Abstract
Light-induced phosphorylation of light-harvesting chlorophyll a/b complex II (LHCII) proteins in plant thylakoid membranes requires an activation of the LHCII kinase via binding of plastoquinol to cytochrome b6f complex. However, a gradual down-regulation of LHCII protein phosphorylation occurs in higher plant leaves in vivo with increasing light intensity. This inhibition is likely to be mediated by increasing concentration of thiol reductants in the chloroplast. Here, we have determined the components involved in thiol redox regulation of the LHCII kinase by studying the restoration of LHCII protein phosphorylation in thylakoid membranes isolated from high-light-illuminated leaves of pumpkin (Cucurbita pepo), spinach (Spinacia oleracea), and Arabidopsis. We demonstrate an experimental separation of two dynamic activities associated with isolated thylakoid membranes and involved in thiol regulation of the LHCII kinase. First, a thioredoxin-like compound, responsible for inhibition of the LHCII kinase, became tightly associated and/or activated within thylakoid membranes upon illumination of leaves at high light intensities. This reducing activity was completely missing from membranes isolated from leaves with active LHCII protein phosphorylation, such as dark-treated and low-light-illuminated leaves. Second, hydrogen peroxide was shown to serve as an oxidant that restored the catalytic activity of the LHCII kinase in thylakoids isolated from leaves with inhibited LHCII kinase. We propose a dynamic mechanism by which counteracting oxidizing and reducing activities exert a stimulatory and inhibitory effect, respectively, on the phosphorylation of LHCII proteins in vivo via a novel membrane-bound thiol component, which itself is controlled by the thiol redox potential in chloroplast stroma.
Light induces the phosphorylation of a number of photosystem II (PSII)-related proteins in the thylakoid membranes of plant chloroplasts (Bennett, 1977, 1991), including two light-harvesting chlorophyll a/b complex II (LHCII) proteins, Lhcb1 and Lhcb2, of the PSII outer antenna (Larsson et al., 1987). Phosphorylation of LHCII proteins has been proposed to balance the excitation energy between PSII and photosystem I (PSI) in plant thylakoid membranes (Bennett, 1991; Allen, 1992). The phosphorylation of LHCII proteins is regulated in response to light via activation of the LHCII kinase with reduced plastoquinone pool and cytochrome b6f complex (Cyt b6f; Vener et al., 1995, 1997; Gal et al., 1997; Zito et al., 1999). Moreover, LHCII protein phosphorylation is down-regulated under high light conditions in vivo, revealing the existence of an inhibitory control mechanism in chloroplasts (Rintamäki et al., 1997). We have recently shown that the chloroplast thioredoxins f and m are effective inhibitors of LHCII protein phosphorylation in vitro (Rintamäki et al., 2000). It has become apparent that the correct thiol redox state is critical for in vitro thylakoid protein phosphorylation (Carlberg et al., 1999; Rintamäki et al., 2000) and that the identification of the thiol redox mediators in the thylakoid membrane is essential for understanding the regulation of the LHCII protein phosphorylation. Although the target molecule involved in the inhibition of LHCII protein phosphorylation has not yet been identified, most of the experimental data suggests that the LHCII kinase contains regulatory thiols controlled by thioredoxin or thioredoxin-like protein (Rintamäki et al., 2000).
The number and identity of thylakoid protein kinases still remain unknown. Snyders and Kohorn (1999) have introduced a protein family called the thylakoid-associated kinases that can phosphorylate LHCII proteins. Later, however, it was suggested that thylakoid-associated kinases may function upstream of the actual LHCII kinase in the kinase cascade leading to the phosphorylation of LHCII proteins (Snyders and Kohorn, 2001). Recently, a novel thylakoid-associated Ser-Thr protein kinase, Stt7, was reported to be required for LHCII protein phosphorylation and for state transition in Chlamydomonas sp. (Depege et al., 2003). A BLAST search revealed two Arabidopsis protein kinases related to Stt7 with one predicted transmembrane domain and a chloroplast-targeting signal in both proteins. Interestingly, Stt7 and one of the related Arabidopsis proteins also contain a putative thioredoxin target site with two Cys residues separated by four amino acids (Depege et al., 2003).
Chloroplast thiol redox mediators regulate the redox-active sites of key proteins in CO2 assimilation, ATP synthesis, and translation of psbA mRNA (Scheibe et al., 1990; Buchanan, 1991; Danon and Mayfield, 1994; Schürmann, 1995; Ruelland and Miginiac-Maslow, 1999). The spectrum of possible thiol redox mediators is wide, because plants differ from other organisms in having a complex thioredoxin profile with more than 20 thioredoxin and thioredoxin-related genes in the Arabidopsis genome (Laloi et al., 2001; Meyer et al., 2002). In the present paper, we have attempted to identify putative components involved in thiol redox regulation of LHCII protein phosphorylation. This was done by studying the restoration of the phosphorylation capacity in vitro in thylakoid membranes isolated from leaves with inhibited LHCII protein phosphorylation. Here, we report the dynamic reactions involved in reversible thiol regulation of LHCII protein phosphorylation. Both the dithiol oxidant and the disulfide reductant reacting with the target regulatory component of LHCII protein phosphorylation are present in illuminated thylakoid membranes. The inhibitory agent capable of inducing disulfide-dithiol exchange in proteins, appears to become associated or activated in vivo within the thylakoid membrane under conditions that lead to the inhibition of LHCII protein phosphorylation. Hydrogen peroxide was shown to be a potent oxidant in the applied in vitro assay system.
