Abstract
We investigated the roles of cortical microtubules in gravity-induced modifications to the development of stem organs by analyzing morphology and orientation of cortical microtubule arrays in hypocotyls of Arabidopsis (Arabidopsis thaliana) tubulin mutants, tua3(D205N), tua4(S178Δ), and tua6(A281T), cultivated under 1g and hypergravity (300g) conditions. Hypocotyls of tubulin mutants were shorter and thicker than the wild type even at 1g, and hypergravity further suppressed elongation and stimulated expansion. The degree of such changes was clearly smaller in tubulin mutants, in particular in tua6. Hypocotyls of tubulin mutants also showed either left-handed or right-handed helical growth at 1g, and the degree of twisting phenotype was intensified under hypergravity conditions, especially in tua6. Hypergravity induced reorientation of cortical microtubules from transverse to longitudinal directions in epidermal cells of wild-type hypocotyls. In tubulin mutants, especially in tua6, the percentage of cells with longitudinal microtubules was high even at 1g, and it was further increased by hypergravity. The twisting phenotype was most obvious at cells 10 to 12 from the top, where reorientation of cortical microtubules from transverse to longitudinal directions occurred. Moreover, the left-handed helical growth mutants (tua3 and tua4) had right-handed microtubule arrays, whereas the right-handed mutant (tua6) had left-handed arrays. There was a close correlation between the alignment angle of epidermal cell files and the alignment of cortical microtubules. Gadolinium ions, blockers of mechanosensitive ion channels (mechanoreceptors), suppressed the twisting phenotype in tubulin mutants under both 1g and 300g conditions. Microtubule arrays in tubulin mutants were oriented more transversely by gadolinium treatment, irrespective of gravity conditions. These results support the hypothesis that cortical microtubules play an essential role in maintenance of normal growth phenotype against the gravitational force, and suggest that mechanoreceptors are involved in modifications to morphology and orientation of microtubule arrays by 1g gravity and hypergravity in tubulin mutants.
The direction of cell expansion is important for determining the shape of whole plant body. Cortical microtubules are assumed to be responsible for anisotropic expansion of plant cells (Wasteneys and Galway, 2003; Lloyd and Chan, 2004; Mathur, 2004; Baskin, 2005; Paredez et al., 2008). The prevailing view is that cortical microtubule arrays direct or constrain the movement of the cellulose synthase complexes and thus align nascent cellulose microfibrils in the same direction in the innermost layer of the cell wall (Baskin, 2001), although some other mechanisms may also be involved (Baskin, 2001; Sugimoto et al., 2003; Wasteneys, 2004).
It is evident that orientation of cortical microtubules plays an essential role in creating the distinct shape of higher plant organs, even if there is uncertainty over the mechanism by which microtubules influence morphogenesis. The importance of cortical microtubule arrays for anisotropic growth has been documented by pharmacological studies and experiments with helical growth mutants of Arabidopsis (Arabidopsis thaliana). Mutants on α- and β-tubulins as well as microtubule-associated proteins show either left-handed or right-handed helical growth (Thitamadee et al., 2002; Nakajima et al., 2004; Sedbrook et al., 2004; Shoji et al., 2004). The rapidly elongating cells of these mutants skew consistently either to the right or to the left and exhibit cortical microtubule arrays that form shallow helices with fixed handedness (Thitamadee et al., 2002; Abe and Hashimoto, 2005; Ishida et al., 2007). Cortical microtubule arrays in the left-handed helical growth mutants form right-handed helix, whereas those in right-handed helical growth mutants form left-handed helix (Thitamadee et al., 2002; Abe and Hashimoto, 2005; Ishida et al., 2007). These results indicate that dysfunctional cortical microtubules are arranged in helical arrays and affect the direction of cell expansion.
The gravitational force is one of the environmental factors that determine the plant body shape. Under hypergravity conditions produced by centrifugation, plants generally have a shorter and thicker body (Soga et al., 2006). Namely, hypergravity modifies growth anisotropy. In Arabidopsis hypocotyls, the expression of most α- and β-tubulin genes was up-regulated by hypergravity (Yoshioka et al., 2003; Matsumoto et al., 2007). In protoplasts of Brassica hypocotyls, hypergravity stimulated the regeneration of cortical microtubules into parallel arrays (Skagen and Iversen, 1999), and in azuki bean (Vigna angularis) epicotyls it increased the percentage of cells with longitudinal cortical microtubules (Soga et al., 2006). The reorientation of cortical microtubules from transverse to longitudinal directions may be involved in modifications by hypergravity to growth anisotropy.
The aim of this study was to clarify the roles of cortical microtubules in gravity-induced modifications to development of stem organs. For this purpose, we examined the changes in growth, morphology, and orientation of cortical microtubule arrays in hypocotyls of Arabidopsis amino acid substitution mutants in α-tubulin structure, tua3, tua4, and tua6, grown under 1g and 300g conditions. We have reported the possible involvement of mechanosensitive ion channels (mechanoreceptors) in hypergravity-induced modifications to growth and cell wall properties (Soga et al., 2004, 2005, 2006). Thus, we also examined the effect of blockers of mechanoreceptors on helical growth and orientation of cortical microtubule arrays in the tubulin mutants.
