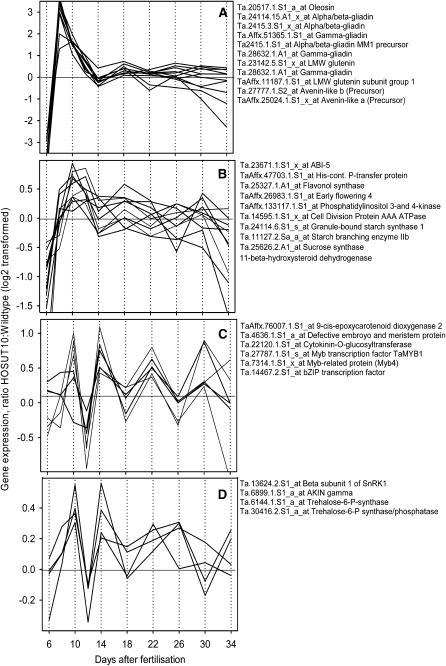
Strikingly, altered gene expression was observed for 10 sequences encoding members of the prolamin class of storage protein genes. Together with an oleosin gene, these candidates show a characteristically altered pattern. At the very early stage (6 DAF), gene expression was down-regulated in HOSUT10. However, at this stage, absolute gene expression levels of prolamins are still very low. This was followed immediately by a strong up-regulation between 8 and 14 DAF. At later stages, there were less distinct changes within this group, with at best slight up-regulation at 18 DAF (Fig. 4A). Such a pattern indicates that SUT1 overexpression strongly stimulates prolamin gene expression, especially at the transition from prestorage and storage phase. The collection of genes with altered expression (Supplemental Table S2) was screened for other members with a pattern similar to that of prolamin genes, explicitly distinct up-regulation between 8 and 14 DAF. Thereby, a group of 10 members was identified (Fig. 4B), among them three from sugar-starch metabolism, Suc synthase, granule-bound starch synthase 1, and starch-branching enzyme, and a His-containing phosphotransfer protein, which is potentially involved in cytokinin signaling in wheat (Ma and Tian, 2005). Two other genes have putative roles in cell proliferation, cell division protein AAA ATPase (Park et al., 2008) and phosphatidylinositol 3- and 4-kinase (Choi et al., 2008). Other sequences have homologies to Early flowering4 involved in oscillatory properties, circadian rhythm, and light signaling (McWatters et al., 2007), and to flavonol synthase, which is inducible by Suc supply and nitrogen depletion (Lillo et al., 2008). One gene encodes 11-β-hydroxysteroid dehydrogenase with a potential role to promote or mediate brassinosteroid effects. Its overexpression in Arabidopsis and canola (Brassica napus) improved growth and seed yield (Li et al., 2007). Finally, one sequence encodes an ABI5 homolog, which in Arabidopsis is involved in ABA signaling and is transcriptionally activated in response to sugars (Arroyo et al., 2003).
Figure 4.
Ratios of gene expression, HOSUT10:wild type. Gene expression was determined by quantitative real-time PCR at 10 stages of development with three biological replicates. Data were extracted from Supplemental Table S2 and are log2 transformed. A, Storage protein and storage-associated genes. B, Genes revealing a cluster similar to that presented in A. C and D, Genes revealing a cluster inverse to that presented in B. For full description of genes, see Supplemental Table S2. Identifier numbers refer to the Affymetrix GeneChip.
Altered Gene Expression in HOSUT10 Grains Related to Metabolism
Increased protein content indicates possible stimulatory effects on amino acid metabolism. Eight genes encoding enzymes of amino acid metabolism were differentially expressed in HOSUT grains (Supplemental Table S2), although without a clear overall tendency. Plastidial Gln synthetase and Asp kinase were strongly up-regulated, especially at later stages (Supplemental Table S2). Asp kinase catalyzes the synthesis of Thr and Lys and is strongly feedback inhibited by these end products (Ben-Tzvi Tzchori et al., 1996). On the other hand, glutamate dehydrogenase (GDH) was clearly down-regulated, especially at 30 and 34 DAF. GDH catalyzes amino acid breakdown and is induced under carbon-deficient conditions (Miyashita and Good, 2008). Its strong down-regulation in HOSUT10 grains may indicate carbon sufficiency. Accumulation of 2-oxoglutarate at DAF 22 to 26 together with decreased Glu but not Gln may also support this assumption (Supplemental Table S2).
Furthermore, there was a preferential up-regulation in HOSUT10 grains of genes encoding mitochondrial enzymes involved in carbohydrate metabolism and respiration, especially during mid and late development, such as uncoupling protein (34 DAF), ATP synthase F1 (22 and 26 DAF), pyruvate dehydrogenase E1 (30 DAF), alternative oxidase (10, 14, and 18 DAF), and malate dehydrogenase (30 and 34 DAF). This may indicate increased usage and respiration of carbohydrates within mitochondria of HOSUT10 grains. There is also a striking up-regulation of pyruvate decarboxylase (PDC), which is normally involved in fermentation pathways. However, under conditions of high sugars and glycolytic fluxes, PDC may bypass pyruvate dehydrogenase toward acetyl-CoA synthesis and therefore could support mitochondrial energy production (Gass et al., 2005).
Deregulated Gene Expression in HOSUT10 Grains Related to Hormonal and Signaling Functions
A subset of genes deregulated in HOSUT10 grains revealed a striking inverse pattern with up-regulation at 10, 14, 22, and 30 DAF and with down-regulation at 8, 12, 18, and 26 DAF (Fig. 4C). Two of these genes encode key enzymes of ABA and cytokinin metabolism, 9-cis-epoxycarotenoid dioxygenase 2 and protein cytokinin-O-glucosyltransferase. Three others encode transcription factors, TaMyb1 and bZIP-like, both with unknown functions in wheat, and Myb4, with possible roles in stress response (Vannini et al., 2004). A set of four genes encoding two proteins, each of the trehalose and the SnRK1 pathways, show a very similar oscillating pattern (Fig. 4D). Signaling pathways of SnRK1 and of trehalose are known to be involved in the regulation of carbohydrate metabolism (Lunn et al., 2006; Radchuk et al., 2006). In summary, these genes, which in HOSUT10 grains show the particular pattern of an oscillatory up- and down-regulation mode inversely to the prolamin genes, mainly encode transcription factors and regulatory genes of hormonal and carbohydrate pathways.
