Abstract
Several proteins of photosystem II (PSII) and its light-harvesting antenna (LHCII) are reversibly phosphorylated according to light quantity and quality. Nevertheless, the interdependence of protein phosphorylation, nonphotochemical quenching, and efficiency of electron transfer in the thylakoid membrane has remained elusive. These questions were addressed by investigating in parallel the wild type and the stn7, stn8, and stn7 stn8 kinase mutants of Arabidopsis (Arabidopsis thaliana), using the stn7 npq4, npq4, npq1, and pgr5 mutants as controls. Phosphorylation of PSII-LHCII proteins is strongly and dynamically regulated according to white light intensity. Yet, the changes in phosphorylation do not notably modify the relative excitation energy distribution between PSII and PSI, as typically occurs when phosphorylation is induced by “state 2” light that selectively excites PSII and induces the phosphorylation of both the PSII core and LHCII proteins. On the contrary, under low-light conditions, when excitation energy transfer from LHCII to reaction centers is efficient, the STN7-dependent LHCII protein phosphorylation guarantees a balanced distribution of excitation energy to both photosystems. The importance of this regulation diminishes at high light upon induction of thermal dissipation of excitation energy. Lack of the STN7 kinase, and thus the capacity for equal distribution of excitation energy to PSII and PSI, causes relative overexcitation of PSII under low light but not under high light, leading to disturbed maintenance of fluent electron flow under fluctuating light intensities. The physiological relevance of the STN7-dependent regulation is evidenced by severely stunted phenotypes of the stn7 and stn7 stn8 mutants under strongly fluctuating light conditions.
Several proteins of PSII and its light-harvesting antenna (LHCII) are reversibly phosphorylated by the STN7 and STN8 kinase-dependent pathways according to the intensity and quality of light (Bellafiore et al., 2005; Bonardi et al., 2005). The best-known phosphorylation-dependent phenomenon in the thylakoid membrane is the state transition: a regulatory mechanism that modulates the light-harvesting capacity between PSII and PSI. According to the traditional view, “state 1” prevails when plants are exposed to far-red light (state 1 light), which selectively excites PSI. Alternatively, thylakoids are in “state 2” when plants are exposed to blue or red light (state 2 light), favoring PSII excitation. In state 1, the yield of fluorescence from PSII is higher in comparison with state 2 (for review, see Allen and Forsberg, 2001). State transitions are dependent on the phosphorylation of LHCII proteins (Bellafiore et al., 2005) and their association with PSI proteins, particularly PSI-H (Lunde et al., 2000). Under state 2 light, both the PSII core and LHCII proteins are strongly phosphorylated, whereas the state 1 light induces dephosphorylation of both the PSII core and LHCII phosphoproteins (Piippo et al., 2006; Tikkanen et al., 2006). In nature, however, such extreme changes in light quality rarely occur. The intensity of light, on the contrary, fluctuates frequently in all natural habitats occupied by photosynthetic organisms, thus constantly modulating the extent of thylakoid protein phosphorylation in a highly dynamic manner (Tikkanen et al., 2008a).
The regulation of PSII-LHCII protein phosphorylation by the quantity of light is much more complex than the regulatory circuits induced by the state 1 and state 2 lights. Whereas changes in light quality induce a concurrent increase or decrease in the phosphorylation levels of both the PSII core (D1, D2, and CP43) and LHCII (Lhcb1 and Lhcb2) proteins, the changes in white light intensity may influence the kinetics of PSII core and LHCII protein phosphorylation in higher plant chloroplasts even in opposite directions (Tikkanen et al., 2008a). Indeed, it is well documented that low light (LL; i.e. lower than that generally experienced during growth) induces strong phosphorylation of LHCII but relatively weak phosphorylation of the PSII core proteins. Exposure of plants to high light (HL) intensities, on the contrary, promotes the phosphorylation of PSII core proteins but inhibits the activity of the LHCII kinase, leading to dephosphorylation of LHCII proteins (Rintamäki et al., 2000; Hou et al., 2003).
Thylakoid protein phosphorylation induces dynamic migrations of PSII-LHCII proteins along the thylakoid membrane (Bassi et al., 1988; Iwai et al., 2008) and modulation of thylakoid ultrastructure (Chuartzman et al., 2008). According to the traditional state transition theory, the phosphorylation of LHCII proteins decreases the antenna size of PSII and increases that of PSI, which is reflected as a quenched fluorescence emission from PSII. Alternatively, subsequent dephosphorylation of LHCII increases the antenna size of PSII and decreases that of PSI, which in turn is seen as increased PSII fluorescence (Bennett et al., 1980; Allen et al., 1981; Allen and Forsberg, 2001). This view was recently challenged based on studies with thylakoid membrane fractions, revealing that modulations in the relative distribution of excitation energy between PSII and PSI by LHCII phosphorylation specifically occur in the areas of grana margins, where both PSII and PSI function under the same antenna system, and the energy distribution between the photosystems is regulated via a more subtle mechanism than just the robust migration of phosphorylated LHCII (Tikkanen et al., 2008b). It has also been reported that most of the PSI reaction centers are located in the grana margins in a close vicinity to PSII-LHCII-rich grana thylakoids (Kaftan et al., 2002), providing a perfect framework for the regulation of excitation energy distribution from LHCII to both PSII and PSI.
When considering the natural light conditions, the HL intensities are the only known light conditions that in higher plant chloroplasts specifically dephosphorylate only the LHCII proteins but not the PSII core proteins. However, such light conditions do not lead to enhanced function of PSII. Instead, the HL conditions strongly down-regulate the function of PSII via nonphotochemical quenching of excitation energy (NPQ) and PSII photoinhibition (for review, see Niyogi, 1999). On the other hand, after dark acclimation of leaves and relaxation of NPQ, PSII functions much more efficiently when plants/leaves are transferred to LL despite strong phosphorylation of LHCII, as compared with the low phosphorylation state of LHCII upon transfer to HL conditions.
The delicate regulation of thylakoid protein phosphorylation in higher plant chloroplasts according to prevailing light intensity is difficult to integrate with the traditional theory of state transitions (i.e. the regulation of the absorption cross-section of PSII and PSI by reversible phosphorylation of LHCII). Moreover, besides LHCII proteins, reversible phosphorylation of the PSII core proteins may also play a role in dynamic light acclimation of plants. Recently, we demonstrated that the PSII core protein phosphorylation is a prerequisite for controlled turnover of the PSII reaction center protein D1 upon photodamage (Tikkanen et al., 2008a). This, however, does not exclude the possibility that the strict regulation of PSII core protein phosphorylation is also connected to the regulation of light harvesting and photosynthetic electron transfer. Moreover, the interactions between PSII and LHCII protein phosphorylation, nonphotochemical quenching, and cyclic electron flow around PSI in the regulation of photosynthetic electron transfer reactions remain poorly understood. To gain a deeper insight into such regulatory networks, we explored the effect of strongly fluctuating white light on chlorophyll (chl) fluorescence in Arabidopsis (Arabidopsis thaliana) mutants differentially deficient in PSII-LHCII protein phosphorylation and/or the regulatory systems of NPQ.