RESULTS
Effects of Hypergravity on Growth
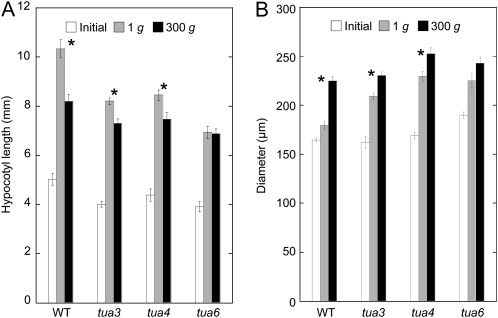
Elongation growth of Arabidopsis hypocotyls was suppressed by hypergravity at 300g (Fig. 1A). In tubulin mutants, elongation growth was suppressed even under 1g conditions: The length of hypocotyls in tubulin mutants was 60% to 70% of that in wild type at 1g. The degree of hypergravity-induced suppression of elongation growth was smaller in tubulin mutants than in wild type. In particular in tua6, elongation growth was not further suppressed by hypergravity (Fig. 1A). On the other hand, lateral thickening of Arabidopsis hypocotyls was stimulated by hypergravity at 300g (Fig. 1B). In tubulin mutants, the thickening of hypocotyls was stimulated even under 1g conditions: The diameter of hypocotyls in tubulin mutants was 115% to 125% of that in wild type at 1g. The degree of hypergravity-induced lateral thickening was smaller in tubulin mutants than in wild type. In particular in tua6, lateral thickening was not further stimulated by hypergravity (Fig. 1B).
Figure 1.
Effects of hypergravity on elongation growth and lateral thickening in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown on agar medium at 1g for 48 h at 25°C. Seedlings were then transferred to 1g or 300g conditions, and grown for a further 24 h at 25°C. A, The length was measured using a scale. B, The diameter of hypocotyls was measured with a digital stereoscopic microscope. Values are means ± se (n = 20). *, Mean values with significant differences between 1g and 300g treatments (P < 0.05).
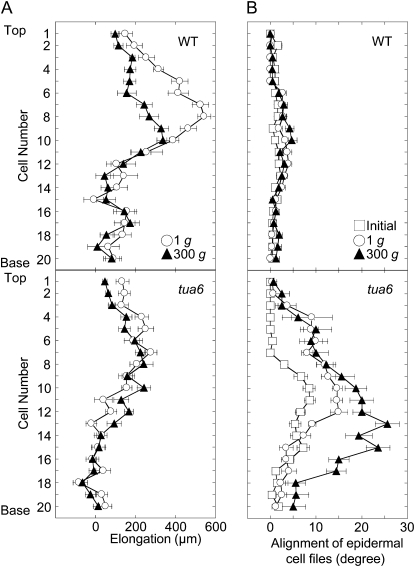
The length of individual epidermal cells within a cell file was measured with a scanning electron microscope (SEM) at 48 and 72 h, and elongation of each cell for 24 h was calculated (Fig. 2A). Elongation occurred in the upper 12 cells, especially at cells five to 10 from the top in wild type and at cells four to eight in tua6. Elongation area was somewhat dispersed under hypergravity conditions.
Figure 2.
Cell elongation and twisting profiles in an epidermal cell file in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tua6 were grown as in Figure 1. A, The length of individual cells from the top (cell 1) to the base (cell 20) was measured with SEM and elongation for 24 h was calculated. B, The alignment angle of individual cells was measured with SEM. Values are means ± se (n = 15).
Effects of Hypergravity on Cell Alignment
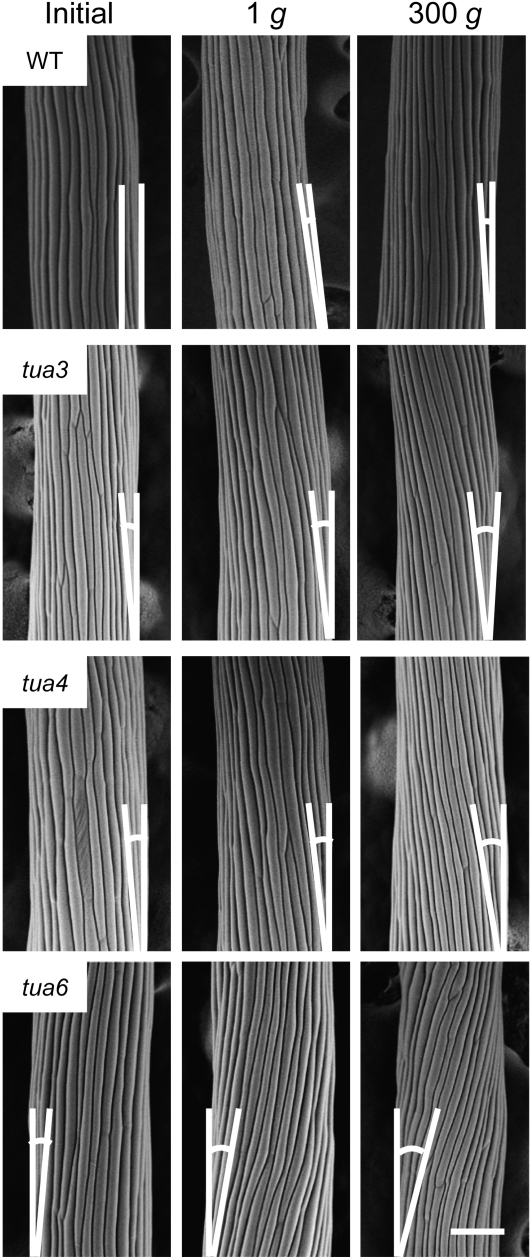
Wild-type hypocotyls grew almost straight irrespective of gravity conditions. On the other hand, hypocotyls of tubulin mutants showed either left-handed (tua3 and tua4) or right-handed (tua6) helical growth (Fig. 3). The alignment angle of individual epidermal cells within a cell file was measured with SEM in tua6 (Fig. 2B). The alignment angle increased from cells eight to 10, and was kept almost constant at the following several cells under both 1g and hypergravity conditions. Hypergravity increased the length of area where cell twisting occurred. The alignment angle of epidermal cells then decreased toward the base, probably because the twisting did not occur in the early growth phase of hypocotyls.