Metabolite Profiling
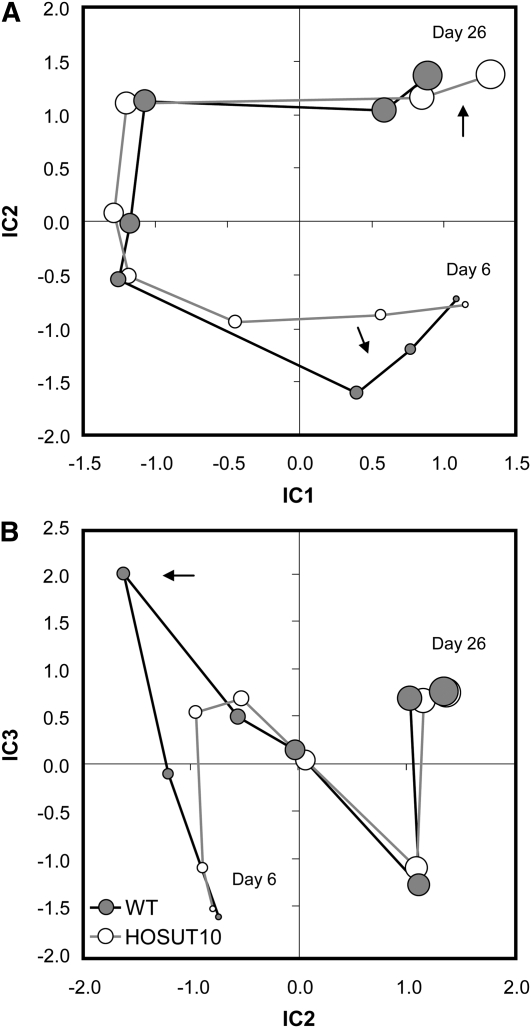
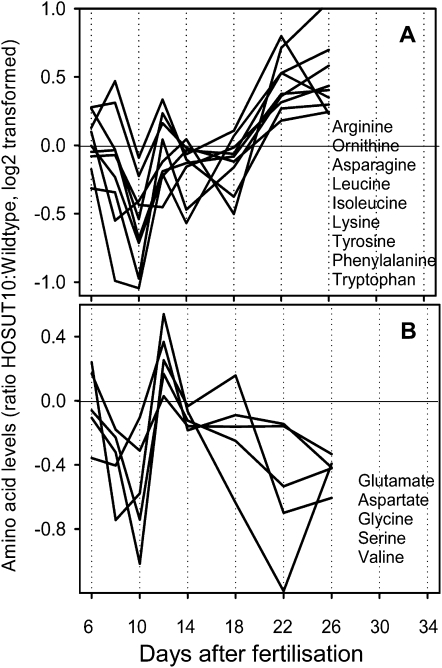
Metabolite profiling was performed from HOSUT10 and wild-type grains at stages 6, 8, 10, 12, 14, 18, 22, and 26 DAF (Supplemental Table S3). Hierarchical clustering of all metabolites (Supplemental Fig. S1) illustrates clusters according to their developmental behavior and clearly distinguishes minor (top) from major (bottom) changes. Steady decreases are more common than steady increases. To identify those stages showing major differences of metabolite composition between HOSUT10 and wild-type grains, we performed an independent component analysis based on median-centered and log10-transformed response ratios (Fig. 5). Major deviations in metabolite steady-state levels occur in early (6–8 DAF) and late (26 DAF) stages. Overall, there were not many differences in the ratios of metabolite contents. Although Suc transporter activity is apparently enhanced in HOSUT10 grains, there was no change in Suc levels in HOSUT10 grains compared with the wild type (Supplemental Table S3). On the contrary, there was a trend to lower levels from 8 DAF onward, although not statistically significant. There were also very little changes in the levels of hexoses and hexose phosphates. We found an increase of malate and 2-oxoglutarate levels at mid and later stages, indicating that carbohydrate acceptors for amino acid biosynthesis are not limited. However, citrate levels were lower in HOSUT grains at 12 and 26 DAF. Changes in amino acid levels were more pronounced. There was a clear trend for higher levels, specifically in later stages, 22 and 26 DAF, for Lys, Leu, Ile, Phe, Tyr, and Trp. On the other hand, levels of Cys, Asp, and Glu were decreased at these later stages. Because Asp and Glu are precursors of a range of other amino acids, such a pattern would indicate a stimulation of amino acid biosynthesis at later stages in HOSUT10 grains. Cluster analysis revealed that changed amino acid profiles roughly fall into two clusters. Levels of group 1 members (Arg, Orn, Asn, Leu, Ile, Lys, Tyr, Phe, Trp) were relatively lower in HOSUT10 grains between 8 and 12 DAF but displayed higher levels at later stages between 22 and 26 DAF (Fig. 6A). Levels of group 2 members (Glu, Asp, Gly, Ser, Val) were relatively decreased in HOSUT10 grains between 8 and 12 DAF and again between 20 and 26 DAF (Fig. 6B). This pattern suggest that most amino acid levels show a relative depression in HOSUT10 grains between 8 and 14 DAF, which coincides with the developmental period when prolamin gene expression is stimulated (Fig. 4A). This is in agreement with the independent component analysis results (Fig. 5).
Figure 5.
