Abstract
The kccDHS1 allele of kazachoc (kcc) was identified as a seizure-enhancer mutation exacerbating the bang-sensitive (BS) paralytic behavioral phenotypes of several seizure-sensitive Drosophila mutants. On their own, young kccDHS1 flies also display seizure-like behavior and demonstrate a reduced threshold for seizures induced by electroconvulsive shock. The product of kcc shows substantial homology to KCC2, the mammalian neuronal K+–Cl− cotransporter. The kccDHS1 allele is a hypomorph, and its seizure-like phenotype reflects reduced expression of the kcc gene. We report here that kcc functions as a K+–Cl− cotransporter when expressed heterologously in Xenopus laevis oocytes: under hypotonic conditions that induce oocyte swelling, oocytes that express Drosophila kcc display robust ion transport activity observed as a Cl−-dependent uptake of the K+ congener 86Rb+. Ectopic, spatially restricted expression of a UAS-kcc+ transgene was used to determine where cotransporter function is required in order to rescue the kccDHS1 BS paralytic phenotype. Interestingly, phenotypic rescue is largely accounted for by targeted, circumscribed expression in the mushroom bodies (MBs) and the ellipsoid body (EB) of the central complex. Intriguingly, we observed that MB induction of kcc+ functioned as a general seizure suppressor in Drosophila. Drosophila MBs have generated considerable interest especially for their role as the neural substrate for olfactory learning and memory; they have not been previously implicated in seizure susceptibility. We show that kccDHS1 seizure sensitivity in MB neurons acts via a weakening of chemical synaptic inhibition by GABAergic transmission and suggest that this is due to disruption of intracellular Cl− gradients in MB neurons.
Mushroom body (MB) expression of the kazachoc (kcc) K+–Cl− cotransporter is shown here to rescue seizure-sensitive phenotypes in Drosophila through an effect on GABAergic fast synaptic inhibition. Heretofore, considerable interest has focused on the MB because of its essential role in olfactory learning and memory (Heisenberg 2003; Davis 2005; Keene and Waddell 2007; Berry et al. 2008). The MB occupies a central position in the fly nervous system, integrating incoming olfactory, mechanical, taste, and visual sensory signals and then sorting the distribution of outgoing motor signals (Heisenberg 2003). Short- and long-term alteration of individual nerve cell physiology in the MB is thought to form the basis of learning and memory (Davis 2005; Keene and Waddell 2007; Berry et al. 2008). A role for the MB in seizure susceptibility has not previously been suspected. Here we suggest that the orderly arrangements of axons and neuropile of MB Kenyon cells (KCs) not only facilitate learning and memory, but also provide the type of anatomical substrate in flies that is thought to be essential for seizure spread in the mammalian brain (Hauser and Hesdorffer 1990; Traub and Miles 1991).
Inhibitory synaptic transmission in Drosophila is thought to be mediated primarily by GABAergic neurons found throughout the CNS at all stages of development (Buchner et al. 1988; Jackson et al. 1990; Harrison et al. 1996; Yasuyama et al. 2002). γ-aminobutyric acid (GABA) is synthesized from glutamate via a conserved glutamic acid decarboxylase encoded by the Drosophila Gad1 gene (Jackson et al. 1990; Buchner 1991). GABAergic activity is limited by sequestering extracellular GABA back into presynaptic neurons by GABA transporters that are sensitive to inhibition by dl-2,4-diaminobutyric acid, nipecotic acid, and valproic acid (Neckameyer and Cooper 1998; Leal et al. 2004). Three ionotropic GABAA receptor subunits have been identified in Drosophila and are encoded by the Rdl, LCCH3, and GRD loci (Hosie et al. 1997). When expressed heterotopically in Xenopus oocytes, the best studied of these, Rdl, forms GABA-gated Cl− channels that are sensitive to block by picrotoxin (ffrench-Constant et al. 1991, 1993; Zhang et al. 1995). Inhibitory Cl− currents are dependent on maintenance of Cl− gradients, particularly in low intracellular Cl− concentrations. In the fly, Cl− gradients appear to be maintained by the kcc K+–Cl− cotransporter (Hekmat-Scafe et al. 2006).
Chemical synaptic transmission onto MB neurons has been examined in dissociated KCs in primary culture (Su and O'dowd 2003). Spontaneous miniature excitatory postsynaptic currents (EPSCs) are mediated mainly by nicotinic acetylcholine (ACh) receptors. Miniature inhibitory postsynaptic currents (IPSCs) appear to be mediated primarily by picrotoxin-sensitive GABAA receptors, probably encoded by Rdl (Su and O'dowd 2003; Harrison et al. 1996). In vivo, cholinergic inputs to the MB are thought to arise primarily from antennal lobe projection neurons (Yasuyama et al. 2002). Two antennal lobe neurons that project to the MB, the anterior paired lateral (APL) neurons, were recently shown to be GABAergic (Liu and Davis, 2009). Additional GABAergic inputs to the Drosophila MB seem likely; in locust they appear to come from a poorly understood region of the brain called the lateral horn, which is itself also driven by antennal lobe projection neurons (Perez-Orive et al. 2002).
Previously, we identified the kccDHS1 partial loss-of-function mutation as a seizure enhancer that also causes increased seizure sensitivity in young flies (Hekmat-Scafe et al. 2006). The kcc product shows homology to the mammalian KCC2 K+–Cl− cotransporter, and we inferred that a decrease in inhibitory synaptic strength is responsible for causing the seizure phenotypes. In this study, we describe our search for identifying the source of these vulnerable inhibitory synapses and report that they appear to lie primarily in the MBs of the Drosophila brain. Further, we speculate on the possibility of their involvement in synaptic plasticity functions of the MB.
MATERIALS AND METHODS
Molecular biology:
The kcc gene has two major alternative splice forms: the B form, which is found in adult heads, and the D form, which is found in embryos (Hekmat-Scafe et al. 2006). Plasmids GH09271 and LD02554, carrying cDNAs for kcc-B and kcc-D, respectively, were obtained from the Drosophila Genomics Resource Center. A 3.6-kb BglII/XhoI kcc-B cDNA fragment and 3.9-kb XbaI/HindIII kcc-D cDNA fragment were subcloned into the Xenopus expression vector pGEMHE (Mount et al. 1999) using standard methods (Sambrook and Russell 2001) to create expression plasmids pDH153 and pDH154, respectively. Expression constructs for human KCC2 (hKCC2) and mouse KCC4 in pGEMHE have been described previously (Song et al. 2002). To prepare cRNA for injection, Qiagen-purified cDNA constructs were linearized at the 3′-end using NheI, and cRNA was transcribed in vitro, using the T7 RNA polymerase mmESSAGE kit (Ambion). RNA integrity was confirmed on agarose gels, and concentration was determined by absorbance reading at 260 nm. cRNA was stored frozen in aliquots at −80° until used.
The P{w+ UAS-kcc-B} Drosophila P-element transformation vector pDH156 was created by subcloning a 3.6-kb BglII/XhoI kcc-B cDNA fragment from GH09271 into pUAST (Brand and Perrimon 1993). The P{w+ UAS-kcc-D} plasmid pDH157 was constructed by subcloning a 3.9-kb NotI/XhoI kcc-D cDNA fragment from LD02554 into pUAST. A P{w+} plasmid carrying the human KCC2 gene (hKCC2) downstream of GAL4 UAS repeats (pDH159) was produced by subcloning a 3.4-kb EcoRI/XbaI fragment of hKCC2 cDNA (Song et al. 2002) into pUAST. Transgenic w1118 flies carrying either pDH156, pDH157, or pDH159 were created by standard P-element-mediated transformation procedures (Spradling and Rubin 1982) using Qiagen-purified DNA.
Measurement of K+–Cl− cotransport:
Adult female Xenopus laevis were purchased from NASCO (Fort Atkinson, MI) and maintained in a Marine Biotech XR3 system (New Bedford, MA) under controlled light conditions at a water temperature of 16–18°. Oocytes were surgically collected from animals anesthetized by 0.17% tricaine immersion; after several such procedures, anesthetized frogs were killed by cardiac puncture. The use and care of the animals in these experiments were approved by the Institutional Animal Care and Use Committee at Harvard Medical School. After extraction, oocytes were incubated for 1 hr with vigorous shaking in a Ca2+-free ND96 medium (96 mm NaCl, 2 mm KCl, 1 mm MgCl, and 5 mm HEPES/Tris, pH 7.4, plus 2 mg/ml collagenase A). Oocytes were then washed four times in regular ND96, defolliculated by hand, and incubated overnight in ND96 at 16°. Mature oocytes were injected with 50 μl of water or with water containing 0.5 μg/μl of cRNA transcribed in vitro from the various KCC constructs. Oocytes were incubated for 4–5 days prior to transport assays, in ND96 at 16° supplemented with 2.5 mm sodium pyruvate and 5 mg/100 ml of gentamicin.