RESULTS
Restoration of the Capacity to Phosphorylate LHCII Proteins in Vitro in Thylakoid Membranes Isolated from High-Light-Illuminated Leaves
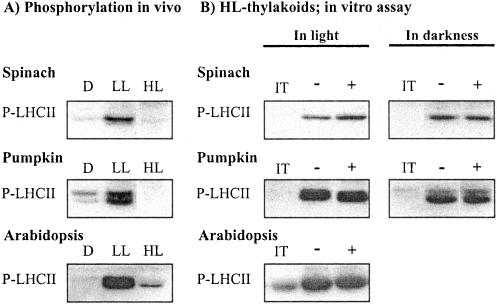
Maximal phosphorylation of the LHCII proteins in leaves occurs at lower light intensities than plants have experienced at growth conditions (Fig. 1A; see also Rintamäki et al., 1997). High light illumination induces inhibition of LHCII protein phosphorylation, which is only slowly restored in vivo upon transfer of plants to low light (Rintamäki et al., 1997). We addressed the molecular mechanisms for the restoration of LHCII protein phosphorylation capacity using thylakoid membranes isolated from three plant species illuminated in high light. When assayed in light, the presence of trans-4,5-dihydroxy-1,2-dithiane (DTTox) in the assay medium doubled the phosphorylation of LHCII proteins in isolated spinach (Spinacia oleracea) thylakoids (Fig. 1B; Table I). When the phosphorylation assay was performed in darkness via activation of the kinase with NADPH and ferredoxin, the extent of LHCII protein phosphorylation was not dependent on the presence of oxidant, and the quantity of the phosphorylated LHCII proteins (P-LHCII) was comparable with the level obtained in light with thiol oxidant (Fig. 1B). However, the capacity to phosphorylate LHCII proteins in thylakoids isolated from high-light-treated spinach never reached the capability observed in vivo under optimal light conditions for phosphorylation (Table I). This was not due to technical problems with the in vitro assay system, because the full recovery of LHCII protein phosphorylation was obtained in thylakoid membranes, which were isolated from low-light-illuminated leaves and subsequently allowed to dephosphorylate at room temperature in a medium without NaF before the in vitro assay (Table I). Contrary to spinach, a nearly complete restoration of LHCII protein phosphorylation occurred without any addition of oxidants in thylakoids isolated from high-light-illuminated pumpkin (Cucurbita pepo) and Arabidopsis leaves and assayed in light or in darkness (Fig. 1B; Table I).
Figure 1.
A, Irradiance-dependent phosphorylation of LHCII proteins in leaves. Plant leaves were incubated in darkness (D) or illuminated at a low (LL) and high (HL) light for2has described in “Materials and Methods.” The leaf samples were frozen in liquid nitrogen, and the phosphorylation level of LHCII proteins in isolated thylakoids was analyzed by immunoblotting. B, Restoration of LHCII protein phosphorylation capacity in vitro in thylakoid membranes isolated from high-light-illuminated leaves. Isolated thylakoid membranes were incubated in darkness for 10 min in the absence (-) or the presence of 2 mm DTTox (+), and subsequently LHCII protein phosphorylation was initiated by the addition of ATP and switching the light on. Phosphorylation of LHCII proteins was also carried out in darkness by activating the kinase with NADPH and ferredoxin. Phosphoproteins were detected by immunoblotting with phospho-Thr antibody. IT indicates phosphorylation level of LHCII proteins in isolated thylakoids before the phosphorylation assay. For quantification of the data, see Table I.
Table I.
Phosphorylation of LHCII proteins in vivo and the effect of thiol oxidant on the restoration of LHCII protein phosporylation in thylakoid membranes isolated from high-light-illuminated leaves
Plant leaves were illuminated at low light (LL) or at high light (HL) as described in “Materials and Methods,” and the phosphorylation level of LHCII proteins in isolated thylakoids was analyzed by immunoblotting. Subsequently, thylakoids isolated from the LL-treated and HL-treated leaves were rephosphorylated in vitro in the absence (−DTTox) or presence (+DTTox) of 2 mm thiol oxidant. Phosphoproteins were detected by immunoblotting with phospho-Thr antibody, and the immunoblots were quantified by scanning. Results are presented as a percentage of the phosphorylation level of LHCII proteins in LL-illuminated leaves in vivo. Results are means of three independent experiments ± se. n.d., Not determined.
| Species | In Vivo Phosphorylation
|
In Vitro Assay in Light
|
||||
|---|---|---|---|---|---|---|
| LL Thylakoids
|
HL Thylakoids
|
|||||
| LL | HL | −DTTox | +DTTox | −DTTox | +DTTox | |
| % | ||||||
| Spinach | 100 | 2 ± 2 | 102 ± 5 | 115 ± 2 | 27 ± 7 | 58 ± 10 |
| Pumpkin | 100 | 9 ± 3 | 98 ± 7 | n.d. | 91 ± 8 | 91 ± 3 |
The results in Figure 1 and Table I indicate that the target regulatory thiols of LHCII protein phosphorylation become oxidized during isolation of thylakoids from high-light-illuminated leaves. It is thus noteworthy that a simple in vitro phosphorylation assay of isolated thylakoids is not an appropriate test to analyze the inhibition level of LHCII protein phosphorylation in vivo. The in vitro phosphorylation assay with spinach thylakoid membranes, however, suggests that the maintenance of the capacity to phosphorylate LHCII proteins upon illumination of thylakoids requires the sustained presence of functional oxidant in thylakoid preparations isolated from high-light-illuminated leaves. Further experiments were designed to characterize the molecular mechanisms involved in sustaining the phosphorylation of LHCII proteins in thylakoids isolated from high-light-illuminated pumpkin leaves.
Effect of Scavengers for Reactive Oxygen Species (ROS) on the Restoration of LHCII Protein Phosphorylation in Thylakoid Membranes Isolated from High-Light-Treated Leaves
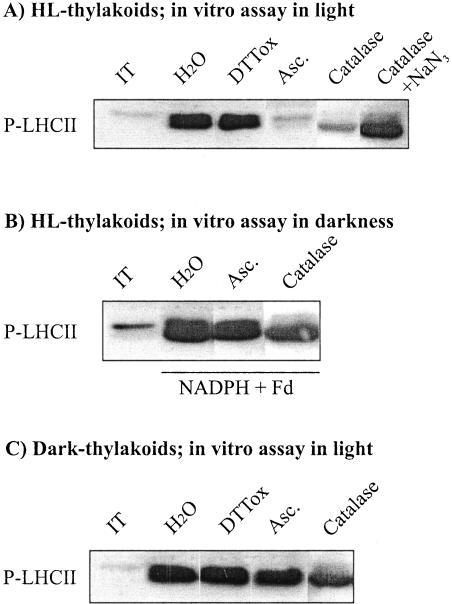
ROS, which are capable of oxidizing dithiols of proteins in plant cells (see König et al., 2002; Neill et al., 2002), are potential thiol oxidants to maintain LHCII protein phosphorylation in the assay system described above. To test the role of ROS in maintaining LHCII protein phosphorylation, we added catalase to the in vitro assay medium. This treatment induced significant inhibition of LHCII protein phosphorylation in thylakoids isolated from high-light-illuminated pumpkin leaves (Fig. 2A, lane marked catalase), which, however, was found to revert when sodium azide, an inhibitor of catalase, was also included in the assay medium containing catalase (Fig. 2A, lane marked catalase + NaN3). This suggests that hydrogen peroxide, produced via reduction of oxygen by PSI electron transport (Alscher et al., 1997; Backhausen et al., 2000), is involved in maintaining the oxidation state of the regulatory thiols in the LHCII kinase. LHCII protein phosphorylation was also inhibited by ascorbate, another scavenger of hydrogen peroxide (Miyake and Asada, 1992; Alscher et al., 1997) when it was present in the phosphorylation assay medium (Fig. 2A, lane marked Asc.), further supporting the role of hydrogen peroxide as a thiol oxidant in thylakoid preparations. This demand for a thiol oxidant is specific to LHCII protein phosphorylation, because the scavengers for ROS did not inhibit the phosphorylation of PSII core proteins in the assay medium (data not shown).
