Abstract
Background
This systematic review with meta-analysis was designed to evaluate the literature and to develop recommendations regarding the use of preoperative radiotherapy in the management of patients with resectable rectal cancer.
Methods
The MEDLINE, CANCERLIT and Cochrane Library databases, and abstracts published in the annual proceedings of the American Society of Clinical Oncology and the American Society for Therapeutic Radiology and Oncology were systematically searched for evidence. Relevant reports were reviewed by four members of the Gastrointestinal Cancer Disease Site Group and the references from these reports were searched for additional trials. External review by Ontario practitioners was obtained through a mailed survey. Final approval of the practice guideline report was obtained from the Practice Guidelines Coordinating Committee.
Results
Two meta-analyses of preoperative radiotherapy versus surgery alone, nineteen trials that compared preoperative radiotherapy plus surgery to surgery alone, and five trials that compared preoperative radiotherapy to alternative treatments were obtained. Randomized trials demonstrate that preoperative radiotherapy followed by surgery is significantly more effective than surgery alone in preventing local recurrence in patients with resectable rectal cancer and it may also improve survival. A single trial, using surgery with total mesorectal excision, has shown similar benefits in local recurrence.
Conclusion
For adult patients with clinically resectable rectal cancer we conclude that:
• Preoperative radiotherapy is an acceptable alternative to the previous practice of postoperative radiotherapy for patients with stage II and III resectable rectal cancer;
• Both preoperative and postoperative radiotherapy decrease local recurrence but neither improves survival as much as postoperative radiotherapy combined with chemotherapy. Therefore, if preoperative radiotherapy is used, chemotherapy should be added postoperatively to at least patients with stage III disease.
Background
Adenocarcinoma of the rectum is a common malignancy that originates in the last 15 cm of the large bowel where most of its external surface is not covered by peritoneum but rather is directly surrounded by connective-adipose tissue. Resection of the rectum and surrounding tissues can cure approximately 50% of patients. The other half will eventually die of the disease. Surgical failure is related to distant micro metastases that are not apparent and / or incomplete local resection. Local recurrences increase in frequency and survival decreases as the tumor penetrates through the rectal wall and extends to regional lymph nodes [1]. These prognostic factors form the basis for the tumor, node, metastases (TNM) staging system widely used to advise therapy (see Additional file 1).
Meticulous dissection of perirectal tissues en bloc with total mesorectal excision (TME) decreases local relapse [2]. Preoperative and postoperative radiotherapy (RT) has also been used to reduce local recurrence, which is associated with pelvic pain and rectal obstruction, and to prevent disease dissemination from the local site, thereby improving survival. Other goals of RT are to convert inoperable tumors into resectable cases, to preserve the anal sphincter and to avert a colostomy [3]. The advantages and disadvantages of preoperative and postoperative RT have been well described [3]. The principal advantage of postoperative RT is that it is given only to patients at high risk of recurrence according to well-investigated prognostic factors [pathological stages II and III (B2 and C)]. Major incentives for preoperative RT originate from a variety of perspectives. Biologically, a tumor with an undisturbed circulation and oxygenation has a better chance for full radiation effects. Moreover, a tumor reduced in size and with the surrounding tissues sterilized facilitates surgery and reduces potential tumor dissemination. A more practical incentive is the possibility of using an equally effective five-day course of high dose fraction preoperative RT instead of a 25 – 30 day standard postoperative course of RT.
Another practice guideline by the Gastrointestinal Cancer Disease Site Group (DSG) first developed in 1997 and updated in 2001 reviewed the effects of postoperative RT and / or chemotherapy in resected stage II / III rectal cancer [4]. This guideline recommended the combined use of postoperative radiation and chemotherapy as the preferred treatment for resected stage II and III rectal cancer. This combined treatment improved the local recurrence rate by 50% and the five-year survival rate by 42%. The same guideline discouraged the use of RT alone as adjuvant treatment because it only decreased local recurrence rates. For the present report, we initially reviewed only the effect of RT given before definitive surgery on survival and local recurrence. Following advice received from practitioner feedback and from our resource group, we have also included a discussion of preoperative RT compared with postoperative combined RT plus chemotherapy. This report does not consider the use of preoperative RT to convert locally advanced, initially unresectable rectal cancer into resectable cases, to preserve the anal sphincter, or to delay the need for colostomy.
Methods
This practice guideline was developed by the Cancer Care Ontario Practice Guidelines Initiative (CCOPGI), using the methodology of the Practice Guidelines Development Cycle [5]. Evidence was selected and reviewed by four members of the CCOPGI's Gastrointestinal Cancer DSG and methodologists. This practice guideline report is a convenient and up-to-date source of the best available evidence on preoperative RT for clinically resectable rectal cancer, developed through systematic reviews, evidence synthesis and input from practitioners in Ontario. External review by Ontario practitioners was obtained through a mailed survey consisting of items that address the quality of the draft practice guideline report and recommendations, and whether the recommendations should serve as a practice guideline. Final approval of the original guideline report was obtained from the Practice Guidelines Coordinating Committee (PGCC).
The report is intended to promote evidence-based practice. The Practice Guidelines Initiative is editorially independent of Cancer Care Ontario and the Ministry of Health and Long-Term Care. The CCOPGI has a formal standardized process to ensure the currency of each guideline report. This consists of periodic review and evaluation of the scientific literature, and where appropriate, integration of this literature with the original guideline information.
Examination of the evidence
Literature search strategy
MEDLINE (1966 to April 2003), CANCERLIT (1983 to April 2002) and the Cochrane Library (Issue 1, 2003) were searched with no language restrictions. "Rectal neoplasms" (Medical subject heading [MeSH]), "colorectal neoplasms" (MeSH) and the text word "rectal cancer" were combined with "radiotherapy" (MeSH) and the following phrases used as text words: "preoperative"; "neoadjuvant"; "radiotherapy"; "radiation"; "irradiation". These terms were then combined with the search terms for the following study designs or publication types: practice guidelines, meta-analyses, and randomized controlled trials. The proceedings of the 1998 to 2002 annual meetings of the American Society of Clinical Oncology (ASCO) and the 1999 to 2002 annual meetings of the American Society for Therapeutic Radiology and Oncology (ASTRO) were searched for reports of new or ongoing trials. Relevant articles and abstracts were selected and reviewed and the reference lists from these sources were searched for additional trials. A search of personal reprint files was also conducted.
Study selection criteria
Trials of preoperative RT in resectable rectal cancer are characterized by multiple methodological problems because two treatments are combined (RT and surgery) to affect a heterogeneous condition (various populations and stages of rectal carcinoma) and to achieve a variety of goals (downstaging, improving resectability, decreasing local and possibly distant recurrences and improving survival). Cummings [6] detailed many of the pitfalls that marred early trials, including deficiencies in trial design, eligibility criteria, treatment standardization and reporting of results. We used this critique to develop standard criteria for the selection of trials of preoperative RT for rectal cancer. Studies were included in this systematic review of the evidence if they met all of the following criteria:
1. Patients were randomly assigned to preoperative RT versus surgery alone or an alternative treatment.
2. The study population was well defined. Studies preferably included only rectal carcinoma, defined by tumors located within 15 cm of the pectinate line or anal verge on sigmoidoscopy, or rectosigmoid tumors. Patients were screened for metastases and co-morbidity by clinical and imaging procedures and were assessed as surgically resectable for cure.