K+–Cl− cotransport was assessed by measuring Cl−-dependent uptake of 86Rb+ (Perkin-Elmer Life Sciences, Norwalk, CT), a congener of K+, in X. laevis oocytes as described previously (Mount et al. 1999; Song et al. 2002; Mercado et al. 2006). Rubidium uptake was assessed 2–3 days after injection under both isotonic and hypotonic conditions, as noted. A 30-min incubation period in a Na+- and Cl−-free medium (50 mm N-methyl-d-glucamine gluconate, 10 mm K+ gluconate, 4.6 mm Ca2+ gluconate, 1 mm Mg2+ gluconate, 5 mm HEPES/Tris, pH 7.4) containing ouabain (1 mm) was followed by a 60-min uptake period in a Na+-free medium (mm: 50 N-methyl-d-glucamine-Cl, 10 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES/Tris, pH 7.4) supplemented with ouabain and 2.5 μCi of 86Rb+ per milliliter (PerkinElmer Life Sciences). Isotonic conditions were generated by supplementing the same solutions with 3.5 g/100 ml sucrose to reach isosmolar conditions for oocytes (∼210 mosmol/kg). Ouabain was added to inhibit 86Rb+ uptake via Na+–K+–ATPase, and removal of extracellular Na+ prevented 86Rb+ uptake via the endogenous Na+–K+−2Cl− cotransporter. All uptakes were performed at 32°. At the end of the uptake period, oocytes were washed three times in ice-cold uptake solution without isotope to remove extracellular fluid tracer. Oocytes were dissolved in 10% SDS, and tracer activity was determined for each oocyte by beta-scintillation counting. The uptake experiments all included at least 15 oocytes in each experimental group; statistical significance was defined as two-tailed P < 0.05, and results were reported as means ±SE. All of the transport experiments shown were performed several times with the appropriate controls (water-, KCC2-, and/or KCC4-injected groups).
Fly stocks:
A list of Drosophila stocks used is given in Table 1. Stocks were maintained on standard cornmeal–molasses medium at ∼24°). The kccDHS1 mutation is a 13-bp insertion in intron 11 of kcc leading to an approximate twofold reduction in transcript and an approximate fourfold reduction in protein; phenotypically it behaves as a hypomorph (Hekmat-Scafe et al. 2006). The X-linked eas gene encodes an ethanolamine kinase; the recessive easPC80 allele carries a frameshift mutation and is believed to be a null (Pavlidis et al. 1994). The bss gene is located at 1-54.6 (corresponding to approximately cytological region 12F); the identity of its gene product has not been published (Ganetzky and Wu 1982). The bss1 allele is a semidominant mutation.
TABLE 1.
Drosophila stocks
| Stock no. | Genotype |
|---|---|
| CS-5 | Wild type |
| D506 | w; kccDHS1/CyO |
| D634 | w; kccDHS1/SM5 Cy; pDH156{w+ UAS-kcc-B}-3/TM6B |
| D635 | w; kccDHS1/SM5 Cy; pDH157{w+ UAS-kcc-D}-7/TM6B |
| D637 | w; kccDHS1/SM5 Cy; pDH159{w+ UAS-hKCC2}-2/TM6B |
| D696 | w; MB-GAL80 kccDHS1/SM5 Cy; pDH156{w+ UAS-kcc-B}-3/TM6B |
| D586 | w; kccDHS1/SM5 Cy; elav-GAL43A |
| D672 | w; 1407 kccDHS1/CyO |
| D587 | w; kccDHS1/SM5 Cy; REPO-GAL4/TM6B |
| D670 | w; kccDHS1/SM5 Cy; Rdl-GAL4-2-1 |
| D590 | w; Gad1-GAL4 kccDHS1/CyO |
| D662 | w; kccDHS1/SM5 Cy; c232 |
| D663 | w; 104Y kccDHS1/CyO |
| D695 | w; GH146 kccDHS1/CyO |
| D589 | w; Cha-GAL4 kccDHS1/CyO |
| D642 | w; kccDHS1/CyO;;OK107/+ |
| D661 | w; kccDHS1/SM5 Cy; MB247 |
| D689 | w; c772 kccDHS1/CyO |
| D588 | w; c739 kccDHS1/CyO |
| D643 | w; c305a kccDHS1/CyO |
| D658 | w; 201Y kccDHS1/CyO |
| 5137 | y1w;P{w[+mC]=UAS-mCD8∷GFP.L}LL5 |
| D629 | w; c739 kccDHS1; +/TM6B |
| D622 | w; c739 kccDHS1; pDH156{w+ UAS-kcc-B}-3/TM6B |
| D623 | w; c739 kccDHS1; pDH159{w+ UAS-hKCC2}-2 |
| D690 | w; kccDHS1/SM5 Cy; P{GD14683=UAS-Ncc69-RNAi}v30000/TM6B |
| D691 | w; kccDHS1/SM5 Cy; Rdl-RNAi-8-10G/TM6B |
| D700 | w; UAS-TNT kccDHS1/CyO |
| D610 | w; c739;; pDH156{w+ UAS-kcc-B}-3/TM6B |
| MR047 | w easPC80 f |
| MR068 |
w bss1 f |
The 1407, Rdl-GAL4-2-1, Gad1-GAL4 (Ng et al. 2002), 104Y and c232 (Renn et al. 1999), GH146 (Stocker et al. 1997), MB247 and c772, and c305a (Krashes et al. 2007) GAL4 drivers were obtained from Nara Muraro and Richard Baines (University of Manchester), Julie Simpson (Janelia Farms), Gero Miesenboeck (Yale University), Roland Strauss (University of Wuerzburg), Gautam Agarwal and Ehud Isacoff (University of California, Berkeley), Christopher Tabone and J. Steven de Belle (University of Nevada, Las Vegas), and Scott Waddell (University of Massachusetts Medical School), respectively; the remaining GAL4 drivers were obtained from the Bloomington Drosophila Stock Center. The kccDHS1 stocks carrying a GAL4 driver on the second chromosome (D672, D590, D663, D695, D589, D689, D588, D643, and D658) were created as follows: D506 virgin females were crossed to males carrying the GAL4 driver. Non-CyO virgin female progeny were then crossed to D506 males and recombinant w; Driver-GAL4 kccDHS1/kccDHS1 male progeny identified by orange eyes and bang sensitivity. A single recombinant male was crossed to D245 virgin females and orange-eyed w; Driver-GAL4 kccDHS1/CyO male and virgin female progeny used to establish a balanced stock. We created kccDHS1 stocks carrying a GAL4 driver on the third chromosome (D586, D587, D670, D662, and D661) by crossing U036 virgin females to Driver-GAL4 males. Several w; +/SM5 Cy; Driver-GAL4/TM3 males were then crossed to D579 virgin females. Finally, w; kccDHS1/SM5 Cy; Driver-GAL4/TM6B male and virgin female progeny from this cross were combined to establish a balanced stock; when possible, a homozygous Driver-GAL4 version of the stock lacking the TM6B balancer was also created. The kccDHS1 stock carrying OK107 (D642) was constructed by first crossing D245 virgin females to a w;;;OK107 stock, then crossing w; +/CyO;;OK107/+ male progeny from this cross to homozygous w; kccDHS1 D506 virgin females and finally combining w; kccDHS1/CyO;;OK107/+ male and virgin female progeny from this cross to establish a stock. The MB-GAL80 flies were from Scott Waddell (University of Massachusetts Medical School); flies carrying the Ncc69 RNAi (Dietzl et al. 2007) and Rdl RNAi (Liu et al. 2007) constructs were obtained from the Vienna Drosophila RNAi Center and from Xu Liu and Ron Davis (Baylor University Medical College), respectively; and UAS-TNT flies were obtained from Christopher Tabone and J. Steven de Belle (University of Nevada, Las Vegas). Stocks carrying kccDHS1 along with each of these transgenes were created essentially as described for the kccDHS1 + Driver-GAL4 lines above. The Rdl1 (ffrench-Constant et al. 1991), RdlMD-RR (ffrench-Constant et al. 1993), Ncc69PL00618, and Df(3L)eygC1 were obtained from the Bloomington Drosophila Stock Center. Strains carrying kccDHS1 along with one of these third chromosomal mutations were created as in the same manner as described for the third chromosomal GAL4 driver stocks.
BS behavioral testing:
Testing for BS paralysis was performed on flies <1 day posteclosion except those involving the late-acting c772 driver (Yang et al. 1995; Armstrong et al. 1998), which were tested at 24–36 hr posteclosion. Flies were typically collected at <17 hr posteclosion, anesthetized with CO2, transferred to fresh food vials in groups of ∼15 for 6–17 hr, and then tested by vortexing at maximum speed for 10 sec. BS flies displayed a characteristic period of paralysis followed by a period of hyperactivity resembling seizure-like behavior.
Crosses were performed at 23° if kccDHS1 progeny carrying a GAL4-driven transgene were being tested for BS behavior. This temperature is a compromise between 22°, which is ideal for discerning the BS phenotype of the cold-sensitive kccDHS1 mutation (Hekmat-Scafe et al. 2006), and 25–29°, which is optimal for GAL4 function (Duffy 2002). Ordinarily, virgin females carrying the kccDHS1 mutation, as well as a particular GAL4 driver, were crossed to males carrying the kccDHS1 mutation and a UAS-kcc transgene over a TM6B balancer chromosome and the percentage bang sensitivity of ∼200 kccDHS1 + Driver-GAL4 + UAS-kcc test progeny compared to that of their kccDHS1 + Driver-GAL4 control siblings. Initially, two to three independent third chromosomal insertions of each of the three transgenic plasmids (pDH156=UAS-kcc-B, pDH157=UAS-kcc-D and pDH159=UAS-hKCC2) were examined in this manner using the elav, REPO, and c739 drivers, and the insertion that gave the greatest degree of suppression (pDH156-3, pDH157-7, and pDH159-2, respectively) used in all subsequent experiments. Crosses used to assay suppression of kccDHS1 by other chromosomal mutations (shown in Figure 7, B–C) were performed at 22°, and those used to monitor suppression of eas and bss/+ by ectopic mushroom body expression of kcc+ (Figure 8) were performed at 25°. The chi-square test was used to determine the P-values for differences in percentage BS between test and sibling control flies. The relative percentage BS of test flies was determined by setting the level for control flies at 100%.