Figure 3.
Surface view of epidermal cell files in Arabidopsis tubulin mutants grown at 1g or 300g. Wild type (WT) and tubulin mutants were grown as in Figure 1. The outer surface of cells 10 to 12 from the top of hypocotyls was observed with SEM. Supplementary lines denote the longitudinal axis of hypocotyls and the alignment of epidermal cell files, for measuring the alignment angle. The bar denotes 100 μm.
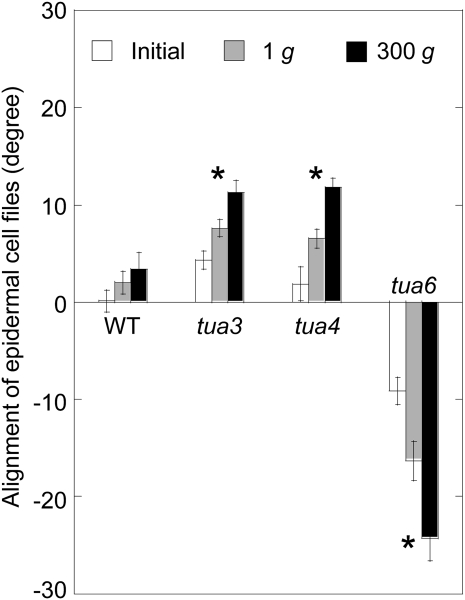
The twisting phenotype was quantified by measuring the alignment angle of cells 10 to 12 where the angle was almost constant (Fig. 4). Hypocotyls of tubulin mutants showed twisting at 1g, and the degree of twisting phenotype was intensified under hypergravity conditions. In particular, the alignment angle of epidermal cell files was large in tua6 even at 1g, which was further increased by hypergravity (Fig. 4).
Figure 4.
Effects of hypergravity on alignment angle of epidermal cell files in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown as in Figure 1, and the angle of cells 10 to 12 to the longitudinal axis was measured using a protractor, as in Figure 3. Values are means ± se (n = 20). *, Mean values with significant differences between 1g and 300g treatments (P < 0.05).
Effects of Hypergravity on Alignment of Cortical Microtubules
In epidermal cells in the upper half of 48-h-old etiolated hypocotyls, cortical microtubules adjacent to the outer tangential wall were predominantly transverse to the long axis of the cell, whereas cells with longitudinal microtubules were predominant in the lower half. During growth for the following 24 h, reorientation of cortical microtubules from transverse to longitudinal directions occurred in just above the middle of hypocotyls (cells 10–12 from the top), with no changes in orientation in other regions. The results match with the report by Le et al. (2005).
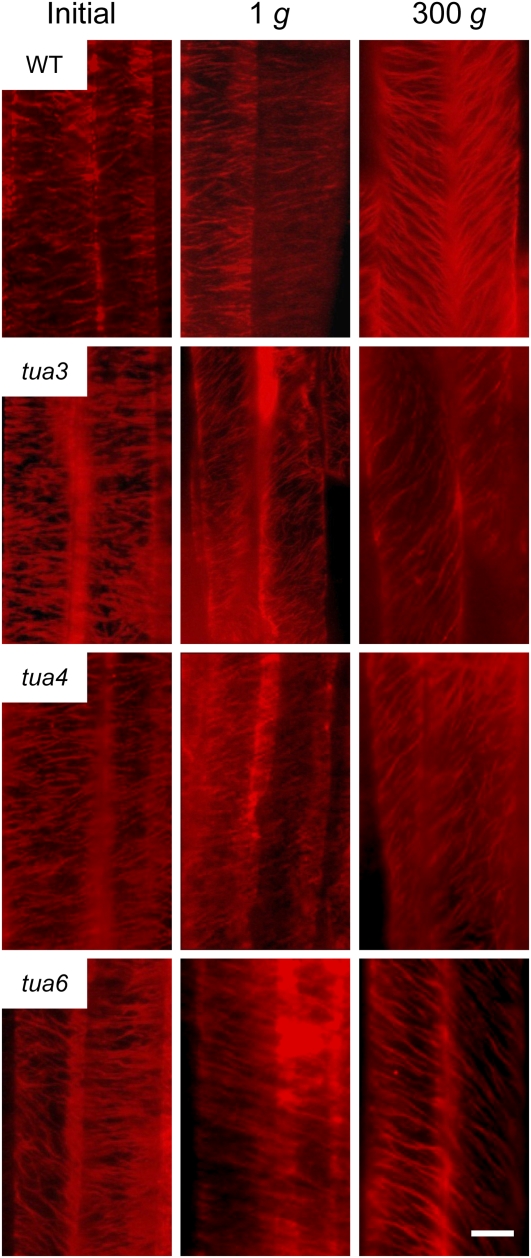
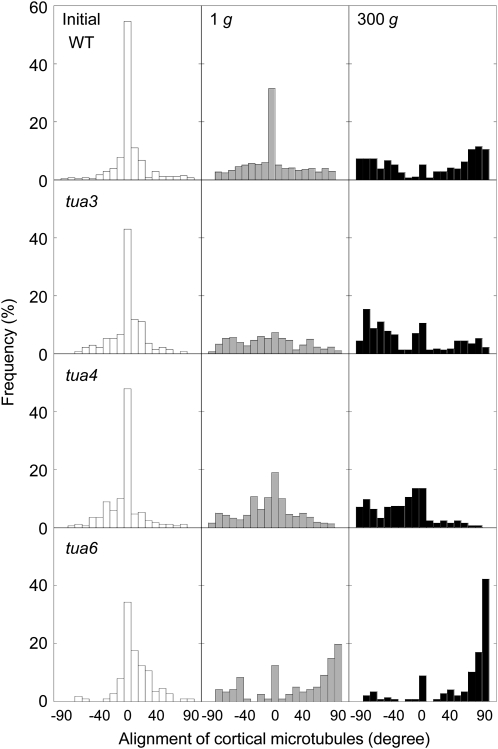
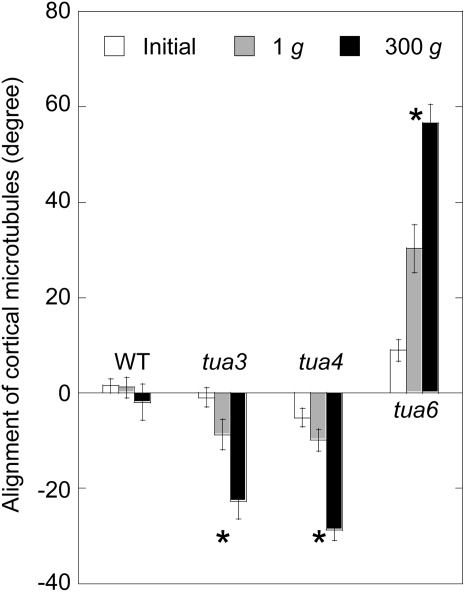
We analyzed the alignment of cortical microtubules at cells 10 to 12 in detail (Figs. 5–7). In wild type, the percentage of cells having transverse microtubules was slightly decreased during growth. Hypergravity greatly stimulated reorientation of microtubules from transverse to longitudinal directions in wild type, without any preference to the left or right direction (Fig. 6). In the left-handed helical growth mutants (tua3 and tua4), the frequency distribution was dispersed with a bias toward right-handed microtubule arrays at 1g (Figs. 6 and 7). The percentage of cells with right-handed microtubule arrays was greatly increased by hypergravity. On the other hand, the frequency distribution of microtubule orientation was dispersed with a bias toward left-handed arrays in the right-handed helical growth mutant tua6 at 1g, which was further stimulated by hypergravity (Figs. 6 and 7).