RESULTS
STN7 and STN8 Kinases Have Mutual Regulation Pathways
Figure 1 demonstrates how the phosphorylation patterns of the PSII core (D1, D2, and CP43) and LHCII proteins are dynamically regulated by the STN7 and STN8 kinases upon varying the light intensity. Wild-type and stn mutant plants were subjected to light treatments changing in intensity from LL (30 μmol photons m−2 s−1) to HL (1,000 μmol photons m−2 s−1) every 30 min for a total duration of 180 min. Phosphorylation level of the PSII core proteins (especially CP43) was low under LL in the wild type and the stn8 mutant lacking the STN8 kinase and having only the STN7 functional, whereas the LHCII protein phosphorylation was relatively strong. In contrast, always when HL was switched on, the phosphorylation of LHCII repeatedly decreased and the phosphorylation level of the PSII core proteins increased. This demonstrated the presence of two differently regulated STN7-dependent phosphorylation pathways, one specific for LHCII proteins and the other specific for CP43. The stn7 mutant, containing only a functional STN8, did not phosphorylate LHCII but phosphorylated the PSII core proteins more strongly than the wild type, especially under LL. This is in accordance with our previous finding indicating that, particularly under LL, the photosynthetic electron transfer chain (ETC) in stn7 is more reduced than in the wild type (Tikkanen et al., 2006). As mentioned above, in the stn8 mutant, the STN7 kinase strongly phosphorylated LHCII but also relatively well the PSII core proteins, especially CP43. However, the STN7 kinase in the stn8 mutant gradually lost its capacity for phosphorylation of the PSII core proteins in the course of the 180-min experiment, probably due to breakdown of the STN7 enzyme (Lemeille et al., 2009). In contrast, the capability of the STN8 kinase to phosphorylate the PSII core proteins in the stn7 mutant remained unaltered throughout the entire experiment under fluctuating light. It is worth noting that light conditions used in our experiments were not high enough (Pursiheimo et al., 2001) to induce the phosphorylation of minor PSII-LHCII proteins like CP29.
Figure 1.
Regulation of thylakoid protein phosphorylation by the STN7 and STN8 kinase pathways. A, Phosphorylation of thylakoid proteins in wild-type (WT) plants and the stn7, stn8, and stn7 stn8 mutants exposed to 30 and 1,000 μmol photons m−2 s−1 in 30-min intervals. B, Phosphorylation of thylakoid proteins in wild-type plants and the stn7, stn8, and stn7 stn8 mutants after 16 h of dark incubation in the absence (−) and presence (+) of 0.25 mm Glc. C, Phosphorylation of thylakoid proteins in the wild type after 1 h of treatment with far-red light (state 1) and red light (state 2). Phosphorylation of thylakoid proteins was determined by immunoblotting with P-Thr antibody, and 0.2 μg of chl was loaded in each well. P-CP43, P-D2, P-D1, and P-LHCII represent phosphorylated forms of the PSII core proteins CP43, D2, and D1 and the LHCII proteins Lhcb1 and Lhcb2. Representative data from three independent experiments are shown.
Feeding of leaves with Glc in darkness activated both the STN7 and STN8 kinases (Fig. 1B). Such metabolic manipulation of the kinase activities in darkness resulted in the wild type in a phosphorylation pattern that was comparable to that induced by the state 2 light (Fig. 1C), which strongly reduces the photosynthetic ETC but does not induce the feedback down-regulation of the STN7 kinase by overreduction of chloroplast stroma (Rintamäki et al., 2000). This experiment highlighted the fact that thylakoid protein phosphorylation is not only regulated by the intensity and quality of light. Under natural conditions, the phosphorylation status of the thylakoid proteins is set by cooperative regulation by the prevailing light conditions and the metabolic status of the chloroplast (Hou et al., 2003).
Effect of Thylakoid Protein Phosphorylation on Antenna Systems of PSII and PSI
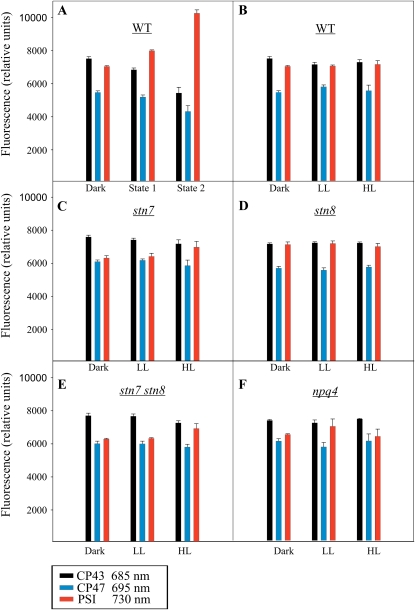
As discussed above, the modifications of PSII-LHCII protein phosphorylation in natural environments critically differ from those occurring upon the well-documented state 1-state 2 transitions induced by artificial light conditions. This prompted us to explore whether the changes in white light intensity, and the associated modifications in PSII-LHCII protein phosphorylation, are coupled to short-term changes in functional properties of the PSI and PSII antenna systems. To this end, we first applied state 1-state 2 lights to study the 77 K chl a fluorescence emission from PSII (CP43 and CP47) and PSI in wild-type plants. As reported earlier (Allen and Forsberg, 2001), the state 2 light, which strongly activates the phosphorylation of both the PSII core and LHCII proteins (Fig. 1C), strongly increased the relative fluorescence from PSI (Fig. 2A).
Figure 2.
The 77 K chl a fluorescence emitted from CP43 (685 nm), CP47 (695 nm), and PSI (730 nm). The 77 K chl a fluorescence was recorded in the wild type (WT; B), stn7 (C), stn8 (D), stn7 stn8 (E), and npq4 (F) after 16 h of dark incubation and subsequent 1-h exposure to 30 or 1,000 μmol photons m−2 s−1. Wild-type plants were also exposed to far-red light (state 1) and red light (state 2) for 1 h following the 16-h dark incubation (A). All thylakoid samples were prepared in the presence of 10 mm NaF and diluted to 10 μg chl mL−1 before the measurements. Data are means of three independent measurements, and error bars represent sd. [See online article for color version of this figure.]
In order to investigate the effects of white light-induced phosphorylation on the antenna systems of PSII and PSI, the dark-acclimated wild-type, stn7, stn8, stn7 stn8, and npq4 plants were subjected to LL and HL for 60 min and the 77 K chl a fluorescence emission from PSII and PSI was recorded from isolated thylakoids (Fig. 2). Opposite to the effects induced by state 1 and state 2 light conditions (Fig. 2A), the effects of different light intensities on the relative fluorescence emission from PSII and PSI in wild-type plants were only minor (Fig. 2B), even though distinct modulations in the phosphorylation of the PSII and LHCII proteins were recorded under these conditions (Fig. 1A; Aro and Ohad, 2003). As compared with the wild type (Fig. 2B), the stn7 single mutant and the stn7 stn8 double mutant showed slightly reduced relative fluorescence from PSI both after dark acclimation and after illumination with LL (Fig. 2, C and E). After exposure to HL, however, no differences from the wild type were recorded (Fig. 2, C and E). The stn8 mutant behaved like the wild type under all light intensities tested (Fig. 2D). Interestingly, the npq4 mutant (Fig. 2F) showed somewhat lower relative fluorescence from PSI than the wild type after treatment in the dark or under HL, whereas LL-exposed npq4 mutant did not differ from the wild type.