Figure 7.—
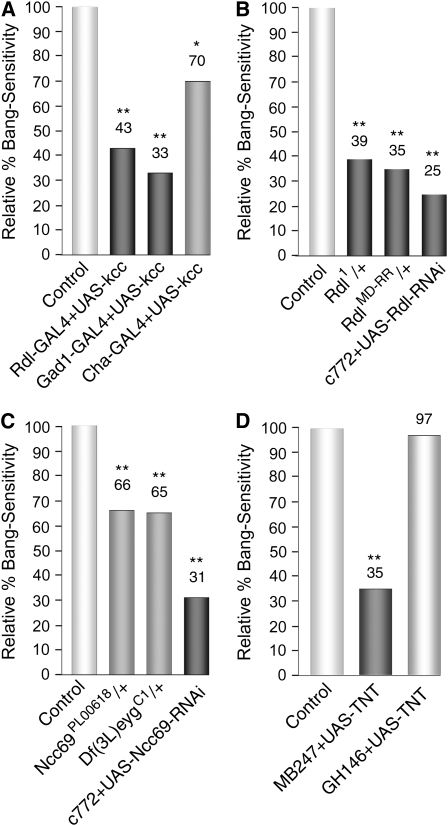
Disruption of GABAergic signaling or synaptic transmission in MB reduces the bang sensitivity of kccDHS1 flies. (A) Expression of the UAS-kcc+ transgene in GABAergic neurons reduces their bang sensitivity. Expression of the wild-type kcc+ gene in the neurons targeted by either of two GAL4 drivers (Rdl, those expressing the Rdl GABAA receptor and Gad1, those producing GABA) produced marked reductions in bang sensitivity. Expression of kcc+ in neurons targeted by the Cha GAL4 driver (neurons producing acetylcholine) resulted in more modest, albeit statistically significant, reductions in bang sensitivity. In these experiments, virgin females carrying the kccDHS1 mutation as well as a particular GAL4 driver (i.e., D670, D590, or D589) were crossed to D634 males carrying the kccDHS1 mutation and a UAS-kcc+ transgene over a TM6B balancer chromosome. The percentage bang sensitivity of the kccDHS1 + Driver-GAL4 + UAS-kcc+ test progeny is given relative to that of their kccDHS1 + Driver-GAL4 control siblings. n > 80; (**) P < 0.001, (*) P < 0.05. (B) Reducing the dosage of the Rdl GABAA receptor by mutation or RNAi reduces the bang sensitivity of kccDHS1 flies. kccDHS1 flies heterozygous for either of two Rdl mutations (Rdl1 or RdlMD-RR) displayed almost one-third the bang sensitivity (39% or 35% relative BS, respectively) of their control siblings. Mushroom body expression of an RNAi construct that reduces the level of Rdl produces an even more marked reduction in the level of bang sensitivity of kccDHS1 mutant flies (25% relative bang sensitivity). In the first set of experiments, D506 virgin females homozygous for kccDHS1 were crossed to males carrying kccDHS1 and the corresponding Rdl mutation over a TM6B balancer. The percentage bang sensitivity of the kccDHS1; Rdl/+ test progeny is presented relative to that of the corresponding kccDHS1 control siblings. In the last experiment, D689 virgin females carrying kccDHS1 and the MB GAL4 driver c772 were crossed to D691 males carrying kccDHS1 as well as an Rdl RNAi construct over a TM6B balancer. The percentage bang sensitivity of the kccDHS1 + c772 + Rdl-RNAi test progeny is presented relative to that of the corresponding kccDHS1 + c772 control siblings. n > 220; (**) P < 0.001. (C) Reducing the level of the Ncc69 Na+–K+–Cl− cotransporter specifically in MB reduces bang sensitivity. kccDHS1 flies carrying either of two lesions in Ncc69 [Ncc69PL00618 or Df(3L)eygC1] displayed a modest reduction in bang sensitivity compared to their control siblings (lightly shaded bars; 65–66% relative bang sensitivity). By contrast, MB expression of an Ncc69 RNAi construct produces a marked reduction in the level of bang sensitivity of kccDHS1 mutant flies (dark-shaded bars; 31% relative bang sensitivity). In the first set of experiments, D506 virgin females homozygous for kccDHS1 were crossed to males carrying kccDHS1 and an Ncc69 mutation over a TM6B balancer. The percentage bang sensitivity of the kccDHS1; Ncc69/+ test progeny is presented relative to that of the corresponding kccDHS1 control siblings (n > 75). In the last experiment, D661 virgin females carrying kccDHS1 and the MB GAL4 driver c772 was crossed to D690 males carrying kccDHS1 as well as an RNAi construct for Ncc69 over a TM6B balance. The percentage bang sensitivity of the kccDHS1 + c772 + Ncc69-RNAi test progeny is presented relative to that of the corresponding kccDHS1 + c772 control siblings. n > 360; (**) P < 0.001. (D) Blocking synaptic transmission in MB ameliorates the bang sensitivity of kccDHS1 flies. Tetanus toxin (TNT) blocks synaptic transmission by cleaving synoptobrevin, which is necessary for synaptic vesicle fusion (Martin et al. 2002). Flies that express tetanus toxin specifically in their mushroom bodies show a significant reduction in bang sensitivity (35% of control levels, dark-shaded bar); no such reduction was observed when synaptic transmission was instead blocked in the antennal neurons that project to the MB (97% of control, lightly shaded bar). In the first experiment, D661 flies carrying kccDHS1 and the MB driver MB247 were crossed to either D700 males carrying kccDHS1 and a UAS-TNT transgene or D506 males carrying only kccDHS1; percentage bang sensitivity of kccDHS1 + MB247 + UAS-TNT progeny from the first cross is presented relative to that of kccDHS1 + MB247 progeny from the second cross. In the second experiment, D695 flies carrying kccDHS1 and the GH146 GAL4 driver were crossed to either D700 or D506; percentage bang sensitivity of kccDHS1 + GH146 + UAS-TNT progeny from the first cross is presented relative to that of kccDHS1 + GH146 progeny from the second cross. n > 110; (**) P < 0.001.
Figure 8.—
Mushroom body expression of kcc functions as a global seizure suppressor. Expression of a UAS-kcc+ transgene in the Drosophila mushroom bodies significantly reduces the behavioral seizure susceptibility of flies carrying either the easily shocked (eas) or bang-senseless (bss) bang-sensitive mutation. Whereas all of the eas + c739 control flies are bang sensitive, only 66% of their eas + c739 + UAS-kcc+ siblings that express kcc+ ectopically in their mushroom bodies display the bang-sensitive phenotype. Similarly, although nearly half (49%) of control bss/+ heterozygotes carrying c739 are bang sensitive, only 14% bang sensitivity is observed in their bss/+ test siblings carrying UAS-kcc+ as well as c739. In the first experiment, eas virgin females (MR047) were crossed to D610 males carrying the c739 MB GAL4 driver and a UAS-kcc+ transgene over a TM6B balancer chromosome, and the percentage bang sensitivity of eas + c739 + UAS-kcc+ male progeny compared to that of their eas + c739 control brothers. In the second experiment, bss virgin females (MR068) were crossed to D610 males and the percentage bang sensitivity of the bss/+ female test progeny carrying c739 and UAS-kcc+ compared to that of their control sisters lacking the UAS-kcc+ transgene. n > 70; (**) P < 0.001.
Electrophysiology:
Flies were raised at 23° and collected less than 24 hr posteclosion for electrophysiological testing. Brain stimulation and recording of both giant fiber (GF)-driven muscle potentials and seizures was performed as described previously (Hekmat-Scafe et al. 2006). Electrical stimuli were delivered to the fly's brain using bipolar tungsten electrodes. Single-pulse stimuli (0.5 ms duration, 0.8 Hz) were used to drive the GF, and the GF-driven muscle potentials were recorded from the dorsal longitudinal muscle (DLM) using a tungsten recording electrode. Seizure-like spiking is observed in at least seven different muscle groups and over 30 muscle fibers in the thorax that reflect the HF firing of the innervating motoneurons (Kuebler and Tanouye 2000). Seizure-like activity was evoked by delivering short wave trains of HF electrical stimuli (0.5-ms pulses delivered at 200 Hz for 300 ms) to the fly's brain. Seizure threshold was defined as the lowest intensity HF stimulus required to elicit seizure-like activity. A Student's two-tailed t-test was used to determine the P-values for differences in seizure threshold of the D622 and D623 test flies relative to that of the D629 control flies.
GFP monitoring of GAL4 expression patterns:
Flies carrying each of the GAL4 drivers in combination with a UAS-mCD8∷GFP reporter were obtained by crossing BL5137 virgin females to males bearing a particular GAL4 driver at 25°. For each Driver-GAL4/GFP combination, progeny were anesthetized with CO2 <24 hr posteclosion and brains from several female progeny were harvested into HL3 buffer (Stewart et al. 1994). The brains were then imaged with an Andor IQ CCD camera (Andor, Belfast, Ireland) and a BX-50WI microscope with a 75-W Xenon lamp and a 10× 0.3 NA objective (Olympus). Excitation was done at 470 ± 20 nm with a 495LP dichroic and images (515 ± 15 nm emission) acquired for 0.2 sec. At least two brains were independently imaged for each GAL4 driver.
