Abstract
Mammalian coat patterns (e.g., spots, stripes) are hypothesized to play important roles in camouflage and other relevant processes, yet the genetic and developmental bases for these phenotypes are completely unknown. The domestic cat, with its diversity of coat patterns, is an excellent model organism to investigate these phenomena. We have established three independent pedigrees to map the four recognized pattern variants classically considered to be specified by a single locus, Tabby; in order of dominance, these are the unpatterned agouti form called “Abyssinian” or “ticked” (Ta), followed by Spotted (Ts), Mackerel (TM), and Blotched (tb). We demonstrate that at least three different loci control the coat markings of the domestic cat. One locus, responsible for the Abyssinian form (herein termed the Ticked locus), maps to an ∼3.8-Mb region on cat chromosome B1. A second locus controls the Tabby alleles TM and tb, and maps to an ∼5-Mb genomic region on cat chromosome A1. One or more additional loci act as modifiers and create a spotted coat by altering mackerel stripes. On the basis of our results and associated observations, we hypothesize that mammalian patterned coats are formed by two distinct processes: a spatially oriented developmental mechanism that lays down a species-specific pattern of skin cell differentiation and a pigmentation-oriented mechanism that uses information from the preestablished pattern to regulate the synthesis of melanin profiles.
PATTERNED coats are typical of many mammalian groups, whose spots, stripes, and other markings have been hypothesized to play important adaptive roles in camouflage, predator evasion, and social communication (Cott 1940; Searle 1968; Ortolani and Caro 1996). Many mammals bear striped or spotted coats, and these phenotypes have historically drawn attention from many fields of human science and culture (e.g., the leopard's spots, or the stripes seen in tigers and zebras). Although several theoretical studies have proposed mathematical models that could underlie the developmental dynamics of coat pattern formation in mammals (Murray and Oster 1984; Oyehaug et al. 2002), no direct investigation of the genetic basis of these phenotypes has yet been performed, so that their mechanistic causes remain a mystery. Recent advances in genomics, molecular biology, and evolutionary developmental biology (Evo-Devo) have revealed genes and pathways involved in skin pattern formation in Drosophila (Schug et al. 1998; Gompel et al. 2005; Prud'homme et al. 2006; Parchem et al. 2007), butterflies (Joron et al. 2006a,b), and zebrafish (Iwashita et al. 2006; Watanabe et al. 2006; Svetic et al. 2007). In contrast, despite the relevance of characterizing equivalent processes in mammals, little progress toward this goal has been accomplished, perhaps due to the lack of adequate mammalian models exhibiting variation in skin pattern and for which genetic and genomic tools were available.
The domestic cat is a very promising model in this regard, as it presents several coat pattern variants and a growing body of genetic and genomic tools suitable for gene identification (Menotti-Raymond et al. 2003; Murphy et al. 2007; Pontius et al. 2007; Pontius and O'Brien 2007; Davis et al. 2009). Classic work on domestic cat coat color (Robinson 1958; Lomax and Robinson 1988) has suggested that there is a monogenic allelic series of coat patterns in the domestic cat, controlled by the Tabby (T) locus: in order of dominance, the four recognized alleles would be Abyssinian or “ticked” (Ta), Spotted (Ts), Mackerel (TM), and Blotched (tb) (Figure 1). Although there has been little doubt among breeders that the “mackerel” and “blotched” forms segregate as a single autosomal locus, this may not be the case for the other two phenotypes (Ta and Ts), which so far have not been tested thoroughly for allelism relative to the more common Tabby variants TM and tb. Some breeding data have suggested that these variants may not be allelic with the main Tabby locus (Lorimer 1995), but further scrutiny is required to test this hypothesis. A recent genetic study (Lyons et al. 2006) considered the Abyssinian variant as an allele of Tabby, reflecting the prevalent perception that they are coded by the same locus. Testing this hypothesis, and identifying the implicated genomic region (or regions), is a first step in the process of dissecting the molecular and developmental basis for these pattern-formation phenotypes.
Figure 1.—
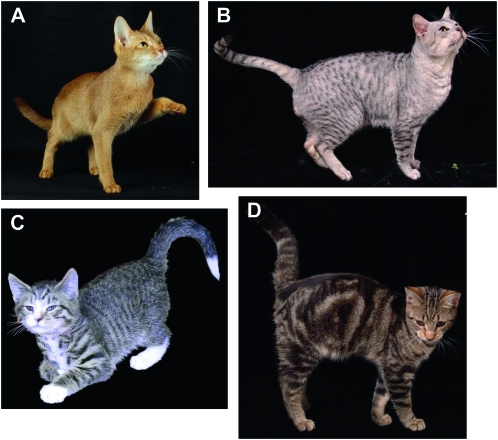
Major coat pattern phenotypes of the domestic cat (Felis silvestris catus). A “hierarchy” of pelage patterns is observed in this species, with the absence of markings seen in Abyssinian cats (A) dominating over a spotted coat (B), which dominates over a “mackerel” (striped) coat (C), itself dominant over the blotched pattern (D). The classical, single-locus model for this phenotypic variation proposed the allelic series Ta > Ts > TM > tb for these respective variants.
Aiming to investigate the genetic basis of pattern formation on the domestic cat pelage by genomic, positional methods, we established three separate pedigrees segregating for different combinations of coat pattern phenotypes. Our results demonstrate that at least three different loci underlie the striping and spotting patterns observed in domestic cats and identify the genomic location of two of them.