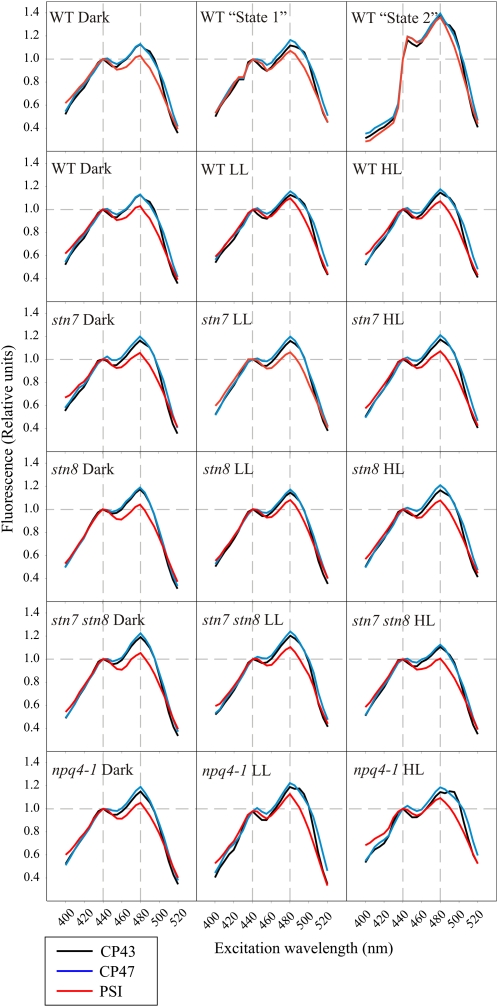
According to the state transition theory (Allen and Forsberg, 2001), illumination by state 2 light induces the migration of phosphorylated (P)-LHCII from PSII in the grana membranes to PSI in the stroma lamellae, which should be detectable by measuring the 77 K chl a fluorescence excitation spectra of PSII (CP43 and CP47) and PSI. Fluorescence excitation spectra give information about pigments associated with the excitation of PSII and PSI and reveal changes in the absorption cross-section of PSII and PSI due to rearrangements of the antenna system upon illumination of leaves with different light qualities and quantities. Accordingly, the phosphorylation of LHCII and migration into contact with PSI should be detected as enhanced relative fluorescence emission from PSI at wavelengths associated with light absorption by LHCII (chl b, 480 nm; carotenoids, <480 nm) as compared with the nonphosphorylated state, where only chl a with maximal absorption at 440 nm takes care of the excitation of PSI.
As expected, the state 1 light-acclimated wild-type leaves showed nearly similar 77 K chl a fluorescence excitation spectra of PSII (CP43 and CP47) and PSI as leaves acclimated to darkness (Fig. 3). State 2 light, in contrast, drastically changed the excitation spectra, and accordingly the absorption cross-sections, of both photosystems. Despite the fact that illumination under state 2 light clearly increased the relative excitation of PSI (i.e. the fluorescence emission from PSI; Fig. 2), the concurrent analysis of the PSI 77 K chl a fluorescence excitation spectrum revealed no specific enhancement in the excitation by wavelengths absorbed by chl b (around 480 nm) and thus the LHCII antenna system (Fig. 3). This result contradicts the idea that the increase in PSI 77 K chl a fluorescence emission was only due to a specific migration of P-LHCII from PSII to PSI (for review, see Allen and Forsberg, 2001). Noteworthy, the illumination by state 2 light changed in a similar way the 77 K chl a fluorescence excitation spectra of both photosystems, CP43 and CP47, as well as PSI (Fig. 3). This provides evidence that the migration of a specific LHCII antenna fraction from one reaction center into contact with the other cannot be the sole mechanistic basis for the red light-induced transition to state 2 (see “Discussion”).
Figure 3.
The 77 K chl a fluorescence excitation spectra of CP43 (685 nm), CP47 (695 nm), and PSI (730 nm) from the wild type (WT), stn7, stn8, stn7 stn8, and npq4 recorded after incubation of plants first for 16 h in darkness and then for 1 h under 30 (LL) or 1,000 (HL) μmol photons m−2 s−1. Wild-type plants were also exposed to far-red light (state 1) and red light (state 2) for 1 h after 16 h of dark incubation. All spectra have been normalized to the excitation by chl a at 440 nm. The peak at 480 nm represents the excitation of the fluorescence emitters by chl b, and the excitation by longer wavelengths represents the excitation by carotenoids. Plant treatments and thylakoid isolation were performed as in Figure 2. [See online article for color version of this figure.]
The 77 K chl a fluorescence of wild-type leaves or any of the mutants acclimated to different white light intensities for 1 h (dark, LL, and HL) did not reveal remarkable changes in the 77 K chl a fluorescence excitation spectra of either PSII or PSI (Fig. 3), despite drastic differences in PSII-LHCII protein phosphorylation levels (Fig. 1). Only a slight increase in the excitation of PSI by wavelengths associated with chlorophyll b (480 nm) absorption was observed at LL in the wild type and stn8 and npq4 mutants, but not in stn7 and stn7 stn8 mutants, indicating the dependence on LHCII phosphorylation (Fig. 3). The excitation properties of PSII and PSI in the npq4 mutant were similar to those in the wild type after treatment of leaves in darkness and LL. However, after HL treatment, the total fluorescence yield was dramatically reduced (data not shown), the shape of the spectrum became less defined, and high variation between the measurements was detected (data not shown). This indicates that excess light damages the structure and function of the light-harvesting machinery if the PsbS protein is missing (npq4 mutant).
Dynamics of Fluorescence Parameters Fm′ and Fs′ under Fluctuating Light in the Wild Type and in Mutants Differing in Thylakoid Protein Phosphorylation, NPQ, and Cyclic PSI Electron Transfer
In order to clarify the roles of LHCII and PSII core protein phosphorylation in dynamic acclimation of the photosynthetic electron transfer reactions, we subjected Arabidopsis wild-type and mutant leaves to changing light intensities. Under such conditions, the capability to dynamically adjust the light-harvesting and electron transfer properties in response to a given light intensity is required. During the experiment, the capability of the photosynthetic machinery for dynamic light acclimation was monitored by measuring the maximal and steady-state fluorescence in the light-acclimated state, denoted as Fm′ and Fs′, respectively. The yield of Fm′ was measured in order to elucidate the quenching state of PSII (NPQ), with high Fm′ indicating high efficiency of excitation energy transfer from LHC to photosystems. Fs′ was measured in order to estimate the redox state of the ETC, with low Fs′ indicating fluent electron flow from PSII to PSI and further to the downstream electron acceptors (Baker, 2008).
Measurements were conducted with the wild type and the stn mutants (stn7, stn8, and stn7 stn8) but also with several other mutants characterized by known disabilities in the light acclimation processes. The npq4 mutant was the most important control for the experiments. It lacks the feedback deexcitation of PSII (qE), which is the main component of NPQ (Niyogi et al., 2005). Another key control was the npq4 stn7 double mutant (Frenkel et al., 2007), which besides the feedback deexcitation also lacks LHCII phosphorylation and is thus deficient in regulatory events originating from cooperation between LHCII phosphorylation and the PsbS-dependent qE. npq1, the third NPQ mutant used here, lacks the deepoxidase enzyme responsible for the conversion reaction of violaxanthin to zeaxanthin (Niyogi et al., 1998) and was used to clarify the relation between the xanthophyll cycle and the other acclimation processes investigated. Regulation of light reactions by protein phosphorylation is based on sensing the redox state of the ETC chain (Aro and Ohad, 2003; Lemeille et al., 2009), whereas the regulation of qE is based on sensing the lumenal protonation (Niyogi, 1999). The development of lumenal protonation can be uncoupled from the water-splitting activity of PSII by cycling electrons around PSI. Such cyclic electron transfer (CET) provides a flexible mechanism to regulate the amount of NPQ independently of PSII function. For this reason, also the pgr5 (Munekage et al., 2002) mutant with impaired CET reactions was included in the measurements.