Figure 5.
Immunofluorescence images of cortical microtubules in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown as in Figure 1. Epidermal cells 10 to 12 were stained as described in “Materials and Methods.” Typical examples of two adjacent cells with distinct orientation of cortical microtubules are shown. The bar denotes 10 μm.
Figure 6.
Frequency distribution of alignment of cortical microtubules in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown as in Figure 1. All values were taken from immunofluorescence micrographs at cells 10 to 12, as shown in Figure 5. Data were obtained from epidermal cells in 10 different hypocotyls and normalized as percentage.
Figure 7.
Effects of hypergravity on alignment of cortical microtubules in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown as in Figure 1. Values are mean alignment angle of cortical microtubules measured in Figure 6 ± se (n = 114–334). *, Mean values with significant differences between 1g and 300g treatments (P < 0.05).
Effects of Gadolinium Ions on Alignments of Cell Files and Cortical Microtubules
We examined the effects of blockers of mechanoreceptors on phenotypes of tubulin mutants. Gadolinium ions up to 30 μm had no effect on elongation growth of hypocotyls in the wild type or tua6 mutant (Supplemental Fig. S1). In the presence of 100 μm gadolinium ions, elongation growth was suppressed and abnormal growth was induced. Gadolinium ions did not clearly influence the alignment angle of epidermal cell files in the wild type, but in tua6 they decreased the alignment angle by increasing the concentrations (Supplemental Fig. S1). Lanthanum ions had similar effects on elongation growth and the alignment of cell files (data not shown). Thus, we used gadolinium ions at 30 μm in the following experiments.
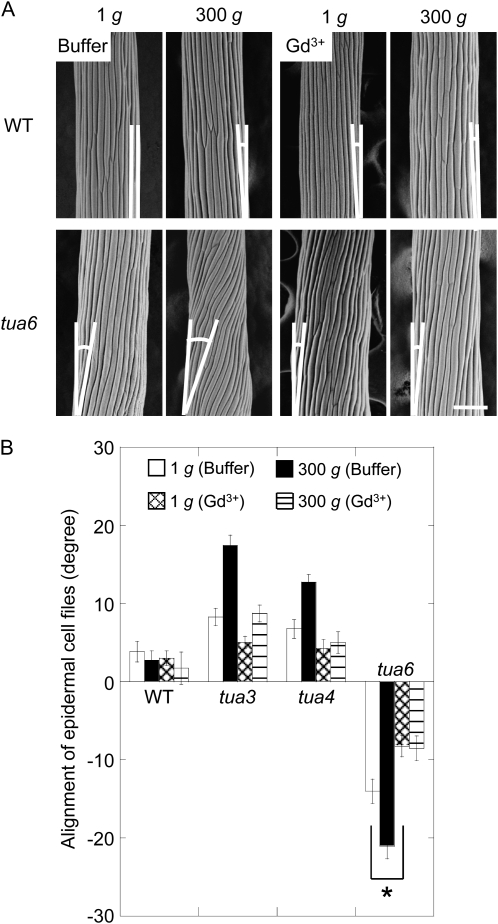
Hypocotyls of tubulin mutants showed either left-handed or right-handed helical growth at 1g. In the presence of gadolinium ions, the alignment angle of epidermal cell files in the mutants was decreased (Fig. 8). The twisting phenotype of tubulin mutants was intensified under hypergravity conditions. Hypergravity had no effect on the alignment angle of epidermal cell files in the presence of gadolinium ions (Fig. 8).