Figure 2.
Effect of scavengers for ROS on LHCII protein phosphorylation in isolated thylakoid membranes. Thylakoids isolated from high-light-illuminated (A and B) or dark-treated (C) pumpkin leaves were phosphorylated in vitro for 20 min in the absence (H2O) or in the presence of the following reagents: 2 mm DTTox,10mm ascorbate (Asc.), 30 units of catalase μg-1 chlorophyll, catalase and 25 μM sodium azide (catalase + NaN3). Phosphorylation of LHCII proteins was carried out both in light (A and C) and in darkness (B) by activating the kinase with light-induced electron transfer or with NADPH and ferredoxin, respectively. The amount of phosphorylated LHCII proteins was detected as described in Figure 1. IT indicates phosphorylation level of LHCII proteins in isolated thylakoid membranes before the phosphorylation assay.
The capacity of LHCII protein phosphorylation was also tested in experiments in which the LHCII kinase was activated in darkness by reduction of plastoquinone pool with NADPH and ferredoxin (Larsson et al., 1987). Pre-incubation of thylakoid membranes with catalase or ascorbate had no inhibitory effect on the capacity for LHCII protein phosphorylation (Fig. 2B), indicating that ROS were not required to keep the LHCII kinase active in darkness in thylakoids isolated from high-light-illuminated leaves. Furthermore, the catalase and ascorbate-induced inhibition of LHCII protein phosphorylation was also completely absent when experiments were conducted with thylakoid membranes isolated from dark-treated leaves (Fig. 2C).
Effect of Artificial Thiol-Modifying Agents on the Restoration of LHCII Protein Phosphorylation in Thylakoid Membranes Isolated from High-Light-Treated Leaves
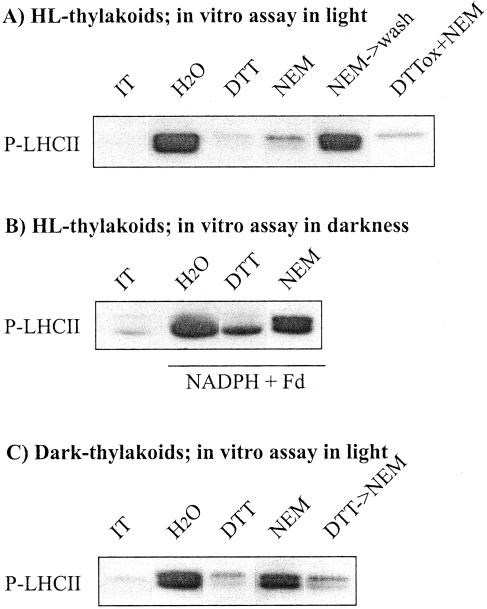
The results presented in Figure 2 indicate an obligatory requirement for hydrogen peroxide in maintaining LHCII protein phosphorylation in thylakoids isolated from high-light-treated leaves when assayed in light, but not in membranes isolated from dark-treated leaves. On the other hand, they also imply the presence of a distinct reductant in the membranes isolated from leaves with inhibited LHCII protein phosphorylation, which upon illumination of thylakoids continuously rereduces the target regulatory component of LHCII protein phosphorylation. We next concentrated on obtaining some insights into the identity of this reducing factor. Thiol-alkylating reagent N-ethylmaleimide (NEM) irreversibly blocks the sulfhydryl-disulfide exchange in proteins. Incubation of the thylakoids isolated from high-light-illuminated pumpkin leaves with low concentration of NEM strongly inhibited the light-induced phosphorylation of LHCII proteins (Fig. 3A, lane NEM). Significantly less inhibition of LHCII protein phosphorylation occurred when NEM was added to the assay medium of thylakoids isolated from high-light-treated leaves and was subsequently washed out before initiating the phosphorylation assay by the addition of ATP and switching the light on (Fig. 3A, lane NEM → wash). This indicates that the regulatory thiols involved in the inhibition of LHCII protein phosphorylation became reduced only during the in vitro phosphorylation assay in light and thus susceptible to alkylation with NEM. NEM seemed to have particularly high affinity to the target thiols of LHCII protein phosphorylation, because the presence of hydrogen peroxide produced endogenously in illuminated thylakoid suspension (see Fig. 2) was not able to block the irreversible alkylation of a target component of NEM. Equally, addition of DTTox together with NEM to assay medium in light (Fig. 3A, lane DTTox + NEM) did not prevent the inhibitory effect exerted by NEM on LHCII protein phosphorylation. Similar inhibition of LHCII protein phosphorylation in light in the presence of NEM was also observed in thylakoid membranes isolated from high-light-treated spinach and Arabidopsis leaves (data not shown).
Figure 3.
Inhibition of LHCII protein phosphorylation by thiol reagents in isolated thylakoid membranes. Thylakoids isolated from high-light-illuminated (A and B) or dark-treated (C) pumpkin leaves were incubated in darkness without (H2O) and with 2 mm DTT or 0.1 mm NEM for 10 min. Thereafter, in vitro phosphorylation was initiated by illumination and addition of ATP (A and C) or by the addition of NADPH + ferredoxin (Fd) and ATP in darkness (B). NEM → wash, NEM was washed out after dark-incubation before starting the phosphorylation assay. DTTox + NEM, Phosphorylation of LHCII proteins, which were assayed in the presence of both 2 mm DTTox and NEM. DTT → NEM, The thylakoid membranes were first incubated in darkness for 5 min with DTT, which was subsequently washed out, and thylakoid pellet was resuspended in the buffer with NEM before the phosphorylation assay. The detection of phosphoproteins and the other abbreviations are as described in Figure 2.
Thiol regulation of LHCII protein phosphorylation in thylakoids isolated from high-light-treated leaves was further tested in experiments in which the LHCII kinase was activated in darkness by the addition of NADPH and ferredoxin. Significant inhibition of LHCII protein phosphorylation was obtained only with DTT in the assay medium (Fig. 3B, lane DTT), whereas like the ROS scavengers (Fig. 2B), NEM had no inhibitory effect on the capacity to phosphorylate LHCII proteins in reactions carried out in darkness (Fig. 3B, lane NEM).