3. Treatments were described clearly, including RT dose, fractionation, duration, field size and portals of irradiation. Timing of surgery after completion of RT was clearly set. General surgical principles were described.
4. Compliance with treatments and follow-up were described.
5. Treatment outcomes were reported for overall survival and / or local failure. Other outcomes such as adverse effects (morbidity and mortality), downstaging (decrease in the proportion of cases with stage III disease), and resectability (total and curative) were recorded if available.
Synthesizing the evidence
Trials of preoperative RT versus surgery alone were pooled using Review Manager 4.2.1 (© Update Software), which is available through the Cochrane Collaboration. Overall mortality, local failure, tumor resectability, tumor downstaging, and adverse effects were pooled in separate analyses for all studies, where data was available. Reported figures or estimates obtained from tables or graphs were used. For calculation of survival and local failure, all eligible patients were considered in the denominator, based on intention to treat. All deaths at the time of reporting, regardless of cause, were included in survival calculations. Patients with local failure included those with non-resected as well as those with recurrent disease. Only resected cases were considered in the calculation of downstaging.
Data were pooled using the random effects model as the more conservative estimate of effect [7]. Results were expressed as relative risk ratios (RR) with 95% confidence intervals (CI), where a RR less than 1.0 favors preoperative RT and a RR greater than 1.0 favors surgery alone. Odds ratios (OR) and absolute risk differences (RD) were also calculated.
Heterogeneity of results among trials was expected in view of the different treatments used and populations tested, as well as the wide time interval and geography across which these trials were conducted. For example, the RT prescription may affect the results. RT doses greater than 30 Gy10 are considered necessary and pelvic fields are as effective as extended fields. Moreover, the use of three or more RT beams will lessen toxicity and short delays of surgery after RT will not demonstrate downstaging. Thus, these factors were investigated with sensitivity analyses to see whether there was an impact on results. Outcomes of predetermined groups of patients were examined initially by the graphic method described by L'Abbe et al. [8] and RR calculated. For sensitivity analyses the following factors were examined:
Treatment effects:
• Biologically effective dose (BED) of RT (less than 30 Gy10 versus equal to or greater than 30 Gy10). BED was calculated using the linear quadratic formula [9] and the parameters suggested for time correction [10]:
BED time = nd (1+d/α/β) - γ/α (T - Tk)
where n = number of fractions, d = dose per fraction, α/β = 10 for tumor effect and acute reactions and α/β = 3 for late reactions, γ/α = repair rate set at 0.6 Gy/day, T = total treatment time and Tk = initial delay time set at 7 days;
• RT fraction size (standard fractions up to 2.5 Gy/day versus high fractions of 5 Gy/day);
• Contemporary RT prescription, defined as studies employing multiple-field technique and target volume confined to the pelvis (i.e. excluding studies employing parallel pair arrangements or including para-aortics); and
• Delay of surgery after completion of RT (less than seven days versus eight or more days).
Population effects:
• Studies including a range of rectal cancer cases versus those including only advanced disease.
Sensitivity analyses were also performed for all five of the meta-analyses (overall survival, local failure, tumor resectability, downstaging, and adverse effects) considering only trials with high design quality. The quality of the 14 eligible randomized trials of preoperative RT versus surgery alone in operable rectal cancer was scored independently. Five assessors assessed each trial using the Detsky instrument [11]. This questionnaire addresses five domains of study quality: randomization process, outcomes measure, patient eligibility, treatment description, and statistical procedures. The 14 questions on the Detsky instrument can be answered "adequate", "inadequate", or "partial" and scored 1, 0, or 0.5, respectively. The final score of each trial is a ratio of the observed points divided by the total number of questions answered. The results from the five assessors were averaged for a final score. Trials with Detsky instrument scores greater than 0.5 were considered to be of high quality.
Results
Literature search results
The literature search identified 24 trials. Nineteen of these trials compared preoperative RT plus surgery to surgery alone [12-29,38]. Five trials compared preoperative RT to alternative treatments [30-34]. Two meta-analyses of preoperative RT versus surgery alone were found in a recent search update [35,36].
Preoperative RT versus surgery alone
Four of the 19 trials of preoperative RT compared with surgery alone were excluded from the review [12-15]. Preliminary results of one trial have been reported in Russian [12], but the report and results were difficult to interpret. This trial was excluded until more mature results are available and it is clear that the trial meets the inclusion criteria. Three trials [13-15] had major violations to the inclusion criteria. The Memorial Hospital trial included both randomized as well as non-randomized patients in the analysis [13]. The Veterans Administration Surgical Oncology Group (VASOG) Trial I included patients who may have had apparent metastases and did not allow for an analysis excluding this group of patients [14]. The Essen trial was described in summary form and was a failed trial of preoperative plus postoperative RT treatment due to difficulties with compliance [15]. The remaining 15 trials are shown in Table 1.
Table 1.
Randomized trials of preoperative RT versus surgery alone in resectable rectal cancer.