Figure 8.
Effects of gadolinium ions on alignment of epidermal cell files in hypocotyls of Arabidopsis tubulin mutants. Arabidopsis seedlings were grown at 1g or 300g in the presence (Gd3+) or absence (buffer) of 30 μm gadolinium chloride for 72 h at 25°C. A, The outer surface of cells 10 to 12 was observed as in Figure 3. The bar denotes 100 μm. B, The angle of epidermal cell files was measured as in Figure 4. Values are means ± se (n = 20). WT, Wild type.
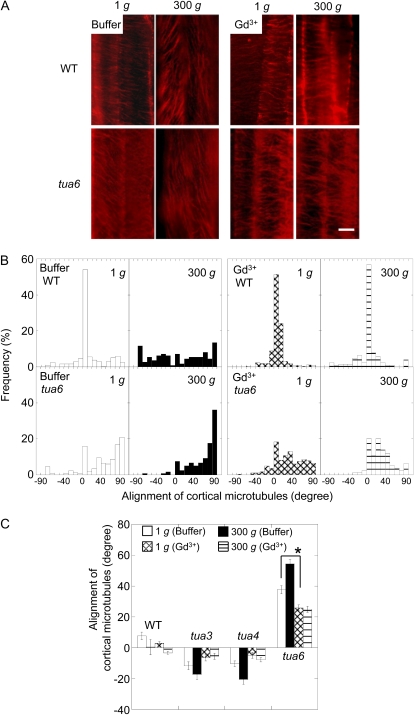
Gadolinium ions did not influence the alignment of cortical microtubules in hypocotyls of wild type at 1g (Fig. 9). Hypergravity-stimulated reorientation of microtubules from transverse to longitudinal directions in the wild type was strongly inhibited by gadolinium ions (Fig. 9). On the other hand, the left-handed helical growth mutants had right-handed microtubule arrays, whereas the right-handed mutant had left-handed arrays, and the alignment angle of microtubule arrays was increased by hypergravity. Gadolinium ions induced reorientation of microtubule arrays from right- or left-handed inclination to transverse directions under both 1g and hypergravity conditions (Fig. 9; Supplemental Fig. S2).
Figure 9.
Effects of gadolinium ions on alignment of cortical microtubules in hypocotyls of Arabidopsis tubulin mutants. Wild type (WT) and tubulin mutants were grown as in Figure 8. A, Immunofluorescence images of cortical microtubules. Epidermal cells 10 to 12 were stained as described in “Materials and Methods.” B, Frequency distribution of alignment of cortical microtubules. All values were taken from immunofluorescence micrographs at cells 10 to 12. C, Mean alignment angle of cortical microtubules measured in B ± se (n = 111–278).
DISCUSSION
Hypocotyls of tubulin mutants showed either left-handed or right-handed helical growth, at 1g (Figs. 3 and 4), as observed in other organs such as flower petals, rosette leaves, and roots (Hashimoto, 2002; Thitamadee et al., 2002). Hypergravity further intensified the twisting phenotype of the mutants, in particular in tua6 (Figs. 3 and 4). Helical growth of Arabidopsis hypocotyls was also induced by propyzamide and colchicine even at 1g (Furutani et al., 2000; Matsumoto et al., 2007), and further intensified by hypergravity (Matsumoto et al., 2007). Thus, helical growth may be induced by disintegration of or modification to the structure of cortical microtubules under influence of the gravitational force. In hypocotyls, the left-handed helical growth mutants had right-handed microtubule arrays, whereas the right-handed mutant had left-handed arrays (Figs. 5–7), as observed in root epidermis of tubulin mutants (Ishida et al., 2007). The inclination of epidermal cell files was most obvious at cells 10 to 12 where reorientation of cortical microtubules from transverse to longitudinal directions occurred. The degree of inclination of microtubule arrays was further increased by hypergravity (Figs. 5–7), and there was a close correlation (r = −0.97) between the degree of helical growth and alignment angle of cortical microtubules. These results suggest that reorientation of cortical microtubules from transverse to longitudinal directions without any bias toward the left or the right induces only simple modification to growth anisotropy, but if reorientation occurs with a bias toward either direction, helical growth may be induced.