We previously reported that NEM in low concentrations (0.1 mm) exerts only a slight effect on LHCII protein phosphorylation in thylakoids isolated from dark-treated leaves (Rintamäki et al., 2000; see also Fig. 3C, lane NEM). Higher concentrations of NEM have been shown to strongly inhibit the phosphorylation of LHCII proteins in isolated thylakoid membranes (Millner et al., 1982). However, these concentrations were shown to nonspecifically inhibit phosphorylation of all PSII phosphoproteins without any preference to LHCII protein phosphorylation (Rintamäki et al., 2000). Using a low concentration of NEM (0.1 mm), the inhibitory effect on LHCII protein phosphorylation was only detected if the membranes isolated from dark-treated leaves were pretreated with DTT, which induces the disulfide-sulfhydryl exchange in thylakoid proteins (Fig. 3C, lane DTT → NEM; DTT was washed out before addition of NEM).
From the results above, it can be deduced that during the illumination of leaves at high light, the thylakoids have acquired a thiol component, which is capable of reducing the target regulatory disulfide of LHCII protein phosphorylation to dithiol. These thiols are then alkylated by NEM. To release the putative thylakoid-bound thiol-reducing component, the membranes were washed with high salt in the presence or absence of Triton X-100. The washed membranes were then phosphorylated in vitro in light in the presence or absence of NEM (Fig. 4). LHCII protein phosphorylation was fully active in thylakoids washed with salt in the presence or absence of Triton X-100, and the phosphorylation was even more intense compared with the control thylakoids. NEM, however, still exerted inhibition on LHCII protein phosphorylation (Fig. 4), indicating that the thiol-reducing inhibitory component must be integral or internal to the thylakoid membrane.
Figure 4.
Effect of NEM on LHCII protein phosphorylation in salt-washed thylakoid membranes isolated from high-light-treated pumpkin leaves. Isolated thylakoids were washed with assay buffer (H2O) or with 1 m NaCl in buffer with or without 0.05% (w/v) Triton, and the washed membranes were phosphorylated in vitro in white light in the presence or absence of NEM. Phosphoproteins were detected as described in Figure 1.
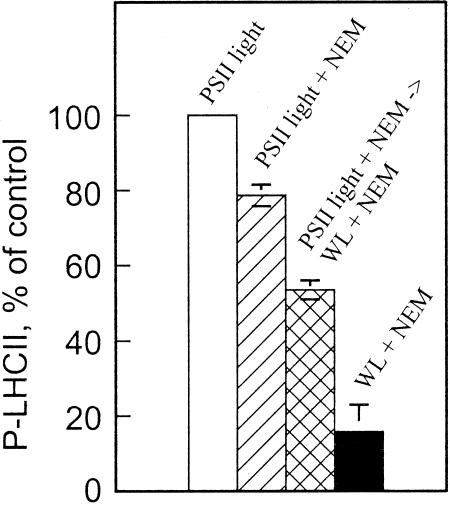
Data presented in Figure 3, A and B, indicate that the putative thiol component in thylakoids isolated from high-light-treated leaves exerts inhibitory effect on LHCII protein phosphorylation only when the membranes were exposed to light. To characterize this light activation process of the putative inhibitory compound, the thylakoids isolated from high-light-illuminated leaves were phosphorylated in PSII light in the presence or absence of NEM. The treatment with NEM combined with the activation of LHCII protein phosphorylation by PSII light resulted only in a slight inhibition of phosphorylation (20%), as compared with 84% inhibition in an assay carried out in white light (Fig. 5). Moreover, when the thylakoids were first phosphorylated in the presence of NEM in PSII light for 5 min and then PSII light was turned off and white light on for 15 min, intermediate inhibition of LHCII protein phosphorylation was recorded after a total in vitro phosphorylation period of 20 min (44% inhibition in Fig. 5). This indicates that the activation of the redox compound that was responsible for reduction and exposure of the regulatory target disulfide of LHCII protein phosphorylation to NEM requires PSI electron transport activity. It is worth noting that corresponding experiments cannot be conducted in PSI light because PSI light deactivates the LHCII kinase via oxidation of the plastoquinone pool and Cyt b6f complex (Vener et al., 1995, 1997).
Figure 5.
Effect of light quality on the sensitivity of LHCII protein phosphorylation to NEM in thylakoid membranes isolated from highlight-illuminated pumpkin leaves. Isolated thylakoids were phosphorylated in PSII light or in white light (WL) for 20 min in the presence or absence of 0.1 mm NEM. In the experiment indicated as PSII light + NEM → WL + NEM, the thylakoids were first phosphorylated in PSII light with NEM for 5 min, and subsequently, the PSII light was turned off and white light on. The phosphorylation was then continued for 15 min in white light. Phosphorylation level of LHCII proteins was identical in control assays without NEM, regardless of whether the activation of the kinase occurred in white light or in PSII light. The phosphorylation level of LHCII proteins was determined as described in Figure 1, and the immunoblots were quantified by scanning. Results are means ± se, n = 2.
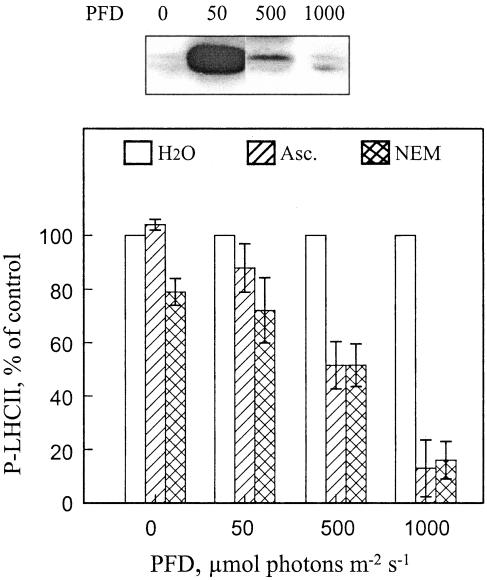
Sensitivity of LHCII Protein Phosphorylation to Ascorbate and NEM in Thylakoid Membranes Isolated from Leaves Illuminated at Different Light Intensities
In thylakoids isolated from dark-treated leaves and contrary to the thylakoid membranes isolated from high-light-illuminated leaves (Figs. 2A and 3A), no significant inhibition of LHCII protein phosphorylation was seen in the presence of ROS scavengers or NEM (Figs. 2C and 3C). In thylakoids isolated from dark-treated leaves, NEM had an inhibitory effect only if, before the addition of NEM, the disulfide bonds of thylakoid proteins were prereduced with DTT and thus made accessible to react with NEM (Fig. 3C, lane DTT → NEM). Thus, we further tested the effect of NEM and ascorbate on the rephosphorylation capacity of LHCII proteins in thylakoid membranes isolated from pumpkin leaves illuminated at different light intensities. LHCII protein phosphorylation was nearly insensitive to these reagents in thylakoid membranes isolated from dark-incubated leaves and from low-light-illuminated leaves (50 μmol photons m-2 s-1) with fully operative LHCII protein phosphorylation in vivo (Fig. 6). The level of inhibition became gradually enhanced with an increasing intensity of leaf pre-illumination. The most severe inhibition of LHCII protein phosphorylation was recorded in thylakoids isolated from leaves, which were illuminated at the light intensity known to induce complete inactivation of LHCII protein phosphorylation in vivo (Fig. 6, 1,000 μmol photons m-2 s-1). Thus, the putative thiol-reducing compound is functional only in thylakoid membranes isolated from leaves with inhibited LHCII protein phosphorylation. Furthermore, the inhibition of phosphorylation was unique to LHCII proteins, because NEM and ascorbate did not significantly affect the phosphorylation of PSII core proteins in thylakoids isolated from differently illuminated leaves (data not shown).