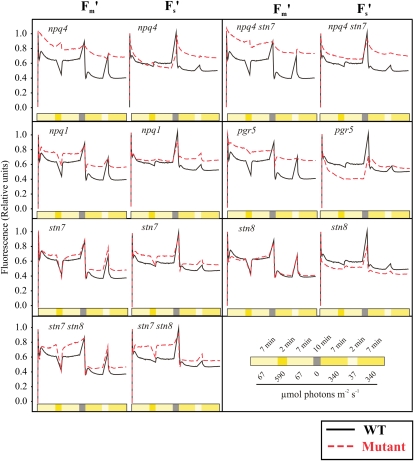
For the experiments, wild-type and mutant plants were grown under 100 μmol photons m−2 s−1 (growth light [GL]). The leaves were detached 2 h after onset of the photoperiod, placed in the measuring holder covered with wet tissue, and acclimated in darkness for 10 min. Thereafter, leaves were first subjected for 7 min to LL (67 μmol photons m−2 s−1), which was below the GL intensity and maintained efficient energy transfer from LHCII to fulfill the requirements of metabolic electron sinks. LL was followed by 2 min under HL (590 μmol photons m−2 s−1), inducing down-regulation of LHCII efficiency, and then again 7 min under LL, which restored the efficiency of LHCII in energy transfer to reaction centers. After this first illumination cycle, the leaves were returned to darkness for 10 min. The second illumination cycle consisted of moderately HL (340 μmol photons m−2 s−1) for 7 min, very LL (37 μmol photons m−2 s−1) for 2 min, and moderately HL again for 7 min to further elucidate the relationship between the efficiency of LHCII in energy transduction and the fluency of electron flow upon varying light intensities and time scales. Fm′ and Fs′, normalized to the initial Fm′ and Fs′ values, were recorded during the illumination cycles.
Wild-type plants demonstrated a highly flexible regulation of chl a fluorescence upon shifts in light intensity, in agreement with earlier reports (for reviews, see Baker, 2008; Eberhard et al., 2008). Briefly, the shift of wild-type leaves from darkness to moderately low actinic light resulted in an approximately 40% decrease in the yield of Fm′. This sudden drop was followed by about 20% restoration of Fm′, after which the Fm′ gradually evened out to the steady-state level of fluorescence characteristic of this light intensity. The following 2-min HL peak further quenched 30% from the remaining fluorescence. However, immediately after turning off the HL, Fm′ was restored to the level preceding the HL pulse. During the subsequent 10-min dark incubation, the Fm′ value recovered to the initial level. After the dark period, when moderately high actinic light (340 μmol photons m−2 s−1) was turned on, the yield of Fm′ was quenched with similar kinetics detected upon the first moderately LL phase of the experiment (67 μmol photons m−2 s−1), but due to a higher light intensity, the fluorescence yield stabilized to a somewhat lower level. During a subsequent 2-min pulse of very LL (37 μmol photons m−2 s−1), Fm′ was mostly restored, but when the moderately HL was turned on, it dropped again to the same level recorded earlier at this light intensity. Fs′ remained fairly constant throughout the illumination cycles of wild-type leaves. Collectively, these experiments demonstrated that the wild type maintained fluent electron flow from PSII to downstream metabolism under fluctuating light intensities, likely due to intact mechanisms to control thermal dissipation of excess energy and the excitation energy distribution between PSII and PSI.
Comparison of the npq4 mutant with the wild type confirmed that the dynamic regulation of Fm′ is primarily dependent on the PsbS protein-dependent qE (Li et al., 2002). The npq4 mutant was nearly inert and did not show dynamic regulation of Fm′. However, despite the incapability for rapid changes in Fm′, the npq4 mutant could gradually decrease the Fm′ during the experiment. This slow induction of quenching was not related to state transition (qT), because the npq4 stn7 double mutant did not differ from the npq4 single mutant in this respect. The yield of Fm′ was similar in npq4 and npq4 stn7 throughout the entire experiment under fluctuating light. This demonstrates that in the absence of the PsbS protein-dependent feedback deexcitation, the phosphorylation of LHCII did not have any effect on the quenching properties of Fm′ (NPQ). Unlike the npq4 mutant, the npq1 mutant showed a wild-type pattern in the regulation of Fm′, although the amplitudes of the changes in fluorescence yield were diminished in the mutant as compared with the wild type (Fig. 4). This was a clear indication that the xanthophyll cycle is not required to induce the PsbS-dependent quenching but that it strongly modulates the strength of thermal dissipation, as was shown recently (Kalituho et al., 2007).
Figure 4.
Chl a fluorescence as a response to changing light intensity. Intact leaves from wild-type (WT), stn7, stn8, and stn7 stn8 plants and npq4, npq4 stn7, npq1, and pgr5 control plants grown under 120 μmol photons m−2 s−1 were detached and placed in a leaf holder 2 to 4 h after GL was turned on in the morning. The measurements were started after 10 min of dark incubation by turning the light on, and the fluorescence parameters Fs′ and Fm′ were monitored during illumination of leaves with programmed changes in light intensity as indicated. Value 1 was given to the first measuring points of Fm′ and Fs′, and all other values were normalized against this initial value. Fluorescence curves were highly reproducible both for wild-type and mutant plants. Representative curves from at least 10 measurements are shown for the wild type and the mutants. [See online article for color version of this figure.]
The npq4 mutant maintained Fs′ at a similar level as the wild type when light intensity did not exceed the GL intensity, thus maintaining fluent electron flow. Moreover, when light intensity increased from 67 to 590 μmol photons m−2 s−1 for 2 min, the npq4 mutant could maintain Fs′ stable, indicating no disturbance in electron flow. However, when leaves were shifted from darkness directly to light intensity above the GL, the npq4 mutant could not maintain Fs′ at a similar level as the wild type. Because Fs′ remained at an equal level in the wild type and npq4 under LL conditions, and neither did the short shifts in light intensity affect the level of Fs′ in the npq4 mutant, it is conceivable that the light-harvesting efficiency of both photosystems is similarly effected by qE, and Fs′ increased only when electron flow was limited by electron acceptors after PSI. Also, the npq1 mutant had difficulties maintaining Fs′ at a similarly stable level as the wild type upon prolonged exposure to light intensity above the GL. This emphasizes the importance under excess light of both the PsbS protein-dependent and the xanthophyll cycle-dependent mechanisms in regulating the efficiency of LHCII to maintain fluent electron flow from light reaction to downstream metabolism.
The stn7, stn8, and stn7 stn8 kinase mutants were all capable of efficiently quenching Fm′ when the light intensity suddenly increased (Fig. 4). The initial quenching of Fm′ was even stronger in the stn mutants than in the wild type. However, despite rapid and efficient quenching of Fm′ in all the kinase mutants, the stn7 and stn7 stn8 mutants had severe difficulties in maintaining the low Fm′ level after the rapid induction phase of Fm′ quenching. Intriguingly, the rapid and strong quenching of Fm′ coupled to the incapability of maintaining the induced quenching level severely amplified the difference between the low and high quenching states of LHCII in the stn7 and stn7 stn8 mutants as compared with the wild type and stn8.