MATERIALS AND METHODS
Pedigrees and phenotyping:
To avoid potential complications stemming from multiple loci affecting coat pattern formation phenotypes, we chose to analyze each variant separately, a strategy that could simultaneously test for allelism and identify the implicated genomic regions. We thus established three independent pedigrees, each of which segregated for a single “Tabby” variant, relative to a standard. Pedigree 1 was a multigenerational outbred (and nonbreed) domestic cat pedigree maintained by the Nestlé Purina PetCare Company for nutrition studies, which we have previously used to build a genetic linkage map of the domestic cat genome (Menotti-Raymond et al. 2009; Schmidt-Küntzel et al. 2009) and also to identify genes involved in coat color and hair length (Eizirik et al. 2003; Ishida et al. 2006; Kehler et al. 2007). It consisted of 287 individuals, 256 of which were genotyped. This pedigree showed segregation for the mackerel and blotched Tabby variants, providing a basis for their mapping using a genome scan.
Pedigrees 2 and 3 were specifically designed and developed for this study, in each case aiming to isolate the inheritance of a single Tabby variant. Both of these pedigrees were developed at the National Institutes of Health Animal Center (NIHAC), using a backcross mating design (Figure 2). Pedigree 2 focused on the inheritance of the “spotted” (Ts) variant and was founded with a pure-bred male cat of the Egyptian Mau breed, which was crossed with three unrelated blotched females (supporting information, Figure S1). Egyptian Mau cats are fully spotted (Figure 3) and breed true for this trait, thus being homozygous for whatever alleles cause this coat pattern. Blotched was selected as the tester phenotype, since it is determined by a recessive allele relative to mackerel, so that these individuals can be confidently assumed to be homozygotes (tb/tb) at the Tabby locus. The founder crosses of this pedigree were therefore set up to be TsTs × tbtb, considering the single-locus model of Tabby inheritance.
Figure 2.—
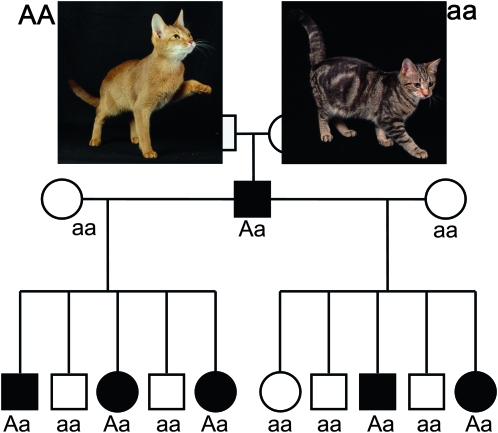
Schematic of the mating strategy employed here to separately investigate the “spotted” and Abyssinian (“ticked”) domestic cat pattern variants. In both cases, individuals known to be homozygous for the allele of interest (symbolized here by “A,” and exemplified by ticked) were bred to tester animals known not to carry this allele, and thus to be homozygous for a recessive variant (symbolized by “a” and exemplified by “blotched”). F1 individuals were necessarily heterozygous at the locus of interest and were subsequently backcrossed to additional, unrelated tester cats. Phenotypic segregation among third-generation offspring was then used to assess Mendelian inheritance, allelism, and genomic mapping of the implicated locus. The drawing is a simplified abstraction (see text and Figure S1 and Figure S2 for details), as the parental generation may be composed of one male mated to multiple females (pedigree 2), or different pairs (pedigree 3).
Figure 3.—
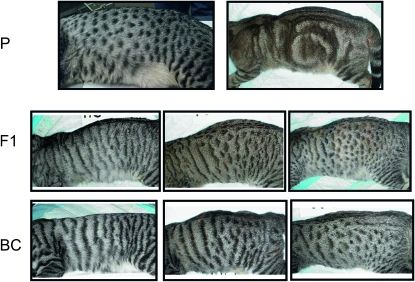
Phenotypic variation observed in pedigree 2, designed to characterize the “spotted” coat variant. P, parental generation; F1, F1 generation; BC, backcross generation. For the F1 and BC generations, only examples of nonblotched patterns are shown to illustrate the range of markings observed in these animals (from spotted to fully striped phenotypes). Some individuals in the BC generation bore complete stripes, to the point that they could be fully categorized as a typical “mackerel” cat (bottom left).
Pedigree 3 focused on the inheritance of the Abyssinian (Ta) variant (known as ticked in breeder terminology), also based on a backcross mating design (Figure 2 and Figure S2). The Abyssinian form is semidominant relative to any of the other variants, with homozygotes Ta/Ta bearing plain color (a homogeneous agouti coat, with banded individual hairs but no body markings) (Figure 1), and heterozygotes exhibiting faint, thin body stripes and banding on the legs and tail (Lomax and Robinson 1988). The founders of this kindred were two males previously generated in an Abyssinian pedigree segregating for retinal atrophy which had been outcrossed to non-Abyssinian European shorthairs (Menotti-Raymond et al. 2007). Given their progenitors (both had different Abyssinian sires, each of which had been crossed to a blotched and a mackerel female, respectively), these two individuals were known to be heterozygotes carrying the TA allele, and indeed displayed stripes on the legs and tail of an otherwise typical Abyssinian (i.e., agouti or ticked) coat. These two F1 individuals were used for backcrossing with 12 non-Abyssinian females (mackerel or blotched), and phenotypic segregation of ticked was analyzed in the resulting third-generation individuals.
