Abstract
Generation of an organ of appropriate size and shape requires mechanisms that coordinate growth and patterning, but how this is achieved is not understood. Here we examine the role of the growth regulator dMyc in this process during Drosophila wing imaginal disc development. We find that dMyc is expressed in a dynamic pattern that correlates with fate specification of different regions of the wing disc, leading us to hypothesize that dMyc expression in each region directs its growth. Consistent with this view, clonal analysis of growth in each region demonstrated distinct temporal requirements for dMyc that match its expression. Surprisingly, however, experiments in which dMyc expression is manipulated reveal that the endogenous pattern has only a minor influence on wing shape. Indeed, when dMyc function is completely lacking in the wing disc over most of its development, the discs grow slowly and are small in size but appear morphologically normal. Our experiments indicate, therefore, that rather than directly influence differential growth in the wing disc, the pattern of dMyc expression augments growth directed by other regulators. Overall, however, an appropriate level of dMyc expression in the wing disc is necessary for each region to achieve a proportionately correct size.
HOW pattern and growth are coordinated during development to produce an organ of correct size and shape is a central question in biology. The Drosophila wing is an elegant, self-organizing system that is ideal for the study of this coordination. Wing growth is coupled to the specification of cell fates, and these processes are regulated by a small number of conserved signaling pathways and selector proteins. The wing develops from the wing imaginal disc, a proliferating epithelium housed in the larva that also gives rise to the dorsal thorax of the adult fly. The adult wing includes the blade, made from wing pouch (WP) cells of the wing disc, and hinge structures, which are formed by cells immediately proximal to the WP.
Wing development proceeds through a series of steps in which regions of fates are specified. Discs begin development composed of cells with either anterior (A) or posterior (P) identity and subsequently undergo several subdivisions. Early in the second larval instar (L2), the action of Wingless (Wg) and the EGF receptor divide the wing disc into large domains that define the body wall and wing (Wang et al. 2000; Zecca and Struhl 2002). A short time later, a second subdivision segregates dorsal (D) and ventral (V) cells. At the D/V boundary, Notch signaling induces expression of Wg and the wing selector gene vestigial (vg) in the boundary cells, initiating the expansion of the WP region (Couso et al. 1993; Williams et al. 1993; Diaz-Benjumea and Cohen 1995; Kim et al. 1995, 1997; Neumann and Cohen 1996, 1997; Zecca et al. 1996). Near the end of L2, the expression of homothorax (hth), a selector gene required for hinge development, becomes specifically expressed in proximal cells (Azpiazu and Morata 2000; Casares and Mann 2000). This is followed in early L3 by the appearance of a ring of Wg expression that circumscribes the WP (the inner ring, IR), and in mid-L3 a second, concentric ring (the outer ring, OR) (Couso et al. 1993; Williams et al. 1993; Neumann and Cohen 1996). Hth is a target of Wg in these cells and is upregulated in cells adjacent to the two rings of Wg expression (Casares and Mann 2000). These latter events mark the hinge specification of proximal wing cells.
Recent work indicates that Wg and Dpp, a BMP/TGF-β family member, regulate wing growth by engaging the Fat/Hippo tumor-suppressor signaling pathway and by controlling a Vg feed-forward loop that expands the WP (Zecca and Struhl 2007a,b; Rogulja et al. 2008). Fat/Hippo signaling regulates the transcription of several genes required for cell survival, cell division, and growth (Harvey et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003; Huang et al. 2005; Cho et al. 2006). The dMyc transcription factor, encoded by the diminutive (dm) gene, also provides an essential role in controlling growth of the fly and is regulated by Wg and Dpp (Johnston et al. 1999; Prober and Edgar 2002). Myc is a conserved protein that is essential for growth in both vertebrates and invertebrates. In both mice and Drosophila, hypomorphic alleles of myc result in animals with a smaller body size (Johnston et al. 1999; Trumpp et al. 2001). Despite being smaller, dm mutant flies appear morphologically normal with no obvious patterning defects (Johnston et al. 1999), suggesting tight linkage between the patterning machinery and dMyc. In the wing, Wg and Notch activity repress dMyc expression in the zone of nonproliferating cells that surrounds the D/V boundary to enforce a cell cycle arrest of these cells (Johnston et al. 1999; Duman-Scheel et al. 2004; Herranz et al. 2008). However, how dMyc contributes to wing development and the nature of the relationship between pattern formation and dMyc expression and activity in the growing wing disc is not understood.
In this study, we examine the role of dMyc in the generation of size and shape of the Drosophila wing. We find that dMyc expression is regionally patterned and dynamic throughout wing development and provide evidence that its spatial and temporal expression pattern corresponds to a functional requirement in the growth of cells in different regions of the disc. Despite this, our data indicate that the spatial pattern of dMyc expression is not necessary for sculpting the shape of the wing. Furthermore, we find that rudimentary wing growth can occur in the complete absence of dMyc, although its absence prevents the wing from reaching the correct size or proportion. Together, our experiments argue that while not essential to produce a wing, dMyc expression and function permits the wing to grow at a rate that is compatible with the rate of larval development and allows each region to reach its correct size and proportion at the end of development.
MATERIALS AND METHODS
Fly strains and husbandry:
The following strains were used: dmP0 (Johnston et al. 1999), yw;Tub>dmyc, y+>Gal4/CyO; +, and yw;+;Tub>dmyc, y+>Gal4 (hereafter called Tub-dmyc) (de la Cova et al. 2004). Sevelin, yw;+;+, FRT19A;ry506, and yw; FRT82B N-myc were obtained from the Bloomington Stock Center. w dmP0 FRT19A/FM7c, w dm4 FRT19A/FM7, and FRT82B Tub-dmycWT were gifts of P. Gallant. yw Ubi-GFP FRT19A;hsflp1 was a gift of G. Struhl. P{neoFRT}82B M(3)96C, arm-lacZ was a gift of E. Bach. yw;VgGal 4, UAS-flp, Tub>CD2>Gal 4 UAS-GFP/CyO was a gift of M. Crickmore (Crickmore and Mann 2006).
Embryos from appropriate crosses were collected on grape plates for 2-hr periods and ≤50 first instar larvae were transferred to freshly yeasted, molasses food vials and raised at 25°.
Growth measurements:
Clonal analysis:
Mutant or control (zero-copies GFP) and sibling (two-copies GFP) clones were induced by Flp/FRT-mediated mitotic recombination after larval heat shock in a 37° incubator. Heat shocks were carried out for 40 min at 48 hr after egg laying (AEL) or for 30 min at 72 hr AEL. Clones induced at 48 hr AEL were allowed to grow until either 81 hr AEL or 112 hr AEL. Clones induced at 72 hr AEL were allowed to grow until 112 hr AEL. Discs were stained for either Wg or Hth protein to define the hinge, pouch, and notum. The clonal area was measured using Axiovision software (Zeiss) as described (de la Cova et al. 2004).
Minute experiments:
Tub-dmycWT (Steiger et al. 2008) was recombined onto FRT82B M(3)96C, arm-lacZ. Experiments were done with dm4 mutant males rescued with Tub-dmycWT. Animals were heat-shocked mid-second instar (72 hr AEL for dm4; FRT82B Tub-dmyc/+ and 76 hr AEL for dm4; FRT82B M Tub-dmyc/+) and dissected 64 hr later (136 and 140 hr). dm4 mutant clones were marked with two copies of pi-myc or lack of arm-lacZ. FRT82B control clones were induced at 48 hr AEL and dissected at 112 hr AEL.
Wing disc size measurements:
The IR and OR of Wg expression were used to demarcate the WP and hinge (Figure 2A). Hinge size was measured at 82, 96, and 110 hr AEL as the area within the IR and OR, and WP size as the area inside the IR. Given that the hinge region of the wing disc becomes increasingly folded between 96 and 110 hr AEL, this method of size measurement underestimates actual hinge size; however, it allows an assessment of relative size trends between genotypes as the discs gain mass.