| Trial (reference) | No. of patients randomized RT / no RT (analyzed) | RT prescription* | Biologically effective dose (Gy10) | Compliance with RT† 0 <1 >1 | Surgical delay after RT (days) | Median follow-up (months) | Study quality score‡ |
| Yale [16] | 15 (15) / 16 (16) | 45 in 25 (31) 2 fields, G | 53.1 | - 7% - | 2–42 | >60 | 0.29 |
| Toronto [17] | 60 (60) / 65 (65) | 5 in 1 (1) 2 fields, P | 7.5 | - - - | 0 | 72 | 0.55 |
| MRC-I [18] | 549 (549) / 275 (275) | 5 in 1 (1) or 20 in10 (14) 2 fields, G | 7.5 or 19.8 | 2% 6% - | <7 | >60 | 0.66 |
| VASOG-II [19] | 180 (180) / 181 (181) | 31.5 in 18 (24) 2 fields, G | 26.8 | 7% 11% 4% | ~40 | 60 | 0.70 |
| Norway [20] | 159 (155) / 150 (145) | 31.5 in 18 (24) 2 fields, G | 26.8 | 1% - - | <21 | 54 | 0.80 |
| EORTC [21] | 236 (231) / 230 (228) | 34.5 in 15 (19) 2 fields, G | 35.2 | 1% - - | 1–69 | >72 | 0.67 |
| Brazil [22] | 34 (34) / 34 (34) | 40 in 20 (25) 2 fields, P | 35.4 | - - - | 7 | 120 | 0.48 |
| Hungary [23] | 171 (171) / 165 (165) | 40 in 20 (26) or 50 in 25 (33) 2 fields, P | 35.4 or 43.2 | - - - | 42 | >60 | 0.44 |
| ICRF-UK [24] | 228 (228) / 239 (239) | 15 in 3 (5–7) 2 fields, P | 22.5 | - 10% - | <2 | 60 | 0.64 |
| Stockholm-I [25] | 424 (424) / 425 (425) | 25 in 5 (5–7) 2 fields, G | 37.5 | - 12% - | <7 | 107 | 0.74 |
| MRC-II [26] | 139 (139) / 140 (140) | 40 in 20 (28) 2 fields, P | 35.4 | 1% 5% 4% | <7 | >60 | 0.83 |
| NW-UK [27] | 143 (143) / 141 (141) | 20 in 4 (4) 3 field, P | 30.0 | - 1% 3% | <7 | 96 | 0.57 |
| Sweden [28] | 583 (573) / 585 (574) | 25 in 5 (5) 3–4 fields, P | 37.5 | 3% 1%§ - | <7 | >60 | 0.82 |
| Stockholm-II [29] | 272 (272) / 285 (285) | 25 in 5 (5–7) 4 fields, P | 37.7 | -------5%------ | <7 | 50 | 0.88 |
| Dutch [38] | 924 (908) / 937 (897) | 25 in 5 (5) 4 fields, P | 37.5 | 3% 2% ? | <7 | 24.9 | 0.81 |
Note: EORTC indicates European Organization for Research and Treatment of Cancer; ICRF-UK, Imperial Cancer Research Fund United Kingdom; MRC, Medical Research Council; NW-UK, Northwest Region Rectal Cancer Group United Kingdom; VASOG, Veterans Administration Surgical Oncology Group. * Total dose in Gy, number of fractions, (duration of treatment in days), number of treatment fields, target volume (G, guitar-shaped; P, pelvic). † 0 indicates per cent of patients receiving no treatment; <1, percent receiving less than planned treatment; >1, percent receiving more than planned treatment. ‡ Based on independent assessment by five reviewers using the Detsky instrument10. § 5% of patients also received RT over >7 days, and 8% of patients received radiation through a two portal beam.
The significant trial coordinated by the Dutch Colorectal Cancer Group report results after a median follow-up of 24.9 months, much shorter than other trials, and numbers of patients with events cannot be determined [38]. Therefore this trial is not included in the meta-analysis. This study, which included patients with rectal cancer not fixed or amenable to local excision, standardized surgery with total mesorectal excision. Patients were randomized to surgery alone or surgery preceded by RT (bottom row, Table 1)[38]. The recurrence rate was significantly lower in patients receiving preoperative RT (2.4% vs. 8.2%; p < 0.001) but overall survival was the same for both treatment groups. In a multivariate subgroup analysis, tests for interaction between tumor location, TNM stage and treatment were not significant, suggesting that treatment effect was similar for all the subgroups analyzed.
This left 14 trials of preoperative RT compared with surgery alone [16-29]. One trial [18] contributed to two comparisons (single fraction preoperative RT versus surgery alone; multiple fractions preoperative RT versus surgery alone). One trial [28] included 316 patients who were also included in another trial [29]; neither report described results for these patients separately from those who were included in only one of the two trials. Reports of three trials [17,22,23] did not provide data on compliance with treatment. Where results have been reported or updated in more than one publication, only the most recent publication is listed.
All trials included patients with resectable rectal cancer. Two trials also included patients with rectosigmoid tumors [16,19]. Some trials excluded patients with small tumors [25,28,29] or were limited to those with locally advanced tumors [23,26,27] or those requiring abdomino-perineal resection [19]. Patients excluded were those with evidence of distant metastases, previous malignancy or previous RT.
Surgery was performed from a few hours up to 40 days following the preoperative RT course, but most surgeries were done within one to four weeks of preoperative RT. The description of surgical procedures was very general except for the distinction between palliative and curative procedures. Radiation was delivered mostly by anterior and posterior portals. Only recent trials used three or four radiation portals [27-29]. Radiation fields were mostly pelvic but some trials used extended guitar-shaped fields up to L2 [16,20,21,25]. The total radiation dose and the fractionation schedules were quite different across studies, ranging from 5 Gy in a single treatment to over 50 Gy in five weeks.
Compliance with treatments was generally well described. Follow-up data were collected prospectively in all but one trial [17]. The follow-up schedule was described for all trials except one [16]. The rate of patients lost to follow-up was 16% in one trial [24], but most other trials seemed to have had greater than 90% compliance with follow-up. The median follow-up at the time of the trial report was five years or more in most trials.
Pooled results of trials comparing preoperative RT to surgery alone
Overall survival
Survival was reported for all trials except one [23]. Survival results for only resected cases were reported for one trial [28]. The overall mortality risk ratio favored preoperative RT (RR, 0.94; 95% CI, 0.89 to 0.99; p = 0.02), results that correspond to an absolute risk difference of 4% (95% CI, 0.7% to 7.5%; p = 0.018). There was significant heterogeneity across trials (X2 = 20.59; p < 0.10). As observed in Figure 1, most heterogeneity derived from a study from Brazil [22]; removal of this study resulted in non-significant heterogeneity and the results were similar (RR, 0.95; 95% CI, 0.91 to 0.99; p = 0.012). Survival benefit was observed only for trials using BED > 30 but there was significant heterogeneity detected.
Figure 1.
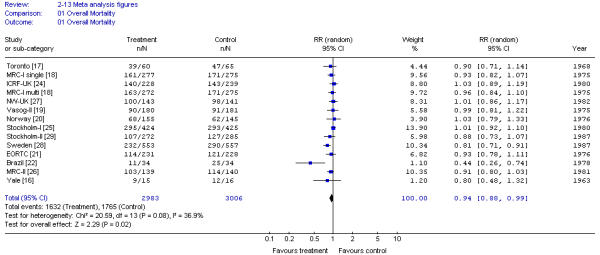
Meta-analysis examining preoperative RT for patients with resectable rectal cancer: overall mortality. Results were reported for only the eligible or evaluable patients for Norway [20] trial. Results were reported for only the eligible patients undergoing surgery for the Sweden [28] trial.
Local failure
Local failure rate was calculated for all trials as the number of patients unable to have tumor removal as well as those with recurrent disease after resection. The overall relative risk ratio favored preoperative RT (RR, 0.71; 95% CI, 0.57 to 0.89; p = 0.0025) with an absolute risk reduction of 8.6% (95% CI, 3.1% to 14.2%; p = 0.0024). There was significant heterogeneity when local failure rates were pooled across trials (X2 = 61.72; p < 0.001). Figure 2 displays the local failure risk ratios with the trials arranged in ascending order of the RT dose (BED) used. There was no treatment effect in the analysis of three trials of preoperative RT using doses of 7.5 to 26.8 BED (RR, 0.95; 95% CI, 0.79 to 1.11; p = 0.58) while trials using doses greater than or equal to 30 BED had evidence of reduced local failures (RR, 0.63; 95% CI, 0.48 to 0.83; p = 0.0011) with significant heterogeneity being detected.