For all three pedigrees, individuals were phenotyped by E. Eizirik and photographs recorded in a central database maintained at the Laboratory of Genomic Diversity, National Institutes of Health (NIH). All cats analyzed in this study were maintained in facilities inspected by the United States Department of Agriculture, under conditions established by the American Association of Laboratory Animal Care in compliance with the federal Animal Welfare Act.
DNA extraction and marker genotyping:
Blood samples were obtained from all individuals in pedigrees 1–3. In addition, for pedigrees 2 and 3, fibroblast cell lines were established as a source of high-quality genomic DNA. DNA was extracted from whole blood or cell lines using a QIAamp DNA Blood Midi kit (QIAGEN). PCR amplification was performed with a touchdown PCR protocol as described previously (Menotti-Raymond et al. 2005). Sample electrophoresis and genotyping, as well as Mendelian inheritance checking, were carried out as previously described (Ishida et al. 2006). See the results section and Table S1 for the microsatellite loci typed in each pedigree.
Development of microsatellites for fine mapping of Tabby and Ticked:
After linkage was established to a known region using previously published cat STR markers, additional microsatellites from candidate regions were mined from the cat 1.9X whole genome sequence (Pontius et al. 2007). Initially microsatellites were selected on the basis of their conserved syntenic position in the dog, following the method described by Ishida et al. (2006). Following the availability of a cat genome assembly (Pontius et al. 2007), microsatellite markers were selected on the basis of their location on cat chromosomes using the algorithm ABCC Retrieve STRs (ABCC STR-centric tools, http://www.abcc.ncifcrf.gov/Genomes/Cat/index.php) (Pontius and O'Brien 2007) (these include all loci used for Ticked mapping with prefix “chrB1,” see below). Primers (see Table S1) were designed with Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi (Rozen and Skaletsky 2000), including an M13 tail for fluorescent labeling of PCR products (Boutin-Ganache et al. 2001).
Genetic linkage mapping:
Single-marker LOD scores were computed using Superlink (Fishelson and Geiger 2002; Fishelson and Geiger 2004), as described in Ishida et al. (2006) and Kehler et al. (2007). Recombination fractions are optimal to within 0.01. Multipoint analyses were performed to clarify which markers belong above or below the intervals of zero recombination. The traits were modeled as fully penetrant. For the LOD scores shown here, the trait-associated allele frequency was set to 0.25, but the LOD scores are insensitive to this value. Marker-allele frequencies were set all equal, but since most of the cats are genotyped, the marker allele frequencies have little effect on the LOD scores.
RESULTS
A detailed inspection of phenotype segregation in pedigree 1 corroborated the expected monogenic inheritance of the mackerel and blotched Tabby variants (Figure 1), with the latter being a fully penetrant, autosomal recessive allele. In the absence of candidate genes for this phenotype, a genome scan was performed using 483 microsatellite markers. Among these established markers, significant linkage to the Tabby locus was observed in a region of chromosome A1 [markers FCA566 (LOD = 31.4, θ = 0.01), FCA90 (LOD = 10.7, θ = 0.05), and FCA1331 (LOD = 42.61, θ = 0)] (Table 1). Fine mapping of Tabby was accomplished by developing additional microsatellite markers for the region (see Table S1 for a list of all new primers), combined with the subsequent addition of meioses from pedigree 2 (see below). This joint approach defined a genomic region of ∼5 Mb for the Tabby locus, on the basis of conserved synteny with the human and dog genomes (Table 1). Although the domestic cat sequence assembly is not yet complete in this region, the current radiation hybrid (RH) map (Davis et al. 2009) shows that this genomic segment is well defined and exhibits conserved synteny to human chromosome 5 (113.0–118.6 Mb) and dog chromosome 11 (6.6–11.5 Mb).
TABLE 1.
Linkage mapping of the domestic cat Tabby locus
| Markera | Cat chr. | GL map positionb | RH map positionb | Peak LODc | Θ of peakc | Position in GARFIELD (start)d | Hsa36 (start)e | Cfa2 (start)e |
|---|---|---|---|---|---|---|---|---|
| FCA566 | A1 | 144.8 | 755.7 | 31.36* | 0.01* | Chr Un8: 85,018 | Unknown | Unknown |
| FLT4 | A1 | — | — | 28.73 | 0.03 | Chr Un11: 349,316 | Chr 5: 179,972,586 | Chr 11: 4,173,747 |
| ChrUn11_369202 | 24.99 | 0.05 | Chr Un11: 369,202 | Chr 5: 179,954,132 | Chr 11: 4,190,117 | |||
| Hsa5:179.47 | A1 | — | — | 23.43* | 0.02* | Chr Un11: 699,876 | Chr 5: 179,470,809 | Chr 11: 4,568,828 |
| Hsa5:178.67 | A1 | — | — | 20.19 | 0.02 | Chr Un11: 1,234,398 | Chr 5: 178,672,817 | Chr 11: 5,312,931 |