Figure 6.
Correlation of the light-intensity-induced inhibition of LHCII protein phosphorylation in vivo with the sensitivity of phosphorylation to the ROS scavenger and NEM in vitro. Pumpkin leaf discs were incubated in darkness or illuminated at a photon flux density (PFD) of 50, 500, or 1,000 μmol m-2 s-1 for 2 h, and the phosphorylation level of LHCII proteins in isolated thylakoids was analyzed by immunoblotting (top panel). Subsequently, thylakoids isolated from the treated leaf discs were rephosphorylated in vitro in the absence (H2O) or presence of 10 mm ascorbate (Asc.) or 0.1 mm NEM (bottom panel). The phosphorylation level of LHCII proteins was determined as described in Figure 1, and the immunoblots were quantified by scanning. Results are means ± se, n = 2-4.
DISCUSSION
The LHCII kinase is under complex regulation by both light reactions, PSII and PSI, in the thylakoid membrane (Figs. 3, 4, 5, 6). PSII excitation activates the kinase via reduction of Cyt b6f complex, whereas PSI excitation induces both deactivation of the enzyme and the production of thiol mediators that inhibit LHCII protein phosphorylation in the chloroplast. Because of this strict regulation of the LHCII kinase by both the electron transfer components in thylakoid membranes and the thiol-redox mediators in the chloroplast, LHCII protein phosphorylation possibly serves as a delicate indicator for sensing the imbalance between light reactions and carbon metabolism in leaf cells (Pursiheimo et al., 2001; Hou et al., 2002).
We have shown that the transfer of a plant to a higher light intensity than the plant has experienced during the growth induces almost total downregulation of LHCII protein phosphorylation, which recovers fairly slowly when plants are transferred to low light intensity (Rintamäki et al., 1997). Our model for the regulation of LHCII protein phosphorylation (Rintamäki et al., 2000; Hou et al., 2002) proposes that the LHCII kinase can exist in three different forms, the prevalence of which depends on the level of redox equivalents in chloroplast: (a) a deactivated form in the absence of reduced plastoquinone in the Qo site of Cyt b6f complex (prevailing in darkness in vivo); (b) an activated form in the presence of reduced plastoquinone in the Qo site of Cyt b6f complex (prevailing in low light); (c) a thiol-inhibited form induced by an increased thiol-redox state in the chloroplast (prevailing in high light). We have shown that only the deactivated form of the LHCII kinase is prone to thiol-redox mediators (Rintamäki et al., 2000; Hou et al., 2002). Thus, the inhibition of the LHCII kinase by thiol mediators presumes that the activated and deactivated forms of the enzyme alternate in illuminated chloroplast with continuous electron flow through plastoquinone and Cyt b6f complex. Oxidation of plastoquinol in the Qo site of Cyt b6f complex deactivates the LHCII kinase (Vener et al., 1995, 1997), which, in the absence of thiol mediators capable of trapping the deactivated form of the enzyme, can become reactivated via binding of the next plastoquinol to the Qo site of Cyt b6f complex. Thus, the concentration of thiol mediators in the chloroplast determines the inhibition state of the LHCII kinase. The role of thiol mediator-induced inhibition of the LHCII kinase is thus to release the enzyme from the single control exerted by the redox status of plastoquinone and Cyt b6f complex, thereby allowing a more flexible regulation of LHCII protein phosphorylation under various environmental conditions and metabolic status of leaves (Pursiheimo et al., 2001; Hou et al., 2002). Furthermore, inhibition of the deactivated form of the enzyme may be beneficial, because oxidation of the thiol-inhibited LHCII kinase produces a deactivated (catalytically inactive) form of the enzyme that can be subsequently controlled by the redox status of plastoquinone and Cyt b6f complex. This may prevent a futile phosphorylation of LHCII proteins under unfavorable conditions (e.g. in darkness).
Our hypothesis of the LHCII kinase as a target protein of thiol mediators is based on a cooperative regulation of LHCII protein phosphorylation via plastoquinol and thiol mediators in chloroplast (see discussion in Rintamäki et al., 2000). A putative thioredoxin target site in a novel LHCII protein kinase Stt7 (Depege et al., 2003) is in line with the hypothesis. However, the Cyt b6f complex as a target of thiol mediators cannot be completely ruled out at the moment; in this case, the reduction of the target disulfide site in the complex would prevent the activation of the LHCII kinase. Nevertheless, such a thiol-induced reduction of Cyt b6f complex should exclusively affect its interaction with the LHCII kinase, because no inhibition of linear electron transfer was observed in NEM-treated thylakoids isolated either from dark-adapted or from high-light-illuminated pumpkin leaves (data not shown).
Chloroplast thioredoxins are reductants, which could conceivably induce inactivation of the LHCII kinase (Rintamäki et al., 2000). Our present results, however, show a copurification of the thiol mediator with thylakoid membranes isolated from high-light-illuminated leaves. This putative thioredoxin-like protein is tightly associated with thylakoids (Fig. 4) and it inhibits LHCII protein phosphorylation in vitro in white light (Figs. 3 and 5) but remains inactive in PSII light (Fig. 5) and in darkness (Fig. 3). However, the inhibitory factor itself turned out to be insensitive to treatments of thylakoid membranes with NEM, at least in the phosphorylation assays conducted in light (Figs. 3, 4, 5, 6). This resistance to NEM may be due to in situ location and/or conformation of the component: As an internal membrane factor (Fig. 4), it is probably not readily attainable by external NEM. It is noteworthy that the activity of this thylakoid-associated thioredoxin-like compound in the thylakoid membrane positively correlates with the level of inhibition of LHCII protein phosphorylation in vivo, whereas such an activity cannot be induced by in vitro illumination of thylakoid membranes isolated from leaves with functional LHCII protein phosphorylation (Fig. 6). Thus, a contact in vivo with the highly reducing stroma is an absolute requirement for induction of this thioredoxin-like activity in thylakoid membranes (Fig. 6).