Independent component (IC) analysis based on the median centered and log10-transformed response ratios. The graph shows that deviations of metabolite levels during grain development occur in the early phase DAF 8 to 10 (arrow in B) and the late phase (26 DAF), indicating a continued development in transgenic grains at day 26 (arrow in A). The size of each circle indicates its developmental stage: smallest circle, 6 DAF; largest circle, 26 DAF. WT, Wild type.
Figure 6.
Ratios of amino acid levels, HOSUT10:wild type. Amino acids were determined by GC-MS, and data were extracted from Supplemental Table S3. A, Cluster with amino acid levels lower at 8 to 12 DAF and higher at later stages, 22 to 26 DAF. B, Cluster with amino acid levels lower at 8 to 12 DAF and lower at later stages, 22 to 26 DAF.
DISCUSSION
Conventional breeding for yield increase in wheat seems to approach an upper limit. Especially grain protein content, although economically important, is difficult to improve because of negative correlations between yield and grain protein content (Sinclair, 1998; Barneix, 2007). Increasing grain sink strength could be a promising approach to overcome this constraint (Reynolds et al., 2009). We here show that overexpression of a Suc transporter increases Suc uptake capacity. We speculate that such changes, via a network of metabolic and hormonal signals, leads to higher grain protein content.
Protein Increase in HOSUT Grains Is Not Compromising Yield
Wheat grains overexpressing HvSUT1 in the endosperm significantly take up more Suc in vitro (Fig. 2, A and B). This indicates that the transgene is functional. Surprisingly, grain Suc as well as hexose levels, as measured by HPLC (Fig. 3A) and gas chromatography-mass spectroscopy (GC-MS; Supplemental Table S3), are not altered, indicating tight control of Suc levels within the endosperm (Jenner et al., 1991). Thus, we speculate that HvSUT1 modifies Suc flux potential rather than metabolite steady-state levels.
Notably, mature grains of three lines show increased percentages of total nitrogen (Fig. 1C), which reflects a higher grain protein content. Starch concentration, on the other hand, was unchanged but was increased on the per seed level due to higher thousand grain weight. Likewise, line HOSUT10, grown in different locations, greenhouses, semicontrolled conditions (Hensel et al., 2009), and in field trails, always displayed higher percentages of total nitrogen, grain protein content, and nitrogen-carbon ratios compared with the wild type (Table I). These data indicate that HvSUT1 expression specifically stimulates grain protein accumulation. Under different growth conditions, there was no evidence for decreased yield of HOSUT lines. Thus, increases in grain protein content seem not to be correlated to lower yield (Table I). This has often been observed and is explained by the fact that high starch content of wheat grains (75%–85%) is almost completely dependent on starch accumulation (Sinclair, 1998). That means that every increase in yield should relatively decrease protein content (Barneix, 2007). Furthermore, because protein biosynthesis is energetically more expensive than that of starch (Vertregt and deVries, 1987), any yield increase would primarily apply to starch content.
Also, for HOSUT lines, a positive impact on yield cannot be supported definitely. Despite the higher protein content of individual seeds, higher seed size, and significant yield increases when grown under semicontrolled conditions, we found no clear overall higher protein yield (milligrams of seed protein per plant) that was statistically significant. A positive impact of HvSUT1 expression on protein yield under field conditions is not yet clear, because the scale of the performed field trials was too small to reliably exemplify significant effects. Nevertheless, the data suggest that increasing Suc uptake potential into grains by HvSUT1 overexpression could be a promising approach and worth pursuing toward increasing grain yield and/or grain protein yield.
HvSUT1 Overexpression in Grains Stimulates Prolamin Accumulation
Fractionation of HOSUT10 grain proteins into albumins/globulins and the prolamin classes gliadins and glutenins clearly shows that only prolamins are significantly increased (Table I), indicating a specific effect of increased Suc uptake capacity on prolamin biosynthesis. Many data reveal stimulatory effects from nitrogen supply and fertilization on grain protein content (Barneix, 2007). This led to the general conclusion that grain protein content is strictly responsive to nitrogen. However, in HOSUT10 grains, a specific effect from Suc or Suc fluxes has to be assumed. Physiological evidence shows different effects of assimilate supply on starch and protein accumulation. Whereas starch is not very sensitive to variations of assimilate supply within normally observed limits, protein content responds more directly (Perez et al., 1989; Jenner et al., 1991). Such a relationship can explain the positive effect only on protein but not on starch. Moreover, in maize kernels, Suc has been reported to increase storage product gene expression (Giroux et al., 1994), and in Arabidopsis, it can induce storage-related genes such as oleosins, 2S and 12S globulins, as well as Lec- and Fus-like transcription factors (Tsukagoshi et al., 2007). In grain legumes, Suc induces and sustains storage product formation, shown by in vitro culture (Weber et al., 1996), mutant analysis (Perez et al., 1993), and transgenic approaches where sugar levels were increased (Weigelt et al., 2009). In all these cases, seed protein content responds positively to sugar supply and/or concentration. Moreover, Suc transporter overexpression in pea cotyledons leads to earlier onset of protein accumulation (Rosche et al., 2002, 2005).
Notably, transcript profiling of HOSUT10 in comparison with wild-type grains shows predominant changes within the cluster of prolamin genes, with strong up-regulation at the transition from prestorage to storage phase, DAF 8 to 12 (Fig. 4A). This is in accordance with higher levels of prolamin-type proteins (Table I). We conclude that, especially at the onset of the storage phase, improved Suc supply stimulates prolamin gene expression and protein accumulation.