Figure 2.—
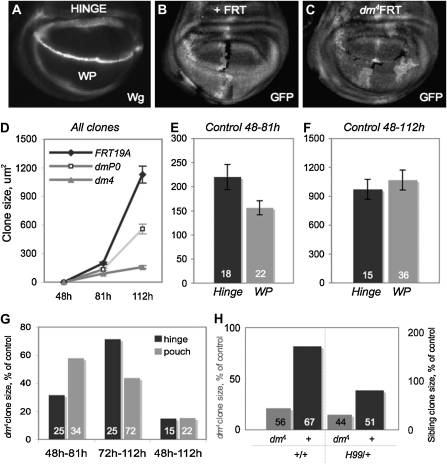
Regional and temporal growth requirements for dMyc reflect its expression pattern during wing disc growth. (A) Analysis of control and dmyc mutant clones in female larvae to determine growth requirements for dMyc. Clones were induced at specific times and scored on the basis of location: hinge (region within Wg rings) and WP (region within inner ring of Wg). Different regions of the disc were scored using either Wg or Hth staining. (B and C). Mitotic recombination produces a GFP-negative clone and a sister clone with two copies of GFP. The GFP-negative dmyc mutant clones (dmP0, hypomorphic allele, or dm4, null allele) (C) were compared to GFP-negative control clones (B). (D) Clonal growth of dmP0 mutant cells and dm4 mutant cells show a dose-dependent requirement for dmyc in cell proliferation and growth. Clones were induced at 48 hr AEL and dissected at either 81 hr AEL or 112 hr AEL to assess growth over time (where clone growth is the product of cellular growth, cell division, and cell survival). (E) Hinge cell clones grow significantly larger than WP clones early (P = 0.04). (F) WP clones later grow more to reach the same size as hinge clones by 112 hr AEL. (G) dm4 mutant clones are significantly smaller than control clones in all regions both early and late in development (P <10−3 for all dm4 mutant clones compared to corresponding control). Hinge cells are more sensitive to loss of dmyc early in development, whereas WP cells are more sensitive to loss of dmyc late in development. Early, dm4 hinge clones grow to 32% of control hinge clone size while dm4 WP clones reach 58% of control WP clone size (Mann–Whitney test, P <10−4). Later, dm4 WP clones grow to only 44% of control WP clone size while dm4 hinge clones reach 71% of control hinge clone size (Mann–Whitney test, P <10−4). By the end of development, dm4 clones in either region are <20% of corresponding control clone size. (H) Wild-type sibling clones (GFP++) of dm4 mutant clones that have grown from 48 hr AEL to 112 hr AEL are significantly bigger than corresponding control GFP++ clones (P <10−9). In a H99/+ background, wild-type sibling clones of dm4 mutant clones are no longer significantly bigger than corresponding control GFP++ clones (P = 0.17). The H99/+ background does not alter the size of the dm4 mutant clone (combined from all regions: P = 0.83). Bar graphs are labeled with number of clones measured.
Adult wing measurements:
Hinge measurements in adult wings were made by tracing the proximal to distal costa, continuing to its intersection with the radius, connecting this intersection to the allula, tracing around the allula and axillary cord, and finishing back at the proximal costa, excluding the tegula. Blade measurements were made by following the distal border of the hinge (as defined above) and encircling the rest of the blade.
Statistics used for size measurements:
For size measurements, Student's t-test was used to determine significance of P < 0.05. When measurements were converted to ratios or normalized to control, a nonparametric test, Mann–Whitney, was used to determine significance of P < 0.05. P-values were calculated using Excel or MiniTab.
Quantitative PCR:
The relative level of dmyc expression was determined by quantitative (real-time) RT–PCR on RNA isolated from 30 to 40 late L3 wing discs of each genotype (Table 1). RNA was isolated using TRIzol (Invitrogen), and single-stranded cDNA was produced from 1 μg RNA using a SuperScript First-Strand Synthesis kit (Invitrogen). PCR reactions were performed using LightCycler FastStart DNA MasterPlus SYBR Green I kit (Roche). dmyc expression levels in each genotype were normalized to act5C or nup44A levels (both genes gave equivalent results) and then normalized to yw control. RNA was isolated from whole wing discs; thus the level of dmyc mRNA expression is the sum of all regions of the disc. Comparisons between discs with patterned vs. ubiquitous dmyc expression are therefore an averaging of high- and low-expressing cells.
TABLE 1.
Modulation of the dmyc expression pattern alters wing disc size in late-L3 wing discs
| Condition | Genotype | % yw WP | % yw H | WP:H ratio | dmyc mRNA | n |
|---|---|---|---|---|---|---|
| Wild type | yw | 100 | 100 | 1.4 | 1.0 | 19 |
| 1 | yw, Tub-dmyc/+ | 105 | 109 | 1.1 | 1.7 | 31 |
| 2 | dm PO | 46 | 56 | 1.2 | 0.2 | 24 |
| 3 | dm4; Tub-dmyc/+ | 63 | 74 | 1.2 | 0.8 | 30 |
|
dm4;+;Tub-dmyc/+ |
62 |
77 |
1.2 |
ND |
19 |
Wing disc size from male larvae at late L3 (110-hr time point in Figure 4). Conditions 1–3 are as described in the text. yw is used as a wild type (WP) and hinge (H). dmyc mRNA level per wing disc cell is relative to yw. dm4;+;Tub-dmyc/+ measurements were measured in a separate experiment; this genotype is delayed by 23 hr compared to yw and dm4; Tub-dmyc/+.
Immunocytochemistry:
RNA in situ hybridizations were carried out using digoxigenin-labeled RNA probes (Johnston and Edgar 1998). Fixation and immunocytochemistry of imaginal discs were carried out as described (Johnston and Edgar 1998). The following antibodies and dilutions were used: mouse anti-digoxigenin-AP, 1:2000 (Roche); rabbit anti-GFP, 1:200 (Invitrogen); mouse anti-Wg 4D4, 1:30 (Developmental Studies Hybridoma Bank); guinea pig anti-Hth, 1:2000 (gift of R. Mann); rabbit anti-β-gal, 1:2000 (MP Biomedicals); and guinea pig anti-dMyc, 1:1000 (gift of G. Morata). Secondary antibodies used were purchased from Jackson Immunoresearch and Invitrogen Molecular Probes. Images were taken using a Zeiss Axioplan 2 microscope with an Orca-100 CCD camera (Hammatsu) or AxioCam (Zeiss) and processed with Photoshop (Adobe) software.
Vg memory experiment:
To selectively remove dMyc from wings of animals that otherwise express dMyc, we used a “memory” experiment with flies of the following genotype: dm4; VgGal 4, UAS-flp, Tub>CD2>Gal 4/+, UAS-GFP; Tub>dmyc>Gal4/+. Cells that express VgGal4 at any point during development will express UAS-Flp recombinase and excise the >CD2> cassette and the >dmyc>cassette. Once the >CD2> cassette is excised, the cells will heritably express GFP from the tubulin promoter. Loss of dmyc expression occurs only from cells that have expressed VgGal4 in their lifetime, while the rest of the animal retains the Tub>dmyc>Gal4 cassette. The VgM driver is activated prior to when dMyc expression becomes patterned in the wing (supporting information, Figure S5, A and B). In addition to the wing disc, GFP-positive cells can be found in the haltere, salivary glands, and a few cells of the leg and brain in the VgM experiment (data not shown).