In Vitro Phosphorylation of LHCII Proteins in Thylakoid Membranes Isolated from Leaves with Inhibited LHCII Kinase: A Hypothetical Model
According to our hypothetical model (Rintamäki et al., 2000; Hou et al., 2002), the functional state of the LHCII kinase is determined by counterbalanced action of kinase activation, deactivation, and thiol-dependent inhibition in illuminated leaves. The results presented in the present paper give strong support to the hypothesis: Continuous activation/deactivation/thiol-dependent inhibition of LHCII protein phosphorylation occurs in thylakoid membranes isolated from high-light-illuminated leaves (Fig. 7). The thiol-inhibited form of the enzyme is highly dominant under high light in vivo, and the LHCII protein phosphorylation is thus downregulated (Fig. 7A). This balance is altered when thylakoid membranes are isolated. Thiol-inhibited LHCII kinase becomes oxidized during thylakoid isolation and/or during incubation of the thylakoid preparation at room temperature (Fig. 7B). The nature of the oxidant involved remains to be elucidated.
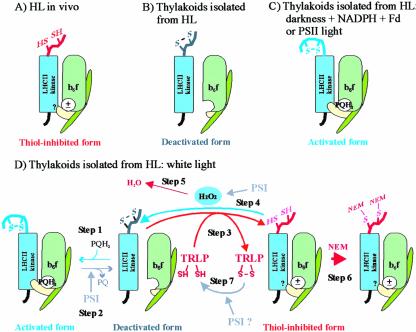
Figure 7.
Schematic presentation of the activation states of the LHCII kinase in thylakoid membranes of high-light-illuminated leaves (A) and in thylakoids isolated from such leaves and treated experimentally as presented in this paper (B-D). A, A thiol-inhibited form of the LHCII kinase predominates in leaves under high redox potential in chloroplasts in vivo. B, The thiol-inhibited LHCII kinase is oxidized during isolation of thylakoids from high-light-illuminated leaves (HL). In these membranes, the plastoquinone pool is oxidized, and thus the LHCII kinase is in deactivated form. C, Reduction of the plastoquinone pool and subsequent binding of plastoquinol (PQH2) to the Qo site of Cyt b6f complex induces activation of the LHCII kinase. The activated form of the LHCII kinase predominates in thylakoid membranes illuminated in PSII light or incubated in darkness in the presence of NADPH and ferredoxin (see “Discussion”). D, Dynamic activation/deactivation and thiol-induced inhibition of the LHCII kinase in thylakoids illuminated in white light. The steps of the model, marked 1 to 7, are described in the “Discussion.” The movement of Rieske protein (yellow subunit of Cyt b6f complex) between proximal and distal positions with respect to the membrane, induced by binding/release of plastoquinone to/from the Qo site, respectively (Darrouzet et al., 2000; Wollman, 2001), is shown. ±, The dithiol form of the LHCII kinase is inhibited despite the presence and absence of PQH2 at the Qo site of Cyt b6f complex.? in the LHCII kinase, Interaction of the thiol-inhibited LHCII kinase with Cyt b6f complex is unknown. TRLP, Thioredoxin-like protein. For simplicity, only the monomeric form of the Cyt b6f complex is presented, and the membrane bilayer structure is not shown.
LHCII proteins can be phosphorylated in vitro in thylakoid membranes isolated from high-light-illuminated leaves, when the plastoquinone pool is reduced either by light or with NADPH and ferredoxin in darkness (Larsson et al., 1987; Bennett, 1991). This phosphorylation is induced by binding of plastoquinol to the Qo site of Cyt b6f complex, which activates the LHCII kinase (Fig. 7, C and D, step 1; Vener et al., 1995, 1997; Gal et al., 1997; Zito et al., 1999). Oxidation and release of plastoquinone from the Qo site of Cyt b6f complex during electron transfer, in turn, deactivates the LHCII kinase (Fig. 7D, step 2; Vener et al., 1997), and this occurs in illuminated thylakoids via electron transfer through PSI to molecular oxygen (Alscher et al., 1997; Backhausen et al., 2000). The deactivated form of the LHCII kinase is a target for reduction by a thioredoxin-like protein, resulting in inhibition of the enzyme (Fig. 7D, step 3). However, the electron transport via PSI also induces a production of hydrogen peroxide (Alscher et al., 1997; Backhausen et al., 2000), which at micromolar concentrations has been shown to efficiently oxidize dithiols in proteins in vivo (Åslund et al., 1999). It is thus conceivable that hydrogen peroxide is responsible for reoxidation of the target dithiols in the LHCII kinase during illumination of thylakoids isolated from high-light-treated leaves (Fig. 7D, step 4), resulting in a recovery of the catalytically active LHCII kinase (Fig. 7D, step 1). Only when this reoxidizing activity is quenched, e.g. with catalase or ascorbate (Fig. 7D, step 5), or when the dithiols in the LHCII kinase are irreversibly trapped by NEM (Fig. 7D, step 6) is the inhibitory effect of thioredoxin-like protein detectable in thylakoid membranes. The complete restoration of LHCII protein phosphorylation in thylakoids isolated from high-light-illuminated spinach leaves did not occur even in the presence of extra thiol oxidant (Table I), suggesting that for an unknown reason, the oxidation of the thiol-inhibited LHCII kinase (Fig. 7D, step 4) is hampered in the spinach membranes assayed in vitro.
We have estimated that the deactivation rate of the LHCII kinase in isolated thylakoid suspension is about 300 times higher in white light than in darkness (Hou et al., 2003), indicating the efficiency of oxygen in accepting electrons from PSI in illuminated thylakoid membranes. The low quantity of the deactivated form of the LHCII kinase (Fig. 7D, step 2), which serves as a substrate for the putative thiol reductant, may explain the ineffectiveness of NEM to inhibit LHCII protein phosphorylation in thylakoid membranes illuminated in PSII light or incubated in darkness (Figs. 3B and 5). Alternatively, electron transport via PSI may be involved in the reduction of thioredoxin-like protein in illuminated thylakoid membranes (Fig. 7D, step 7).