Increased Suc Uptake into HOSUT10 Grains Exerts Metabolic Effects
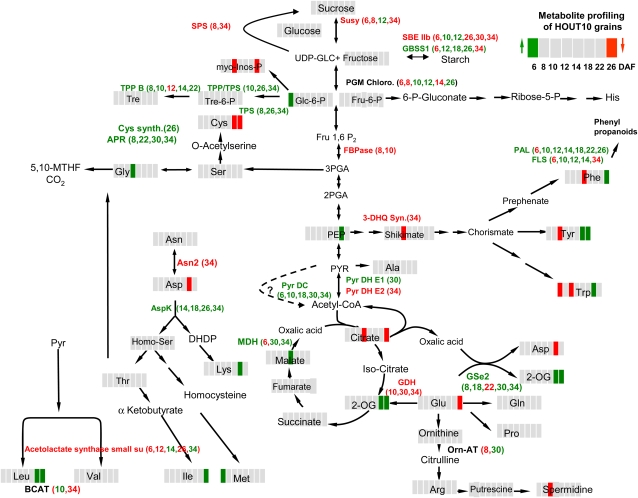
Significant changes in gene expression and metabolite levels related to central carbon metabolism and amino acid biosynthesis are summarized in Figure 7. Plastidial Gln synthetase and Asp kinase were strongly up-regulated, especially at later stages, suggesting increased synthesis of Gln and of amino acids of the Asp branch in HOSUT grains (Fig. 6). Such up-regulation is accompanied by higher levels of certain amino acids between 22 and 26 DAF and may indicate that prolonged seed filling is due to improved availability of amino acids. There is no indication for altered gene expression of amino acid transporters in the endosperm. However, it cannot be excluded that such stimulation occurs in leaf phloem. Together, the alterations argue for certain deregulation of the carbon-nitrogen balance in HOSUT grains, which ultimately indicates carbon sufficiency and relative depletion of nitrogen. First, adenosine 5′-phosphosulfate reductase, a key enzyme of sulfate reduction, is up-regulated at 8, 22, 30, and 34 DAF. In Arabidopsis, adenosine 5′-phosphosulfate reductase transcripts and activity respond positively to sugar supply (Hesse et al., 2003). This indicates that increased Suc uptake capacity in HOSUT10 activates sulfate assimilation. Second, GDH is strikingly down-regulated, especially at 30 and 34 DAF. Its major role is deamination of Glu and feeding of carbon into the tricarboxylic acid cycle to adjust high nitrogen-carbon ratios (Miyashita and Good, 2008). Accordingly, Suc can suppress GDH gene expression in tobacco (Nicotiana tabacum; Masclaux-Daubresse et al., 2005). In contrast, under conditions of excess amino acid availability such as remobilization, GDH is up-regulated (Thiel et al., 2009). Thus, GDH down-regulation in HOSUT grains indicates adequate carbon availability and relative depletion of nitrogen. Third, preferential up-regulation of gene expression related to mitochondrial carbohydrate metabolism and respiration (Supplemental Table S2) indicates increased usage and respiration of carbohydrates, which can be expected under conditions of increased carbon flux. Striking up-regulation of PDC throughout HOSUT10 grain development is surprising at first view. However, PDC may bypass pyruvate dehydogenase and therefore could support carbon flux into the tricarboxylic acid cycle. Such a role for PDC has been described, especially under conditions of high sugars and glycolytic fluxes (Gass et al., 2005). Fourth, higher 2-oxoglutarate and malate levels at mid/late stages indicate ample availability of carbon acceptors for amino acid biosynthesis, indicating that HvSUT1 overexpression stimulates both amino acid metabolism and the synthesis of organic acids as carbon skeletons for amino acid biosynthesis. Such stimulation was observed after overexpression of a phosphoenolpyruvate carboxylase and repression of ADP-Glc pyrophosphorylase (Radchuk et al., 2007; Weigelt et al., 2009).
Figure 7.
Summary of changed transcript and metabolite levels in HOSUT10 caryopses. Data are derived from Supplemental Tables S2 (transcripts) and S3 (metabolites). Color code is as follows: red, down-regulated; green, up-regulated in HOSUT10 caryopses with respect to the wild type.
Most amino acid levels show relative depression in HOSUT10 grains between 8 and 14 DAF, which coincides with the period when prolamin gene expression is strongly stimulated (Fig. 6). Thus, amino acids in HOSUT grains become transiently limited (Fig. 6) at the stage of the pronounced burst of prolamin gene expression (Fig. 4A). Decreased levels of Asp and Glu as precursors of a range of other amino acids indicate stimulated amino acid biosynthesis at later stages in HOSUT10 grains. It can be concluded that endosperm-specific SUT1 expression leads to stimulation of prolamin gene expression at earlier stages and activates and maintains amino acid biosynthesis during late maturation. Both effects can be related to signaling and metabolic effects from improved sugar supply. Probably an enhanced Suc flux potential will only become effective at later stages, when Suc uptake normally becomes limited. Bypassing such limitation may lead to prolonged fresh weight accumulation.
HvSUT1 Overexpression Stimulates a Network of Sugars and Hormonal Signals
Assuming that Suc or its increased flux capacity in HOSUT grains stimulates prolamin gene expression, it can be hypothesized that genes that show a similar pattern of up-regulation are coregulated and therefore might be also under metabolic control and potentially involved in sugar signaling. Such a cluster, similar to that of the prolamin genes, includes genes involved in sugar-starch metabolism, some of which were up-regulated in Arabidopsis seedlings and pea seeds with increased sugar levels (Osuna et al., 2007; Weigelt et al., 2009). Up-regulated gene expression related to cytokinin and brassinosteroid functions as well as cell proliferation reveals positive regulation of growth and cell proliferation due to assimilate supply (Rosche et al., 2002, 2005; Hartig and Beck, 2006; Li et al., 2007; Weigelt et al., 2009). Other members include Early flowering4 and flavonol synthase, which are inducible by Suc and nitrogen depletion (Lillo et al., 2008). The transcription factor ABI5 is potentially involved in ABA signaling and transcriptionally activated in response to sugars (Arroyo et al., 2003). Notably, coregulation of ABI5 together with storage protein genes, as observed in HOSUT10 grains, suggests a positive response to sugars and points to a key role of ABI5 in metabolic signaling of prolamin gene expression in wheat grains.