Upon the initial induction phase of Fm′ quenching (NPQ), the wild type and stn7, stn8, and stn7 stn8 mutants showed equal levels of Fs′. However, immediately when Fm′ became partially recovered, the stn7 mutant and even more clearly the stn7 stn8 mutant showed distinctly higher Fs′ as compared with the wild type and stn8. This demonstrated that the lack of STN7-dependent PSII-LHCII protein phosphorylation led to a redox imbalance of ETC, especially under LL when NPQ was low and the excitation energy transfer from LHCII to photosystems was highly efficient. Thus, the importance of the LHCII protein phosphorylation-dependent regulation of excitation energy distribution to the two photosystems becomes manifested under low NPQ and, accordingly, the high efficiency state of LHC.
The impact of cyclic electron flow on the regulation of Fm′ and Fs′ was tested with the pgr5 mutant (Munekage et al., 2002; DalCorso et al., 2008), which has reduced capacity to perform cyclic electron flow around PSI as compared with the wild type. Even though the exact role of the PGR5 protein in cyclic electron flow is not fully understood, the use of the pgr5 mutant was expected to provide important information concerning the roles of multiple electron transfer routes in the regulation of PSII via NPQ and thylakoid protein phosphorylation. Interestingly, the pgr5 mutant was nearly as inert as npq4 in performing rapid changes in Fm′ levels in fluctuating light. However, despite the enhanced efficiency of LHCII (high Fm′) under LL, the level of Fs′ in pgr5 was low, indicating oxidized ETC as compared with the wild type under limiting light intensities. Even under HL, the induction of NPQ in pgr5 was very low, but in contrast to LL Fs′ stabilized at a higher level in pgr5 as compared with the wild type. These experiments indicated that under LL, the wild-type plants required the PGR5-dependent CET to reduce intersystem ETC. However, when the light intensity increased, PGR5-dependent activity was required for the development of NPQ, thus decreasing the rate of electron flow.
Phosphorylation-Dependent Regulation of Electron Transfer Has a Great Impact on Plant Growth under Fluctuating Light
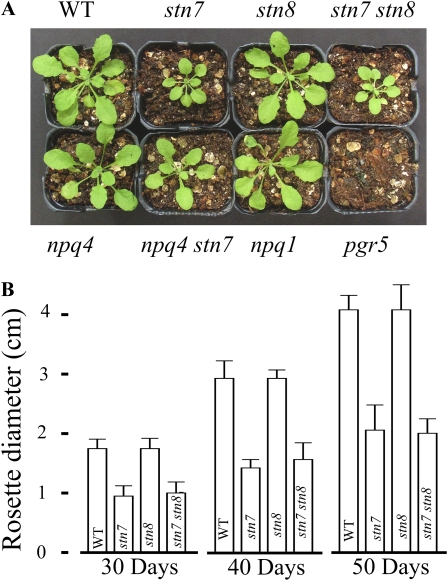
None of the mutants used here showed any visible growth phenotype under constant LL, moderate light, or HL conditions (data not shown). To test whether the misregulation of excitation energy distribution and electron transfer, as observed for some mutants in the short-term fluctuating light experiments (Fig. 4), has any specific impact on the phenotype when plants are grown under fluctuating light intensity, we placed wild-type plants and stn7, stn8, stn7 stn8, npq4, npq4 stn7, npq1, and pgr5 mutant plants to grow under LL, which was interrupted by frequent HL peaks. The purpose of using such a growth condition was to maximally challenge the dynamic regulation of the excitation energy distribution and electron transfer reactions and to relate the impact of these capacities on the growth of plants. The stn7 and stn7 stn8 plants grew very slowly as compared with the wild type, and pgr5 showed a lethal phenotype (Fig. 5). In contrast, the stn8, npq4, and npq1 mutants did not show any visible phenotype, and stn7 npq4 showed a less profound phenotype than stn7 (Fig. 5). As shown in Figure 4, short interruptions of LL illumination phases with HL pulses strongly disturb the redox balance of ETC in stn7 and stn7 stn8 but not in stn8, npq4, and npq1 mutants, providing evidence that the regulation of excitation energy distribution by the STN7-dependent phosphorylation cascade has a vital role for plant growth under fluctuating light. The pgr5 mutant showed severe difficulties in maintaining redox balance of electron transfer reactions, yet the influence of short HL pulses was not apparent. Nevertheless, the pgr5 mutant had a lethal phenotype under fluctuating light, suggesting that the genotype probably has more influence on the stromal redox state than on the redox state of ETC.
Figure 5.
A, Visual phenotype of wild-type (WT) plants and stn7, stn8, stn7 stn8, npq4, stn7 npq4, npq1, and pgr5 mutant plants grown under fluctuating light for 50 d. B, Rosette diameter of wild-type, stn7, stn8, and stn7 stn8 plants measured 30, 40, and 50 d after sowing. Data are means of five plants, and error bars represent sd. Similar differences in growth rates of wild-type and mutant plants were obtained also in two other growth experiments. Five minutes of moderate LL (50 μmol photons m−2 s−1) and 1 min of NPQ-inducing HL (500 μmol photons m−2 s−1) were continuously following each other during the light period of 12 h followed by a 12-h dark period. [See online article for color version of this figure.]
DISCUSSION
Reversible PSII-LHCII Protein Phosphorylation Induced by White Light of Different Intensity Does Not Result in State Transitions
Photosynthetic organisms cope with constantly changing light conditions via highly flexible regulation mechanisms that adjust the light absorption and photosynthetic processes according to metabolic and environmental cues. On the one hand, plants have mechanisms to enhance light harvesting when photosynthesis and growth are limited by the availability of light. On the other hand, when the light intensity exceeds the capacity by which the reducing energy can be consumed by metabolic reactions, the excess excitation energy becomes dissipated as heat via NPQ. Moreover, even though the balancing of the light-harvesting capacity between PSII and PSI via state transitions has long been recognized as a regulatory mechanism to optimize the relative function of PSII and PSI (Allen and Forsberg, 2001; Haldrup et al., 2001; Rochaix, 2007; Kargul and Barber, 2008), its biological significance under different natural light conditions has remained controversial. The mechanistic basis of state transitions is not fully understood, yet recent data (Tikkanen et al., 2008b) indicated that regulation takes place in grana margins, where the majority of PSI reaction centers are located in close vicinity to PSII-LHCII-rich grana thylakoids (Kaftan et al., 2002).
In early studies, the induction of state transitions by differential excitation of the two photosystems was found to correlate with changes in the level of LHCII protein phosphorylation (Bennett et al., 1980; Allen et al., 1981). More recently, studies with the stt7 kinase mutant of Chlamydomonas reinhardtii and the stn7 kinase mutant of Arabidopsis demonstrated that LHCII phosphorylation indeed is a prerequisite for state transitions to occur (Depege et al., 2003; Bellafiore et al., 2005). These classical state transition studies, however, are based on selective excitation of the two reaction centers by different qualities of light and therefore are hard to integrate with studies on the modulation of light harvesting and excitation of the two photosystems under natural environments, where thylakoid protein phosphorylation is dually controlled by the intensity of light and the downstream metabolic reactions. Indeed, we found no correlation between white light-induced modulations in the level of LHCII protein phosphorylation and the extent of state transition (Figs. 1 and 2).