RESULTS
Drosophila kcc functions as a K+–Cl− cotransporter:
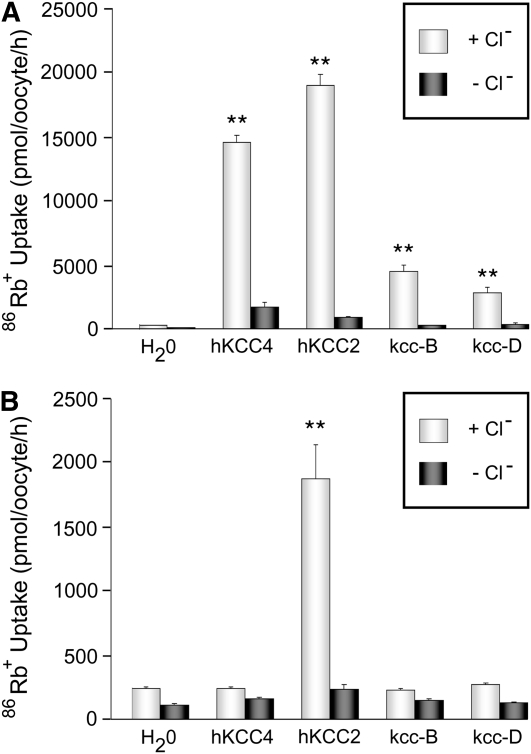
Drosophila carry a single gene, kcc whose product displays structural features common to mammalian K+–Cl− cotransporters, KCC1-4 (Filippov et al. 2003). Alternative splicing of the kcc gene results in two major kcc isoforms: kcc-B, which displays widespread expression in the adult brain, and kcc-D, which is enriched in embryos and larvae (Hekmat-Scafe et al. 2006). The kcc-B and kcc-D isoforms differ in their N termini; kcc-D also encodes 31 extra amino acids near its C terminus in a region corresponding roughly to that of a 100-amino-acid insertion present in KCC2, but not the other mammalian KCCs (Hekmat-Scafe et al. 2006). To demonstrate that Drosophila kcc encodes functional cotransporters, kcc cRNAs were injected and their products heterologously expressed in Xenopus oocytes (Figure 1). Oocytes injected with kcc-B cRNA showed robust transport, as indicated by Cl−-dependent uptake of the K+ congener, 86Rb+ (Figure 1A). 86Rb+ uptake by kcc-B is comparable to, although somewhat less than, that of human KCC2 and KCC4 positive controls (Figure 1A). As with the positive controls, kcc-B cotransport activity is induced under hypotonic conditions that induce oocyte swelling indicating stretch-activated activity. Cotransport activity is similarly observed by heterologous expression of the kcc-D isomer. Neither Drosophila kcc isoform conferred constitutive K+–Cl− cotransport activity on microinjected oocytes subjected to isotonic conditions (Figure 1B). As reported previously, control oocytes expressing human KCC2 exhibited considerable K+–Cl−- cotransport activity under isotonic conditions (Figure 1B; Song et al. 2002).
Figure 1.—
Drosophila kcc mediates K+–Cl− cotransport when expressed in Xenopus oocytes. (A) Xenopus oocytes microinjected with kcc-B, kcc-D, or control (human KCC2 or KCC4) cRNA all demonstrate uptake of 86Rb+ (a congener of K+) under hypotonic conditions. This 86Rb+ uptake is Cl−-dependent as it is observed in the presence (+Cl−, lightly shaded bars), but not in the absence (−Cl−, dark-shaded bars), of extracellular Cl−. Control oocytes microinjected with H20 (left) showed no Cl−-dependent uptake of 86Rb+ under hypotonic conditions. (**) P < 0.0001 vs. water-injected control oocytes. (B) Xenopus oocytes microinjected with neither the kcc-B nor the kcc-D cRNA displayed uptake of 86Rb+ under isotonic conditions that was significantly higher in the presence of Cl− (+Cl−, light-gray bar) than in its absence (−Cl−, dark-shaded bar). Control oocytes microinjected with either H20 or human KCC4 cRNA also showed no Cl−-dependent uptake of 86Rb+. In contrast, Xenopus oocytes microinjected with cRNA for human KCC2 demonstrate a robust Cl−-dependent uptake of 86Rb+ under isotonic conditions. (**) P < 0.0001 vs. water-injected control oocytes
Ectopic kcc+ expression in neurons rescues BS phenotypes:
GAL4 drivers (Brand and Perrimon 1993) were used to ectopically express UAS-kcc+ to determine where cotransporter function is required to rescue the BS paralytic phenotype of kccDHS1 mutants. The BS phenotype is substantially reduced (<50% BS relative to their respective sibling control groups) by expression of kcc-B using a pan-neuronal driver, such as elav-GAL43A or 1407, whereas expression of kcc-B in glia (REPO-GAL4) produces no apparent change in BS phenotype (Figure 2A). We interpret this to mean that kcc-B is required in neurons, but not in glia, to reduce BS behavior. Rescue of BS phenotypes by kcc-D expression was notably weaker than that of kcc-B (Figure 2B), and subsequent experiments described in this article focus only on the kcc-B splice form, utilizing “kcc+” to refer to this product.
Figure 2.—
Neuroanatomic mapping of kcc function in the Drosophila brain. (A) Expression of a UAS-kcc-B transgene in all neurons of an otherwise kccDHS1 fly using either of two pan-neuronal GAL4 drivers (elav or 1407, dark-shaded bars) markedly reduces its bang-sensitive paralytic phenotype, a behavioral indicator of seizure susceptibility. On the other hand, expression of the UAS-kcc-B transgene in all gila using the REPO GAL4 driver (lightly shaded bar, right) does not produce any significant change in the degree of bang sensitivity. This suggests that kcc normally functions in neurons, rather than glia, to reduce susceptibility to seizures. (B) Expression of a UAS-kcc-D transgene in all neurons of a kccDHS1 fly using a pan-neuronal GAL4 driver (dark-gray bars) produces modest rescue of its bang-sensitive phenotype; expression in glia (lightly shaded bar, right) produces slight, but statistically significant, suppression. (C) Expression of the UAS-kcc-B transgene in various subpopulations of brain neurons reduces their bang sensitivity. Expression of the wild-type kcc-B gene in the neurons targeted by either of two GAL4 drivers (OK107, mushroom body, MB, neurons; and c232, ellipsoid body, EB, neurons) produced marked reductions in the bang sensitivity (dark-shaded bars; 30 and 17% relative bang sensitivity, respectively). Expression of kcc+ in neurons targeted by two other GAL4 drivers (104Y, fan-shaped body, FSB; GH146, antennal projection neurons, APN) resulted in more modest reductions in bang sensitivity (right bars; 76 and 63% relative bang sensitivity, respectively). In all of these experiments, virgin females carrying the kccDHS1 mutation as well as a particular GAL4 driver (i.e., D586, D672, D587, D662, D663, D695, D642, D661, D689, D588, D643, or D658) were crossed to either D634 or D635 males carrying the kccDHS1 mutation and a UAS-kcc-B or UAS-kcc-D transgene, respectively, over a TM6B balancer chromosome. The percentage bang sensitivity of the kccDHS1 + Driver-GAL4 + UAS-kcc+ test progeny are given relative to that of their kccDHS1 + Driver-GAL4 control siblings. N > 180; (**) P < 0.001.
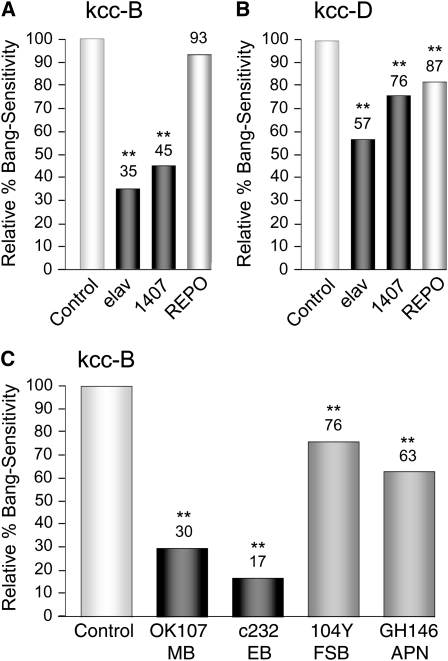
Expression of kcc+ in discrete neuronal subpopulations provides further insight into the neuronal circuitry underlying seizure susceptibility (Figures 2C and 3). Expression of kcc+ in either of two brain regions, the MB (OK107 driver) and the ellipsoid body (EB) of the central complex (c232 driver), produces marked reductions in BS phenotype (30% BS for OK107, 17% BS for c232, relative to sibling controls). Expression of kcc+ in the nearby fan-shaped body of the central complex (104Y driver) or in antennal neurons projecting to the MB (GH146 driver) produces only modest reductions in BS phenotype (76% BS for 104Y, 63% BS for GH146, relative to sibling controls). These results suggest that expression of kcc+, and by extension, the maintenance of low intracellular Cl− concentrations, in the MB and EB is especially important in reducing the overall seizure susceptibility of the Drosophila brain. The MBs are plastic structures associated with learning and memory (reviewed in, e.g., Heisenberg 2003; Davis 2005; Keene and Waddell 2007; Berry et al. 2008), analogous to the mammalian hippocampus, a frequent site of epileptic foci (Hauser and Hesdorffer 1990). In subsequent experiments, we have examined more closely the link between expression of kcc+ in the Drosophila MB and seizure sensitivity.