Gadolinium and lanthanum ions have been used as blockers of mechanosensitive ion channels in various materials (Ding and Pickard, 1993; Fasano et al., 2002). Both blockers are capable of nullifying hypergravity-induced modifications to growth anisotropy and cell wall properties, as well as reorientation of cortical microtubules (Soga et al., 2004, 2005, 2006). If mechanoreceptors are involved in gravity-dependent modifications to morphology and orientation of microtubule arrays in tubulin mutants, these phenotypes are expected to be suppressed by the blockers. Actually, gadolinium ions decreased the alignment angle of epidermal cell files and that of cortical microtubules of tubulin mutants at 1g (Figs. 8 and 9). Moreover, hypergravity had no effect on the alignment angles of cell files or cortical microtubules in the presence of gadolinium ions (Figs. 8 and 9). These results suggest that mechanoreceptors are involved in modifications to morphology and orientation of microtubule arrays by 1g gravity and hypergravity in tubulin mutants.
Hypergravity suppressed elongation growth and increased lateral expansion of hypocotyls in wild-type Arabidopsis (Fig. 1), as reported in azuki bean epicotyls (Soga et al., 2006). Hypocotyls of tubulin mutants were shorter and thicker than the wild type even at 1g, and hypergravity further suppressed elongation and stimulated expansion (Fig. 1). However, the degree of such changes was clearly smaller in tubulin mutants; in particular in tua6 no further changes were induced by hypergravity, even if they still elongate significantly at 1g (Fig. 1). It is possible that suppression of elongation growth and stimulation of lateral expansion were simply caused by an inclination of cell files. However, inclination of cell files as well as reorientation of cortical microtubules was most obvious at cells 10 to 12 from the top, where active elongation growth has almost ceased (Fig. 2). In addition, the calculated decrease in longitudinal length of hypocotyls based on the pitch angle of inclined cell files (maximally 7%) is too small for the measured decrease (maximally 33%). Moreover, gadolinium ions suppressed the twisting growth phenotype and orientation of microtubule arrays toward the left or the right (Figs. 8 and 9), but they did not influence elongation growth in tubulin mutants (Supplemental Fig. S3). Thus, helical growth may not be the direct cause of modification to growth anisotropy in tubulin mutants. The mechanism of modification to growth anisotropy in tubulin mutants remains to be clarified.
In the mutants used in this study, mutant tubulin proteins are incorporated into the microtubule polymer and produce dominant-negative effects (Ishida et al., 2007). The left-handed helical growth mutant tua4(S178Δ) has one amino acid deletion in a loop preceding helix 5 at the intradimer between α- and β-tubulins (Löwe et al., 2001). On the other hand, the lateral association between the protofilaments is mediated largely by the M loop and its interacting regions of the adjacent protofilament (Nogales et al., 1999), and mutations at the region, such as tua6(A281T), led mostly to right-handed helical growth (Ishida et al., 2007). In addition, when GFP or hemaglutinin epitope tag was fused to the N terminus of α- or β-tubulin molecules, plants expressing the modified β-tubulins were phenotypically normal and possessed transversely oriented cortical arrays in the epidermal cells of root elongation zone, whereas the expression of modified α-tubulins caused helical growth (Abe and Hashimoto, 2005). N terminus of α-tubulin is positioned close to loop T7 at the tubulin interdimer interface, where α-tubulin acts to stimulate GTP hydrolysis in β-tubulin (Nogales et al., 1999; Nogales, 2000; Li et al., 2002). The twisting phenotype of these mutants was intensified by hypergravity (Figs. 3 and 4). Thus, the longitudinal interface between α- and β-tubulins and the lateral interface between two adjacent protofilaments may be important for normal organization of microtubules and sensitivity to the gravitational force. Out of three mutants used in this study, the twisting phenotype and inclination of cortical microtubules were most clearly observed in tua6. The expression of TUA6 was most prominently up-regulated under hypergravity conditions among six α-tubulin genes (Matsumoto et al., 2007), which may be related to strong phenotype in tua6.
Plants generally have a shorter and thicker body under hypergravity conditions (Soga et al., 2006). On the other hand, elongation growth of Arabidopsis hypocotyls and rice (Oryza sativa) coleoptiles was stimulated under microgravity conditions in space (Hoson et al., 2002; Soga et al., 2002). Growth parameters such as elongation rate of Arabidopsis hypocotyls varied in proportion to the logarithm of the magnitude of gravity in the range from microgravity to hypergravity (Soga et al., 2001; Hoson and Soga, 2003). Plant stems are rather resistant to gravity stimuli, and in this study we used 300g to induce clear effects. However, hypergravity of 300g brings about only half of the changes in growth parameters, as compared with microgravity in space (approximately 10−4g). Moreover, exposure to 300g had no effect on growth in the presence of lanthanum or gadolinium ions, which are blockers of mechanosensitive ion channels (Soga et al., 2004, 2005; Supplemental Fig. S3). These results indicate that 300g is not an excessive stimulus, because hypocotyls respond to this magnitude of force physiologically normal (Hoson and Soga, 2003). Some other organs are more sensitive to gravity stimuli; for instance, growth of pollen tubes was clearly suppressed by hypergravity at 4g (Musgrave et al., 2009). According to the nature of dose response mentioned above, hypergravity at 4g is estimated to bring about a quarter of the changes in growth parameters, as compared with 300g.