While the state 1 and state 2 lights have similar effects on the phosphorylation of both the PSII core and LHCII proteins, either decreasing or enhancing the phosphorylation of both of them at the same time (Piippo et al., 2006; Tikkanen et al., 2006), the changes in light intensity sometimes have completely opposite effects on the PSII core and LHCII protein phosphorylation (Fig. 1), also suggesting that one phosphorylation pathway may negatively regulate the other. Under white light, the strongest level of LHCII protein phosphorylation is typically observed upon a shift of plants from normal GL to LL conditions (Pursiheimo et al., 2001). On the contrary, when the light absorption by pigments exceeds the capacity of carbon assimilation to utilize converted light energy, the stromal side of PSI becomes overreduced and induces the down-regulation of LHCII phosphorylation at the time when the PSII core protein phosphorylation reaches maximal intensity (Rintamäki et al., 2000; see model in Fig. 6). Moreover, the stromal overreduction not only inactivates the STN7 kinase but also accelerates the degradation of the enzyme (Lemeille et al., 2009), thus providing an additional level of regulation in light harvesting.
Figure 6.
Model depicting the differential roles of PSII-LHCII protein phosphorylation in the regulation of excitation energy distribution between PSII and PSI. Such regulation mostly occurs in grana margins where PSII and PSI are in close proximity (Tikkanen et al., 2008a) and depends on light conditions used for the induction of LHCII phosphorylation. A, Under LL, the PsbS protein is deprotonated and the xanthophyll cycle is inactivated, allowing efficient transfer of excitation energy from LHCII to PSII and PSI. B, Under such conditions, the STN7-dependent high LHCII and low PSII core protein phosphorylation are prerequisites for balanced excitation energy distribution to PSII and PSI, and the lack of the STN7 kinase leads to overexcitation of PSII as compared with PSI. C, Upon increase in light intensity, PsbS gets protonated and the xanthophyll cycle is activated, leading to efficient thermal dissipation of excitation energy. LHCII is dephosphorylated under such a low efficiency state of LHCII, and no STN7 kinase-dependent mechanism to maintain excitation balance between PSII and PSI is required due to the fact that the majority of the absorbed excitation energy becomes dissipated as heat. D, Under traditional state 1 light (far red), both the PSII core and LHCII proteins are dephosphorylated, PSII excitation by LHCII is favored, and thermal dissipation of excitation energy is at a minimum. Nevertheless, in natural conditions under the canopy, where far-red light is enriched, the excitation of PSII and PSI is in balance due to the fact that PSI absorbs far-red light more efficiently than PSII. hv, Light energy. E, Shift to state 2 light (red) induces strong phosphorylation of both the PSII core and LHCII proteins, yet the efficiency of LHCII is as high as under state 1 light (low NPQ). This unnatural state with strong overall phosphorylation of both the PSII core and LHCII proteins combined with high-efficiency LHCII leads to very efficient excitation energy transfer from LHCII to PSI and thus to a transfer to state 2, where PSI excitation is strongly favored over PSII.
Despite of strong fluctuations in phosphorylation of the PSII core and LHCII proteins with respect to changes in light intensity (Fig. 1), the relative 77 K chl a fluorescence emission (Fig. 2) and the 77 K chl a fluorescence excitation spectra (Fig. 3) of PSII and PSI remained nearly unaffected, implying no induction of state transitions. In the stn7 and stn7 stn8 mutants, the relative 77 K chl a fluorescence from PSI as compared with the wild type was slightly reduced under LL, yet the normalized 77 K chl a fluorescence excitation spectra remained quite similar to that in the wild type. These findings demonstrate that in the wild type, when the proper control of the PSII-LHCII protein phosphorylation is present, the relative distribution of excitation energy between the two photosystems is quantitatively rather similar in intact thylakoid systems under any natural light condition with or without phosphorylation of LHCII. However, the abnormal phosphorylation of the PSII-LHCII proteins in the stn7 and stn7 stn8 mutants decreases the excitation energy flow from the entire antenna system, covering a wide range of excitation wavelengths, to the PSI reaction center. Thus, the fluctuations in phosphorylation of the PSII-LHCII proteins, as induced by changes in light intensity, do not remarkably change the relative excitation of PSII and PSI but more likely function to maintain equal excitation of both reaction centers.
Role of the STN7 Kinase in the Regulatory Network of Excitation Energy Distribution and Electron Transfer Reactions
Using the stn kinase mutants, we demonstrate here that under moderate light intensities, plants adjust NPQ to slightly different levels depending on the phosphorylation status of the PSII-LHCII proteins (Fig. 4). Thus, the phosphorylation is possibly involved in regulation of the extent of excitation energy donated from the light-harvesting antenna to both reaction center complexes. Under natural fluctuating light environments, the efficiency of light reactions needs nonstop regulation in order to keep the balance with the needs and restrictions of downstream metabolism. This can occur either by regulation of light absorption efficiency or by regulation of the efficiency by which the absorbed light energy is used in photosynthesis. The PsbS protein-dependent NPQ gives the mechanistic framework for regulation of the efficiency to use the absorbed light energy (Niyogi, 1999).
Using the stn and npq mutants, we addressed the question of how the PSII-LHCII protein phosphorylation-dependent balancing of excitation energy distribution between the reaction centers cooperates with the PsbS-dependent quenching process (Fig. 6). Under LL, the stn7 mutant has a high-light-mimicking phosphorylation pattern of LHCII, which leads to a situation where PSII excitation is favored over PSI excitation (Fig. 6B) and plants exhibit a strongly reduced intersystem ETC (Fs′ in Fig. 4; Bellafiore et al., 2005; Tikkanen et al., 2006). In contrast to the stn7 mutant, the stn8 mutant strongly phosphorylates LHCII, weakly phosphorylates the PSII core proteins (Fig. 1), and has slightly less reduced ETC (Fs′) as compared with the wild type (Fig. 4). Despite the opposing behavior of the stn single mutants, the stn7 stn8 double mutant resembled the stn7 mutant, but the incapability of maintaining the redox balance was even more obvious, thus stressing the importance of both kinase pathways in the regulation of electron transfer reactions.
The STN7-dependent regulation to balance the excitation energy distribution between PSII and PSI is required particularly under LL intensities, when thermal dissipation of excitation energy is low and LHCII transfers excitation energy efficiently to reaction centers (Fig. 6, A and B). When light intensity increases and concomitantly the efficiency of LHCII becomes down-regulated via NPQ, the STN7 kinase-dependent regulation loses its importance in regulation of excitation energy distribution (Fig. 6C). In the stn7 npq4 mutant, incapable of down-regulating the antenna efficiency, the stn7 mutation-induced excitation imbalance is not dependent on light intensity and exists also at HL conditions.
The absence of NPQ, as such, did not affect the redox balance of ETC under LL or upon short HL pulses, indicating that NPQ modulates the efficiency of both photosystems to the same extent and does not affect the relative distribution of excitation energy between PSII and PSI. However, when the exposure of npq4 to HL intensity is prolonged, the capacity of metabolic sinks to accept electrons becomes crucial for electron flow and the situation of overreduction of ETC is easily attained (Fig. 4). This brings up a question about the “unbalancing” force that makes the STN7 kinase-dependent regulation so necessary in maintaining the balance of equal excitation energy distribution to both photosystems under low and moderate photon fluence rates. It is well known that the distribution of excitation energy between PSII and PSI changes depending on salt concentration, which also modulates the stacking-unstacking-restacking dynamics of the thylakoid membrane (Murata, 1969, 1971). Changes in grana width and extent of stacking also occur depending on light conditions and are closely related to the kinetics of PSII core and LHCII protein phosphorylation (Rozak et al., 2002; Chuartzman et al., 2008), yet the physiological significance of such a relationship has remained elusive. It is conceivable that the changes in granal stacking in chloroplasts regulate the light absorption capacity of the thylakoid membrane according to the light intensity. As an irrevocable consequence of such a modulation of thylakoid ultrastructure, the lateral segregation of PSII, LHCII, and PSI also changes and affects the excitation balance between PSII and PSI. This is reflected in the redox state of the electron transfer components, which in turn regulates the phosphorylation of the PSII-LHCII proteins (Vener et al., 1998; Zito et al., 1999; Aro and Ohad, 2003). It is likely that the dynamic regulation of PSII core and LHCII protein phosphorylation by the STN7 kinase facilitates the movements of PSII, LHCII, and PSI-LHCI complexes along each other and the thylakoid membrane, enabling the maintenance of excitation balance between the two reaction centers upon any white light-induced configuration of the thylakoid ultrastructure.