Figure 3.—
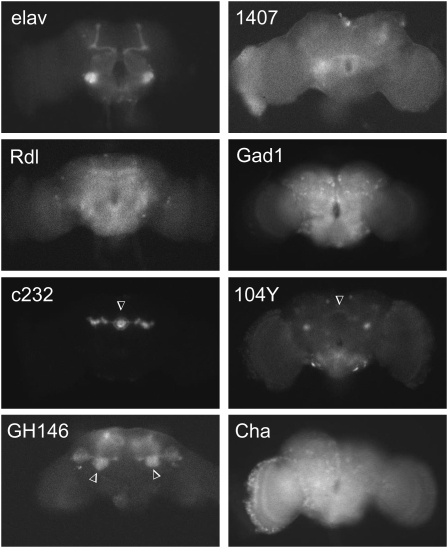
Expression patterns of neuronal GAL4 drivers used for neuroanatomical mapping of kcc function. Shown is the fluorescence observed upon excitation at 470 nm when a UAS-mCD8∷GFP reporter is combined with each of the GAL4 drivers indicated. Both elav and 1407 display pan-neuronal expression; the apparent enrichment of elav-GAL4 signal in mushroom bodies is an artifact of the mCD8-based membrane targeting and has been observed previously (Ito et al. 2003). The Rdl driver produces relatively widespread expression in neurons carrying the Rdl GABAA receptor. The Gad1 and Cha drivers display abundant expression in GABAergic and cholinergic interneurons, respectively (Salvaterra and Kitamoto 2001; Ng et al. 2002)). Three of the other GAL4 drivers produce relatively circumscribed expression patterns (arrowheads): c232 (ellipsoid body; Renn et al. 1999), 104Y (scatterned neurons, including those in the fan-shaped body; Renn et al. 1999) and GH146 (antennal lobe neurons, which project to the mushroom body) (Stocker et al. 1997). The expression pattern for the OK107 driver is shown with those of the other mushroom body drivers in Figure 5B. The representative brains are from 1- to 2-day-old female flies and are positioned such that the anterior side is facing the camera; dorsal is up and ventral down.
kcc functions in MB neurons to reduce the BS phenotype:
Much of the reduction in seizure susceptibility of kccDHS1 flies observed when a kcc+ transgene is expressed in all neurons appears to be solely attributable to its effect in MB neurons (Figure 4). This is shown most clearly by utilizing the GAL4 inhibitor, GAL80, to exclude kcc+ expression from MB neurons, but to allow it in other neurons. When this is done in kccDHS1 flies with kcc+ concurrently driven by elav-GAL4, suppression of BS behavioral paralysis is significantly compromised (35% BS without GAL80 inhibition, 53% BS with GAL80 inhibition, relative to sibling controls).
Figure 4.—
kcc's neuronal function reflects a significant contribution in mushroom bodies. (A) The reduction in seizure susceptibility of kccDHS1 flies observed when a kcc+ transgene is expressed in all neurons with an elav GAL4 driver (35% relative bang sensitivity, dark-shaded bar) is significantly reduced when kcc+ induction in mushroom body is blocked due to the presence of a MB-GAL80 construct (53% relative bang sensitivity, medium-shaded bar). In these experiments virgin females carrying the kccDHS1 mutation and elav-GAL4 +/− MB-GAL80 (D586 or D696, respectively) were crossed to D634 males carrying the kccDHS1 mutation and a UAS-kcc+ transgene over a TM6B balancer chromosome. The percentage bang sensitivity of the kccDHS1 + elav-GAL4 +/− MB-GAL80 + UAS-kcc+ test progeny is given relative to that of their kccDHS1 + elav-GAL4 control +/− MB-GAL80 siblings. n > 230; (**) P < 0.001. (B) The MB-GAL4 construct (described in Krashes et al. 2007) largely eliminates GAL4-driven expression in the MB. Combination of the OK107 driver with the UAS-mCD8∷GFP reporter (top) reveals robust expression in the MB (arrows); MB expression (arrows) is markedly reduced in the presence of MB-GAL4 (bottom), which represses GAL4 function specifically in the MB.
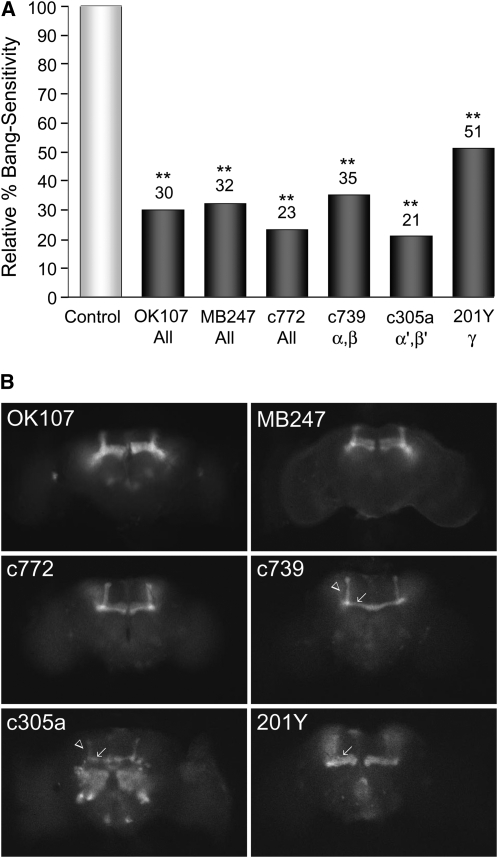
Each MB consists of ∼2000 KC neurons with dendrites in the calyx region and axonal extensions into α/β, α′/β′ or γ lobes (Berry et al. 2008; Aso et al. 2009). Ectopic expression of kcc+ in the entire MB using any of three GAL4 drivers OK107, MB247, or c772 markedly reduces the BS phenotype of kccDHS1 mutant flies (30, 32, or 23% relative BS, respectively). Similar reductions in BS phenotype are observed when kcc+ expression is restricted to KCs displaying specific axonal branches (c739—α/β lobes, 35% BS; c305a—α'/β' lobes, 21% BS; and 201Y—γ lobe, 51% BS, relative to sibling controls). The c772 driver is expressed later in development (late pupa) than the other MB drivers, which express in late larva–early pupa (Yang et al. 1995; Armstrong et al. 1998; Aso et al. 2009). The results for c772 are not substantially different from those of other drivers suggesting that kcc+ is reducing the BS phenotype through an effect on MB function, rather than on MB development.
MB expression of either Drosophila kcc+ or human KCC2 ameliorates the seizure susceptibility of kccDHS1 flies behaviorally and electrophysiologically:
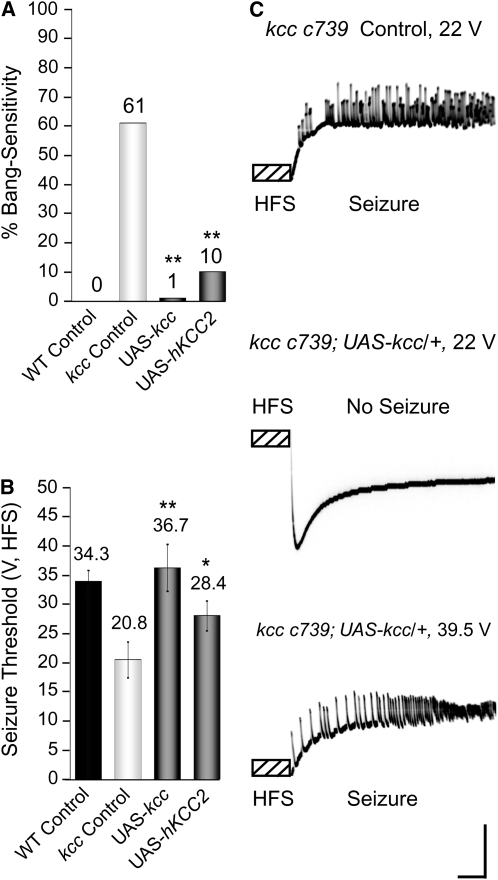
Significant, albeit incomplete, rescue of the kccDHS1 BS phenotype is observed after expressing one copy of UAS-kcc+ and one copy of the MB GAL4 driver (Figure 5). By contrast, nearly complete rescue is observed in kccDHS1 flies carrying a second copy of the MB GAL4 driving UAS-kcc+ (1% BS for two copies of c739) (Figure 6A). Flies carrying two copies of the c739 MB GAL4 driver, but no UAS-kcc+, still display significant bang sensitivity (61% BS). This indicates that the kcc+ level in the kccDHS1 fly's MB critically affects the BS phenotype. We infer that the somewhat higher residual BS behavior observed in experiments employing only one MB-GAL4 driver (Figure 5) reflects inadequate expression of the kcc+ transgene at the lowered temperatures required to score the kccDHS1 phenotype (Duffy 2002; Hekmat-Scafe et al. 2006), although other brain regions, such as the ellipsoid body (Figure 2C) may also be contributing to the phenotype.
Figure 5.—
Expression of kcc in all mushroom body subregions markedly reduces the behavioral seizure susceptibility of kcc mutant flies. (A) Expression of wild-type kcc+ in the entire MB using either of three GAL4 drivers markedly reduces the relative bang sensitivity of kccDHS1 mutant flies (OK107 30%, MB247 32%, and c772 23%), as does more restricted induction of the UAS-kcc+ transgene in Kenyon cells with distinct axonal branches (c379—α/β lobes, 35%; c305a—α'/β' lobes, 21%; and 201Y—γ lobe, 51%). In these experiments, virgin females carrying the kccDHS1 mutation and one of the MB GAL4 drivers (i.e., D642, D661, D689, D588, D643, or D658) were crossed to D634 males, which carry the kccDHS1 mutation and the UAS-kcc+ transgene over a TM6B balancer. The percentage bang sensitivity of the kccDHS1 + MB-GAL4 + UAS-kcc+ test progeny is shown relative to that of their kccDHS1 + MB-GAL4 control siblings lacking the UAS-kcc+ transgene. n > 220; (**) P < 0.001. (B) MB expression patterns of the varying MB-GAL4 drivers are revealed by examining the green fluorescence in brains of flies carrying a UAS-mCD8∷GFP reporter in combination with each of the MB-GAL4 drivers. Three of the GAL4 drivers (OK107, MB247, and c772) are expressed in all five axonal branches of the MB, whereas three others show more restricted expression patterns: c739 in the α (arrow)/β (arrowhead) lobes, c305a in the α′ (arrow)/β′ (arrowhead) lobes, and 201Y in the γ lobe (arrow). Some GFP fluorescence is observed in regions outside of the MB; in particular, c305a is also expressed in the antennal lobe. The brains are positioned so that the anterior side is facing the camera; dorsal is up and ventral down.