In this study, we found that hypocotyls of Arabidopsis tubulin mutants showed helical growth even at 1g, which was further intensified by 300g (Figs. 3 and 4). Such modifications to development of hypocotyls by gravity and by tubulin mutation were accompanied by reorientation of cortical microtubules from transverse to longitudinal directions with a bias toward the left or the right direction (Figs. 5–7). Furthermore, these phenotypes of tubulin mutants were clearly suppressed by blockers of mechanoreceptors not only at 300g but also at 1g (Figs. 8 and 9). These results suggest that tubulin mutants are hypersensitive to the gravitational force and the effects of gravity are saturated at lower doses. To confirm this possibility, we carried out a space experiment termed Resist Wall in the European Modular Cultivation System on the International Space Station. It was expected that under microgravity condition in space, the defects of growth in tubulin mutants were rescued and they could grow and develop more or less normally (Hoson et al., 2007). Unfortunately, we faced great difficulties in initial watering because of serious anomalies of water supply system and no plants developed to the expected developmental stage (Hoson et al., 2009). We are preparing for the next space experiment to verify the hypothesis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Three mutants of Arabidopsis (Arabidopsis thaliana ‘Columbia’) were used in this study: tua3 has the Asp-205-to-Asn mutation in the TUA3, tua4 has the deletion of Ser-180 in the TUA4, and tua6 has the Ala-281-to-Thr mutation in the TUA6 protein. The details of these mutations were as described previously (Ishida et al., 2007). The mutation of TUA4 is a precise one-amino acid deletion in a loop preceding Helix 5 (Löwe et al., 2001) and situates very close to the mutated residue in lefty1 and lefty2 (Thitamadee et al., 2002). The mutation of TUA6 is at the lateral contact regions between the microtubule protofilaments mediated largely by the M loop and its interacting regions of the adjacent protofilament (Nogales et al., 1999). The mutation of TUA3 did not map to particular functional regions defined by the structural analysis. These seeds were sterilized in 2% (v/v) sodium hypochlorite solution for 1 min, and then washed thoroughly with water. The sterilized seeds were planted on 1.5% (w/v) agar medium in a 25 mL centrifuge tube, kept at 4°C for 2 d, and exposed to white light (5 W m−2 at seed level) for 6 h to induce germination. For gadolinium treatment, the sterilized seeds were planted on 1.5% (w/v) agar containing 1 mm MES-KOH buffer (pH 6.0) with various concentrations of gadolinium chloride. Seeds were then incubated in the dark at 25°C.
To analyze the growth behavior, we exposed the seedlings to hypergravity at 300g for 24 h at 25°C in the dark with a centrifuge (H-28-F; Kokusan). After the treatment, we measured the length of hypocotyls using a scale, and the thickness of hypocotyls with a digital multiangle stereoscopic microscope system (VB-G25; Keyence). SEM (TM-1000; Hitachi) was used to measure the angle of epidermal cell files to the longitudinal axis of hypocotyls. Because cells 10 to 12 from the top showed typical helical growth, we used this region for measurements of the angle of the cortical cell line. In the measurement, the longitudinal axis of hypocotyls was defined as 0°. The cortical cell lines aligned at angles between 0° and 90° (counterclockwise from the top) form left-handed helical growth, whereas those aligned at angles between 0° and −90° form right-handed helical growth.
Immunofluorescence Microscopy
Whole seedlings were fixed with a mixture of 1.5% (v/v) formaldehyde and 0.5% (v/v) glutaraldehyde in PEMT buffer (50 mm PIPES, 2 mm EGTA, 2 mm MgSO4, 0.05% [v/v] Triton X-100, pH 7.2) for 40 min and rinsed three times in PEMT buffer. They were subsequently treated with 0.05% (w/v) Pectolyase Y-23 (Kyowa Chemical Products) and 0.05% (w/v) Cellulase Y-C (Kyowa Chemical Products) in PEMT buffer with 0.4 m mannitol for 20 min at 30°C, and then rinsed three times in PEMT buffer. Seedlings were treated with methanol at −20°C for 10 min, and rehydrated in phosphate-buffered saline (PBS) for 10 min. Autofluorescence caused by free aldehydes from glutaraldehyde fixation was reduced with 1 mg mL−1 NaBH4 in PBS for 15 min, followed by the treatment with 1% (w/v) bovine serum albumin (BSA) in incubation buffer (50 mm glycine in PBS) for 30 min. After incubating in 0.5% Triton X-100 for 1 h, seedlings were incubated with primary antibodies against α-tubulin (product T6199, Sigma) diluted 1:1,000 (v/v) in 1% BSA at 30°C for 16 h, and rinsed three times with incubation buffer. Seedlings were then incubated with Cy3-conjugated anti-mouse IgG (product C2181, Sigma) diluted 1:100 in (v/v) in 1% BSA for 3 h at 37°C, and rinsed three times with PBS. They were mounted on a slide glass and covered with a solution containing 90% (v/v) glycerol. Immunofluorescence images were collected with a fluorescence microscope (Axioskop 2 plus; Carl Zeiss) equipped with a cooled CCD camera (VB-7000, Keyence), and processed with bundled image processing software (BZ-H1A, Keyence).
Quantitative analysis of cortical microtubule alignment was carried out, as reported by Sugimoto et al. (2003). In measurement of cortical microtubule alignment, transverse orientation was defined as 0° to the long axis of the hypocotyls. The cortical microtubules aligned at angles between 0° and 90° (clockwise from 0°) form left-handed helix, whereas those aligned at angles between 0° and −90° (counterclockwise from 0°) form right-handed helix.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers M17189 (TUA3), M84697 (TUA4), and M84699 (TUA6).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of gadolinium ions on elongation growth and alignment of epidermal cell files in hypocotyls of Arabidopsis tubulin mutants.
Supplemental Figure S2. Effects of gadolinium ions on alignment of cortical microtubules in hypocotyls of Arabidopsis tubulin mutants.