Similar to the stn7 single mutant and stn7 stn8 double mutant, the pgr5 mutant with impaired cyclic electron flow (Munekage et al., 2002; DalCorso et al., 2008) could not maintain the wild-type level of NPQ. However, besides low NPQ, the pgr5 mutant also had very low Fs′, indicating oxidized ETC as compared with the wild type. In contrast to this, stn7 and stn7 stn8 had very high Fs′, indicating strongly reduced ETC. Thus, these data suggest that the stn kinase mutants may not be impaired in PGR5-dependent cyclic electron flow; therefore, the phosphorylation-dependent modulation of NPQ is not likely to occur by CET-dependent lumenal protonation but rather via regulation of linear electron transfer or some other mechanism. Common for both the stn7 and pgr5 mutants is a severe difficulty in managing the electron transfer under fluctuating light intensity (Fig. 4), and both mutants also show strongly retarded growth under fluctuating light, pgr5 even showing a lethal phenotype. Slow growth of the stn7 and stn7 stn8 mutants indicates that the regulation of electron transfer via the STN7-dependent mechanism is important for plant growth under fluctuating light. Proper distribution of excitation energy from LHCII to both photosystems seems to have more drastic effects on plant growth than the regulation of light-harvesting efficiency of LHCII via NPQ. Indeed, the growth of npq4 and npq1 was not retarded under fluctuating light. The drastic phenotype of pgr5 is evidently not related to its inability to induce NPQ.
CONCLUSION
Plants grown under constant light conditions have a high capacity to compensate the consequences of mutations in genes encoding the proteins required for proper acclimation to different light intensities. Under continuously fluctuating light, however, the straightforward acclimation is not possible for mutants that lack the protein(s) responsible for dynamic regulation of the light-harvesting system and energy distribution between the two photosystems. The stn7 mutant lacking the STN7 LHCII kinase belongs to such mutants and has a strongly stunted phenotype only when grown under strongly fluctuating light intensity.
It is well known that LHCII phosphorylation changes the relative excitation of the two reaction centers in the traditional state transition experiment involving different qualities of light to induce reversible LHCII phosphorylation. It is conceivable that in tandem with LHCII protein phosphorylation, the PSII core protein phosphorylation also takes part in this process (Fig. 6). Phosphorylation of LHCII and PSII is also dynamically regulated according to light intensity. However, the physiological role of such “white light phosphorylation” differs from traditional state transitions. We have demonstrated here that although the white light-induced changes in thylakoid protein phosphorylation are strong, they do not change the relative excitation of PSII and PSI but instead guarantee equal excitation of both photosystems under varying light intensities. In line with this observation, the lack of LHCII phosphorylation in the stn7 and stn7 stn8 mutants leads to overexcitation of PSII.
Efficient electron transfer reactions require a balanced excitation energy distribution between PSII and PSI, particularly in grana margin regions. Our results provide evidence that the STN7-dependent PSII-LHCII protein phosphorylation functions in balancing the excitation energy distribution from LHCII to PSII and PSI, thus ensuring the functional stoichiometry between the photosynthetic protein complexes, especially under LL when NPQ is low and LHCII efficiently transfers energy to photosystems. Thermal dissipation of excitation energy (NPQ), as such, does not affect the excitation balance between PSII and PSI. However, in the absence of the STN7 kinase, the high-efficiency LHCII (low NPQ) overexcites PSII relative to PSI, leading to strong reduction of ETC. Due to such a domination of PSII excitation by LHCII in stn7, changes in LHCII efficiency (NPQ) according to light intensity also predominantly affect the function of PSII. Thus, strongly fluctuating light that constantly changes the efficiency of LHCII (NPQ) leads to drastic fluctuations in the redox state of ETC when the STN7 kinase is missing, causing the stunted growth of the stn7 mutant.
MATERIALS AND METHODS
Growth of Plants and Short-Term Light and Glc Treatments
Wild-type Arabidopsis (Arabidopsis thaliana) ecotype Columbia and the stn7 (Tikkanen et al., 2006), stn8 (Vainonen et al., 2005), stn7 stn8 (Bonardi et al., 2005), npq4 stn7 (Frenkel et al., 2007), npq4 npq1 (Breitholtz et al., 2005), and pgr5 (Munekage et al., 2002) mutant plants were grown in a Phytotron under 120 μmol photons m−2 s−1, 8-h photoperiod, and relative humidity of 70%. OSRAM PowerStar HQIT 400/D Metal Halide Lamps served as a light source both upon plant growth and in HL and LL treatments. For the growth of plants under fluctuating light, an electronically controlled shading system was used. Five minutes of moderate LL (50 μmol photons m−2 s−1) and 1 min of HL (500 μmol photons m−2 s−1) were continuously following each other during the light period of 12 h followed by a 12-h dark period. In HL and LL treatments, leaves were placed in a temperature-controlled chamber at 23°C and light was passed through a heat filter. Mature rosette leaves from 4- to 6-week-old plants were used for experiments.
Wild-type plants were exposed to light favoring the excitation of PSII (state 2 light) and that of PSI (state 1 light) to ensure the maximal phosphorylation and dephosphorylation of thylakoid proteins, respectively. A fluorescent tube (GroLux F58W/GROT8; Sylvania) covered with an orange filter (Lee 105; Lee Filters) served as state 2 light, and state 1 light was obtained from halogen lamps (500 W) covered with an orange filter (Lee 105; Lee Filters) and a median blue filter (Roscolux 83; Rosco Europe). Temperature was maintained at 23°C by a water-cooled glass chamber between the fluorescence tube and the plants.
Glc (0.25 m) treatment was given through cut petioles overnight in darkness.
SDS-PAGE and Immunoblotting
Proteins were separated on 15% polyacrylamide gels with 6 m urea. The content of chl was determined according to Porra et al. (1989). Samples equivalent to 0.2 μg of chl were loaded in each well. After electrophoresis, the polypeptides were transferred to an Immobilon-P membrane (Millipore), and the membrane was blocked with 5% fatty acid-free bovine serum albumin (Sigma-Aldrich). Western blotting was performed with standard techniques (Vainonen et al., 2009) using D1-, D2-, CP43-, Lhcb1-, and Lhcb2-specific antibodies and P-Thr antibodies (New England Biolabs), and for visualization, the Phototope-Star Chemiluminescent kit was used (New England Biolabs). PSII-LHCII phosphoproteins D1, D2, CP43, Lhcb1, and Lhcb2 were identified both by protein-specific antibodies and by mass spectrometry as described earlier (Tikkanen et al., 2006; Vainonen et al., 2009).