Figure 6.—
Expression of either Drosophila kcc or human KCC2 in MB significantly reduces the seizure susceptibility of kccDHS1 flies both behaviorally and electrophysiologically. (A) Expression of Drosophila kcc+ or human KCC2 (hKCC2) in mushroom bodies ameliorates the bang sensitivity of kccDHS1 mutant flies. The kccDHS1 control flies (D269, w; c739 kccDHS1; +/TM6B) display significant bang sensitivity (61%) as compared to CS-5 controls, which are not bang sensitive. Expression of the UAS-kcc+ transgene in mushroom bodies with two copies of the c739 driver (D622, w; c739 kccDHS1; UAS-kcc/TM6B) almost fully rescues the bang-sensitive phenotype (1% BS). Expression of human KCC2 in mushroom bodies (D623, w; c739 kccDHS1; UAS-hKCC2) partially resues the bang-sensitive phenotype (10% BS). n > 200; (**) P < 0.001. (B) Mushroom body expression of either Drosophila kcc+ or human KCC2 raises the seizure threshold of kccDHS1 mutant flies. Our kccDHS1 control flies (D629) show a seizure threshold of 20.8 ± 3.1 V (n = 7), which is significantly lower than the 34.3 ± 1.9 seizure threshold typically seen in wild-type CS-5 flies (Tan et al. 2004). On the other hand, kccDHS1 test flies that express the UAS-kcc transgene specifically in mushroom bodies (D622) have a seizure threshold of 36.7 ± 4.1 V (n = 8), which is comparable to that of wild-type CS-5 flies. Our kccDHS1 flies that express human KCC2 in their mushroom bodies (D623) display an intermediate seizure threshold of 28.4 ± 2.6 V (n = 5). (*) P < 0.05, (**)P < 0.001. (C) Top: A seizure elicited in a kccDHS1 control fly (D629) by a high-frequency stimulus of 22 V, which is above the fly's seizure threshold. The high-frequency stimulus (HFS) is a short wave train (0.5-ms pulses at 200 Hz for 300 ms) of electrical stimuli delivered to the brain. Middle: By contrast, a 22 V HFS fails to elicit a seizure in a kccDHS1 test fly which express the UAS-kcc+ transgene specifically in mushroom bodies (D622) because it is below the fly's seizure threshold (32.7 +/− 0.23 V). Bottom: However, when this fly is given a higher intensity HFS of 39.5 V, which is above its seizure threshold, it sustains a seizure. The vertical calibration bar is 20 mV and the horizontal bar is 200 ms.
Interestingly, we find that human KCC2 can substitute for fly kcc+ and is surprisingly effective at rescuing the kccDHS1 phenotypes. Flies mutant for kccDHS1 and carrying two copies of a UAS-hKCC2 transgene plus two copies of c739 display only 10% BS (Figure 6A). This rescue suggests functional and structural conservation between human KCC2 and fly kcc and is consistent with the notion that kcc, like KCC2, functions as a neuronal K+–Cl− cotransporter (Figures 1 and 2).
Electrophysiology shows that MB expression of both Drosophila kcc and human KCC2 raise the seizure threshold of kccDHS1 mutant flies, an indication that reduced seizure susceptibility is responsible for rescue of the BS phenotype (Figure 6B-C). Control kccDHS1 flies show a seizure threshold of 20.8 ± 3.1 V, significantly lower than the 34.3 ± 1.9 V seizure threshold typically seen in wild-type CS-5 flies (Tan et al. 2004). By contrast, kccDHS1 test flies expressing the UAS-kcc+ transgene in their MBs show a 36.7 ± 4.1 V seizure threshold, comparable to wild type. Thus, MB expression of kcc+ rescues the seizure susceptibility of kccDHS1 mutant flies electrophysiologically as well as behaviorally. The kccDHS1 flies expressing human KCC2 in their mushroom bodies display an intermediate seizure threshold (28.4 ± 2.6 V) indicating partial rescue of the seizure-susceptibility phenotype.
Disruption of GABAergic signaling or synaptic transmission in MB reduces the seizure susceptibilty of kccDHS1 flies:
The mammalian KCC2 cotransporter maintains intracellular Cl− gradients, and changes in KCC2 expression or function alter the strength of GABAergic synaptic inhibition (Ben-Ari et al. 2007). Similarly, we find interaction between Drosophila kcc and the GABAergic system in determining the severity of the kccDHS1 BS phenotype, supporting the notion that MB neurons, through GABAergic signaling, play an important role in the circuitry mediating seizure genesis or spread. The link between kcc function and GABAergic inhibition was initially examined by expressing UAS-kcc+ exclusively in neurons that contain the Rdl GABAA receptor (Figure 7A). The BS phenotype of kccDHS1 is substantially reduced (43% relative BS) by driving kcc+ expression with Rdl-GAL4-2-1; in the same range as for pan-neuronal drivers (Figure 2A). Significant rescue of the BS phenotype is also observed with the Gad1-GAL4 driver suggesting that GABA transmitting cells may also play a role in the BS phenotype. By contrast, targeting kcc+ expression to all cholinergic neurons with Cha-GAL4 produces only a modest reduction in the BS phenotype (70% relative BS). The implication is that GABAergic inputs play a larger role than cholinergic ones in modulating the kccDHS1 BS phenotype. Consistent with this notion, the kccDHS1 BS paralytic phenotype is suppressed by a reduction in the level of the Rdl GABAA receptor either globally or specifically in the MB (Figure 7B). The kccDHS1 BS paralytic phenotype is greatly reduced in heterozygous Rdl1/+ or RdlMD-RR/+ flies (39% BS for Rdl1/+, 35% BS for RdlMD-RR/+, relative to sibling controls). In addition, specifically reducing Rdl levels in the MB utilizing an Rdl RNAi construct reduces the kccDHS1 BS paralytic phenotype to an even greater extent (25% BS, relative to sibling controls).
In mammals, the Na+–K+–Cl− cotransporter, NKCC1, and the neuronal K+–Cl− cotransporter, KCC2, have opposing effects on intracellular Cl− gradients (Farrant and Kaila 2007). A BLASTp (Altschul et al. 1997) search of the Drosophila genome using human NKCC1 reveals four potential Na+–K+–Cl− cotransporter genes (Ncc69, CG31547, CG10413, and CG12773). Ncc69 is the most likely NKCC1 homolog as it has significant brain expression (Chintapalli et al. 2007) and is known to interact with fray (Leiserson et al. 2000), a homolog of the SPAK kinase, which modulates NKCC1 (W. Leiserson and H. Keshishian, personal communication). Here we show that reducing the level of Ncc69 by mutation has only a modest effect on decreasing the BS of kccDHS1 flies (Figure 7C). However, reducing Ncc69 expression exclusively in the MB of kccDHS1 flies by combining a MB driver and Ncc69 RNAi construct produces a marked decrease in the BS phenotype: 31% BS, relative to sibling controls (Figure 7C).
Blocking synaptic transmission from MB neurons by directed expression of Tetanus toxin (TNT) dramatically reduces the bang sensitivity of kccDHS1 flies (35% BS, relative to sibling controls). By contrast, blocking synaptic transmission in a control neuronal population (antennal neurons projecting to the MB targeted by the GH146 GAL4 driver) produces no significant difference in the bang sensitivity of kccDHS1 flies (97% BS, relative to sibling controls).
MB expression of kcc functions as a global seizure suppressor:
Expression of UAS-kcc+ not only rescues the BS phenotype of kccDHS1 mutants, it also suppresses the phenotype of other, unrelated BS mutants (Figure 8). Whereas all control flies carrying the easily shocked (eas) mutation are BS, only two-thirds of their siblings that express kcc+ ectopically in their MBs are BS. Similarly, although nearly half (49%) of control flies heterozygous for the semidominant bang-senseless (bss) mutation are BS, only 14% of their bss/+ test siblings that express kcc+ ectopically in their MBs show the BS phenotype. These observations suggest that GABAergic signaling in MB neurons is a fundamental determinant of seizure susceptibility in the Drosophila brain.
DISCUSSION
We reported previously that Drosophila kccDHS1 mutants, with reduced levels of kcc, display an increased susceptibiilty to epileptic-like seizures in a manner similar to that seen in mice with reduced KCC2 levels (Woo et al. 2002; Hekmat-Scafe et al. 2006; Zhu et al. 2008). Here we confirm that Drosophila kcc and mammalian KCC2 are functional homologs. Furthermore, we show that expression specifically in the MB of either Drosophila kcc or human KCC2 largely alleviates kccDHS1 seizure sensitivity, highlighting the importance of this brain structure in seizure genesis. Finally, we report a general role for MB kcc function in seizure susceptibility on the basis of observations that phenotypes of other BS mutants are ameliorated by MB expression of kcc+. We propose a Drosophila model for investigating the roles of kcc and GABAergic inhibition in epileptogenesis.
Drosophila kcc is a functional homolog of mammalian KCC2:
The KCC2 and NKCC1 ion cotransporters regulate mammalian nervous system excitability by modulating the strength of GABAergic synaptic inhibition (reviewed in Ben-Ari et al. 2007). Regulation occurs because the cotransporters exert opposing effects on the GABA reversal potential (EGABA) by differentially affecting intracellular Cl−, [Cl−]in. NKCC1 transports Cl− into the neuron, thereby increasing [Cl−]in, whereas KCC2 transports Cl− out of the neuron, thereby decreasing [Cl−]in. Thus, EGABA varies according to the differential expression of these two cotransporters. Ordinarily, in most adult neurons, a relatively negative EGABA arises from the presence of KCC2 and absence of NKCC1. Opening of the GABAA receptor Cl− conductance then results in a typical inhibitory membrane hyperpolarization. In most fetal neurons, EGABA is at a relatively more positive potential than for adult neurons due to the presence of NKCC1 and the absence of KCC2. Consequently, GABA activation can result in a depolarizing postsynaptic potential. This depolarization, sometimes referred to as “excitatory GABA,” can overcome the inhibitory shunting effects of channel opening, initiate action potentials, and elicit Ca2+-dependent synaptic transmitter release (Luhmann and Prince 1991; Yuste and Katz 1991; Wang et al. 1994; Obrietan and van den Pol 1995; Chen et al. 1996; Owens et al. 1996). During nervous system development, differential expression of NKCC1 and KCC2 underlies a so-called “GABA switch”: a change in GABA synaptic transmission from the fetal excitatory form of transmission to the mature adult inhibitory form (Ben-Ari et al. 2007).