Figure 2.
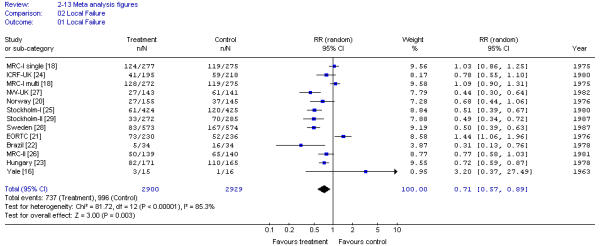
Meta-analysis examining preoperative RT for patients with resectable rectal cancer: local failure. Results were reported for only the eligible or evaluable patients for the Norway [20] trial.
Tumor resectability
Total tumor resectability between the treatment arms was not significantly different when 12 trials (14 comparisons) involving 5 923 patients were pooled (RR, 1.00; 95% CI 0.99 to 1.00; p = 0.36). Pooling of 14 trials (16 comparisons) involving 6 816 patients detected no significant difference in curative resections for preoperative RT compared with surgery alone (RR, 0.99; 95% CI, 0.98 to 1.01; p = 0.59). There was no significant heterogeneity among trials in either pooled analysis.
Downstaging
There was an overall significant decrease in the incidence of stage III rectal cancer among patients randomized to preoperative RT compared with surgery alone, but there was significant heterogeneity among the pooled results (X2 = 38.57; p < 0.001) (Figure 3). Neither radiation dose or its fractionation, nor timing of surgery affected results.
Figure 3.
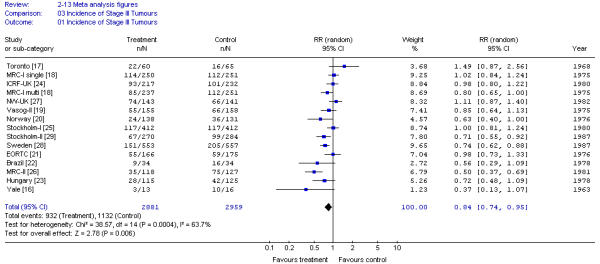
Meta-analysis examining preoperative RT for patients with resectable rectal cancer: incidence of stage III tumors.
Adverse effects
Preoperative RT did not significantly increase 30-day postoperative mortality compared with surgery alone (RR, 1.33; 95% CI, 0.87 to 2.05; p = 0.19). These results showed significant heterogeneity of trial results (X2 = 23.35; p < 0.05) (Figure 4). Results were not affected by radiation dose.
Figure 4.
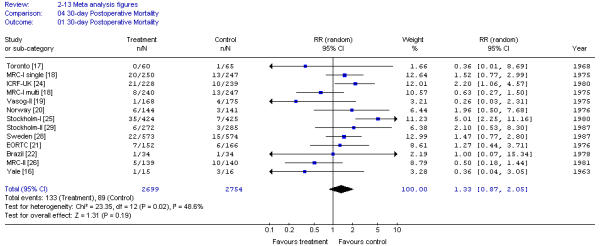
Meta-analysis examining preoperative RT for patients with resectable rectal cancer: 30-day postoperative mortality.
Postoperative morbidity was similar across trials and consisted mainly of delay of perineal wound healing and infection. The pooled results for postoperative morbidity also demonstrated significant heterogeneity (X2 = 62.74; p < 0.001) (Figure 5). Results were not different for patients receiving high or low dose RT or delay to surgery of < 7 or > 8 days.
Figure 5.
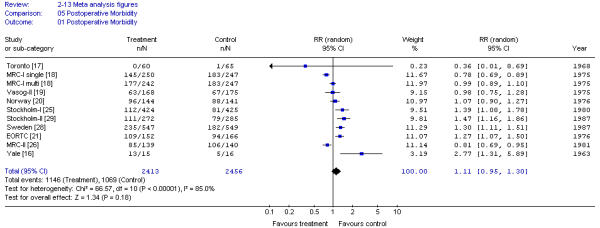
Meta-analysis examining preoperative RT for patients with resectable rectal cancer: postoperative morbidity.
Sensitivity analyses
Sensitivity analyses were conducted to test the robustness of the conclusions of the five pooled analyses above (overall survival, local failure, tumor resectability, downstaging, adverse effects) when excluding the three studies with quality scores of less than 0.5 [16,22,23]. Results of the pooled analyses of the best quality studies were not different from the results considering all studies (data not shown). Details of the quality assessment results using the Detsky instrument can be found in Additional file 2.
Published meta-analyses
After completing our analysis, two literature-based meta-analyses of trials comparing preoperative RT to surgery alone for resectable rectal cancer were published [35,36]. The Camma et al. [35] meta-analysis followed a methodology similar to ours. In the analysis, however, there was also an investigation of patient subgroups (Dukes' stages A, B and C, and male sex) and a regression analysis for overall survival (but not for other outcomes). The following regression variables were used: BED, stage of disease, male sex, study publication year, study size, allocation concealment and handling of withdrawals. This meta-analysis considered 14 published trials [14,16-22,24-28]: it included the VASOG-I trial [14], which we excluded, and it did not include a trial from Hungary [23] or the single-fraction RT arm from the MRC-I trial [18]. All comparisons were made using odds ratios. Camma et al. [35] detected a significant reduction in overall mortality with preoperative RT (Table 2). In a subgroup analysis, the decrease in mortality occurred in patients with Dukes' stages B and C but not in patients with stage A disease. No interaction was found between RT dose and survival (BED < 30 or > 30; p = 0.53). In the regression analysis none of the factors used as variables had a significant impact on survival. Cancer-specific mortality and local recurrences were also reduced by the use of preoperative RT (Table 2) but not distant metastases. The overall rate of post-operative adverse events was higher in patients receiving preoperative RT (57.4% versus 42.4%; p < 0.001). The 30-day postoperative mortality was not significantly different between patients receiving preoperative RT and those having only surgery. Camma et al. [35] concluded that preoperative RT reduced overall and cancer-specific mortality rates, and particularly local recurrence rates, while not affecting distant metastases. Postoperative mortality was not affected by the use of preoperative RT in spite of a higher rate of adverse events in patients.
Table 2.