Another cluster of deregulated genes with striking oscillations (Fig. 4, C and D) reveals a pattern that is inverse to that observed in the clusters shown in Figure 4, A and B. Such members include 9-cis-epoxycarotenoid dehydrogenase, a key enzyme of ABA synthesis found to be down-regulated in pea embryos accumulating excess sugars (Weigelt et al., 2009), and cytokinin-O-glycosyltransferase, which inactivates cytokinins and causes growth retardation when overexpressed in maize (Pineda Rodo et al., 2008). Three other members encode Myb- and bZIP-like transcription factors, whereas four are related to trehalose and SnRK1 pathways, with potential involvement in the regulation of carbohydrate metabolism (Lunn et al., 2006; Radchuk et al., 2006). In Arabidopsis, trehalose-6-phosphate synthase and a SnRK1 subunit rapidly decreased upon Suc feeding (Osuna et al., 2007). It could be that in wheat grain development, such gene products (Fig. 4, C and D) modulate and/or counterbalance the effect from increased carbon flux or sugar and as such function as negative regulators of Suc signaling.
In summary, the analysis of specific clusters (Fig. 4) leads to the conclusion that overexpression of HvSUT1 on the one hand stimulates grain protein accumulation but partially deregulates the metabolic status of wheat grains. This is accompanied by an inverse oscillatory behavior of gene expression of positive and negative regulators related to assimilate supply and sugar signaling. As such, metabolic alterations can be effectively balanced by different signaling components (Fig. 4, C and D), which together have the capacity to adjust grain storage metabolism throughout development in response to metabolic alterations. Alternating stimulation of positive and negative regulation due to HvSUT1 expression may cause such an oscillatory pattern of gene expression and show the high flexibility of wheat grain storage metabolism.
MATERIALS AND METHODS
Generation of Transgenic Wheat Plants
The HvSUT1 cDNA (1,894 bp; accession no. AJ272309) was fused to the HorB1 promoter (550 bp; accession no. X87232) and the HorB1 terminator (1,663 bp; accession no. FN643080). The HorB1P∷HvSUT1:HorT construct was cloned into binary vector pPZP200 (Hajdukiewicz et al., 1994). Plant transformation was carried out either by particle bombardment of immature wheat (Triticum aestivum) embryos (HOSUT10) coated with the plasmids pJFBar and HorB1∷HvSUT1/pPZP200 in equimolar ratios as described by Varshney and Altpeter (2001) or by Agrobacterium tumefaciens-mediated gene transfer using plasmid HorB1∷HvSUT1/pPZP200 (HOSUT20 and -11) largely following the protocol of Hensel et al. (2009).
Plant Growth Conditions
Seeds of winter wheat (cv Certo) were germinated in greenhouses (20°C) in trays with substrate mix (www.klasmann-deilmann.de/) for 2 weeks, followed by 8 weeks of incubation in vernalization chambers (4°C/4°C, 8-h/16-h day/night regime). Plantlets were stepwise adapted to an 18°C/14°C, 16-h/8-h day/night regime (SON-T-Agro 400 high-pressure sodium bulbs with 25,000 lux) in 2.5-L pots containing cultivation substrate (substrate 2; www.klasmann-deilmann.de/). Fertilization of greenhouse plants was conducted as described by Hensel et al. (2009). To create field-related conditions, plants were grown in soil beds under glass without supplemental light or temperature regulation from October to July. Soil bed plants were fertilized with 0.3% Hakaphos blau (www.compo.de/) once a week. Field trials were performed on a 106-m2 area at IPK Gatersleben, designed as a 4 × 4 Latin square, with 200 plants per line and single planting with 0.2-m distance. Nitrogen fertilization was performed as recommended by Agrolab (www.agrolab.de).
For mRNA expression studies and metabolite measurements, immature seeds were collected 6 to 34 DAF from the first four emerged spikes per plant, separated into maternal and filial fractions, and frozen at −80°C.
Nucleic Acid Isolation and Hybridization
High-quality genomic DNA was prepared from 150 mg of leaf material as described by Palotta et al. (2000). Fifteen micrograms of total DNA was digested with HindIII or EcoRI. DNA gel-blot analysis was performed as described by Hensel et al. (2009). PCR primers to prove transgenic HvSUT1 integration and copy number were 5′-GCCGGGCGGTCGCAGCTCGCGTCTA-3′ and 5′-GCTGCTGAAATTTCACCATCCATTC-3′ (accession no. AM055812).
Gene Expression Analysis by Quantitative Real-Time PCR
Total RNA was isolated using phenol-chloroform extraction as described by Heim et al. (1993) and treated with Turbo DNase I (Invitrogen). First-strand cDNA was synthesized with oligo(dT) primer and SuperScript III reverse transcriptase (Invitrogen). Real-time PCR from three biological replicates was performed using 2× SybrGreen PCR Mastermix on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). A total of 109 genes from the Affymetrix GeneChip Wheat Genome hybridization results (Supplemental Table S1) that obviously showed altered gene expression were selected. Primer design and reaction parameters were as described by Czechowski et al. (2005). Amplification efficiency was assessed with the LinRegPCR program (Ramakers et al., 2003). Determined ΔΔct-values from “transgenics” were related to that of “wild-type” plants at respective stages. Wheat actin (accession no. AB181991) was used as housekeeping gene for normalization. Primer sequences are given in Supplemental Table S4.
Determination of Suc, Starch, Proteins, Total Carbon and Nitrogen, and Suc Uptake Studies
Starch and Suc were determined as described by Weigelt et al. (2009). Total carbon and nitrogen in dried wheat flour were determined with the Vario EL Elementar analyzer (www.elementar.de), and protein content was calculated from percentages of total nitrogen by multiplication by conversion factor 5.7 for wheat grains (Sosulski and Imafidon, 1990). Protein fractions albumin/globulin and gliadin were sequentially extracted as described by Wieser (1997). Extracted wheat protein fractions were quantified using the colorimetric Pierce BCA Protein Assay (Thermo Scientific). Bovine serum albumin and gliadin from wheat (G3375; Sigma-Aldrich) were used as standards. Glutenin fractions were determined from the difference of total protein and albumin/globulin/gliadin fractions. In vitro Suc uptake was determined as described by Wardlaw et al. (1995).