In summary, the present paper demonstrates the experimental separation of two dynamic reactions involved in thiol regulation of LHCII protein phosphorylation in isolated thylakoid membranes, the dithiol oxidation and disulfide reduction. The identity of these regulatory factors in vivo remains to be determined. It is shown here that hydrogen peroxide can serve as an oxidant in isolated thylakoid membranes. Hydrogen peroxide is known to induce stress tolerance in plants (Foyer et al., 1997), thus making it an attractive candidate also for regulating LHCII protein phosphorylation in vivo. Furthermore, the results indicate that the thioredoxin-like compound responsible for regulation of the LHCII kinase associates with thylakoid membranes or becomes active upon illumination of leaves at high light intensities. Several transmembrane proteins involved in thioldisulfide metabolism have been documented recently (Rietsch and Beckwith, 1998; Matsuo et al., 2001; Collet and Bardwell, 2002). The thioredoxin-like protein HCF164 has been found in chloroplasts anchored to the thylakoid membrane at its luminal side (Lennartz et al., 2001). HCF164 has been suggested to have a role in the maturation or the assembly of the Cyt b6f complex. Besides the periplasmic components, the disulfide bond formation in proteins in prokaryotic periplasm also comprises a cytoplasmic thioredoxin and a transmembrane thiol-disulfide oxidoreductase, which is oxidized by ubiquionone, a component of the respiratory electron transfer chain (Collet and Bardwell, 2002). An analogous cascade may exist also for regulation of the LHCII kinase in thylakoid membranes, involving a dithiol oxidant and a disulfide reductant, both being strictly regulated by redox poise in the chloroplast via the electron transfer chain and stromal factors.
MATERIALS AND METHODS
Plant Material and Light Treatment of Leaf Discs
Pumpkin (Cucurbita pepo) plants were grown in a greenhouse at 20°C with a 16-h photoperiod at a PFD of 200 μmol photons m-2 s-1. Spinach (Spinacia oleracea) was grown in a greenhouse at 400 μmol photons m-2 s-1 with 10-h-light/14-h-dark rhythm at 25°C. Arabidopsis ecotype Wassilewskija was grown in a growth chamber with an 8-h photoperiod at a PFD of 230 μmol m-2 s-1. Fully expanded leaves were used in the experiments. Leaf discs (diameter 2.7 cm), punched from dark-adapted pumpkin and spinach leaves and floating on distilled water in a petri dish, were illuminated for 2 h in a growth chamber at 23°C at a PFD of 50 (optimal light intensity for LHCII protein phosphorylation in pumpkin and Arabidopsis leaves; low light), 100 (optimal light intensity for LHCII protein phosphorylation in spinach leaves; low light), 500, or 1,000 (high light) μmol m-2 s-1 (see Rintamäki et al., 1997). Intact leaves of Arabidopsis were used in light treatments. A metal halide lamp (HQI-T, 250 W for daylight) served as a light source. For analysis of LHCII protein phosphorylation in vivo, the leaf samples were rapidly frozen in liquid nitrogen and stored at -80°C until isolation of thylakoid membranes. For in vitro phosphorylation assay, the thylakoid membranes were isolated immediately after light treatments without freezing the leaf materials.
In Vitro Phosphorylation Assay of Thylakoid Membranes Isolated from Differentially Illuminated Leaves
Thylakoid membranes were isolated from dark-incubated or illuminated leaves or leaf discs as described previously (Rintamäki et al., 1996). Thylakoids were resuspended in assay buffer consisting of 50 mm HEPES-NaOH, pH 7.5, 100 mm Suc, 5 mm NaCl, 10 mm MgCl2, and 10 mm NaF at a final chlorophyll concentration of 0.4 mg mL-1. Before phosphorylation assays, isolated thylakoid membranes were pre-incubated at 23°C for 5 or 10 min in darkness in the presence of the following chemicals: l-ascorbic acid (Sigma-Aldrich, St. Louis), DTT (Roche Diagnostics, Mannheim, Germany), DTTox (Sigma-Aldrich), NEM (Sigma-Aldrich), catalase (Sigma-Aldrich), and sodium azide (Sigma-Aldrich). The concentrations of the chemicals are indicated in the figures. When the binding of the thiol-reducing mediator to the thylakoid membrane was studied, thylakoids were incubated with either 1 m NaCl or a combination of 1 m NaCl and 0.05% (w/v) Triton X-100, and the membranes were subsequently pelleted and resuspended in the assay medium. Thylakoid protein phosphorylation was initiated by the addition of 0.4 mm ATP under a PFD of 100 μmol m-2 s-1, and thylakoid samples for immunoblot analysis were frozen in liquid nitrogen after illumination of 20 min. When PSII light (650 ± 10 nm, 100 μmol photons m-2 s-1) was used, the thylakoids were illuminated through an S25-650 filter (Corion, Franklin, MA) with a slide projector (250-W lamp) as a light source. Phosphorylation of thylakoid proteins in darkness was assayed in the presence of 1 mm NADPH (Roche Diagnostics) and 10 μm ferredoxin (Sigma-Aldrich) as described by Larsson et al. (1987).
Detection of Thylakoid Phosphoproteins by Polyclonal Phospho-Thr Antibody
Isolated thylakoid membranes were solubilized in the presence of 6 m urea, and the polypeptides were separated by SDS-PAGE using 15% (w/v) acrylamide gels with 6 m urea (Laemmli, 1970). Routinely, 1.0 μg of chlorophyll was loaded into each well. The polypeptides were transferred to an Immobilon-P membrane (Millipore, Bedford, MA), and the membrane was blocked with 1% (w/v) bovine serum albumin (BSA; with antibody from Zymed Laboratories [San Francisco]) or 5% (w/v) BSA (with antibody from New England Biolabs, Beverly, MA). The BSA was fatty-acid free (Sigma-Aldrich). Phosphoproteins were immunodetected using a Phototope-Star Chemiluminescent kit (New England Biolabs) with rabbit polyclonal phospho-Thr antibodies (from Zymed or New England Biolabs) as described by Rintamäki et al. (1997). For quantification of phosphoproteins, the immunoblots were scanned using an IMAGE program (Imaging Research, St. Catharine's, Ontario, Canada). Phosphorylation of the two light-harvesting proteins Lhcb1 and Lhcb2 (designated P-LHCII) is shown in the immunoblots presented in the figures.
Chlorophyll Determinations
Chlorophyll was extracted in 80% (v/v) buffered acetone and was determined as described previously (Porra et al., 1989).
Acknowledgments
We thank Mika Keränen for skillful technical assistance.
This work was supported by the Academy of Finland.