| Col23A1 | A1 | — | — | 21.32 | 0.06 | Chr A1: 124,220,448 | Chr 5: 177,617,085 | Chr 11: 6,306,204 |
| YTHDC2 | A1 | — | — | 37.78 | 0.01 | Chr A1: 123,872,511 | Chr 5: 112,958,230 | Chr 11: 6,617,669 |
| Cfa11:7.68 | A1 | — | — | 30.27 | 0 | Chr A1: 123,080,103 | Chr 5: 113,730,035 | Chr 11: 7,288,271 |
| FCA1331 | A1 | 147.3 | — | 42.61 | 0 | Chr A1: 122,968,298 | Chr 5: 113,814,901 | Chr 11: 7,382,372 |
| Hsa 5-116.83 | A1 | — | — | 32.45 | 0 | Chr Un11: 2,154,347 | Chr 5: 116,836,837 | Chr 11: 10,069,791 |
| Hsa-5-118.59 | A1 | — | — | 40.85 | 0.01 | Chr Un11: 3,108,430 | Chr 5: 118,596,950 | Chr 11: 11,556,021 |
| Hsa5-118.72 | A1 | — | — | 34.57 | 0.01 | Chr Un11: 3,208,206 | Chr 5: 118,721,887 | Chr 11: 11,656,633 |
| Cfa11:11.26 | A1 | — | — | 22.92* | 0.01* | Chr Un11:253,059 | Chr 5: 119,149,336 | Chr 11: 13,698,383 |
| Cfa11:12.55 | A1 | — | — | 30.58* | 0.01* | Chr Un11: 4,235,006 | Chr 5: 120,543,823 | Chr 11: 14,227,481 |
| FCA90 |
A1 |
154.7 |
947.3 |
10.67* |
0.05* |
Unknown |
Chr 5: 123,155,913 |
Chr 11: 16,596,908 |
Markers are shown in genomic order along the domestic cat chromosome A1, on the basis of the most recent genetic linkage (GL) (Menotti-Raymond et al. 2009) and radiation hybrid (RH) (Davis et al. 2009) maps of the domestic cat. Markers with prefix other than FCA represent loci that were developed for fine mapping after multipoint linkage analysis demonstrated that FCA566 and FCA90 flanked the Tabby interval.
Positions of markers with a blank in these columns were not determined in those versions of the RH or GL maps.
Peak LOD score and estimated recombination fraction (θ) for linkage between each polymorphic marker and the Tabby locus. Asterisks indicate loci that were genotyped only in the Nestlé-Purina colony (pedigree 1); all other loci were additionally genotyped in pedigree 2 and reflect cumulative LOD scores from the two pedigrees.
Position in the domestic cat whole genome sequence assembly as visualized in the GARFIELD browser (Pontius and O'Brien 2007) (http://lgd.abcc.ncifcrf.gov/cgi-bin/gbrowse/cat).
Columns 8 and 9 show the positions in the human (HSA build 36) (http://www.ncbi.nlm.nih.gov/projects/mapview/stats/BuildStats.cgi?taxid=9606&build=36&ver=3) and dog (CFA build 2) (http://www.ncbi.nlm.nih.gov/projects/mapview/stats/BuildStats.cgi?taxid=9615&build=2&ver=1) genome assemblies found by BLAT analysis to be orthologous to sequences flanking the respective domestic cat markers.
The parental crosses of pedigree 2 (spotted vs. blotched phenotypes) yielded seven F1 individuals, none of which were fully spotted, but rather exhibiting intermediate patterns between spotted and mackerel (i.e., they would be considered to be “broken mackerel,” due to a mixture of spots and broken stripes; Figure 3). These F1 individuals were backcrossed to nine tester blotched cats, producing a third-generation progeny of 39 animals, 35 of which could be confidently phenotyped (Figure S1). These backcross offspring conformed to a 1:1 ratio of blotched (n = 19) to nonblotched (n = 16) pattern, supporting an allelic relationship at the Tabby locus. However, the nonblotched animals exhibited a full range from spotted to striped phenotypes, including individuals that could be fully categorized as mackerel (see Figure 3). Since the mackerel form was not present in any of the original crosses, this result demonstrates that it was represented by the spotted individuals, modified by the epistatic action of other genes. The segregation we observed was therefore between the two common alleles at the Tabby locus, TM and tb, with the former originally masked as the spotted phenotype by modifier loci. Given these findings, we added the meioses from this pedigree to the analysis of the Tabby locus (TM and tb), described above, to refine the mapping of its genomic position (Table 1).
In the case of pedigree 3 (Figure S2), the two male founders (F1 individuals) were backcrossed to multiple non-Abyssinian females, producing a total of 85 offspring, 73 of which could be phenotyped with confidence (the remaining 12 were stillborn and did not allow for reliable recognition of coat color features). These third-generation individuals exhibited an almost perfect 1:1 ratio of Ta carriers (n = 36) vs. noncarriers (mackerel or blotched) (n = 37), in accordance to Mendelian expectations for a single locus. To verify whether this segregation in pedigree 3 occurred at the same locus as the TM and tb alleles mentioned above, we initially genotyped the members of this kindred for six microsatellites linked to the Tabby locus (mapped to chromosome A1 using pedigrees 1 and 2, see Table 1), as well as three additional markers in the same region of chromosome A1. The results from this experiment excluded this chromosomal segment, refuting Tabby as the implicated locus in the Ta variant (Table S2).