Statistical analyses were performed with Student's t test or, alternatively, with the Wilcoxon rank-sum test with Sigma-Stat software (www.systat.com/).
Determination of ABA
ABA was extracted from plant material and analyzed according to Miersch et al. (2008) with the following modifications. Fresh material (10–500 mg) was homogenized with 10 mL of methanol and appropriate amounts (1 ng of standard per 5 mg of plant material) of [2H6]cis,trans-ABA as internal standard. Homogenates were filtered, placed on columns filled with 3 mL of DEAE-Sephadex A25 (Amersham Pharmacia Biotech; Ac− form; methanol), followed by washing with 3 mL of methanol and 3 mL of 0.1 m acetic acid in methanol. Fractions were eluted with 3 mL of 1 m acetic acid in methanol and 3 mL of 1.5 m acetic acid in methanol, combined, evaporated, and separated by preparative HPLC (Eurospher 100-C18, 5 μm, 250 × 4 mm; www.knauer.net/) using a gradient of 40% to 100% methanol in 0.2% (v/v) aqueous acetic acid in 25 min. The fraction at retention time 10 to 12 min was collected, evaporated, derivatized into pentafluorobenzyl esters, and analyzed by GC-negative chemical ionization-MS. Retention times of pentafluorobenzyl esters were as follows: [2H6]cis,trans-ABA, 17.70 min; cis,trans-ABA, 17.78 min. Fragments with mass-to-charge ratio 269 (standard ABA) and mass-to-charge ratio 263 (ABA) were used for quantification.
Transcript Profiling by 55k Microarray Analysis
Total RNA was isolated as described by Radchuk et al. (2007), and integrity and concentration were examined on the Agilent 2100 Bioanalyzer using the RNA 6.000 LabChip Kit (www.agilent.com). Five micrograms of total RNA was used to prepare double-stranded cDNA (SuperScript II; www.invitrogen.com) primed with oligo(dT) containing a T7 RNA polymerase promoter site. cDNA was purified by phenol-chloroform extraction before in vitro transcription using the in vitro transcription labeling kit (www.affymetrix.com) to synthesize copy RNA. Unincorporated nucleotides were removed using the RNeasy kit (Qiagen). Copy RNA was fragmented and hybridized to the Affymetrix GeneChip Wheat Genome array according to the manufacturer's instructions. The array was scanned with a third generation Affymetrix GeneChipScanner 3000. Affymetrix GeneChip data representing approximately 55,000 transcripts with complete Wheat Genome coverage were extracted from fluorescence intensities and scaled in order to normalize data for interarray comparison using the MicroArray Suite software package MAS 5.0 (Affymetrix). Data quality control, normalization of raw data, estimation of signal intensity, average expression values, and P values was as described by Sreenivasulu et al. (2008).
Metabolite Profiling by GC-MS
Frozen filial preparations from developing grain tissue were extracted using conventional methanol/chloroform extraction. A conventional polar fraction was prepared by liquid partitioning into water-methanol, and a 0.5-mL fraction was dried and derivatized for GC-MS profiling (Lisec et al., 2006; Erban et al., 2007). GC coupled to electron impact ionization/time-of-flight MS was performed using an Agilent 6890N24 gas chromatograph with splitless injection connected to a Pegasus III time-of-flight mass spectrometer (www.leco.de/). Metabolites were quantified using at least three specific mass fragments. GC data preprocessing and compound identification were performed using TagFinder software (Luedemann et al., 2008) and mass spectral and retention time index collection of the Golm metabolome database (Kopka et al., 2005). Six biological replicates of each sample were analyzed by GC coupled to electron impact ionization/time-of-flight MS, and numerical processing was performed as described previously (Van Dongen et al., 2009).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Hierarchical clustering using Euclidian distance and complete linkage.
Supplemental Table S1. Subset of sequences with altered gene expression between HOSUT10 and wild-type grains.
Supplemental Table S2. Quantitative real-time PCR analysis of a subset of 109 sequences with clear annotations.
Supplemental Table S3. Metabolite profiling based on GC-MS.
Supplemental Table S4. List of primers used for quantitative real-time PCR; results are given in Supplemental Table S3.
Supplementary Material
Acknowledgments
We thank Gabriele Einert, Elsa Fessel, Uta Siebert, Ines Fehrle, Birgit Ortel, and Angela Stegmann for excellent technical assistance.
This work was supported by the Federal Ministry of Education and Research (grant nos. 0310610 and 0310638A) and the Ministry for Cultural Affairs (grant no. 0045KL/0805T).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hans Weber (weber@ipk-gatersleben.de).
The online version of this article contains Web-only data.