Mammals carry four KCC K+–Cl− cotransporters (KCC1–4) encoded by separate genes (Blaesse et al. 2009). Of these, KCC2 is neuron specific; the others display more widespread expression (Mercado et al. 2004). Neurons lacking KCC2 do not regulate [Cl−]in despite co-expression of other KCCs (Zhu et al. 2008). The Drosophila genome includes only a single, alternatively spliced, K+–Cl− cotransporter gene, kcc (Hekmat-Scafe et al. 2006). Despite the absence of constitutive activity under isotonic conditions (Figure 1B), Drosophila kcc is evidently involved in GABAergic neuronal physiology (Figure 7), consistent with a role in regulating neuronal [Cl−]in. Our expression studies in Drosophila (Figure 6) as well as a heterologous system (Figure 1) indicate that the kcc-B isoform is a functional homolog of mammalian KCC2. Our ectopic expression experiments reveal that Drosophila kcc-B acts in neurons, rather than glia, to reduce BS (Figure 2A); the kcc-D isoform was less effective and specific (Figure 2B). Furthermore, human KCC2 functionally complements Drosophila kccDHS1 (Figure 6).
Reduced kcc expression in MBs underlies seizure sensitivity in kcc mutants:
Although kcc, like human KCC2, is normally expressed throughout the brain (Song et al. 2002; Hekmat-Scafe et al. 2006), expression of either wild-type kcc+ or human KCC2 specifically in the MBs of a kccDHS1 fly's brain greatly reduces its seizure susceptibility both behaviorally and electrophysiologically (Figures 5 and 6). Ectopic expression of kcc+ in kccDHS1 MB KCs, which extend their axons into either the α and β, α′ and β′, or γ lobes are all effective at rescuing the BS phenotype (Figure 5). This result, combined with our earlier observation that kcc protein is normally found at levels significantly higher in the MB calyx (dendritic region) than in the peduncle (axon tracts) (Hekmat-Scafe et al. 2006), suggests that kcc likely acts in the KC dendrites rather than in their axons. The degree of rescue may reflect some cell specificity, rather than simply the number of KCs expressing kcc+, as we observe far greater suppression with the c739 than the 201Y driver, which targets the larger number of KCs (Wang et al. 2007). Expression of kcc+ in neuronal regions outside the MB, particularly the ellipsoid body (Figure 2C), can also modulate seizure susceptibility.
We suggest that kccDHS1 causes seizure sensitivity because underexpression of its K+–Cl−-cotransporter results in a higher [Cl−]in that compromises GABAA inhibitory synaptic strength. The decrease in inhibition would change the overall balance between excitation and inhibition making the kccDHS1 brain hyperexcitable and thus, seizure sensitive. One possibility is that [Cl−]in might be sufficiently high that some neurons, such as MB KCs, might display excitatory GABAergic responses. That is, these mutant neurons may come to resemble mammalian fetal neurons and depolarize in response to GABA transmission. Although EGABA has not been measured in kccDHS1 MB neurons to allow a direct test of this, observations on Rdl expression make us think that excitatory GABA may be a possibility. Reducing the level of the Rdl GABAA receptor in the MB by RNAi ameliorates seizure sensitivity in kccDHS1 flies (Figure 7B) suggesting a reduction in excitability. Ordinarily, reduction of GABAA receptor is expected to decrease inhibition resulting in an overall increase in excitability. This resembles a previous finding (Hekmat-Scafe et al. 2006) that the GABAA blocker picrotoxin is a seizure suppressant in kccDHS1 mutants, but is a convulsant for normal flies. Our results are most consistent with the notion that excitatory GABAergic signaling in the MBs influences the maintenance, rather than the development, of an epileptic state in the brain. We observed that kccDHS1 flies in which kcc+ was induced in the MBs during the late pupal stage using the c772 driver (Armstrong et al. 1998) displayed identical levels of behavioral seizure susceptibility to control flies 24-hr postecclosion (A. Fajilan and D. Hekmat-Scafe, data not shown), but significantly reduced levels 12 hr later (Figure 5). This suggests that the effect of kcc in MB occurs during the late pupal–early adult period.
Perhaps it is the MB's neuronal plasticity that makes it particularly vulnerable to the development of epileptic-like seizures. The mammalian hippocampus, which is also critical for learning and memory, is a frequent site of epileptic foci (Hauser and Hesdorffer 1990). One possibility is that the plasticity of these brain regions reflects the type of excitatory GABAergic signaling normally observed during early neuronal development (Ben-Ari et al. 2007). Excitatory GABAergic signaling has been observed in the adult hippocampus, where rhythmic firing is believed to promote neuronal plasticity (Obrietan and van den Pol 1996; Ge et al. 2006), but may also make this structure particularly susceptible to epileptic seizures.
Inhibitory synaptic strength and Drosophila MB function:
MB kcc+ rescues seizure sensitivity not only in kccDHS1 mutants, but also in other BS mutants, such as bss and eas. These genes encode different products and appear to cause Drosophila seizure sensitivity through different mechanisms (Ganetzky and Wu 1982; Pavlidis et al. 1994). Nevertheless, a common feature must account for phenotypic suppression of these different mutations: we propose that this is a kcc+-mediated increase in inhibitory synaptic strength. There are several implications arising from this proposition. The first is that inhibitory synaptic strength must normally be capable of being strengthened in MB neurons. We infer that this is the case because we believe that the mechanisms underlying bss and eas seizure sensitivity are not directly related to a damaged inhibitory signaling system; their synapses are more likely to reflect a fairly normal inhibitory synaptic situation in the MB. Nevertheless, synaptic inhibition is apparently strengthened by kcc+ expression in the MB of these mutants, producing seizure suppression. Second, this strengthening of inhibitory synaptic strength by kcc+ is likely generated by decreasing [Cl−]in, suggesting that normally, [Cl−]in could be relatively high in MB neurons. The normally high [Cl−]in might also be facilitated by the presence of substantial Ncc69, since a decrease in its level by RNAi can also markedly suppress seizures (Figure 7C).
Overall, these observations suggest that normally, inhibitory synaptic strength in the MB may have a relatively large working range due to the presence of both kcc and Ncc69: a working range that is apparently revealed by mutation or RNAi utilizing seizure sensitivity as an indirect assay. This could be an artifact of the experimental methodologies used especially given the vagaries of GAL4/UAS ectopic expression and exactly how to interpret seizure enhancement and seizure suppression as a measure of synaptic strength. Nevertheless, loss-of-function mutations and RNAi experiments indicate clearly the importance of kcc and Ncc69 functions in the MB. Taken together all of the observation are very suggestive of high [Cl−]in in the MB. Finally, if, given these caveats, it remains true that inhibitory synaptic strength in the MB has a large working range, we wonder what is its normal function? It is tempting to speculate that inhibitory synaptic modulation might be critical in some aspect of fly learning or memory, the best studied and most interesting aspect of MB function (Heisenberg 2003; Davis 2005; Keene and Waddell 2007; Berry et al. 2008). Inhibition and its modulation by upstream regulators such as fray, the fly homolog of SPAK (Leiserson et al. 2000), are generally not considered as important aspects of current models of learning and synaptic plasticity. However, they may ultimately play an important role in sprouting and connectivity, aspects that could contribute significantly to the importance of MB function.
Acknowledgments
We are most grateful to Scott Waddell (University of Massachusetts Medical School), Julie Simpson (Janelia Farms), Gero Miesenboeck (Yale University), Christopher Tabone and J. Steven de Belle (University of Nevada, Las Vegas), Xu Liu and Ron Davis (Baylor University Medical College), Roland Strauss (University of Wuerzburg), Nara Muraro and Richard Baines (University of Manchester), Gautam Agarwal and Ehud Isacoff (University of California, Berkeley), the Bloomington Drosophila Stock Center, and the Vienna Drosophila RNAi Center for Drosophila stocks. Gautam Agarwal and Grant Kauwe provided helpful advice and assistance with the GFP imaging of Drosophila brains. This work was supported by grants from the McKnight Foundation and the National Institute of Neurological Disorders and Stroke (NS-31231) to M.T., by R01 DK57708 to D.M., and by a summer research grant to R.H. from the Biology Fellows Program, which is funded by the Howard Hughes Medical Institute for undergraduate biological science majors at the University of California, Berkeley.