RESULTS
dMyc is expressed in a dynamic pattern during wing disc development:
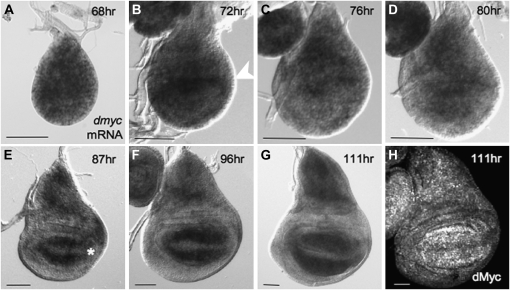
To determine how dMyc contributes to the growth of proximal and distal regions of the wing, we first examined its mRNA and protein expression during the developmental transitions that specify the fates of each region. dmyc mRNA is expressed in all cells early in L2 (Figure 1A). As hinge development begins at the transition to L3, dmyc expression transiently increases in proximal cells that also initiate expression of the IR of Wg (Figure 1B, Figure S1). As L3 proceeds, dmyc expression gradually decreases throughout the dorsal and ventral hinge region, while remaining at high levels in the dorsal body-wall primordium, the notum. At the same time, dmyc expression increases in distal cells (Figure 1, C–F). By the end of larval development dmyc expression is very low in hinge cells but high in cells of the notum and WP. With the onset of proneural specification at the wing margin, dmyc expression is repressed by the activities of Wg and Notch in cells flanking the D/V boundary as these cells arrest growth and division (Figure 1G) (Johnston et al. 1999; Johnston and Sanders 2003; Duman-Scheel et al. 2004; Herranz et al. 2008). dMyc protein expression is similar to dmyc mRNA at all stages examined (Figure 1H, Figure S1, and data not shown). These observations indicate that the expression of dmyc is closely allied with the subdivision of the disc into regions of proximal and distal cell fates.
Figure 1.—
dMyc is expressed in a dynamic pattern during wing disc development. (A–G) dmyc mRNA by RNA in situ hybridization. (A) dmyc is fairly uniformly expressed at early stages of wing disc development. (B and C) Early in the third instar, dmyc expression increases in hinge cells (arrowhead in B; also see Figure S1). (D–G) Later, dmyc expression gradually decreases in hinge cells. At the same time, dmyc expression in WP cells intensifies. Still later, dmyc expression is repressed in cells at the D/V boundary as they undergo cell cycle and growth arrest (asterisk in E). (H) dMyc protein expression is similar to that of dmyc mRNA. Bars, 50 μm.
The dynamic expression of dMyc correlates with changes in disc growth:
The dynamic temporal and regional expression pattern of dMyc led us to hypothesize that the functional requirement for dMyc changes during growth of the wing disc. To test this idea, we used Flp/FRT-mediated mitotic recombination to remove dmyc function in cell clones at specific times during disc growth (Xu and Rubin 1993). Clones of wing disc cells mutant for either the hypomorphic allele dmP0 or the null dm4 allele were marked by the absence of GFP, as were control clones induced in parallel experiments. The area of each mutant clone was measured after defined periods of growth and compared to controls. As cells remain in close proximity after division, this provides a reasonable measure of growth (Neufeld et al. 1998; Johnston et al. 1999; de la Cova et al. 2004).
We used the transitions in dmyc expression as a guide for the initiation and duration of clone growth. To examine the dMyc requirement from the early period of uniform dmyc expression to its upregulation in hinge cells, clones were induced at the onset of L2, 48 hr AEL, and allowed to grow until 81 hr AEL, early in L3. The requirement for dMyc during later transitions in dmyc expression was examined with mutant and control clones grown from the onset of L3 at 72 or 81 hr AEL until 112 hr AEL, late in L3. Comparison of mutant and control clone size revealed a clear requirement for dmyc function at each time point (Figure 2D, Figure S2). Throughout L2 and L3, dmP0 and dm4 mutant clones grew at substantially reduced rates compared to control clones and were significantly smaller than controls at the end of the growth phase (Figure 2D). The amount of growth that occurred was dMyc dose dependent, such that dmP0 clones grew more at each time point than dm4 clones. Despite their significantly reduced size, dm4 null mutant clones proliferate to some extent (median number of cells = 17, 48–112 hr AEL clones), indicating that cells lacking dmyc grow at a rate set by other growth regulatory mechanisms.
We then examined the functional requirement for dMyc in distal and proximal regions of the disc at specific times. Control and dm4 mutant clones were generated as above and scored on the basis of their location in either hinge or WP (Figure 2A). We focused on these regions in particular because their counterparts are easily measured in adults. The early L3 increase in dmyc expression in cells fated to be hinge (Figure 1, B–D; Figure S1) predicted that its loss at that time would compromise the growth of these cells. We therefore allowed dm4 and control clones to grow from 48 to 81 hr AEL and compared the extent of their growth (Figure 2D). During this period, control clones in the hinge grew significantly larger than those in the WP (Figure 2E). dm4 hinge clones grew slowly and reached only 32% of control clone size at the end of the growth period (Figure 2G). dm4 clones located in the WP also grew slowly, but were not as compromised as hinge clones and reached 58% of control WP clone size (Figure 2G). Thus, hinge cells are particularly sensitive to loss of dmyc during L2–early L3. This suggests that the increase in dmyc expression in hinge cells during this time contributes to their relatively faster rate of growth (Figure 2G).
Midway through L3, dmyc expression again changes: it increases in WP cells and is reduced in hinge cells (Figure 1, E–G). These changes suggest that the requirement for dMyc in hinge cells is reduced as development proceeds, whereas in WP cells it increases. Whereas significantly smaller at 81 hr AEL (P = 0.04), control WP cell clones reached the same size as hinge clones at the growth period's end, suggesting that they grew more during 81–112 hr AEL (Figure 2, E and F). Growth of dm4 mutant WP clones was significantly impaired and reached only 44% of control clone size by the end of the growth period (Figure 2G). In contrast, dm4 clones in the hinge were less compromised and reached 71% of control hinge clone size (Figure 2G). These data indicate that the temporal changes in the pattern of dmyc mRNA and protein expression are accompanied by dynamic regional requirements for dMyc function during wing development.
Even though hinge and WP cells appear to have temporally different requirements for dMyc, proliferation of cells in both regions was significantly impaired by its loss. The average size of a dm4 mutant clone that grew from 48 to 112 hr AEL was <20% of the size of a corresponding control clone (Figure 2G). The reduced growth could be due to cell-autonomous loss of dmyc function or to nonautonomous cell death induced by cell competition (Johnston et al. 1999; de la Cova et al. 2004). We assessed the contribution of cell competition in these experiments by examining the size of wild-type sibling clones, marked by two copies of GFP (GFP++), that were generated along with the mutant clones by recombination. Sibling GFP++ clones of dm4 mutant clones (GFP−) were considerably larger than control GFP++ clones induced in parallel (Figure 2H). Stimulation of faster growth of “winner” cells in response to slow growth of “loser” cells is a hallmark of cell competition (Johnston 2009); thus these results indicate that competition occurs between wild-type sibling cells and dm4 mutant cells (and also the nonclonal dm4/+ cells). This was confirmed by removing one copy of the proapopotic genes hid, grim, and rpr with the H99 deficiency (H99/+), which prevents 90% of competition-induced cell death due to dMyc overexpression (de la Cova et al. 2004). dm4 clones generated in a H99/+ background prevented the extra sibling clone growth. However, it did not appreciably alter the ability of dm null mutant cells to proliferate (P = 0.83) or to increase the frequency of their recovery (16% of GFP++ sibling clones were not accompanied by dm4 clones; this frequency was 14% in the H99/+ background) (Figure 2H). We also assessed the growth potential of dm4 mutant clones by inducing them in a Minute heterozygous background (M/+). We found that, although the additional growth advantage in a M/+ background significantly increased dm4 mutant clone size (P = 1.0 × 10−4), these clones were still only an eighth of the size of a control clone grown for the same period of time (Figure S3). We interpret these results collectively to mean that cells carrying the null dm4 allele have a very limited growth potential. We therefore conclude that the slow growth of dm4 clones is primarily due to a cell-intrinsic requirement for dMyc activity.