References
- Ambrose MJ, Wang TL, Cook SK, Hedley CL (1987) An analysis of seed development in Pisum sativum L. IV. Cotyledon cell population in vitro and in vivo. J Exp Bot 38 1909–1920 [Google Scholar]
- Aoki N, Whitfield P, Hoeren F, Scofield G, Newell K, Patrick J, Offler C, Clarke B, Rahman S, Furbank RT (2002) Three sucrose transporter genes are expressed in the developing grain of hexaploid wheat. Plant Mol Biol 50 453–462 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Bossi F, Finkelstein RR, León P (2003) Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol 133 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall N, Wang XD, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. J Plant Physiol 27 1009–1020 [Google Scholar]
- Balconi CE, Rizzi E, Manzocchi L, Soave C, Motto M (1991) Analysis of in vivo and in vitro grown endosperms of high and low protein strains of maize. Plant Sci 73 9–18 [Google Scholar]
- Barneix AJ (2007) Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J Plant Physiol 164 581–590 [DOI] [PubMed] [Google Scholar]
- Ben-Tzvi Tzchori I, Perl A, Galili G (1996) Lysine and threonine metabolism are subject to complex patterns of regulation in Arabidopsis. Plant Mol Biol 32 727–734 [DOI] [PubMed] [Google Scholar]
- Borrás L, Slafer GA, Otegui ME (2003) Seed dry weight response to source-sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Res 86 131–146 [Google Scholar]
- Bush DR (1989) Proton-coupled sucrose transport in plasmalemma vesicles isolated from sugar beet (Beta vulgaris L. cv Great Western) leaves. Plant Physiol 89 1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Lee Y, Jeon BW, Staiger CJ, Lee Y (2008) Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ 31 366–377 [DOI] [PubMed] [Google Scholar]
- Corke FMK, Hedley CL, Wang TL (1990) An analysis of seed development in Pisum sativum. XI. Cellular development and the position of storage protein in immature embryos grown in vivo and in vitro. Protoplasma 155 127–135 [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erban A, Schauer N, Fernie AR, Kopka J (2007) Non-supervised construction and application of mass spectral and retention time index libraries from time-of-flight gas chromatography-mass spectrometry metabolite profiles. Methods Mol Biol 358 19–38 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signalling in seeds and seedlings. Plant Cell (Suppl) 14 S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N, Glagotskaia T, Mellema S, Stuurman J, Barone M, Mandel T, Roessner-Tunali U, Kuhlemeier C (2005) Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 17 2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux MJ, Boyer C, Feix G, Hannah LC (1994) Coordinated transcriptional regulation of storage product genes in the maize endosperm. Plant Physiol 106 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25 989–994 [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol 8 389–396 [DOI] [PubMed] [Google Scholar]
- Heim U, Manteuffel R, Bäumlein H, Stenbiss HH, Wobus U (1995) Transient expression of a lysine-rich vicilin gene of Vicia faba in barley endosperm detected by immunological tissue printing after particle bombardment. Plant Cell Rep 15 125–128 [DOI] [PubMed] [Google Scholar]
- Heim U, Weber H, Bäumlein H, Wobus U (1993) A sucrose-synthase gene of Vicia faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta 191 394–401 [DOI] [PubMed] [Google Scholar]
- Hensel G, Kastner C, Oleszczuk S, Riechen J, Kumlehn J (2009) Agrobacterium-mediated gene transfer to cereal crop plants: current protocols for barley, wheat, triticale, and maize. Int J Plant Genomics. 2009 835608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Sebastià C, Marsolais F, Saravitz C, Israel D, Dewey RE, Huber SC (2005) Free amino acid profiles suggest a possible role for asparagine in the control of storage-product accumulation in developing seeds of low- and high-protein soybean lines. J Exp Bot 56 1951–1963 [DOI] [PubMed] [Google Scholar]
- Hesse H, Trachsel N, Suter M, Kopriva S, von Ballmoos P, Rennenberg H, Brunold C (2003) Effect of glucose on assimilatory sulphate reduction in Arabidopsis thaliana roots. J Exp Bot 54 1701–1709 [DOI] [PubMed] [Google Scholar]
- Jenner CF, Ugalde TD, Aspinall D (1991) The physiology of starch and protein deposition in the endosperm of wheat. Aust J Plant Physiol 18 211–226 [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmuller E, Dörmann P, Weckwerth W, Gibon Y, Stitt M, et al (2005) GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21 1635–1638 [DOI] [PubMed] [Google Scholar]
- Li F, Asami T, Wu X, Tsang EW, Cutler AJ (2007) A putative hydroxysteroid dehydrogenase involved in regulating plant growth and development. Plant Physiol 145 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31 587–601 [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1 387–396 [DOI] [PubMed] [Google Scholar]
- Luedemann A, Strassburg K, Erban A Kopka J (2008) TagFinder for the quantitative analysis of gas chromatography-mass spectrometry (GC-MS) based metabolite profiling experiments. Bioinformatics 24 732–737 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QH, Tian B (2005) Characterization of a wheat histidine-containing phosphotransfer protein (HP) that is regulated by cytokinin-mediated inhibition of leaf senescence. Plant Sci 168 1507–1514 [Google Scholar]
- Masclaux-Daubresse C, Carrayol E, Valadier MH (2005) The two nitrogen mobilisation- and senescence-associated GS1 and GDH genes are controlled by C and N metabolites. Planta 221 580–588 [DOI] [PubMed] [Google Scholar]
- McWatters HG, Kolmos E, Hall A, Doyle MR, Amasino RM, Gyula P, Nagy F, Millar AJ, Davis SJ (2007) ELF4 is required for oscillatory properties of the circadian clock. Plant Physiol 144 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonate and contribute to a partial switch-off in jasmonate signaling. New Phytol 177 114–127 [DOI] [PubMed] [Google Scholar]
- Miflin BJ, Lea PJ (1977) Amino acid metabolism. Annu Rev Plant Physiol 28 299–329 [Google Scholar]
- Miyashita Y, Good AG (2008) NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J Exp Bot 59 667–680 [DOI] [PubMed] [Google Scholar]
- Müller M, Knudsen S (1993) The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. Plant J 4 343–355 [DOI] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible WR, et al (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49 463–491 [DOI] [PubMed] [Google Scholar]