References
- Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang et al., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. D., J. S. de Belle, Z. Wang and K. Kaiser, 1998. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn. Mem. 5 102–114. [PMC free article] [PubMed] [Google Scholar]
- Aso, Y., K. Grubel, S. Busch, A. B. Friedrich, I. Siwanowicz et al., 2009. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenetics 23 156–172. [DOI] [PubMed]
- Ben-Ari, Y., J.-L. Gaiarsa, R. Tyzio and R. Khazipov, 2007. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87 1215–1284. [DOI] [PubMed] [Google Scholar]
- Berry, J., W. C. Krause and R. Davis, 2008. Olfactory memory traces in Drosophila. Prog. Brain Res. 169 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse, P., M. S. Airaksinen, C. Rivera and K. Kaila, 2009. Cation-chloride cotransporters and neuronal function. Neuron 61 820–838. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Buchner, E., 1991. Genes expressed in the adult brain of Drosophila and effects of their mutations on behavior: a survey of transmitter- and second messenger-related genes. J. Neurogenet. 7 153–192. [DOI] [PubMed] [Google Scholar]
- Buchner, E., R. Bader, S. Buchner, J. Cox, P. C. Emson et al., 1988. Cell-specific immuno-probes for the brain of normal and mutant Drosophila melanogaster. I. Wildtype visual system. Cell Tissue Res. 253 357–370. [DOI] [PubMed] [Google Scholar]
- Chen, G., P. Q. Trombley and A. N. van den Pol, 1996. Excitatory actions of GABA in developing rat hypothalmic neurones. J. Physiol. 494 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli, V. R., J. Wang and J. A. Dow, 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39 715–720. [DOI] [PubMed] [Google Scholar]
- Davis, R. L., 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28 275–302. [DOI] [PubMed] [Google Scholar]
- Dietzl, G., D. Chen, F. Schnorrer, K. C. Su, Y. Barinova et al., 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 151–156. [DOI] [PubMed] [Google Scholar]
- Duffy, J. B., 2002. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis 34 1–15. [DOI] [PubMed] [Google Scholar]
- Farrant, M., and K. Kaila, 2007. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog. Brain Res. 160 59–87. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant, R. H., D. P. Mortlock, C. D. Shaffer, R. J. Macintyre and R. T. Roush, 1991. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc. Natl. Acad. Sci. USA 88 7209–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant, R. H., J. C. Steichen, T. A. Rocheleau, K. Aronstein and R. T. Roush, 1993. A single-amino acid substitution in a gamma-aminobutyric acid subtype A receptor locus is associated with cyclodiene insecticide resistance in Drosophila populations. Proc. Natl. Acad. Sci. USA 90 1957–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov, V., K. Aimanova and S. S. Gill, 2003. Expression of an Aedes aegypti cation-chloride cotransporter and its Drosophila homologoues. Insect Mol. Biol. 12 319–331. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., and C.-F. Wu, 1982. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, S., E. L. Goh, K. A. Sailor, Y. Kitabatake, G. L. Ming, and H. Song, 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. R., H. H. Chen, E. Satelle, P. J. Barker, N. S. Huskisson et al., 1996. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system of Drosophila melanogaster. Cell Tissue Res. 284 269–278. [DOI] [PubMed] [Google Scholar]
- Hauser, W. A., and D. C. Hesdorffer, 1990. Epilepsy: Frequency, Causes, and Consequences. Demos, New York, NY.
- Heisenberg, M., 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4 266–275. [DOI] [PubMed] [Google Scholar]
- Hekmat-Scafe, D. S., M. Y. Lundy, R. Ranga and M. A. Tanouye, 2006. Mutations in the K+/Cl− cotransporter gene kazachoc (kcc) increase seizure susceptibility in Drosophila. J. Neurosci. 26 8943–8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, A. M., K. Aronstein, D. B. Sattelle and R. H. ffrench-Constant, 1997. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 20 578–583. [DOI] [PubMed] [Google Scholar]
- Ito, K., R. Okada, N. K. Tanaka and T. Awasaki, 2003. Cautionary observations on preparing and interpreting brain images using molecular biology-based staining techniques. Microsc. Res. Tech. 62 170–186. [DOI] [PubMed] [Google Scholar]
- Jackson, F. R., L. M. Newby and S. J. Kulkarni, 1990. Drosophila GABAergic systems: sequence and expression of glutamic acid decarboxylase. J. Neurochem. 54 1068–1078. [DOI] [PubMed] [Google Scholar]
- Keene, A. C., and S. Waddell, 2007. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8 341–354. [DOI] [PubMed] [Google Scholar]
- Krashes, M. J., A. C. Keene, B. Leung, J. D. Armstrong and S. Waddell, 2007. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler, D., and M. A. Tanouye, 2000. Modifications of seizure susceptibility in Drosophila. J. Neurophysiol. 83 998–1009. [DOI] [PubMed] [Google Scholar]
- Leal, S. M., N. Kumar and W. S. Neckameyer, 2004. GABAergic modulation of motor-driven behaviors in juvenile Drosophila and evidence for a nonbehavioral role for GABA transport. J. Neurobiol. 61 189–208. [DOI] [PubMed] [Google Scholar]
- Leiserson, W. M., E. W. Harkins and H. Keshishian, 2000. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28 793–806. [DOI] [PubMed] [Google Scholar]
- Liu, X., and R. L. Davis, 2009. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat. Neurosci. 12 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., W. C. Krause and R. L. Davis, 2007. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron 56 1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann, H. J., and D. A. Prince, 1991. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophysiol. 65 247–263. [DOI] [PubMed] [Google Scholar]
- Martin, J. R., A. Keller and S. T. Sweeney, 2002. Targeted expression of tetanus toxin: a new tool to study the neurobiology of behavior. Adv. Genet. 47 1–47. [DOI] [PubMed] [Google Scholar]
- Mercado, A., V. Broumand, K. Zandi-Nejad, A. H. Enck and D. B. Mount, 2006. A carboxy-terminal domain in KCC2 confers constitutive K+−Cl− cotransport. J. Biol. Chem. 281 1016–1026. [DOI] [PubMed] [Google Scholar]
- Mercado, A., G. Gamba and D. B. Mount, 2004. Molecular physiology of mammalian K(+)−Cl− cotransporters. Adv. Exp. Med. Biol. 559 29–41. [PubMed] [Google Scholar]
- Mount, D. B., A. Mercado, L. Song, J. Xu, A. L. George Jr. et al., 1999. Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J. Biol. Chem. 274 16355–16362. [DOI] [PubMed] [Google Scholar]
- Neckameyer, W. S., and R. L. Cooper, 1998. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert. Neurosci. 3 279–294. [DOI] [PubMed] [Google Scholar]
- Ng, M., R. D. Roorda, S. Q. Lima, B. V. Zemelman, P. Morcillo et al., 2002. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36 463–474. [DOI] [PubMed] [Google Scholar]
- Obrietan, K., and A. N. van den Pol, 1995. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J. Neurosci. 15 5065–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan, K., and A. N. van den Pol, 1996. Growth cone calcium elevation by GABA. J. Comp. Neurol. 372 167–175. [DOI] [PubMed] [Google Scholar]
- Owens, D. F., L. H. Boyce, M. B. Davis and A. R. Kriegstein, 1996. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 16 6414–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis, P., M. Ramaswami and M. A. Tanouye, 1994. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79 23–33. [DOI] [PubMed] [Google Scholar]
- Perez-Orive, J., O. Mazor, G. C. Turner, S. Cassenaer, R. I. Wilson et al., 2002. Oscillations and sparsening of odor representations in the mushroom body. Science 297 359–365. [DOI] [PubMed] [Google Scholar]
- Renn, S. C., J. D. Armstrong, M. Yang, Z. Wang, X. An et al., 1999. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J. Neurobiol. 41 189–207. [PubMed] [Google Scholar]
- Salvaterra, P. M., and T. Kitamoto, 2001. Drosophila cholinergic neurons and processes visualized with Gal4/UAS–GFP. Brain Res. Gene Expr. Patterns 1 73–82. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russell, 2001. Molecular Cloning: A Laboratory Manual, Ed. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Song, L., A. Mercado, N. Vazquez, Q. Xie, R. Desai et al., 2002. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Mol. Brain Res. 103 91–105. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., and G. M. Rubin, 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218 341–347. [DOI] [PubMed] [Google Scholar]
- Stewart, B. A., H. L. Atwood, J. J. Renger, J. Wang and C. F. Wu, 1994. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 175 179–191. [DOI] [PubMed] [Google Scholar]
- Stocker, R. F., G. Heimbeck, N. Gendre and J. S. de Belle, 1997. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 32 443–456. [DOI] [PubMed] [Google Scholar]
- Su, H., and D. K. O'Dowd, 2003. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J. Neurosci. 8 9246–9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, J. S., F. Lin and M. A. Tanouye, 2004. Potassium bromide, an anticonvulsant, is effective at alleviating seizures in the Drosophila bang-sensitive mutant bang senseless. Brain Res. 1020 45–52. [DOI] [PubMed] [Google Scholar]
- Traub, R. D., and R. Miles, 1991. Neuronal networks of the hippocampus. Cambridge University Press, Cambridge, UK.
- Wang, J., D. B. Reichling, A. Kyrozis and A. B. MacDermott, 1994. Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur. J. Neurosci. 6 1275–1280. [DOI] [PubMed] [Google Scholar]
- Wang, X., D. S. Green, S. P. Roberts and J. S. de Belle, 2007. Thermal disruption of mushroom body development and odor learning in Drosophila. PLoS One 2 e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, N.-S., J. Lu, R. England, R. McClellan, S. Dufour et al., 2002. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus 12 258–268. [DOI] [PubMed] [Google Scholar]
- Yang, M. Y., J. D. Armstrong, I. Vilinsky, N. J. Strausfeld and K. Kaiser, 1995. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron 15 5–54. [DOI] [PubMed] [Google Scholar]
- Yasuyama, K., I. A. Meinertzhagen and F. W. Schürmann, 2002. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J. Comp. Neurol. 445 211–226. [DOI] [PubMed] [Google Scholar]
- Yuste, R., and L. C. Katz, 1991. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron 6 334–344. [DOI] [PubMed] [Google Scholar]
- Zhang, H. G., H. J. Lee, T. Rocheleau, R. H. ffrench-Constant and M. B. Jackson, 1995. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol. Pharmacol. 48 835–840. [PubMed] [Google Scholar]
- Zhu, L., N. Polley, G. C. Matthews and E. Delpire, 2008. NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res. 79 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]