Results of three meta-analyses of preoperative RT versus surgery alone in resectable rectal cancer.
| Outcome | Pooled Results Odds Ratio, 95% Confidence Interval and p value | ||
| Camma et al [35] | CRCCG [36]* | Present Review | |
| Overall Mortality | 0.84 (0.72, 0.98) p = 0.03 |
0.95 (0.86, 1.06) p = 0.34 |
0.85 (0.74, 0.97) p = 0.019 |
| Local Failure | 0.49 (0.38, 0.62) p < 0.001 |
0.58 (0.42, 0.80) p = 0.00008 |
0.62 (0.45, 0.85) p = 0.0029 |
| 30-day Postoperative Mortality | 1.38 (0.86, 2.32) p = 0.22 |
- - |
1.42 (0.90, 2.23) p = 0.13 |
The Colorectal Cancer Collaborative Group (CCCG) [36] identified trials of adjuvant preoperative and postoperative RT started before January 1st, 1987. The CCCG search yielded 19 trials of preoperative RT which included five trials not included in our review: two trials using preoperative plus postoperative RT [15,31], one trial including patients with metastatic disease [14], another combining RT with 5-fluorouracil (5-FU) administration [33] and a Japanese trial published in 1989. Analysis was done by the log-rank method for overall and disease-specific mortality and for all and isolated recurrences. The overall yearly death rate was 5.6% (standard error ± 3.3). All recurrences and isolated recurrences were significantly decreased by preoperative RT at 5 years (45.9% vs. 52.9% and 12.5% vs. 22.2%, respectively) and at 10 years (55.1% vs. 60.8% and 16.7% vs. 25.8%, respectively). There was significant heterogeneity between the results of the 12 trials analyzed (p = 0.002), which was explained by the greater efficacy of RT at higher biologically effective doses (> 30 Gy). The reduction in local recurrence was proportionally similar for the various stages of the disease, and not affected by either sex or age. A multivariate analysis was not done [36].
The results of the published meta-analyses [35,36] and the one conducted for this systematic review are shown in Table 2.
Preoperative RT versus alternative treatments
Preoperative RT versus postoperative adjuvant RT in high-risk cases
In a multi-institutional randomized trial in Sweden, patients with operable rectal cancer were randomized to preoperative RT or selective postoperative RT if the pathological stage was II or III [30,37]. Overall survival was the same for both treatment groups. When only patients with radical resection were considered, local recurrence was less likely for those receiving preoperative RT (11% vs. 22%; p = 0.02) (Table 3). Postoperative complications, both early [30] and late [37], were significantly more frequent after higher-dose postoperative RT. The investigators emphasized that a short course of high fraction preoperative RT is preferable to a standard course of postoperative RT. Preoperative RT was better in reducing local recurrence rates and was associated with lower morbidity.
Table 3.
Randomized trials of preoperative RT compared to alternative treatments in rectal cancer.
| Trial (Reference) | Treatment | No. Patients | Local Failure (%) | Survival Rate at 5 years (%) | ||
| Preoperative RT | Surgery Delay | Postoperative RT (Delay) | ||||
| Pahlman & Glimelius [30] | 5.1 Gy × 5 - |
1 week - |
- 60 Gy in 54* (4–6 weeks) |
236 235 |
22% 33% p = 0.012 |
43% 37% p = 0.43 |
| RTOG [31] | 0.5 Gy × 1 - |
1 day - |
45–51 Gy* (2–6 weeks) |
175 178 |
32% 32% p = NS |
43% 32% p = NS |
| Herrmann et al. [32] | 3.3 Gy × 5 - |
1–2 days - |
41.5 Gy in 48* 59.8 Gy in 56* (6–14 weeks) |
48 46 |
25% 39% p = 0.142 |
49% 37% p = NS |
| EORTC [33] | 2.3 × 15 2.3 × 15 + 5-FU |
2 weeks 2 weeks |
- - |
121 126 |
15% 15% p = NS |
59% 46% p = 0.06 |
| Francois et al. [34] | 3.0 Gy × 13 3.0 Gy × 13 |
2 weeks 6–8 weeks |
- - |
99 102 |
9% 9% p = NS |
78%† 73%† p = NS |
Note: NS indicates not statistically significant; RTOG, Radiation Therapy Oncology Group. * Radiation given only to high-risk cases (stages B2 and C). † 3-year survival rate.
Two other randomized trials have investigated the benefit of preoperative RT given to patients who also received postoperative RT if the pathological stage was II or III [31,32] (Table 3). In a study by the Radiation Therapy Oncology Group (RTOG), investigators randomized patients to a single dose of 5 Gy preoperatively and gave 45 Gy postoperatively to all high-risk patients [31]. After more than five years of follow-up, survival and local failure were similar in patients with or without preoperative RT. The preoperative RT dose in this trial was very small and has been shown to be ineffective [17,18]. German investigators performed a similar but smaller trial using a higher preoperative RT dose [32]. Patients were randomized to immediate surgery or to receive 16.5 Gy in 5 fractions preoperatively. After surgery, patients at high risk of local recurrence (T4 stage, R1-2 or intraoperative tumor perforation) also received 41.4 Gy if they had preoperative RT and 59.8 Gy if they did not have preoperative RT. In a multivariate analysis of local recurrence, the only significant variable was staging (International Union Against Cancer [UICC]; p = 0.0003) while preoperative RT and T4 stage had non-significant effects (p = 0.08 and 0.07, respectively). In a similar analysis of survival, three variables were significant: age (p = 0.0003), UICC stage (p = 0.001) and residual disease status (p = 0.01). Preoperative RT had a non-significant effect (p = 0.078). These trials [31,32] indicate that selective postoperative RT annuls any potential positive effect of preoperative RT in low dose.
Preoperative RT alone versus preoperative RT plus chemotherapy
An early randomized trial by the European Organization for Research and Treatment of Cancer (EORTC) [33] compared preoperative RT with or without chemotherapy. 5-FU by bolus injection was given for four days during the first week of the radiation course. The trial was marred by many difficulties, with 27% of the cases being ineligible or not evaluable. The combined treatment did not reveal any advantage over RT alone (Table 3). Of interest was a marginally significant decrease in liver metastases for patients receiving preoperative combined treatment (p = 0.06).
Preoperative RT with surgery at different intervals
One trial by French investigators tested whether the delay of surgery after the completion of preoperative RT is important [34] (Table 3). Operable patients with rectal tumors accessible to digital rectal examination (stage T2-3, NX, M0) received preoperative RT (39 Gy in 13 fractions over 17 days through 3 fields) and were randomized to surgery after a short (two-week) or a long (six- to eight-week) interval. The only significant difference in outcomes, favoring the long over the short delay, was the higher proportion of patients with a clinical tumor response (partial plus complete) (53.1% versus 71.7%; p = 0.007) and pathological downstaging (10.3% versus 26%; p = 0.005). The three-year local failure and survival rates were not significantly different.