The sum of these experiments suggest that during L2 and L3 the distal and proximal regions of the wing disc grow with distinct characteristics that correlate with changes in dMyc expression. After an initial period in which dMyc is expressed uniformly in the disc, its expression transiently increases in cells fated to become hinge. This increase correlates with an increase in the proliferation rate of hinge cells compared to WP cells. A short time later, dMyc expression changes again, now decreasing in hinge cells but increasing in cells of the WP. This change, which occurs mid-L3, is correlated with a relative increase in WP cell proliferation. Also during this period, WP cells straddling the D/V boundary lose dMyc expression and exit the cell cycle (Johnston and Edgar 1998; Johnston et al. 1999). We conclude that the level of dMyc expression is correlated with the differential growth rates of cells in the wing disc.
The endogenous pattern of dMyc expression is dispensable for wing growth:
Thus far our results indicate that the patterned expression of dMyc is correlated with the regional growth rate differences observed during wing disc development and suggest that dMyc expression might contribute growth instructions for wing shape. We tested this hypothesis by experimentally manipulating the expression pattern and the level of dMyc expression during wing development. We created three genetic conditions in which the pattern and/or intensity of dmyc expression differed from our wild type control (yw, Figure 3, A, E, and I), using dm mutants and strains containing a transgene that ubiquitously expresses dMyc at low levels under the Tubulin α-1 promoter (Tub-dmyc) (de la Cova et al. 2004) as follows:
Condition 1 (yw;Tub-dmyc/+): These flies express the wild-type dmyc expression pattern plus additional ubiquitous expression driven by Tub-dmyc (Figure 3, B, F, and J). The transgene increased dmyc expression ∼65% over control wing discs (percentages of each condition were measured by quantitative RT–PCR and normalized to control yw wing discs; Table 1).
Condition 2 (dmP0): dmyc is expressed in the wild-type pattern but at 15% of control wing disc levels (Figure 3, C, G, K; Table 1).
Condition 3 (dm4;Tub-dmyc/+;+, or dm4;+;Tub-dmyc/+): The endogenous pattern of expression is abolished due to the dm4 null mutation. This is replaced by ubiquitous expression of dmyc at ∼77% of control wing disc levels (Figure 3, D, H, and L; Table 1). The results of each of these experiments are summarized in Table 1 and described below.
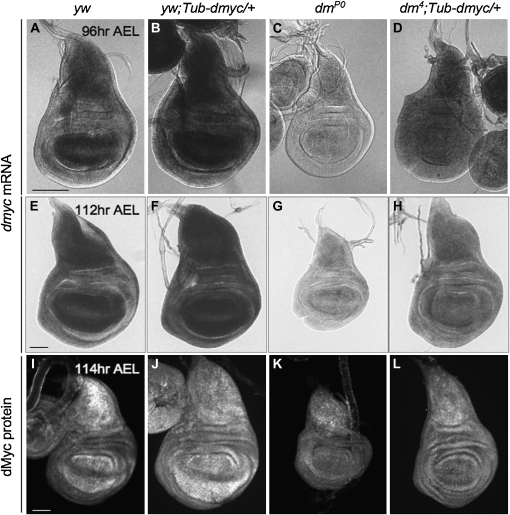
Figure 3.—
dMyc expression after experimental manipulation. dmyc mRNA in wing discs at 96 hr AEL (A–D) and 112 h AEL (E–H) of male larvae. (A and E) yw: wild-type level and patterned dmyc expression. (B and F) yw; Tub-dmyc/+; +: wild type pattern plus additional ubiquitous expression driven by Tub-dmyc (condition 1). (C and G) dmP0: low-level, but wild-type pattern, dMyc expression (condition 2). (D and H) dm4; Tub-dmyc/+;+: low-level, ubiquitous, dMyc expression (condition 3). The additional staining in the WP appears to coincide with the folds in this region and is less evident at 96 hr when the disc is less folded. (I–L) dMyc protein in wing discs at 114 hr AEL. (I) yw. (J) yw;Tub-dmyc/+; +. (K) dmP0. (L) dm4;Tub-dmyc/+; +.
The Tub-dmyc transgene rescues the L2 lethality of hemizygous dm4 male larvae to pharate adulthood, but only 2% of these animals eclosed (Table S1). Expression of mRNA and protein from the Tub-dmyc transgene was verified by RNA in situ hybridization and by immunofluorescence, respectively (Figure 3), and the relative level of dmyc mRNA was quantified by RT–PCR for each genetic condition (Table 1). We measured hinge and WP size in wing discs from each condition at three time points during L3 and in adult wings (see materials and methods for details). Although absolute scale differs between the genetic conditions, during their growth the discs of each condition take on appropriate and characteristic folds in the hinge and pleural regions, allowing size comparisons between them (Figure 3, Figure S4).
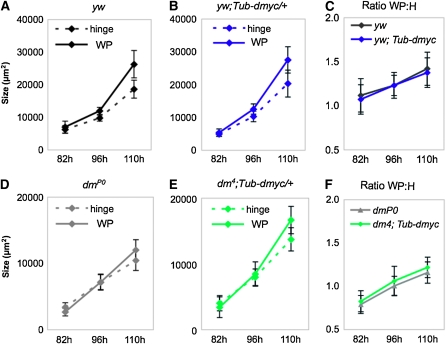
In control wing discs, the hinge and WP are similar in size at 82 hr AEL (Figure 4, A and C). During the subsequent 28 hr, isometry is lost and WP size increases faster than hinge size (Figure 4A), resulting in a significant rise in the ratio of WP to hinge (WP:H) size over time from 1.1 to 1.4 at the latest time point (Figure 4C). The late increase in WP:H ratio coincides with the upregulation of dmyc expression in WP cells and the concomitant expression decrease in hinge cells (Figure 1).