Chl Fluorescence Measurements
The 77 K chl a fluorescence emission and fluorescence excitation spectra were determined as described (Tikkanen et al., 2008b). Briefly, plants were light treated, and immediately after treatments the leaves were frozen in liquid nitrogen. Thylakoids were isolated and diluted in buffer containing 50 mm HEPES/KOH, pH 7.5, 100 mm sorbitol, 10 mm MgCl2, and 10 mm NaF to a chl concentration of 10 μg mL−1 and immediately subjected to measurements of 77 K chl a fluorescence emission spectra using a diode array spectrophotometer (S2000; Ocean Optics) equipped with a reflectance probe. To record the 77 K fluorescence emission curves, the samples were excited with white light below 500 nm, defined by LS500S and LS700S filters (Corion). The emission between 600 and 800 nm was recorded, and the amplitudes of 685-, 695-, and 730-nm chl a fluorescence emission peaks were measured for Figure 2. To obtain the 77 K chl a fluorescence excitation spectra, the samples were excited with wavelengths from 400 to 540 nm with 5-nm steps using f/3.4 Monochromator (Applied Photophysics).
Chl fluorescence parameters Fm′ and Fs′ were determined from intact leaves exposed to actinic light of 67, 590, 340, and 37 μmol photons m−2 s−1 provided by green light-emitting diodes using the JTS-10 spectrometer (Bio-Logic SAS). Fm′ was induced by a 0.25-s saturating flash (7,900 μmol m−2 s−1) of green light. Value 1 was given to the first measuring points of Fm′ and Fs′, and all other values were normalized against this initial value. Fluorescence curves were recorded from at least seven leaves of each mutant and wild-type plant with high repeatability.
Acknowledgments
We thank Prof. Krishna Niyogi for the kind gift of npq4 and npq1 mutants, Prof. Jean-David Rochaix for the stn7 npq4 mutant, Prof. Dario Leister for the stn7 stn8 mutant, Prof. Toshiharu Shikanai for the pgr5 mutant, and Prof. Alexander Vener for the stn8 mutant. Mika Keränen is acknowledged for valuable help in data processing of fluorescence curves.
This work was supported by the Academy of Finland (project no. 118637), the Finnish Graduate School in Plant Biology, and the European Union (FP7 Marie Curie Initial Training Network project no. 215174).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Eva-Mari Aro (evaaro@utu.fi).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Allen JF, Bennett J, Steinback KE, Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291 25–29 [Google Scholar]
- Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6 317–326 [DOI] [PubMed] [Google Scholar]
- Aro EM, Ohad I (2003) Redox regulation of thylakoid protein phosphorylation. Antioxid Redox Signal 5 55–67 [DOI] [PubMed] [Google Scholar]
- Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59 89–113 [DOI] [PubMed] [Google Scholar]
- Bassi R, Giacometti GM, Simpson DJ (1988) Changes in the organization of stroma membranes induced by in vivo state I-state 2 transition. Biochim Biophys Acta 935 152–165 [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433 892–895 [DOI] [PubMed] [Google Scholar]
- Bennett J, Steinback KE, Arntzen CJ (1980) Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci USA 77 5253–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437 1179–1182 [DOI] [PubMed] [Google Scholar]
- Breitholtz HL, Srivastava R, Tyystjärvi E, Rintamäki E (2005) LHC II protein phosphorylation in leaves of Arabidopsis thaliana mutants deficient in non-photochemical quenching. Photosynth Res 84 217–223 [DOI] [PubMed] [Google Scholar]
- Chuartzman SG, Nevo R, Shimoni E, Charuvi D, Kiss V, Ohad I, Brumfeld V, Reich Z (2008) Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20 1029–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schunemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132 273–285 [DOI] [PubMed] [Google Scholar]
- Depege N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299 1572–1575 [DOI] [PubMed] [Google Scholar]
- Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42 463–515 [DOI] [PubMed] [Google Scholar]
- Frenkel M, Bellafiore S, Rochaix J, Jansson S (2007) Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol Plant 129 455 [Google Scholar]
- Haldrup A, Jensen PE, Lunde C, Scheller HV (2001) Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci 6 301–305 [DOI] [PubMed] [Google Scholar]
- Hou CX, Rintamäki E, Aro EM (2003) Ascorbate-mediated LHCII protein phosphorylation: LHCII kinase regulation in light and in darkness. Biochemistry 42 5828–5836 [DOI] [PubMed] [Google Scholar]
- Iwai M, Takahashi Y, Minagawa J (2008) Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii. Plant Cell 20 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftan D, Brumfeld V, Nevo R, Scherz A, Reich Z (2002) From chloroplasts to photosystems: in situ scanning force microscopy on intact thylakoid membranes. EMBO J 21 6146–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalituho L, Beran KC, Jahns P (2007) The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol 143 1861–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargul J, Barber J (2008) Photosynthetic acclimation: structural reorganisation of light harvesting antenna. Role of redox-dependent phosphorylation of major and minor chlorophyll a/b binding proteins. FEBS J 275 1056–1068 [DOI] [PubMed] [Google Scholar]
- Lemeille S, Willig A, Depege-Fargeix N, Delessert C, Bassi R, Rochaix JD (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Niyogi KK (2002) Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent nonphotochemical quenching in photosystem II. J Biol Chem 277 33590–33597 [DOI] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408 613–615 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110 361–371 [DOI] [PubMed] [Google Scholar]
- Murata N (1969) Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta 189 171–181 [DOI] [PubMed] [Google Scholar]
- Murata N (1971) Control of excitation transfer in photosynthesis. V. Correlation of membrane structure to regulation of excitation transfer between two pigment systems in isolated spinach chloroplasts. Biochim Biophys Acta 245 365–372 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Li XP, Rosenberg V, Jung HS (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56 375–382 [DOI] [PubMed] [Google Scholar]
- Piippo M, Allahverdiyeva Y, Paakkarinen V, Suoranta UM, Battchikova N, Aro EM (2006) Chloroplast-mediated regulation of nuclear genes in Arabidopsis thaliana in the absence of light stress. Physiol Genomics 25 142–152 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Pursiheimo S, Mulo P, Rintamäki E, Aro EM (2001) Coregulation of light-harvesting complex II phosphorylation and lhcb mRNA accumulation in winter rye. Plant J 26 317–327 [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix JD (2007) Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett 581 2768–2775 [DOI] [PubMed] [Google Scholar]
- Rozak PR, Seiser RM, Wacholtz WF, Wise RR (2002) Rapid, reversible alterations in spinach thylakoid appression upon changes in light intensity. Plant Cell Environ 25 421 [Google Scholar]
- Tikkanen M, Nurmi M, Kangasjärvi S, Aro EM (2008. a) Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim Biophys Acta 1777 1432–1437 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Nurmi M, Suorsa M, Danielsson R, Mamedov F, Styring S, Aro EM (2008. b) Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim Biophys Acta 1777 425–432 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Piippo M, Suorsa M, Sirpio S, Mulo P, Vainonen J, Vener AV, Allahverdiyeva Y, Aro EM (2006) State transitions revisited: a buffering system for dynamic low light acclimation of Arabidopsis. Plant Mol Biol 62 779–793 [DOI] [PubMed] [Google Scholar]
- Vainonen JP, Hansson M, Vener AV (2005) STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J Biol Chem 280 33679–33686 [DOI] [PubMed] [Google Scholar]
- Vainonen JP, Vener AV, Aro EM (2009) Determination of in vivo protein phosphorylation in photosynthetic membranes. Methods Mol Biol 479 133–146 [DOI] [PubMed] [Google Scholar]
- Vener AV, Ohad I, Andersson B (1998) Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr Opin Plant Biol 1 217–223 [DOI] [PubMed] [Google Scholar]
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J 18 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]