Discussion
Results of three meta-analyses (Table 2) indicate that preoperative RT compared to surgery alone significantly decreases the risk of local failure and overall mortality. The absolute reduction in local failure is 8.6% (95% CI, 3.1% to 14.2%) while the absolute reduction in overall mortality at five years is 3.5% (95% CI, 1.1% to 6.0%). Early results of the Dutch trial [38] confirm the decrease in local recurrence with preoperative RT even after optimal surgery with total mesorectal excision. The improved results of recent trials can be explained by better patient selection and radiation prescription. Swedish investigators, comparing the results of the Stockholm-I [25] and Stockholm-II [29] trials of preoperative RT, showed that the overall survival of patients is significantly affected by the 60-day postoperative mortality rate [39]. This early fatality rate is due to an excess of infectious, cardiovascular and thromboembolic causes. This excess in mortality is attributed to the delivery of similar radiation doses to larger versus smaller volumes and by two rather than multiple radiation portals, and to patient characteristics such as evidence of ischemia by ECG and poor performance status. An increase in morbidity was also observed and consisted of venous thromboembolism, femoral neck and pelvic fractures, and intestinal obstruction. A subgroup of patients who participated in the Swedish trial [28] completed a questionnaire about anorectal dysfunction. Bowel disturbances led to social restrictions in 30% of patients who received preoperative RT compared with 10% of patients who received surgery alone (p < 0.01). The abnormalities included more frequent bowel movements, urgency and incontinence. No single factor could be identified to explain the complications, but the authors postulated the radiation effect on the anal sphincter itself or on its nerve supply [40]. Similar anorectal dysfunction has been reported after postoperative RT combined with chemotherapy [41,42].
Preoperative RT in high fractions has been compared with standard low-fraction postoperative adjuvant RT [30]. The overall survival was the same for both treatment groups but the local recurrence rate was lower and the morbidity less for preoperative RT. While preoperative RT was given to all cases, postoperative RT was given only to high-risk cases (Dukes' stages B2 and C), a group equal to half the number of cases treated with the preoperative approach. The increased morbidity of the postoperative RT must be related in part to the higher radiation dose given. In a retrospective analysis of preoperative and postoperative RT trials, Glimelius et al. [10] observed that for a similar reduction in local failure the dose of radiation must be higher in the postoperative than in the preoperative setting.
The use of preoperative RT in small doses did not decrease the indications for postoperative adjuvant RT [31,32]. Patients who received four bolus injections of 5-FU during the preoperative RT in one old trial increased postoperative mortality and decreased overall survival [33]. The cause for this toxicity is not known and has not been observed in subsequent trials of combined therapy. The delay of surgery after preoperative RT for more than two weeks decreased the rate of stage III disease but had no impact on resectability, local recurrence or survival [34].
Development of a clinical practice guideline
Gastrointestinal Cancer DSG consensus
When presented with the reviewed evidence, the discussion of the Gastrointestinal Cancer DSG focused on results from recent trials of preoperative RT in Europe that demonstrated significant improvements in local failure and survival rates. These results, achieved with a short course of radiation (five fractions) and with less toxicity than standard longer courses of radiation, have prompted the widespread use of this treatment modality in Europe and more recently in North America. Some treatment centers in Ontario have started phase II studies of preoperative RT, in some cases with concurrent chemotherapy.
There are, however, some concerns about the widespread use of preoperative RT. Some potential risks of the treatment seem preventable. The use of radiation given to smaller volumes and multiple fields, instead of the past practice of two fields, has been shown to decrease both early postoperative morbidity and mortality [25,28,29]. The exclusion of patients with poor performance status and those with ischemic changes in the electrocardiogram (ECG) [39] reduced both mortality and morbidity in the first two months. More difficult to predict is the long-term anorectal dysfunction, which restricts the social life of one third of survivors in some series following both preoperative and postoperative adjuvant RT [40,42]. Another concern is that some of the preoperatively irradiated patients would not have required this treatment based on the postoperative staging of the disease. Furthermore, the prognostic value of the postoperative staging of irradiated patients remains uncertain, although downstaging does not occur after the short course of preoperative RT. The postoperative pathological staging is important to determine the need for adjuvant chemotherapy, which improves survival and reduces local recurrence [4].
Should preoperative RT be recommended for adjuvant treatment in resectable rectal cancer? The common practice in North America for patients with resected stages II and III rectal cancer has been postoperative RT plus chemotherapy. In a previous guideline, it was demonstrated that this combined treatment significantly reduced local failure by 50% (95% CI, 8% to 73%) and improved patient survival by 42% (95% CI; 8% to 63%) for patients with stage II and III rectal cancer when compared to postoperative RT alone [4]. In similar patients, postoperative RT alone compared to observation after surgery decreased local recurrences by 27% (95% CI, 4% to 45%) but did not improve survival. Postoperative RT alone has been, therefore, discouraged [4]. Preoperative RT alone, when compared to surgery, has been shown to decrease local failure by approximately 50% and to improve survival by approximately 15% (Table 2). The improvement in local recurrence has occurred after optimal surgery with TME [38]. In a single trial [30,37], preoperative short-course RT has induced less local recurrence (11% versus 22%; p = 0.02) and less morbidity than conventional postoperative RT alone. From these results it can be inferred that preoperative RT is a better treatment choice than postoperative RT with less local failures and less morbidity. A comparison of preoperative RT followed by postoperative chemotherapy versus combined postoperative RT plus chemotherapy is presently being investigated in clinical trials but mature results are not yet available for review.
Based on the evidence from the Swedish and Dutch trials [28,38] and the meta-analyses data, preoperative RT (followed by chemotherapy for at least patients with stage III) is an alternative to our previous recommendation for combined postoperative RT plus chemotherapy for resected patients with stage II and III rectal cancer. Patients must be made aware of the potential benefits and drawbacks of both approaches. Benefits of short-course preoperative RT are lower local failure and less treatment morbidity. Local failure is an important outcome in rectal cancer as recurrences are associated with significant disability. Drawbacks are the need to use preoperative RT in more patients compared to RT administered according to postoperative staging and the possibility that patients not requiring radiation may develop treatment associated complications.
Physicians should encourage patients to participate in clinical trials of the primary treatment of rectal cancer. These trials should require the best possible surgery, the confirmation of the accuracy of clinical staging versus pathological staging, and the use of measures of quality of life. Patients must also be clearly advised of the differences between treatment approaches.
External review
Practitioner feedback was obtained through a mailed survey. The survey consisted of items evaluating the methods, results, and interpretive summary used to inform the draft recommendations and whether the draft recommendations should be approved as a practice guideline. Written comments were invited. Follow-up reminders were sent at two weeks (post card) and four weeks (complete package mailed again). The Gastrointestinal Cancer DSG reviewed the results of the survey.
1. Number surveyed: 155 practitioners in Ontario (30 medical oncologists, 21 radiation oncologists, 100 surgeons, and four gastroenterologists).
2. Return rate: 63%
3. Written comments attached: 54%
4. Agreement with the summary of the evidence: 87%
5. Agreement with the recommendation: 85%
6: Approval of the recommendation as a practice guideline: 68%
Summary of main findings
Written comments provided by practitioners varied. Several respondents expressed concern with the statement in the draft recommendations that "Postoperative radiotherapy for patients with stage II / III rectal cancer is as effective in prolonging survival as preoperative radiotherapy for all rectal cancer patients regardless of stage of disease". These respondents noted that preoperative versus postoperative RT was not the topic of this guideline and randomized trials addressing this issue are still ongoing.