Figure 4.—
Proportional growth in the wing disc does not require patterned expression of dMyc, but growth rate is reduced in its absence. Hinge and WP sizes throughout development in male larvae. (A) Our wild type strain, yw. (B) yw plus Tub-dmyc/+ wing discs (condition 1). (A and B) At 82 hr AEL, hinge and WP sizes do not differ in either yw or yw;Tub-dmyc/+ wing discs (hinge vs. WP 82 hr: yw, P = 0.18; yw;Tub-dmyc/+, P = 0.23), but are different at 96 and 110 hr for both genotypes (hinge vs. WP 96 hr: yw, P = 4.0 × 10−8; yw;Tub-dmyc/+, P = 2.2 × 10−4; hinge vs. WP 110 hr: yw, P = 2.2 × 10−7; yw;Tub-dmyc/+, P = 2.2 × 10−9). (C) Ratios of wing WP to hinge (WP:H) in control yw and yw;Tub-dmyc wing discs throughout development. WP:H significantly increases over time in both yw and yw;Tub-dmyc/+ wing discs (Mann–Whitney test—yw: 82 vs. 96 hr, P = 0.027; 96 vs. 110 hr, P = 5.0 × 10−4; yw;Tub-dmyc/+: 82 vs. 96 hr, P = 4.8 × 10−3; 96 vs. 110 hr, P = 8.0 × 10−3), but WP:H ratios of yw and yw;Tub-dmyc/+ wing discs are not different from each other at any time point (Mann–Whitney test—yw vs. yw;Tub-dmyc/+: 82 hr, P = 0.47; 96 hr, P = 0.91; 110 hr, P = 0.41). (D) dmP0 (condition 2). (E) dm4;Tub-dmyc/+ (condition 3). (D and E) At 82 hr, hinge and WP size are significantly different in dmP0 wing discs (P <10−3) but are not different in dm4;Tub-dmyc/+ wing discs (P = 0.27). At 96 hr AEL, hinge and WP size are not significantly different in either dmP0 or dm4;Tub-dmyc/+ wing discs (hinge vs.WP at 96 hr: dmP0, P = 0.99; dm4; Tub-dmyc/+, P = 0.26) but are different at 110 hr in both genotypes (hinge vs. WP at 110 hr: dmP0, P = 8.0 × 10−4; dm4; Tub-dmyc/+, P = 2.2 × 10−7). (F) Ratios of WP:H in dmP0 and dm4;Tub-dmyc/+ wing discs throughout development. WP:H ratios of dmP0 and dm4;Tub-dmyc/+ wing discs significantly increase over time (Mann–Whitney test—dmP0: 82 vs. 96 hr and 96 vs. 110 hr, P = 1.0 × 10−4; dm4;Tub-dmyc/+: 82 vs. 96 hr and 96 vs. 110 hr, P < 10−4) and were consistently smaller than wild type at every time point (Mann–Whitney test—yw v dmP0 at 82, 96, 110 hr: P <10−4; yw v dm4;Tub-dmyc/+ at 82 and 96 hr: P = 1.0 × 10−4 and at 110 hr: P = 2.0 × 10−4). WP:H ratios of dmP0 and dm4;Tub-dmyc/+ wing discs were not significantly different at any time point (Mann–Whitney test—dmP0 vs. dm4;Tub-dmyc/+ at 82 hr: P = 0.44; at 96 hr: P = 0.91; and at 110 hr: P = 0.41). Measurements were done with animals carrying a Tub-dmyc cassette on chromosome 2. All P-values are derived from Student's t-tests unless otherwise indicated. Error bars show standard deviation. Three additional experiments showed similar trends.
Condition 1:
Although the Tub-dmyc transgene in yw wing discs increases dMyc expression by 65%, the growth of the discs does not deviate from the normal trend. Hinge and WP size are similar at 82 hr AEL but subsequently diverge due to faster growth of the WP (Figure 4B). As in controls, the WP:H size ratio increased from 1.1 to 1.4 over the 28 hr of growth in our experiments (Figure 4C).
Condition 2:
Wing discs from the hypomorphic mutant dmP0 express dmyc in the endogenous pattern but at substantially reduced levels (Figure 3, C, G, and K; Table 1). The reduced dmyc expression significantly impairs disc growth and leads to a smaller overall disc size at each time point (Figure 4D). We noted several differences in the kinetics of growth of these discs. In contrast to controls, hinge size is significantly larger than WP size at 82 hr AEL (P = 0.007), and parity of size between the two regions is reached sometime after 82 hr but prior to 96 hr AEL (Figure 4D). Moreover, WP size does not exceed hinge size until 110 hr AEL, ∼12 hr later than controls (Figure 4D). Although the WP:H size ratio of dmP0 discs increases incrementally (0.8 at 81 hr, 1.2 at 110 hr), it is consistently smaller than controls (Figure 4, C and F). In general, the hinge region grows more than the WP at all time points, but both regions are ∼50% smaller than yw controls at 110 hr. Larvae of this genotype are significantly delayed in their development and grow for an additional 10 hr beyond our last measuring point (data not shown). These observations suggest that the 85% reduction in dmyc expression in dmP0 wing discs leads to a growth program whose trend is virtually identical to controls, but which occurs at a significantly slower rate.
Condition 3:
Wing discs in which endogenous expression of dmyc is completely replaced by a low, ubiquitous level of dmyc expression (dm4; Tub-dmyc; Figure 3, D, H, and L) are severely reduced in size at all stages (Figure 4E). As with discs from condition 2, the relative growth changes between hinge and WP in these discs are delayed. In this case, isometry between hinge and WP size exists at 82 hr and also at 96 hr AEL. Regional growth rates diverge only after 96 hr AEL, and by 110 hr AEL the WP is significantly larger than the hinge (P = 2 × 10−7). The increase in WP:H ratio of these discs is similar to dmP0 wing discs (0.8 at 81 hr to 1.2 at 110 hr; Figure 4F) and is significantly smaller than the ratio of yw controls at all time points (Figure 4, F and C; Table 1).
Taken together, these experiments demonstrate that wing development can occur in the absence of the endogenous pattern of dmyc expression. In wild type, distal and proximal regions of the wing disc switch from isometric to allometric growth late in L3, and this switch is not prevented when the normal pattern of dmyc expression is altered. This switch occurs even when the endogenous pattern of dmyc expression is completely replaced by ubiquitous expression, although reduced levels of dmyc expression significantly delay its onset. These data suggest that the dynamic pattern of dmyc expression does not instruct the regional growth changes in the wing disc. Instead, they imply that the absolute level of dmyc expression is critical to set the rate at which wing disc growth proceeds and that this rate determines the size of each region at the end of larval development.
The complete absence of dMyc slows growth of wing discs and delays wing patterning:
The dm4 allele is lethal during late L1, primarily due to the requirement for dMyc in endoreplication of larval cells (Pierce et al. 2008). The Tub-dmyc transgene in dm4;Tub-dmyc larvae rescues the entire animal, including the endoreplicating cells. To determine the role of dMyc in wing growth while avoiding animal lethality, we engineered animals in which larval cells express dMyc while in wing imaginal discs it is completely absent (see materials and methods for details). We used the dm4;Tub-dmyc animal and selectively removed the dmyc transgene from wing discs with Flp/FRT-mediated recombination by taking advantage of FRT sites that flank the dmyc cDNA (De La Cova et al. 2004). Recombination is induced in wing discs upon expression of UAS-Flp recombinase under control of Vestigial-Gal4 (Vg-Gal4), a wing driver (Crickmore and Mann 2006). This driver is expressed from ∼50 hr AEL throughout the rest of disc development (Figure S5; Figure 5, A and B). As a wing disc cell expresses Vg-Gal4, UAS-Flp is expressed and excision of the dmyc-FRT cassette occurs. The progeny of every Vg-Gal4-expressing cell will heritably express UAS-GFP and create a permanent “memory” of Vg-Gal4 expression in the disc (Crickmore and Mann 2006). For simplicity, we call this the Vg-memory experiment (VgM). In dm4;VgM;Tub-dmyc flies, WP and hinge cells excise the dmyc cDNA and therefore lose all dmyc expression, while the rest of the animal retains the intact Tub-dmyc cassette. Monitoring this process with expression of UAS-GFP indicates that dmyc is excised by 56 hr AEL (Figure S5B). The half-life of dMyc protein is ∼30 min (Galletti et al. 2009); thus it is presumably lost soon after the excision.