This finding indicated that the Abyssinian phenotype is not coded by an allele of Tabby, but rather by a separate genetic locus, which we henceforth call Ticked. Before initiating a whole genome scan for this locus, we proceeded to genotype three microsatellites in a candidate region linked to “brindling” (the “K” locus) in dogs (G. S. Barsh, personal communication; Candille et al. 2007; Kerns et al. 2007), as this canine variant might be homologous to some aspect of the cat pelage patterning. Microsatellites were selected for a region on cat chromosome B1 with conserved synteny to the brindling locus, at 34.8 Mb on dog chromosome 19 (Candille et al. 2007; Kerns et al. 2007). Although Ticked demonstrated significant linkage to the K region [markers FCA522 (LOD = 11.4, θ = 0.09) and FCA519 (LOD = 11.6, θ = 0.05)], recombination between the markers and this trait was demonstrated within this genomic segment of cat chromosome B1 (Table 2). Although this result indicated that the dog brindling and cat ticked coat color variants were not caused by the same gene, this initial finding allowed us to restrict all further marker development and genotyping to chromosome B1. Additional markers were thus designed to identify a region of zero recombination with Ticked (see materials and methods). The genomic region for Ticked was ultimately defined as a segment of ∼3.8 Mb, with conserved synteny to Hsa 8: 23–19.7, 43.1–42.9 Mb. This region is equivalent to that reported by Lyons et al. (2006) as linked to the Tabby locus. The defined segment occurs within a region showing an intrachromosomal break in synteny between cat and human, and an interchromosomal break between cat and dog (Table 2).
TABLE 2.
Linkage mapping of the domestic cat Ticked locus
| Markera | Cat chr. | GL map positionb | RH map positionb | Peak LODc | Θ of peakc | Position in GARFIELD (start)d | Hsa36 (start)e | Cfa2 (start)e |
|---|---|---|---|---|---|---|---|---|
| FCA522 | B1 | 47.9 | 197.8 | 11.39 | 0.09 | B1: 20,954,176 | Chr 8: 12,886,556 | Chr 16: 39,395,506 |
| FCA519 | B1 | 60.2 | 247.2 | 11.60 | 0.05 | B1: 26,480,800 | Chr 8: 32,537,942 | Chr 16: 34,857,499 |
| ChrB1_36137001 | B1 | 14.36 | 0.05 | B1: 36,137,001 | Chr 8: 25,348,288 | Chr 25: 35,009,943 | ||
| ChrB1_38697912 | B1 | 7.53 | 0.03 | B1: 38,697,912 | Chr 8: 23,191,720 | Chr 25: 37,192,502 | ||
| ChrB1_38976563 | B1 | 20.32 | 0 | B1: 38,976,563 | Chr 8: 22,766,523 | Chr 25: 37,435,625 | ||
| ChrB1_39401113 | B1 | 19.41 | 0 | B1: 39,401,113 | Chr 8: 22,400,723 | Chr 25: 37,774,501 | ||
| ChrB1_39665480 | B1 | 12.34 | 0 | B1: 39,665,480 | Chr 8: 22,151,140 | Chr 25: 38,010,313 | ||
| ChrB1_39873803 | B1 | 18.52 | 0 | B1: 39,873,803 | Chr 8: 21,963,551 | Chr 25: 38,177,122 | ||
| ChrB1_40183201 | B1 | 11.53 | 0 | B1: 40,183,201 | Chr 8: 21,646,142 | Chr 25: 38,463,167 | ||
| ChrB1_41254204 | B1 | 16.10 | 0 | B1: 41,254,204 | Chr 8: 20,606,466 | Chr 25: 39,390,344 | ||
| ChrB1_41701042 | B1 | 16.40 | 0 | B1: 41,701,042 | Chr 8: 20,198,053 | Chr 25: 39,762,212 | ||
| ChrB1_41772882 | B1 | 17.89 | 0 | B1: 41,772,882 | Chr 8: 20,127,674 | Chr 25: 39,821,805 | ||
| ChrB1_41799674 | B1 | 10.02 | 0 | B1: 41,799,674 | Chr 8: 20,104,102 | Chr 25: 39,846,768 | ||
| ChrB1_41829103 | B1 | 17.00 | 0 | B1: 41,829,103 | Chr 8: 20,085,589 | Chr 25: 39,870,873 | ||
| ChrB1_42060627 | B1 | 2.86 | 0 | B1: 42,060,627 | Chr 8: 19,843,732 | Chr 25: 40,092,520 | ||
| FCA559 | B1 | 381.0 | 17.33 | 0 | unknown | Chr 8: 19,546,837 | Chr 16: 25,388,799 | |
| ChrB1_53277193 | B1 | 18.77 | 0 | B1: 53,277,193 | Chr 8: 19,709,004 | Chr 16: 25,555,047 | ||
| ChrB1_53355916 | B1 | 13.86 | 0 | B1: 53,355,916 | Chr 8: 43,120,843 | Chr 16: 25,623,485 | ||
| ChrB1_53581031 | B1 | 14.81 | 0.02 | B1: 53,581,031 | Chr 8: 42,881,429 | Chr 16: 25,839,630 | ||
| CFA16:29.97 | B1 | 14.09 | 0.03 | unknown | Chr 8: 38,458,010 | Chr 16: 29,972,613 | ||
| FCA23 | B1 | 83.6 | 463.3 | 11.90 | 0.05 | Un: 77,729,549 | Chr 8: 31,307,618 | Chr 16: 32,706,503 |
| FCA809 | B1 | 84.5 | 476.7 | 12.43 | 0.05 | B1: 68,657,050 | Chr 8: 27,646,753 | Chr 25: 32,866,052 |
| FCA811 | B1 | 84.5 | 488.6 | 1.15 | 0.10 | B1: 68,391,021 | Chr 8: 27,433,306 | Chr 25: 33,063,420 |
| FCA700 |
B1 |
108.1 |
571.9 |
7.24 |
0.10 |
B1: 74,811,696 |
Chr 8: 174,967,845 |
Chr 25: 27,356,441 |
Markers are shown in genomic order along the domestic cat chromosome B1, on the basis of the most recent genetic linkage (GL) (Menotti-Raymond et al. 2009) and radiation hybrid (RH) (Davis et al. 2009) maps. Markers with prefix “chrB1” represent loci that were developed for fine mapping after multipoint linkage analysis demonstrated that FCA23 and FCA519 flanked the Ticked interval.