Other respondents also questioned the recommendation that "postoperative radiotherapy combined with chemotherapy should remain the standard treatment". They argued that if the results of the on-going trials demonstrate that preoperative and postoperative RT are equally effective, then one treatment should not be recommended over the other as the standard treatment. Several respondents raised the question of the role of total mesorectal excision.
Modifications or actions
Practitioner feedback indicated a need to clarify the role of preoperative RT in the context of the companion guideline [4] recommending postoperative RT plus chemotherapy for stage II and III rectal cancer. In this context, the magnitude of benefits and drawbacks of preoperative and postoperative RT with and without chemotherapy are further discussed in the Gastrointestinal Cancer DSG Consensus section.
Practice guidelines coordinating committee approval process
The guideline was circulated to 11 members of the Practice Guideline Coordinating Committee. Seven members returned ballots; five approved the guideline report as written, and two members approved the guideline conditional on the Gastrointestinal Cancer DSG addressing suggestions for revision. The suggestions referred to the recommendations and the meta-analyses. Changes were made to the guideline based on these suggestions, and a revised version was resubmitted to the PGCC for further consideration. The practice guideline was approved with one dissenting vote.
Conclusions
This practice guideline reflects the integration of a review of the evidence, the Gastrointestinal Cancer DSG draft recommendations and the feedback obtained from the external review process. It has been approved by the Gastrointestinal Cancer DSG and the Practice Guidelines Coordinating Committee.
Target population
These recommendations apply to adult patients with clinically resectable rectal cancer. This report does not consider the use of preoperative RT to convert locally advanced, initially unresectable rectal cancer to resectable cases, to preserve the anal sphincter, or to delay the need for colostomy.
Recommendations
Preoperative RT is an acceptable alternative to the previous practice of postoperative RT for patients with stage II and III resectable rectal cancer.
Both preoperative and postoperative RT decrease local recurrence but neither improves survival as much as postoperative RT combined with chemotherapy. Therefore, if preoperative RT is used, chemotherapy should be added postoperatively to at least patients with stage III disease.
Qualifying statement
Patients must be made aware of the potential benefits and drawbacks of both approaches. Benefits of short-course preoperative RT are lower local failure and less treatment morbidity. Local failure is an important outcome in rectal cancer as recurrences are associated with significant disability. Drawbacks are the need to use preoperative RT in more patients compared to RT administered according to postoperative staging and the possibility that patients not requiring radiation may develop treatment associated complications.
List of abbreviations used
TNM, tumor, node, metastases (the staging system of the UICC); RT, radiotherapy; DSG, disease site group; CCOPGI, Cancer Care Ontario Practice Guidelines Initiative; MeSH, medical subject heading; ASCO, American Society of Clinical Oncology; ASTRO, American Society for Therapeutic Radiation and Oncology; RR, relative risk ratio; CI, confidence interval; OR, odds ratio; RD, risk difference; Gy, Gray; BED, biologically effective dose; VASOG, Veterans Administration Surgical Oncology Group; MRC, Medical Research Council; CCCG, Colorectal Cancer Collaborative Group; RTOG, Radiation Therapy Oncology Group; UICC, International Union Against Cancer; EORTC, European Organization for Research and Treatment of Cancer; ECG, electrocardiogram; TME, total mesorectal excision; 5-FU, 5-fluorouracil; PGCC, Practice Guidelines Coordinating Committee.
Competing interests
None declared.
Authors' contributions
AF and BR participated in the statistical analysis and did the majority of the writing of the manuscript with input from LZ, KSW, OA, VT, and other Gastrointestinal Cancer DSG members. KSW, OA, and VT participated in study selection and study quality assessments, and provided feedback on all drafts.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Acknowledgments
Acknowledgements
Additional members of Cancer Care Ontario's Practice Guidelines Initiative's Gastrointestinal Cancer DSG include: Dr. J. Maroun MD (Chair), Dr. B. Cummings MD (Vice-Chair), Mr. M. Citron (Community Representative), Dr. F.G. DeNardi MD, Dr. C. Earle MD, Dr. S. Fine MD, Dr. B. Fisher MD, Dr. C. Germond MD, Dr. D. Jonker MD, Dr. K. Khoo MD, Dr. W. Kocha MD, Ms. M. Lethbridge (Community Representative), Dr. W. Lofters MD, Dr. R. Malthaner MD, and Dr. M. Moore MD. Please see the CCOPGI web site http://www.cancercare.on.ca/ for a complete list of current Gastrointestinal Cancer DSG members.
Contributor Information
Alvaro Figueredo, Email: alvaro.figueredo@hrcc.on.ca.
Lisa Zuraw, Email: zurawl@mcmaster.ca.
Rebecca KS Wong, Email: rebecca.wong@rmp.uhn.on.ca.
Olusegun Agboola, Email: olusegun.agboola@orcc.on.ca.
R Bryan Rumble, Email: rumbleb@mcmaster.ca.
Ved Tandan, Email: tandanv@mcmaster.ca.
the members of Cancer Care Ontario's Program in Evidence-based Care's Gastrointestinal Cancer Disease Site Group, Email: rumbleb@mcmaster.ca.