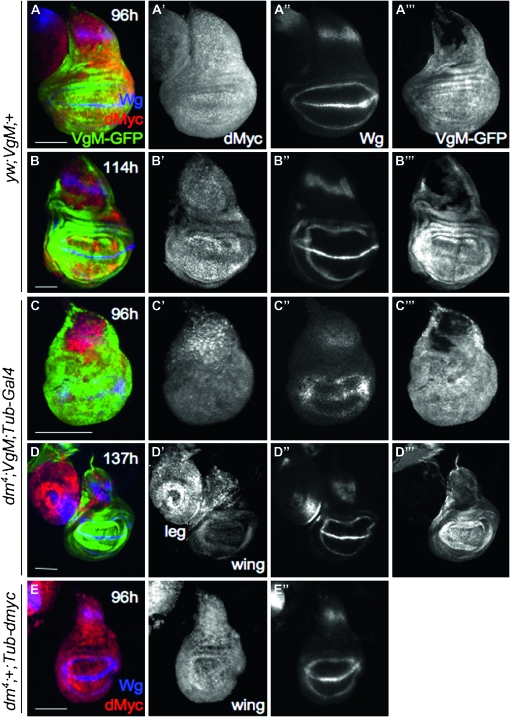
Figure 5.—
Loss of dMyc in wing discs slows their growth and patterning. dMyc (red) and Wg (blue) antibody staining and VgM-GFP expression (green) in 96-hr and late-L3 wing discs. (A) Ninety-six-hour yw;VgM;+ wing disc: (A′) dMyc, (A″) Wg, and (A″′) GFP; (B) 114-hr (late-L3) yw;VgM;+ wing disc: (B′) dMyc, (B″) Wg, and (B″′) GFP; (C) 96-hr dm4;VgM; Tub-Gal4/+ wing disc: (C′) dMyc and (C″) Wg expression in 96-hr dm4;VgM; Tub-Gal4/+ wing discs (immature relative to dm4; Tub-dmyc/+ wing discs of the same chronological age; compare to E″). The pattern in C″ is instead comparable to control yw wing discs at 72 hr AEL (Figure S1, A″–C″); (C″′) GFP; (D) 137-hr (late-L3) dm4;VgM;Tub-Gal4/+ wing disc: (D′) dMyc and (D″). By late-L3, Wg expression has matured (compare to B″): (D″′) GFP; (E) 96-hr AEL dm4;Tub-dmyc/+ wing disc: (E′) dMyc and (E″) Wg expression for comparison with discs in C–C″.
We examined the development of these dm4 mutant wings (dm4;VgM;Tub-Gal4 in Figure 5, C and D, and Table 2A) by dissecting them from L3 larvae at 96 and 137 hr AEL. By 96 hr, GFP fills the entire WP and hinge regions of both control and experimental discs, indicating that VgGal4 had at one time been expressed in all of these cells (only a portion of the notum remains GFP-negative; Figure 5, A″′ and C″′). In wing discs from dm4;VgM;Tub-dmyc animals, dMyc is undetectable in all GFP-positive cells in the wing, although still present in some notum cells and in the metathoracic leg (Figure 5D′). Notably, the null mutant wing discs are smaller than both yw;VgM and dm4;+;Tub-dmyc controls (Table 2A), and their development is even more delayed. This delay is evident from the expression of Wingless, which is dynamic during wing development (Figure 5, A″, C″, and E″, and Figure S1). Null wing discs from dm4;VgM;Tub-dmyc animals express Wg in an immature pattern that consists of only the IR in the WP (Figure 5C″; compare to A″ and E″). Their pattern and small size is more typical of younger discs, for example, yw wing discs at 72 hr AEL (Figure S1). Since expression of dMyc in the larval cells of dm4;VgM;Tub-dmyc animals is identical to that of dm4;Tub-dmyc animals, we conclude that the specific loss of dmyc in the wing disc further slows disc growth and concomitantly delays its developmental patterning.
TABLE 2.
Rudimentary, disproportionate wings form in the absence of dMyc
| A. Male wing discs | ||||
|---|---|---|---|---|
| Genotype | % yw WP | % yw H | WP:H ratio | n |
| yw;+;+ | 100 | 100 | 1.5 | 6 |
| dm4;+;Tub-dmyc/+ | 62 | 77 | 1.2 | 9 |
| dm4;VgM/+;Tub-Gal4/+ | 41 | 56 | 1.1 | 24 |
| B. Adult male wings | ||||
| Genotype |
% yw blade |
% yw hinge |
B:H ratio |
n |
| yw:+;+ | 100 | 100 | 9.6 | 34 |
| yw;VgM/+;+ | 97 | 95 | 9.7 | 37 |
| yw;VgM/+Tub-Gal 4/+ | 94 | 94 | 9.5 | 30 |
| dm4;+Tub-dmyc/+ | 72 | 69 | 9.9 | 28 |
|
dm4;VgM/+;Tub-Gal 4/+ |
45 |
55 |
7.8** |
8a |
Wing specific loss of Tub>dmyc results in disproportionately smaller wing blades. **P = 9.3 × 10−11 (vs. yw;VgM); P = 2.0 × 10−8 vs. yw); P = 6.7 × 10−13 (vs. dm4;Tub>dmyc>Gal 4/+.)
Similar trends were obtained in three independent experiments; n = 15 for dm4;VgM; Tub-Gal4/+.
We considered the possibility that loss of dMyc in other tissues in which the VgM driver is transiently expressed, such as the haltere, and a few cells in the brain and leg discs (data not shown), might contribute nonautonomously to the delay in maturation of Wg expression in the wing disc. To control for this, we examined the patterning rate of eye discs, which never express VgM, by following the progression of the morphogenetic furrow. dm4;VgM;Tub-dmyc eye discs do not show a delay in size or pattern maturation comparable to wing discs from the same genotype (data not shown). These results indicate that the smaller size and delayed patterning are wing disc-autonomous responses to loss of dMyc.
Interestingly, although the dm4; VgM; Tub-Gal4 null wing discs grow more slowly than wing discs from dm4;+; Tub-dmyc larvae, animals of both genotypes stop feeding and enter the wandering stage at the same time, 1 day later than yw; VgM controls. At this stage, null mutant wing discs are still 30% smaller than those from dm4;+;Tub-dmyc control larvae (Table 2A); however, the expression pattern of Wg has matured normally (compare Figure 5B″ with D″). This suggests that the additional day of larval development allows Wg expression to reach a mature pattern, but is not sufficient to overcome the growth defect.
Wing size is determined by the level of dMyc expression:
Although our experiments suggest that the endogenous pattern of dMyc expression is nonessential, they indicate that the overall level of its expression is a critical regulator of wing disc size. The majority of wing growth occurs during larval development through cell proliferation, which ceases ∼24 hr after puparium formation (Schubiger and Palka 1987). The amount of growth that occurs prior to this point largely determines the final size of the wing. However, in the pupa, the wing undergoes extensive morphologic changes that extend and flatten the blade (B) and cause compaction of the hinge (H) region, resulting in a 10-fold larger wing blade than hinge in adults (yw B:H size ratio = 9.6; Table 2B). To determine whether loss of dMyc expression affects these shape changes, we examined the B:H ratio of null mutant adult wings.
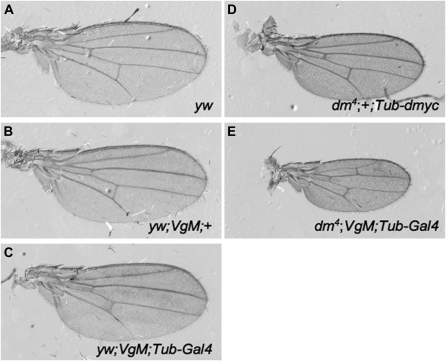
Wings from control animals (yw;VgM and yw;VgM;Tub-dmyc) are similar in size to each other and to yw, indicating that the VgM condition per se does not alter growth and the early removal of Tub-dmyc in flies overexpressing dMyc leads to normal wing size (Table 2B, Figure 6, A–C). Consistent with the small wing disc size, complete loss of dmyc from dm4;VgM;Tub-dmyc wing discs results in significantly smaller adult wings that are only half the size of yw controls. However, only 1% of animals with dmyc mutant wings eclose (dm4;VgM;Tub-dmyc, 3 of 270 total; yw;VgM;Tub-dmyc, 104 of 223). The size of both blade and hinge is affected, but not equally: the blade is 55% smaller, and the hinge 45% smaller than yw controls. Loss of dmyc thus alters the B:H ratio significantly (Table 2B, Figure 6E). The reduction in blade and hinge size in adult wings is of similar magnitude to late L3 wing discs, suggesting that most of the effect of the loss of dMyc occurs during the larval stage. These results indicate that, although a rudimentary amount of wing growth can occur in the complete absence of dMyc, its loss during wing development compromises growth and alters both the size and proportions of the wing.