References
- Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275-335 [DOI] [PubMed] [Google Scholar]
- Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100: 224-233 [Google Scholar]
- Åslund F, Zheng M, Beckwith J, Storz G (1999) Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA 96: 6161-6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhausen JE, Kitzmann C, Horton P, Scheibe R (2000) Electron acceptors in isolated spinach chloroplasts act hierarchically to prevent over-reduction and competition for electrons. Photosynth Res 64: 1-13 [DOI] [PubMed] [Google Scholar]
- Bennett J (1977) Phosphorylation of chloroplast membrane polypeptides. Nature 269: 344-346 [Google Scholar]
- Bennett J (1991) Protein phosphorylation in green plant chloroplasts. Annu Rev Plant Physiol Plant Mol Biol 42: 281-311 [Google Scholar]
- Buchanan B (1991) Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys 288: 1-9 [DOI] [PubMed] [Google Scholar]
- Carlberg I, Rintamäki E, Aro EM, Andersson B (1999) Thylakoid protein phosphorylation and the thiol redox state. Biochemistry 38: 3197-3204 [DOI] [PubMed] [Google Scholar]
- Collet JF, Bardwell JCA (2002) Oxidative protein folding in bacteria. Mol Microbiol 44: 1-8 [DOI] [PubMed] [Google Scholar]
- Danon A, Mayfield SP (1994) Light regulated translation of chloroplast messenger RNAs through redox potential. Science 266: 1717-1719 [DOI] [PubMed] [Google Scholar]
- Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F (2000) Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc Natl Acad Sci USA 97: 4567-4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depege N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572-1575 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100: 241-254 [Google Scholar]
- Gal A, Zer H, Ohad I (1997) Redox-controlled thylakoid protein phosphorylation: news and views. Physiol Plant 100: 863-868 [Google Scholar]
- Hou CX, Pursiheimo S, Rintamäki E, Aro EM (2002) Environmental and metabolic control of LHCII phosphorylation: revealing the mechanism for dual regulation of the LHCII kinase. Plant Cell Environ 25: 1515-1525 [Google Scholar]
- Hou CX, Rintamäki E, Aro EM (2003) Ascorbate-mediated LHCII protein phosphorylation: LHCII kinase regulation in light and in darkness. Biochemistry 42: 5828-5836 [DOI] [PubMed] [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schürmann P, Dietz KJ (2002) The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the hierarchy of photosynthetic electron flux. Proc Natl Acad Sci USA 99: 5738-5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685 [DOI] [PubMed] [Google Scholar]
- Laloi C, Rayapuram N, Chartier Y, Grienenberger JM, Bonnard G, Meyer Y (2001) Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci USA 98: 14144-14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson UK, Sundby C, Andersson B (1987) Characterization of two different subpopulations of spinach light-harvesting chlorophyll a/b-protein complex (LHCII): polypeptide composition, phosphorylation pattern and association with photosystem II. Biochim Biophys Acta 894: 59-68 [Google Scholar]
- Lennartz K, Plucken H, Seidler A, Westhoff P, Bechtold N, Meierhoff K (2001) HCF164 encodes a thioredoxin-like protein involved in the biogenesis of the cytochrome b6f complex in Arabidopsis. Plant Cell 13: 2539-2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Akiyama N, Nakamura H, Yodoi J, Noda M, Kizaka-Kondoh S (2001) Identification of a novel thioredoxin-related transmembrane protein. J Biol Chem 276: 10032-10038 [DOI] [PubMed] [Google Scholar]
- Meyer Y, Vignols F, Reichheld JP (2002) Classification of plant thioredoxins by sequence similarity and intron position. Methods Enzymol 347: 394-402 [DOI] [PubMed] [Google Scholar]
- Millner PA, Widger WR, Abbott MS, Cramer WA, Dilley RA (1982) The effect of adenine nucleotides on inhibition of the thylakoid protein kinase by sulfhydryl-directed reagents. J Biol Chem 257: 1736-1742 [PubMed] [Google Scholar]
- Miyake C, Asada K (1992) Thylakoid bound ascorbate peroxidase scavenges hydrogen-peroxide photoproduced photoreduction of monodehydroascorbate radical. Photosynth Res 34: 156 [Google Scholar]
- Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5: 388-395 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficient and simultaneous equations for assaying chlorophyll a and b with four different solvents: verification of the concentration of chlorophyll by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384-394 [Google Scholar]
- Pursiheimo S, Mulo P, Rintamäki E, Aro EM (2001) Coregulation of light-harvesting complex II phosphorylation and Lhcb mRNA accumulation in winter rye. Plant J 26: 317-327 [DOI] [PubMed] [Google Scholar]
- Rietsch A, Beckwith J (1998) The genetics of disulphide bond metabolism. Annu Rev Genet 32: 163-184 [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Kettunen R, Aro EM (1996) Differential D1 dephosphorylation in functional and photodamaged photosystem II centres: Dephosphorylation is a prerequisite for degradation of damaged D1*. J Biol Chem 271: 14870-14875 [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via plastoquinol and ferredoxin-thioredoxin system in chloroplast. Proc Natl Acad Sci USA 97: 11644-11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro EM (1997) Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo: application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem 272: 30476-30482 [DOI] [PubMed] [Google Scholar]
- Ruelland E, Miginiac-Maslow M (1999) Regulation of chloroplast enzyme activities by thioredoxins: activation or relief from inhibition? Trends Plant Sci 4: 136-141 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Rudolph R, Reng W, Jaenickem R (1990) Structural and catalytic properties of oxidized and reduced chloroplast NADP-malate dehydrogenase upon denaturation and renaturation. Eur J Biochem 189: 581-587 [DOI] [PubMed] [Google Scholar]
- Schürmann P (1995) Ferredoxin-thioredoxin system. Methods Enzymol 252: 274-283 [DOI] [PubMed] [Google Scholar]
- Snyders S, Kohorn BD (1999) TAKs, thylakoid membrane protein kinases associated with energy transduction. J Biol Chem 274: 9137-9140 [DOI] [PubMed] [Google Scholar]
- Snyders S, Kohorn BD (2001) Disruption of thylakoid-associated kinase 1 leads to alteration of light harvesting in Arabidopsis. J Biol Chem 276: 32169-32176 [DOI] [PubMed] [Google Scholar]
- Vener AV, van Kan PJM, Gal A, Andersson B, Ohad I (1995) Activation/deactivation cycle of redox-controlled thylakoid protein phosphorylation: role of plastoquinol bound to the reduced cytochrome bf complex. J Biol Chem 270: 25225-25232 [DOI] [PubMed] [Google Scholar]
- Vener AV, van Kan PJM, Rich PR, Ohad I, Andersson B (1997) Plastoquinol at the quinol oxidation site of the reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by single turnover flash. Proc Natl Acad Sci USA 94: 1585-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623-3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollmann FA (1999) The Qo site of cytochrome b6f complex controls the activation of the LHCII kinase. EMBO J 18: 2961-2969 [DOI] [PMC free article] [PubMed] [Google Scholar]