References
- Cohen AM, Minsky BD, Schilsky RL. Cancer of the rectum. In: DeVita VT, Hellman S, Rosenberg SA, editor. Cancer: Principles and Practice of Oncology. 5. Philadelphia-New York: Lippincott-Raven; 1997. pp. 1197–1233. [Google Scholar]
- MacFarlane JK, Ryall RDH, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-W. [DOI] [PubMed] [Google Scholar]
- Mohiuddin M. Radiation: The preoperative adjuvant strategy. In: Cohen AM, Winawer SJ, editor. Cancer of the Colon, Rectum, and Anus. New York, McGraw-Hill; 1995. pp. 657–668. [Google Scholar]
- Figueredo A, Germond C, Taylor B, Maroun J, Agboola O, Wong R, Zwaal C, Micucci S, for the Gastrointestinal Cancer Disease Site Group Post-operative adjuvant radiotherapy or chemotherapy for resected stage II or III rectal cancer. Curr Oncol. 2000;7:37–51. [Google Scholar]
- Browman GP, Levine MN, Mohide EA, Hayward RSA, Pritchard KI, Gafni A, Laupacis A. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–512. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- Cummings BJ. A critical review of adjuvant pre-operative radiation therapy for adenocarcinoma of the rectum. Br J Surg. 1986;73:332–338. doi: 10.1002/bjs.1800730503. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Int Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- Glimelius B, Isacsson U, Jung B, Pahlman L. Radiotherapy in addition to radical surgery in rectal cancer: evidence for a dose-response effect favouring preoperative treatment. Int J Radiat Oncol Biol Phys. 1997;37:281–287. doi: 10.1016/S0360-3016(96)00510-X. [DOI] [PubMed] [Google Scholar]
- Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- Knysh VI, Alieve BM, Barsukov YA. Combined treatment of rectal cancer using two variants of preoperative concentrated radiotherapy [Russian] Med Radiol Mosk. 1983;28:12–17. [PubMed] [Google Scholar]
- Stearns MW, Jr, Deddish MR, Quan SHQ, Leaming RH. Preoperative roentgen therapy for cancer of the rectum and rectosigmoid. Surg Gynecol Obstet. 1974;138:584–586. [PubMed] [Google Scholar]
- Higgins GA, Jr, Conn JH, Jordan PH, Jr, Humphrey EW, Roswit B, Keehn RJ. Preoperative radiotherapy for colorectal cancer. Ann Surg. 1975;181:624–630. doi: 10.1097/00000658-197505000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebel W, Schulz U, Ried M, Erhard J, Beersiek F, Blöcher G, Nier H, Halama H, Scherer E, Zeller G, Eigler FW, Tacken J. Five-year results of a prospective randomized study: experience with combined radiotherapy and surgery of primary rectal carcinoma. Recent Results Cancer Res. 1988;110:111–113. doi: 10.1007/978-3-642-83293-2_16. [DOI] [PubMed] [Google Scholar]
- Kligerman MM, Urdaneta N, Knowlton A, Vidone R, Hartman PV, Vera R. Preoperative irradiation of rectosigmoid carcinoma including its regional lymph nodes. Am J Roengenol Radium Ther Nucl Med. 1972;114:498–503. doi: 10.2214/ajr.114.3.498. [DOI] [PubMed] [Google Scholar]
- Rider WD, Palmer JA, Mahoney LJ, Robertson CT. Preoperative irradiation in operable cancer of the rectum: report of the Toronto trial. Can J Surg. 1977;20:335–338. [PubMed] [Google Scholar]
- Second Report of an MRC Working Party The evaluation of low dose pre-operative x-ray therapy in the management of operable rectal cancer; results of a randomly controlled trial. Br J Surg. 1984;71:21–25. doi: 10.1002/bjs.1800710107. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Humphrey EW, Dwight RW, Roswit B, Lee LE, Jr, Keehn RJ. Preoperative radiation and surgery for cancer of the rectum. Veterans Administration Surgical Oncology Group Trial II. Cancer. 1986;58:352–359. doi: 10.1002/1097-0142(19860715)58:2<352::aid-cncr2820580226>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Dahl O, Horn A, Morild I, Halvorsen JF, Odland G, Reinertsen S, Reisaeter A, Kavli H, Thunold J. Low-dose preoperative radiation postpones recurrences in operable rectal cancer. Results of a randomized multicenter trial in western Norway. Cancer. 1990;66:2286–2294. doi: 10.1002/1097-0142(19901201)66:11<2286::aid-cncr2820661106>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Gérard A, Buyse M, Nordlinger B, Loygue J, Pene F, Kempf P, Bosset J-F, Gignoux M, Arnaud J-P, Desaive C, Duez N. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC) Ann Surg. 1988;208:606–614. doi: 10.1097/00000658-198811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Neto JA, Quilici FA, Reis JA., Jr A comparison of nonoperative vs. preoperative radiotherapy in rectal carcinoma. A 10-year randomized trial. Dis Colon Rectum. 1989;32:702–710. doi: 10.1007/BF02555778. [DOI] [PubMed] [Google Scholar]
- Illényi L, Grexa E, Gecser G, Kött I. Local recurrence of rectal cancer following preoperative irradiation. Acta Chir Hung. 1994;34:333–347. [PubMed] [Google Scholar]
- Goldberg PA, Nicholls RJ, Porter NH, Love S, Grimsey JE. Long-term results of a randomized trial of short-course low-dose adjuvant pre-operative radiotherapy for rectal cancer: reduction in local treatment failure. Eur J Cancer. 1994;30A:1602–1606. doi: 10.1016/0959-8049(94)00312-s. [DOI] [PubMed] [Google Scholar]
- Stockholm Rectal Cancer Study Group Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomized trial. Cancer. 1990;66:49–55. doi: 10.1002/1097-0142(19900701)66:1<49::aid-cncr2820660111>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Rectal Cancer Working Party Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet. 1996;348:1605–1610. doi: 10.1016/S0140-6736(96)05348-2. [DOI] [PubMed] [Google Scholar]
- Marsh PJ, James RD, Schofield PF. Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma. Results of a prospective, randomized trial. Dis Colon Rectum. 1994;37:1205–1214. doi: 10.1007/BF02257783. [DOI] [PubMed] [Google Scholar]
- Swedish Rectal Cancer Trial Improved survival with preoperative radiotherapy in resectable rectal cancer. New Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- Stockholm Colorectal Cancer Study Group Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996;3:423–430. doi: 10.1007/BF02305759. [DOI] [PubMed] [Google Scholar]
- Pahlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma: report from a randomized multicenter trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sause WT, Pajak TF, Noyes RD, Dobelbower R, Fischbach J, Doggett S, Mohiuddin M. Evaluation of preoperative radiation therapy in operable colorectal cancer. Ann Surg. 1994;220:668–675. doi: 10.1097/00000658-199411000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T, Petersen S, Hellmich G, Baumann M, Ludwig K. Delayed toxicity of brief preoperative irradiation and risk-adjusted postoperative radiotherapy of operative rectal carcinoma. Results of a randomized prospective study [German] Strahlentherapie und Onkologie. 1999;175:430–436. doi: 10.1007/s000660050032. [DOI] [PubMed] [Google Scholar]
- Boulis-Wassif S, Gerard A, Loygue J, Camelot D, Buyse M, Duez N. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone and in combination with 5-fluorouracil, followed by radical surgery. Trial of the European Organization on Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Cancer. 1984;53:1811–1818. doi: 10.1002/1097-0142(19840501)53:9<1811::aid-cncr2820530902>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine , Gerard J-P. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxi A, Cottone M. Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- Colorectal Cancer Collaborative Group Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomized trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–572. doi: 10.1007/BF02049863. [DOI] [PubMed] [Google Scholar]
- Kapiteijn E, Marijnen CAM, Nagtegaal ID, Putter H, Steup WH, Wiggers T, van Krieken JHJM, Hermans J, Leer JWH, van de Velde CJH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- Holm T, Singnomklao T, Rutqvist LE, Cedermark B. Adjuvant preoperative radiotherapy in patients with rectal carcinoma. Adverse effects during long term follow-up of two randomized trials. Cancer. 1996;78:968–976. doi: 10.1002/(SICI)1097-0142(19960901)78:5<968::AID-CNCR5>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dahlberg M, Glimelius B, Graf W, Pahlman L. Preoperative irradiation affects functional results after surgery for rectal cancer. Results from a randomized study. Dis Colon Rectum. 1998;41:543–551. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- Kollmorgen CF, Meagher AP, Wolff BG, Pemberton JH, Martenson JA, Ilstrup DM. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg. 1994;220:676–682. doi: 10.1097/00000658-199411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi BS, Tjandra JJ, Green MD. Morbidities of adjuvant chemotherapy and radiotherapy for resectable rectal cancer: an overview. Dis Colon Rectum. 1999;42:403–418. doi: 10.1007/BF02236362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