Figure 6.—
In the complete absence of dmyc, wing size is severely reduced. (A–E) Adult wings (all to scale) from males of the following genotypes: (A) yw, (B) yw;VgM;+, and (C) yw;VgM;Tub-Gal4/+. The additional dmyc from the Tub-dmyc cassette is excised early in L2. (D) dm4;+;Tub-dmyc/+. These wings express dmyc solely from the Tub-dmyc transgene. (E) dm4;VgM;Tub-Gal4/+. dmyc expression is completely absent from these wings, which are significantly smaller than those in D (hinge, P = 2.0 × 10−4; blade, P = 5.4 × 10−9).
DISCUSSION
By examining the expression of dMyc over the course of wing development, we demonstrate that dmyc mRNA and protein are expressed in a temporally and spatially dynamic manner that corresponds to the subdivision of the wing-blade primordium from the hinge primordium. This relationship raised the possibility that dMyc is specifically deployed, presumably by factors that specify regional fates, to control the growth of each region as it develops, thereby contributing to sculpting the adult wing shape. In this work, we make three major findings. First, our experiments indicate that the intricate pattern of dMyc expression in the wing disc helps cells proliferate at an appropriate rate at any given time during wing development. Second, an adult wing can form in the absence of this pattern, although it is mis-proportioned and rudimentary in size. Finally, the absolute level of dMyc expression determines the rate at which the developing wing grows and also the rate of pattern maturation. Each aspect of dMyc's role in wing development is discussed below.
Patterned expression of dMyc permits, but does not instruct, morphological growth of the wing:
The expression pattern of dMyc in the wing is strikingly dynamic. Prior to the subdivision of the distal wing into hinge and blade, dMyc is expressed fairly uniformly, but as these regions are specified its expression undergoes transient up- and downregulation before stabilizing in a WP predominant pattern that prevails until the end of L3. Our clonal experiments indicate that the level of dMyc expression in a wing disc cell at any given time determines its rate of proliferation, and the changes in the dMyc expression pattern correlate well with changes in relative functional need. We detected clear region-specific differences in the functional requirement for dMyc that corresponded to the specification of proximal and distal wing fates. Moreover, we found that once the wing blade and hinge primordia are specified, they grow with distinct kinetics, such that midway through L2 these regions of the disc switch from isometric to allometric growth, resulting in a considerably larger WP than hinge by late L3.
Despite these correlations, however, modification of the endogenous expression pattern in whole animals demonstrated that the spatial and temporal components are less important than the absolute level of dMyc expressed. The conservative interpretation of our data is that dMyc's role in wing growth is permissive rather than instructive and that it augments a growth rate set by other mechanisms. However, it is puzzling why dMyc is expressed in an extravagant pattern that is not necessary. This pattern could be merely a remnant of evolution. Alternatively, compensatory post-transcriptional control of dMyc could occur. dMyc protein is highly regulated (Galletti et al. 2009) and is notably increased in the absence of Archipelago, a homolog of the vertebrate Fbw7 F-box protein (Moberg et al. 2004). However, within our limits of detection, we observed no difference between the expression patterns of dMyc mRNA and protein at any time during wing development in our experiments. Given the high degree of flexibility during wing growth, it is possible that redundancy among growth regulatory factors that function in the wing allows formation of a small but correctly shaped wing when dMyc is expressed ubiquitously or not at all. Indeed, as a whole, our results illustrate the inherent robustness of wing development.
dMyc levels determine wing scale and proportions and allow larval and imaginal growth to keep pace:
The permissive role of dMyc ensures that cells proliferate at stage-appropriate rates, determines overall size, and allows the development of a wing of correct proximal and distal proportions. Our results complement those of Pierce et al. (2008), who reported that wing discs carrying null mutations of both dmyc and dmnt, the dMyc antagonist, reach a size comparable to wild type after an extended L3 (3–7 days longer than wild type). In that case, loss of dMnt derepressed a subset of genes that rescued the dm4 mutant phenotype. The fact that wings grow reasonably well under those conditions supports our hypothesis that the growth program of the disc is augmented rather than determined by dMyc. The larval delay in those and in our experiments is due to reduced endoreplication of larval cells, which is dMyc dependent (Pierce et al. 2004, 2008; data not shown). In the VgM experiments, we maintained dMyc expression in most tissues while selectively removing it from the wing. Under these conditions, larval development progressed at the same rate as Tub-dmyc-rescued dm4 mutants (condition 3), but wing disc growth was significantly slowed. The uncoupling of larval and disc growth rates resulted in an altered size relationship between the wing and hinge, implying that coordination between larval growth and imaginal growth is important for wing size and shape. Growth regulators such as dMyc thus contribute to body and organ proportionality by promoting a rate of wing disc growth that is compatible with the rate of endoreplication and growth of larval cells. Moreover, control of wing size by dMyc is dose dependent. Together, the data suggest that the dm gene could be an evolutionary target that contributes to the wide variability of wing size among Drosophila species (Garcia-Bellido et al. 1994). Consistent with this possibility, evidence of strong selection at the dm locus has been documented (Jensen et al. 2007).
How is dMyc expression connected to pattern formation?:
Although the pattern of dmyc expression does not appear to instruct overall wing shape, wing cell requirements for dMyc change throughout development, possibly reflecting region-specific responsiveness to dMyc function or expression. What predisposes hinge or WP cells to respond to dMyc differently during wing disc development? Understanding how growth is governed in the different regions of the wing disc should help answer this question. Region-specific cues may be provided by the hinge selector Hth and the wing selector Vg and by Fat/Hippo signaling. The cadherins Fat and Dachsous regulate proximal (hinge) wing growth via Hippo signaling, whereas a feed-forward auto-regulatory loop Vg brings about expansion of distal wing (blade) fates (Cho and Irvine 2004; Cho et al. 2006; Zecca and Struhl 2007b; Rogulja et al. 2008). Regulation in both cases appears to be in response to signaling from Wg and Dpp. Wing growth appears therefore to be controlled quite indirectly. One possibility is that the amplitude of dMyc expression or activity is changed in response to modulation of Hippo and/or Vg activity by signals such as Wg and Dpp. This idea is supported by results showing that dmyc transcripts are significantly upregulated in fat mutant eye discs, in which Hippo activity is deregulated (Garoia et al. 2005). Experiments to address how dMyc expression is directly regulated in the wing disc are an important goal for the future.
A growth delay in wing discs affects pattern maturation:
A striking finding of our experiments is that the rate of wing disc patterning is directly influenced by the rate of its growth: complete loss of dmyc in the wing disc dramatically slows its growth and also slows the rate at which pattern formation matures. It is generally assumed that growth occurs downstream of patterning. This assumption is based on a variety of experimental models in which reorganization of pattern is always accompanied by growth (French et al. 1976). Consistent with this idea, Myc expression is regulated by several conserved factors that control pattern formation (He et al. 1998; Herranz et al. 2008; Johnston et al. 1999; Prober and Edgar 2002), whereas Myc itself controls the growth and proliferation of cells by regulating numerous genes required for ribosome biogenesis and protein synthesis (Grewal et al. 2005; Hulf et al. 2005). However, our experiments suggest that the hierarchy between pattern and growth is not absolute. Impaired cellular biosynthesis when dMyc is limiting may affect a cell's ability to produce proteins required for pattern specification as well as those required for cell division, cell survival, and mass accumulation. Our studies reveal an unappreciated relationship between patterning and growth that influences their coordination and is worthy of further study.