Positions of markers with a blank in these columns were not determined in those versions of the RH or GL maps.
Peak LOD score and estimated recombination fraction (θ) for linkage between each polymorphic marker and the Ticked locus.
Position in the domestic cat whole genome sequence assembly as visualized in the GARFIELD browser (Pontius and O'Brien 2007). “Un” refers to a sequence block unassigned to a chromosome.
Columns 8 and 9 show the positions in the human (HSA build 36) (http://www.ncbi.nlm.nih.gov/projects/mapview/stats/BuildStats.cgi?taxid=9606&build=36&ver=3) and dog (CFA build 2) (http://www.ncbi.nlm.nih.gov/projects/mapview/stats/BuildStats.cgi?taxid=9615&build=2&ver=1) genome assemblies found by BLAT analysis to be orthologous to sequences flanking the respective domestic cat markers.
DISCUSSION
The results presented here indicate that there are at least three different loci determining the pattern of coat markings in the domestic cat: (1) the Tabby locus (for which we propose the symbol “Ta”), mapped to chromosome A1 and containing alleles TaM and tab (mackerel/blotched); (2) one or more modifier loci that create a spotted coat by altering the mackerel stripes and that possibly also influence variation in the blotched pattern; and (3) the Ticked locus (for which we propose the symbol “Ti”), mapping to chromosome B1 and containing alleles TiA (Abyssinian) and Ti+ (non-Abyssinian). The TiA allele is semidominant and has an epistatic effect on the expression of Tabby and its shape-altering modifiers (Table 3).
TABLE 3.
Summary of dominance and epistasis relationships leading to genotype–phenotype correspondence at the domestic cat pattern-forming loci Ticked and Tabby (see text for details)
| Composite genotypes at the Ticked (Ti) and Tabby (Ta) loci | Resulting phenotype |
|---|---|
| TiA/TiA; __/__ | Abyssinian or “ticked” (plain agouti coat) |
| TiA/Ti+; __/__ | Banded legs and tail, along with faint body stripes on an otherwise plain agouti coat |
| Ti+/Ti+; TaM/__ | Mackerel tabby (vertically striped pattern)a |
|
Ti+/Ti+; tab/tab |
Blotched tabby (circular markings and broad stripes) |
One or more modifier genes transform the “mackerel” striping into a “spotted” pattern (previously attributed to the TS allele in the classical single-locus model).
Since our results seem to be discrepant with those presented by Lyons et al. (2006), who reported the mapping of Tabby to the same location that we have defined as the Ticked locus, we offer the following clarification. Lyons et al. (2006) mapped Tabby under the assumption that a single locus was responsible for all coat pattern phenotypes in the domestic cat. Their utilization of a single pedigree that segregated for all but four meiotic events for the ticked phenotype identified the B1 locus that we report here to be responsible for the alleles TiA (Abyssinian) and Ti+ (non-Abyssinian), and have elected to call the Ticked locus. The results of the two studies are therefore congruent, but we show that two different genetic loci are implicated in this particular set of coat patterning variants. In this context, we point out that the name Tabby should be applied to the locus on A1, which specifies the mackerel and blotched alleles, as these phenotypes are classically identified with the Tabby locus.
In Table S3, we list all the human genes (human genome build 36.3) located in the syntenic segments corresponding to the Tabby and Ticked linkage intervals. For the case of Tabby, we noted that the interval includes the gene AP3S1, which may be a good candidate since the AP3 complex transports pigment and the gene AP3B1, encoding another protein in this complex, is mutated in the hypopigmentation-inducing Hermansky-Pudlak syndrome type 2 (Dell'Angelica et al. 1997). Another possible candidate gene for Tabby, TYRP1, is involved in dog coat color phenotypes (Schmutz et al. 2002; Cargill et al. 2005) and maps to dog chromosome 11, 20 Mb distal from the conserved syntenic canine interval identified in Table 1. However, in the domestic cat TYRP1 has been mapped to chromosome D4, and is associated with brown and cinnamon coat color phenotypes (Lyons et al. 2005; Schmidt-Küntzel et al. 2005), and not to patterning traits such as Tabby. Even though the actual genes corresponding to Tabby and Ticked have not been identified at the molecular level, the clarification that multiple loci are involved and the delimitation of two implicated regions enable the design of detailed studies targeting the identification and characterization of these loci and their functions.
We hypothesize that mammalian patterned coats are formed by two distinct processes: (i) a spatially oriented developmental mechanism that lays down a species-specific pattern of skin cell differentiation; and (ii) a pigmentation-oriented mechanism that uses information from the preestablished pattern to regulate the synthesis of particular melanin profiles. Our results, in combination with phenotype-based observations, indicate that the Tabby locus is involved in establishing the shape of the pattern (process “i” above), and so are the modifier loci that produce a spotted coat. The Ticked locus may control process “ii” defined above, i.e., the coupling between pigmentation pathways and the preexisting pattern laid down by Tabby, so that variants may exhibit differing amounts of pigmentation on the coat areas destined to be spots or stripes. As an alternative hypothesis, however, the Ticked locus may also be involved in process i, affecting the shape of markings, by leading to progressively thinner and more numerous stripes that lead them to “disappear” amid the agouti banding of individual hairs. This idea fits the observation of very thin but discernible flank stripes in heterozygous animals TiA/Ti+.