- Palotta MA, Graham RD, Langridge P, Sparrow DHB, Barker SJ (2000) RFLP mapping of manganese efficiency in barley. Theor Appl Genet 101 1100–1108 [Google Scholar]
- Park S, Rancour DM, Bednarek SY (2008) In planta analysis of the cell cycle-dependent localization of AtCDC48A and its critical roles in cell division, expansion, and differentiation. Plant Physiol 148 246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MD, Chambers SJ, Bacon JR, Lambert N, Hedley CL, Wang TL (1993) Seed protein content and composition of near-isogenic and induced mutant pea lines. Seed Sci Res 3 187–194 [Google Scholar]
- Perez P, Martinez-Carrasco R, Martín Del Molino IM, Rojo B, Ulloa M (1989) Nitrogen uptake and accumulation in grains of three winter wheat varieties with altered source-sink ratios. J Exp Bot 40 707–710 [Google Scholar]
- Pineda Rodo A, Brugière N, Vankova R, Malbeck J, Olson JM, Haines SC, Martin RC, Habben JE, Mok DW, Mok MC (2008) Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tasselseed formation. J Exp Bot 59 2673–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radchuk R, Radchuk V, Götz KP, Weichert H, Richter A, Emery RJN, Weschke W, Weber H (2007) Ectopic expression of PEP carboxylase in Vicia narbonensis seeds: effects of improved nutrient status on seed maturation and transcriptional regulatory networks. Plant J 51 819–839 [DOI] [PubMed] [Google Scholar]
- Radchuk R, Radchuk V, Weschke W, Borisjuk L, Weber H (2006) Repressing the expression of the SUCROSE NONFERMENTING-1-RELATED PROTEIN KINASE gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid-insensitive phenotype. Plant Physiol 140 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339 62–66 [DOI] [PubMed] [Google Scholar]
- Reynolds M, Foulkes MJ, Slafer GA, Berry P, Parry MA, Snape JW, Angus WJ (2009) Raising yield potential in wheat. J Exp Bot 60 1899–1918 [DOI] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJ, Frommer WB, Offler CE, Patrick JW (2002) Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30 165–175 [DOI] [PubMed] [Google Scholar]
- Rosche EG, Blackmore D, Offler CE, Patrick JW (2005) Increased capacity for sucrose uptake leads to earlier onset of protein accumulation in developing seeds. Funct Plant Biol 32 997–1007 [DOI] [PubMed] [Google Scholar]
- Salon C, Munier-Jolain NG, Duc G, Voisin AS, Grandgirard D, Larmure A, Emery RJN, Ney B (2001) Grain legume seed filling in relation to nitrogen acquisition: a review and prospects with particular reference to pea. Agronomie 21 539–552 [Google Scholar]
- Scofield GN, Hirose T, Gaudron JA, Upadhyaya NM, Ohsugi R, Furbank RT (2002) Antisense suppression of the rice sucrose transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct Plant Biol 29 815–826 [DOI] [PubMed] [Google Scholar]
- Simmonds NW (1995) The relation between yield and protein in cereal grain. J Food Agric 67 309–315 [Google Scholar]
- Sinclair TR (1998) Historical changes in harvest index and crop nitrogen accumulation. Crop Sci 38 638–643 [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Sosulski FW, Imafidon GI (1990) Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J Agric Food Chem 38 1351–1356 [Google Scholar]
- Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, et al (2008) Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol 146 1738–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder M, Wang XD, Frommer WB, Offler EO, Patrick JW (1999) Sucrose transport into developing seeds of Pisum sativum. Plant J 18 151–161 [DOI] [PubMed] [Google Scholar]
- Thiel J, Müller M, Weschke W, Weber H (2009) Amino acid metabolism at the maternal-filial boundary of young barley seeds: a microdissection-based study. Planta 230 205–213 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Morikami A, Nakamura K (2007) Two B3 domain transcriptional repressors prevent sugar-inducible expression of seed maturation genes in Arabidopsis seedlings. Proc Natl Acad Sci USA 104 2543–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen JT, Fröhlich A, Ramírez-Aguilar SJ, Schauer N, Fernie AR, Erban A, Kopka J, Clark J, Langer A, Geigenberger P (2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Ann Bot (Lond) 103 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C, Locatelli F, Bracale M, Magnani E, Marsoni M, Osnato M, Mattana M, Baldoni E, Coraggio I (2004) Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J 37 115–127 [DOI] [PubMed] [Google Scholar]
- Varshney A, Altpeter F (2001) Stable transformation and tissue culture response in current European winter wheats (Triticum aestivum, L.). Mol Breed 8 295–309 [Google Scholar]
- Vertregt N, de Vries P (1987) A rapid method for determining the efficiency of biosynthesis of plant biomass. J Theor Biol 128 109–119 [Google Scholar]
- Wardlaw IF, Moncur L, Patrick JW (1995) The response of wheat to high temperature following anthesis. II. Sucrose accumulation and metabolism by isolated kernels. Aust J Plant Physiol 22 399–407 [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U (1997. a) A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in faba bean seeds. Plant Cell 9 895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1996) Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J 10 823–834 [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1997. b) Sugar import and metabolism during seed development. Trends Plant Sci 22 169–174 [Google Scholar]
- Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56 253–279 [DOI] [PubMed] [Google Scholar]
- Weber H, Heim U, Golombek S, Borisjuk L, Wobus U (1998) Assimilate uptake and the regulation of seed development. Seed Sci Res 8 331–345 [Google Scholar]
- Weigelt K, Küster H, Radchuk R, Müller M, Weichert H, Fait A, Fernie AR, Saalbach I, Weber H (2008) Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism and highlights the importance of mitochondrial metabolism. Plant J 55 909–926 [DOI] [PubMed] [Google Scholar]
- Weigelt K, Küster H, Rutten T, Fait A, Fernie AR, Miersch O, Wasternack C, Emery RJN, Desel C, Hosein F, et al (2009) ADP-glucose pyrophosphorylase deficient pea embryos reveal specific transcriptional and metabolic changes of carbon-nitrogen metabolism and stress responses. Plant Physiol 149 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterisation of two transporters and implications for seed development and starch accumulation. Plant J 21 455–467 [DOI] [PubMed] [Google Scholar]
- Wieser H (1997) Turbidimetrische Bestimmung einzelner Kleberproteintypen in Weizenmehl. Getreide Mehl und Brot 51 333–334 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.