Acknowledgments
We thank the Bloomington Stock Center, the Developmental Studies Hybridoma Bank, Peter Gallant, Gines Morata, Michael Crickmore, Richard Mann, Erika Bach, and Gary Struhl for reagents; Cleo Tsanis for technical support; and Andrew Tomlinson and members of the Johnston lab for comments on the manuscript. This work was supported by grants from the National Institutes of Health (RO1HD042770 and RO1GM078464).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.110379/DC1.
References
- Azpiazu, N., and G. Morata, 2000. Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development 127 2685–2693. [DOI] [PubMed] [Google Scholar]
- Casares, F., and R. S. Mann, 2000. A dual role for homothorax in inhibiting wing blade development and specifying proximal wing identities in Drosophila. Development 127 1499–1508. [DOI] [PubMed] [Google Scholar]
- Cho, E., and K. D. Irvine, 2004. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131 4489–4500. [DOI] [PubMed] [Google Scholar]
- Cho, E., Y. Feng, C. Rauskolb, S. Maitra, R. Fehon et al., 2006. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38 1142–1150. [DOI] [PubMed] [Google Scholar]
- Couso, J. P., M. Bate and A. Martinez-Arias, 1993. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science 259 484–489. [DOI] [PubMed] [Google Scholar]
- Crickmore, M. A., and R. S. Mann, 2006. Hox control of organ size by regulation of morphogen production and mobility. Science 313 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova, C., M. Abril, P. Bellosta, P. Gallant and L. A. Johnston, 2004. Drosophila myc regulates organ size by inducing cell competition. Cell 117 107–116. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J., and S. M. Cohen, 1995. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121 4215–4225. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel, M., L. A. Johnston and W. Du, 2004. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc. Natl. Acad. Sci. USA 101 3857–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, V., P. J. Bryant and S. V. Bryant, 1976. Pattern regulation in epimorphic fields. Science 193 969–981. [DOI] [PubMed] [Google Scholar]
- Galletti, M., S. Riccardo, F. Parisi, C. Lora, M. K. Saqcena et al., 2009. Identification of domains responsible for ubiquitin-dependent degradation of dMyc by glycogen synthase kinase 3beta and casein kinase 1 kinases. Mol. Cell. Biol. 29 3424–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido, A., F. Cortes and M. Milan, 1994. Cell interactions in the control of size in Drosophila wings. Proc. Natl. Acad. Sci. USA 91 10222–10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoia, F., D. Grifoni, V. Trotta, D. Guerra, M. C. Pezzoli et al., 2005. The tumor suppressor gene fat modulates the EGFR-mediated proliferation control in the imaginal tissues of Drosophila melanogaster. Mech. Dev. 122 175–187. [DOI] [PubMed] [Google Scholar]
- Grewal, S. S., L. Li, A. Orian, R. N. Eisenman and B. A. Edgar, 2005. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat. Cell Biol. 7 295–302. [DOI] [PubMed] [Google Scholar]
- Harvey, K. F., C. M. Pfleger and I. K. Hariharan, 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114 457–467. [DOI] [PubMed] [Google Scholar]
- He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel et al., 1998. Identification of c-MYC as a target of the APC pathway. Science 281 1509–1512. [DOI] [PubMed] [Google Scholar]
- Herranz, H., L. Perez, F. A. Martin and M. Milan, 2008. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 27 1633–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., S. Wu, J. Barrera, K. Matthews and D. Pan, 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122 421–434. [DOI] [PubMed] [Google Scholar]
- Hulf, T., P. Bellosta, M. Furrer, D. Steiger, D. Svensson et al., 2005. Whole-genome analysis reveals a strong positional bias of conserved dMyc-dependent E-boxes. Mol. Cell. Biol. 25 3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, J. D., V. L. Bauer DuMont, A. B. Ashmore, A. Gutierrez and C. F. Aquadro, 2007. Patterns of sequence variability and divergence at the diminutive gene region of Drosophila melanogaster: complex patterns suggest an ancestral selective sweep. Genetics 177 1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, L. A., 2009. Competitive interactions between cells: death, growth and geography. Science 324 1679–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, L. A., and B. A. Edgar, 1998. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 394 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, L. A., and A. L. Sanders, 2003. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 5 827–833. [DOI] [PubMed] [Google Scholar]
- Johnston, L. A., D. A. Prober, B. A. Edgar, R. N. Eisenman and P. Gallant, 1999. Drosophila myc regulates cellular growth during development. Cell 98 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., K. D. Irvine and S. B. Carroll, 1995. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell 82 795–802. [DOI] [PubMed] [Google Scholar]
- Kim, J., K. Johnson, H. J. Chen, S. Carroll and A. Laughon, 1997. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature 388 304–308. [DOI] [PubMed] [Google Scholar]
- Moberg, K. H., A. Mukherjee, A. Veraksa, S. Artavanis-Tsakonas and I. K. Hariharan, 2004. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr. Biol. 14 965–974. [DOI] [PubMed] [Google Scholar]
- Neufeld, T. P., A. F. de la Cruz, L. A. Johnston and B. A. Edgar, 1998. Coordination of growth and cell division in the Drosophila wing. Cell 93 1183–1193. [DOI] [PubMed] [Google Scholar]
- Neumann, C. J., and S. M. Cohen, 1996. A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122 3477–3485. [DOI] [PubMed] [Google Scholar]
- Neumann, C. J., and S. M. Cohen, 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124 871–880. [DOI] [PubMed] [Google Scholar]
- Pantalacci, S., N. Tapon and P. Leopold, 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5 921–927. [DOI] [PubMed] [Google Scholar]
- Pierce, S. B., C. Yost, J. S. Britton, L. W. Loo, E. M. Flynn et al., 2004. dMyc is required for larval growth and endoreplication in Drosophila. Development 131 2317–2327. [DOI] [PubMed] [Google Scholar]
- Pierce, S. B., C. Yost, S. A. Anderson, E. M. Flynn, J. Delrow et al., 2008. Drosophila growth and development in the absence of dMyc and dMnt. Dev. Biol. 315 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober, D. A., and B. A. Edgar, 2002. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 16 2286–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja, D., C. Rauskolb and K. D. Irvine, 2008. Morphogen control of wing growth through the Fat signaling pathway. Dev. Cell 15 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger, M., and J. Palka, 1987. Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev. Biol. 123 145–153. [DOI] [PubMed] [Google Scholar]
- Steiger, D., M. Furrer, D. Schwinkendorf and P. Gallant, 2008. Max-independent functions of Myc in Drosophila melanogaster. Nat. Genet. 40 1084–1091. [DOI] [PubMed] [Google Scholar]
- Trumpp, A., Y. Refaeli, T. Oskarsson, S. Gasser, M. Murphy et al., 2001. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414 768–773. [DOI] [PubMed] [Google Scholar]
- Udan, R. S., M. Kango-Singh, R. Nolo, C. Tao and G. Halder, 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5 914–920. [DOI] [PubMed] [Google Scholar]
- Wang, S. H., A. Simcox and G. Campbell, 2000. Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev. 14 2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. A., S. W. Paddock and S. B. Carroll, 1993. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117 571–584. [DOI] [PubMed] [Google Scholar]
- Wu, S., J. Huang, J. Dong and D. Pan, 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114 445–456. [DOI] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223–1237. [DOI] [PubMed] [Google Scholar]
- Zecca, M., and G. Struhl, 2002. Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129 1357–1368. [DOI] [PubMed] [Google Scholar]
- Zecca, M., and G. Struhl, 2007. a Control of Drosophila wing growth by the vestigial quadrant enhancer. Development 134 3011–3020. [DOI] [PubMed] [Google Scholar]
- Zecca, M., and G. Struhl, 2007. b Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development 134 3001–3010. [DOI] [PubMed] [Google Scholar]
- Zecca, M., K. Basler and G. Struhl, 1996. Direct and long-range action of a wingless morphogen gradient. Cell 87 833–844. [DOI] [PubMed] [Google Scholar]