The logic behind this two-step process stems from observations such as the following: (a) coat patterns are species specific, with instances of intraspecific polymorphism that also seem to be heritable; (b) many variants affect the spatial conformation of the pattern (process i), but do not seem to affect the mechanism that “reads” this pattern to promote differential pigmentation (process ii) (e.g., the case of the domestic cat mackerel and blotched variants); (c) conversely, there are variants that affect pigmentation pathways (e.g., X-linked Orange in domestic cats; Schmidt-Küntzel et al. 2009; melanism in several species) but do not change the underlying pattern, which remains constant and often visible in a different color; (d) the instructions to produce darker pigment on stripes/spots overrides the dorsal-ventral patterning often seen on mammalian coats (e.g., the black stripes present on the whitish tiger ventrum); and (e) two felids [lion (Panthera leo) and puma (Puma concolor)] exhibit a developmentally regulated fading of body markings, which are present in juveniles and essentially disappear in adults. The latter observation suggests that it is process ii that is developmentally regulated, gradually decoupling the pigmentation pathways from the underlying pattern (which was evidently formed in the juvenile). Many such comparisons are possible within and among the extant 37 felid species, highlighting the potential of this mammalian family as a model for investigating the evolutionary genetics of coat pattern formation. Although the understanding of the molecular basis of this phenomenon is still in its infancy, we propose that comparative genetic analyses of the domestic cat and its wild relatives hold promise for unraveling these complex and potentially revealing developmental pathways for mammals in general.
Acknowledgments
We thank Audrey Law for making available the male Egyptian Mau cat used as the founder of pedigree 2, as well as Lyn Colenda and Kevin J. Cogan at the National Institutes of Health Animal Center for their efforts in maintaining and managing pedigrees 2 and 3. We thank the Nestlé Purina PetCare Center and personnel for providing us with samples of DNA, as well as assistance in phenotyping the cats from pedigree 1. We thank Marti Welch, Advanced Technology Program, SAIC, Frederick, MD, for assistance in taking photographs. We additionally thank the Frederick County Animal Control Center, Frederick, MD, which allowed us to take photographs of a mackerel cat. David Wells, Ali Wilkerson, Jan Martenson, and William Murphy assisted with various aspects of project design and execution. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), and National Library of Medicine. This project has been funded in whole or in part with federal funds from the NCI and NIH under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.109629/DC1.
References
- Boutin-Ganache, I., M. Raposo, M. Raymond and C. F. Deschepper, 2001. M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechniques 31 24–26, 28. [PubMed] [Google Scholar]
- Candille, S. I., C. B. Kaelin, B. M. Cattanach, B. Yu, D. A. Thompson et al., 2007. A B-defensin mutation causes black coat color in domestic dogs. Science 318 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill, E. J., T. R. Famula, R. D. Schnabel, G. M. Strain and K. E. Murphy, 2005. The color of a Dalmatian's spots: linkage evidence to support the TYRP1 gene. BMC Vet. Res. 1 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cott, H. B., 1940. Adaptive Coloration in Animals. Methuen, London.
- Davis, B. W., T. Raudsepp, A. J. Pearks Wilkerson, R. Agarwala, A. A. Schäffer et al., 2009. A high-resolution cat radiation hybrid and integrated FISH mapping resource for phylogenomic studies across Felidae. Genomics 93 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., C. E. Ooi and J. S. Bonifacino, 1997. β3A-adaptin, a subunit of the adaptor-like complex AP-3. J. Biol. Chem. 272 15078–15084. [DOI] [PubMed] [Google Scholar]
- Eizirik, E., N. Yuhki, W. E. Johnson, M. Menotti-Raymond, S. S. Hannah et al., 2003. Molecular genetics and evolution of melanism in the cat family. Curr. Biol. 13 1–20. [DOI] [PubMed] [Google Scholar]
- Fishelson, M., and D. Geiger, 2002. Exact genetic linkage computations for general pedigrees. Bioinformatics 18(Suppl 1): S189–S198. [DOI] [PubMed] [Google Scholar]
- Fishelson, M., and D. Geiger, 2004. Optimizing exact genetic linkage computations. J. Comput. Biol. 11 263–275. [DOI] [PubMed] [Google Scholar]
- Gompel, N., B. Prud'homme, P. J. Wittkopp, V. A. Kassner and S. B. Carroll, 2005. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433 481–487. [DOI] [PubMed] [Google Scholar]
- Ishida, Y., V. A. David, E. Eizirik, A. A. Schäffer, B. A. Neelam et al., 2006. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics 88 698–705. [DOI] [PubMed] [Google Scholar]
- Iwashita, M., M. Watanabe, M. Ishii, T. Chen, S. L. Johnson et al., 2006. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: implications for the regulation of melanosome movement. PLoS Genet. 2 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron, M., C. D. Jiggins, A. Papanicolaou and W. O. McMillan, 2006. a Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity. Heredity 97 157–167. [DOI] [PubMed] [Google Scholar]
- Joron, M., R. Papa, M. Beltrán, N. Chamberlain, J. Mavárez et al., 2006. b A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol. 4 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler, J. S., V. A. David, A. A. Schäffer, E. Eizirik, D. K. Ryugo et al., 2007. Four separate mutations in the feline Fibroblast Growth Factor 5 gene determine the long-haired phenotype in domestic cats. J. Hered. 98 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns, J. A., E. J. Cargill, L. A. Clark, S. I. Candille, T. G. Berryere et al., 2007. Linkage and segregation analysis of black and brindle coat color in domestic dogs. Genetics 176 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax, T. D., and R. Robinson, 1988. Tabby pattern alleles of the domestic cat. J. Hered. 79 21–23. [DOI] [PubMed] [Google Scholar]
- Lorimer, H. E., 1995. Variations on the theme or how to paint a cat, pp. 193–200 in The Cat Fanciers' Association, Inc. Yearbook. The Cat Fanciers' Association, Inc., Manasquan, NJ.