Supplemental Figure S3. Effects of gadolinium ions on elongation growth in hypocotyls of Arabidopsis tubulin mutants.
Supplementary Material
Acknowledgments
We thank Dr. Sumiko Kaihara for helpful advice in preparing the manuscript.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (grant no. 17510159) and a Grant for Ground-Based Research for Space Utilization from the Japan Space Forum (to T. Hoson).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Takayuki Hoson (hoson@sci.osaka-cu.ac.jp).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe T, Hashimoto T (2005) Altered microtubule dynamics by expression of modified α-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants. Plant J 43 191–204 [DOI] [PubMed] [Google Scholar]
- Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215 150–171 [DOI] [PubMed] [Google Scholar]
- Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21 203–222 [DOI] [PubMed] [Google Scholar]
- Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3 83–110 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Massa GD, Gilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21 71–88 [DOI] [PubMed] [Google Scholar]
- Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T (2000) The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 127 4443–4453 [DOI] [PubMed] [Google Scholar]
- Hashimoto T (2002) Molecular genetic analysis of left-right handedness in plants. Philos Trans R Soc Lond B Biol Sci 357 799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T, Matsumoto S, Soga K, Wakabayashi K, Hashimoto T, Sonobe S, Muranaka T, Kamisaka S, Kamada M, Omori K, et al (2007) The outline and significance of the Resist Wall experiment: role of microtubule-membrane-cell wall continuum in gravity resistance in plants. Biol Sci Space 21 56–61 [Google Scholar]
- Hoson T, Matsumoto S, Soga K, Wakabayashi K, Hashimoto T, Sonobe S, Muranaka T, Kamisaka S, Kamada M, Omori K, et al (2009) Growth and cell wall properties in hypocotyls of Arabidopsis tua6 mutant under microgravity conditions in space. Biol Sci Space 23 71–76 [Google Scholar]
- Hoson T, Soga K (2003) New aspects of gravity responses in plant cells. Int Rev Cytol 229 209–244 [DOI] [PubMed] [Google Scholar]
- Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, Kamisaka S (2002) Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol 43 1067–1071 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kaneko Y, Iwano M, Hashimoto T (2007) Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc Natl Acad Sci USA 104 8544–8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, De Cnodder T, Van der Straeten D, Verbelen JP (2005) Cell elongation and microtubule behavior in the Arabidopsis hypocotyl: responses to ethylene and auxin. J Plant Growth Regul 24 166–178 [Google Scholar]
- Li H, DeRoiser DJ, Nicholson WV, Nogales E, Dowinng KH (2002) Microtubule structure at 8 Å resolution. Structure 10 1317–1328 [DOI] [PubMed] [Google Scholar]
- Lloyd C, Chan J (2004) Microtubules and the shape of plants to come. Nat Rev Mol Cell Biol 5 13–22 [DOI] [PubMed] [Google Scholar]
- Löwe J, Li H, Downing KH, Nogales E (2001) Refined structure of αβ-tubulin at 3.5 A resolution. J Mol Biol 313 1045–1057 [DOI] [PubMed] [Google Scholar]
- Mathur J (2004) Cell shape development in plants. Trends Plant Sci 9 583–590 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Saito Y, Kumasaki S, Soga K, Wakabayashi K, Hoson T (2007) Up-regulation of expression of tubulin genes and roles of microtubules in hypergravity-induced growth modification in Arabidopsis hypocotyls. Adv Space Res 39 1176–1181 [Google Scholar]
- Musgrave ME, Kuang A, Allen J, van Loon JJWA (2009) Hypergravity prevents seed production in Arabidopsis by disrupting pollen tube growth. Planta 230 863–870 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Furutani I, Tachimoto H, Matsubara K, Hashimoto T (2004) SPIRAL1 encodes a plant-specific microtubule localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16 1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E (2000) Structural insights into microtubule function. Annu Rev Biochem 69 277–302 [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Dowing KH (1999) High-resolution model of the microtubules. Cell 96 79–88 [DOI] [PubMed] [Google Scholar]
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR (2008) Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol 147 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Ehrhardt DW, Fisher SE, Scheible WR, Somerville CR (2004) The Arabidopsis sku6/spiral1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 16 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Narita N, Hayashi K, Asada J, Hamada T, Sonobe S, Nakajima K, Hashimoto T (2004) Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol 136 3933–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagen EB, Iversen TH (1999) Simulated weightlessness and hyper-g results in opposite effects on the regeneration of the cortical microtubule array in protoplasts from Brassica napus hypocotyls. Physiol Plant 106 318–325 [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Hoson T, Kamisaka S (2001) Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Adv Space Res 27 1011–1016 [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T (2002) Stimulation of elongation growth and xyloglucans breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 215 1040–1046 [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T (2004) Graviperception in growth inhibition of plant shoots under hypergravity conditions produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta 218 1054–1061 [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T (2005) Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Funct Plant Biol 32 175–179 [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T (2006) Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta 224 1485–1494 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO (2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15 1414–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T (2002) Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417 193–196 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO (2004) Progress in understanding the role of microtubules in plant cells. Curr Opin Plant Biol 7 651–660 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Galway ME (2003) Remodeling the cytoskeleton for growth and form: an overview with some new views. Annu Rev Plant Biol 54 691–722 [DOI] [PubMed] [Google Scholar]
- Yoshioka R, Soga K, Wakabayashi K, Takeba G, Hoson T (2003) Hypergravity-induced changes in gene expression in Arabidopsis hypocotyls. Adv Space Res 31 2187–2193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.