- Lyons, L. A., I. T. Foe, H. C. Rah and R. A. Grahn, 2005. Chocolate coated cats: TYRP1 mutations for brown color in domestic cats. Mamm. Genome 16 356–366. [DOI] [PubMed] [Google Scholar]
- Lyons, L. A., S. J. Bailey, K. C. Baysac, G. Byrns, C. A. Erdman et al., 2006. The Tabby cat locus maps to feline chromosome B1. Anim. Genet. 37 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, M. E. Roelke, Z. Q. Chen, K. A. Menotti et al., 2003. Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus). J. Hered. 94 95–106. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, A. A. Schäffer, R. Stephens, D. Wells et al., 2007. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered 98 211–220. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond, M., V. A. David, A. A. Schäffer, J. F. Tomlin, E. Eizirik et al., 2009. An autosomal genetic linkage map of the domestic cat, Felis silvestris catus. Genomics 93 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti-Raymond, M. A., V. A. David, L. L. Wachter, J. M. Butler and S. J. O'Brien, 2005. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. J. Forensic Sci. 50 1061–1070. [PubMed] [Google Scholar]
- Murphy, W. J., B. Davis, V. A. David, R. Agarwala, A. A. Schäffer et al., 2007. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics 89 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. D., and G. F. Oster, 1984. Generation of biological pattern and form. IMA J. Math. Appl. Med. Biol. 1 51–75. [DOI] [PubMed] [Google Scholar]
- Ortolani, A., and T. M. Caro, 1996. The adaptive significance of color patterns in carnivores: phylogenetic test of classic hypotheses, pp. 132–188 in Carnivore Behavior, Ecology and Evolution, edited by J. L. Gittleman. Cornell University Press, New York.
- Oyehaug, L., E. Plahte, D. I. Vage and S. W. Omholt, 2002. The regulatory basis of melanogenic switching. J. Theor. Biol. 215 449–468. [DOI] [PubMed] [Google Scholar]
- Parchem, R. J., M. W. Perry and N. H. Patel, 2007. Patterns on the insect wing. Curr. Opin. Genet. Dev. 17 300–308. [DOI] [PubMed] [Google Scholar]
- Pontius, J. U., J. C. Mullikin, D. R. Smith, A. S. Team, K. Lindblad-Toh et al., 2007. Initial sequence and comparative analysis of the cat genome. Genome Res. 17 1675–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius, J. U., and S. J. O'Brien, 2007. Genome Annotation Resource Fields–GARFIELD: A Genome Browser for Felis catus. J. Hered. 98 386–389. [DOI] [PubMed] [Google Scholar]
- Prud'homme, B., N. Gompel, A. Rokas, V. A. Kassner, T. M. Williams et al., 2006. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440 1050–1053. [DOI] [PubMed] [Google Scholar]
- Robinson, R., 1958. Mosaicism in mammals. Genetica 29 120–145. [DOI] [PubMed] [Google Scholar]
- Rozen, S., and H. Skaletsky, 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132 365–386. [DOI] [PubMed] [Google Scholar]
- Schmidt-Küntzel, A., E. Eizirik, S. J. O'Brien and M. Menotti-Raymond, 2005. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. J. Hered. 96 289–301. [DOI] [PubMed] [Google Scholar]
- Schmidt-Küntzel, A., G. Nelson, V. A. David, A. A. Schäffer, E. Eizirik et al., 2009. A domestic cat X-chromosome linkage map and the sex-linked orange locus- mapping of orange, multiple origins, and epistasis over non-agouti. Genetics 181 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz, S. M., T. G. Berryere and A. D. Goldfinch, 2002. TYRP1 and MC1R genotypes and their effects on coat color in dogs. Mamm. Genome 13 380–387. [DOI] [PubMed] [Google Scholar]
- Schug, M. D., C. M. Hutter, K. A. Wetterstrand, M. S. Gaudette, T. F. Mackay et al., 1998. The mutation rates of di-, tri- and tetranucleotide repeats in Drosophila melanogaster. Mol. Biol. Evol. 15 1751–1760. [DOI] [PubMed] [Google Scholar]
- Searle, A. G., 1968. Comparative Genetics of Coat Color in Mammals. Logos Press, London.
- Svetic, V., G. E. Hollway, S. Elworthy, T. R. Chipperfield, C. Davison et al., 2007. Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects in choker mutants. Development 134 1011–1022. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., M. Iwashita, M. Ishii, Y. Kurachi, A. Kawakami et al., 2006. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 7 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]